Abstract
The prognosis for patients with pancreatic cancer is extremely poor, as they are resistant to first line chemotherapy. The long-term goal of this study was to identify effective combination chemotherapy for pancreatic cancer using pancreatic cancer surgical specimens in the histoculture drug response assay (HDRA) based on three-dimensional culture of tumour fragments, which maintains nature tumour histology in vitro. From 2015 to 2017, the HDRA was performed with tumour specimens from 52 pancreatic cancer patients from Asan Medical Hospital. First, combination drug regimens showed higher drug efficacy and less patient variation than single drugs. Initially, 5-Fluorouracil(5-FU)/Belotecan/Oxaliplatinum and Tegafur/Gimeracil (TS-1)/Oxaliplatinum/Irinotecan were found to be effective. Second, we were able to correlate the efficacy of some drugs with tumour stage. Third, when designing new combination regimens containing 5-FU or gemcitabine, we could identify more effective drug combinations. This is the first study to demonstrate usefulness of the HDRA for pancreatic cancer. Using this technique, we could identify novel candidate combination drug regimens that should be effective in treating pancreatic cancer.
Subject terms: Biological techniques, Cancer, Drug discovery
Introduction
The prognosis of patients with pancreatic cancer is poor; the 5-year survival rate is less than 10%1,2, and it is predicted to be the second most common cause of cancer-related deaths by 20303. In particular, pancreatic cancers is characterized by rapid tumour growth, frequent recurrence, and early metastasis, which require intensive adjuvant chemotherapy4,5. During the last decades, adjuvant chemotherapy has been developed for pancreatic cancer. As a representative example, the survival rate of patients receiving mFOLFIRINOX has been reported to be more than doubled in selected cases6,7. In addition, numerous clinical trials are underway with various combination regimens based on 5-FU, gemcitabine, and nab-paclitaxel6,8. However, pancreatic cancer is recalcitrant to first-line chemotherapy. Due to the aggressive progression of the disease, rapid selection of the most efficacious chemotherapy for each patient could improve patient outcome9,10. Numerous clinical studies have been performed on pancreatic cancer using a combination of radiation therapy, conventional chemotherapy, or various immunotherapies11–13. In addition, clinical studies on targeted and personalized therapy based on tumour biomarkers such as CCR2, PARP, PDH, STAT3, and mesothelin are also ongoing14,15. Especially, attempts to overcome the extensive extracellular matrices of pancreatic cancer have been made16,17. However, unlike lymphoma, breast cancer, and lung cancer, whose survival rate has improved through the discovery of novel treatment, pancreatic cancer remains recalcitrant18–20.
The histoculture drug response assay (HDRA) is based on three dimensional culture of tumour fragments that maintain the original cancer–stromal histological architecture21,22. The HDRA can identify effective chemotherapy drugs based on inhibition of tumour viability. Unlike other in vitro assays, the HDRA can maintain the tumour original microenvironment. Therefore, the HDRA correlated with patient survival in many clinical trials and may be more useful for cancers that have an extensive stromal component in the tumour microenvironment. A common characteristic of pancreatic cancer is desmoplasia, which involves the deposition of fibroblasts and extracellular matrix components into the tumour microenvironment23. Since the desmoplastic stroma acts as a physiological barrier for anticancer drugs, it is essential to consider the desmoplasia of pancreatic cancer in order to improve chemotherapy efficacy16,24.
In the present study, we used the HDRA to identify candidate effective chemotherapy drug combinations using pancreatic cancer surgical specimens. This is the first HDRA study for pancreatic cancer.
Results
Patient characteristics
Of the 52 patients enrolled in this study, 32 were male (61.5%) and the average age was 63.7 years (Table 1). The average values of preoperative blood tests were WBC 6.2 (× 103/µl), Hb 12.77 (g/dl), albumin 3.69 (g/dl), and CA 19–9 228.1 (U/ml). Only 5 patients (9.6%) received chemotherapy before surgery. Distal pancreatectomy was the most common surgical technique (57.7%), and the average tumour size measured after surgery was 3.72 cm. Forty-nine cases (94.2%) were diagnosed as ductal adenocarcinoma, and advanced stages (T2, N1/2) were more frequent than early stages (T1, N0).
Table 1.
Host and tumor factors of enrolled patients.
| Factors | Values |
|---|---|
| Sex (female/male), N (%) | 20/32 (38.5%/61.5%) |
| Age, years, Avg ± SD (range) | 63.69 ± 7.93 (46–79) |
| BMI (kg/m2), Avg ± SD (range) | 24.3 ± 3.56 (17.63–35.13) |
| Preoperative laboratory, Avg ± SD (range) | |
| WBC (× 103/µl) | 6.2 ± 1.79 (3.8–12.2) |
| Hb (g/dl) | 12.77 ± 1.68 (8.9–16.2) |
| Albumin (g/dl) | 3.69 ± 0.36 (2.8–4.3) |
| Total bilirubin (mg/dl) | 1.01 ± 2.29 (0.1–13.6) |
| CA 19-9 (U/ml) | 228.1 ± 436.9 (0.6–2003.0) |
| Preoperative chemotherapy (yes/no), N (%) | 5/47 (9.6%/90.4%) |
| Operation (PPPD/DPS/TPS), N (%) | 20/30/2 (38.5%/57.7%/3.8%) |
| Tumor size (cm), Avg ± SD (range) | 3.72 ± 2.22 (0.3–14.8) |
| Tumor_differentiation (wel/mod/por), N (%) | 1/41/7 (2.0%/83.7%/14.3%) |
| Lymphovascular invasion (absent/present), N (%) | 15/37 (28.8%/71.2%) |
| Perineural invasion (absent/present), N (%) | 11/41 (21.2%/78.8%) |
| T stage (T1/T2/T3), N (%) | 9/29/14 (17.3%/55.8%/26.9%) |
| N stage (N0/N1/N2), N (%) | 24/17/11 (46.2%/32.7%/21.2%) |
| Pathologic diagnosis, N (%) | |
| Ductal adenocarcinoma | 49 (94.23%) |
| Adenosquamous carcinoma | 1 (1.92%) |
| Neuroendocrine carcinoma | 1 (1.92%) |
| Hepatoid adenocarcinoma | 1 (1.92%) |
BMI body mass index, WBC white blood cell, Hb hemoglobin, PPPD pylorus preserving pancreaticoduodenectomy, DPS distal pancreatectomy with splenectomy, TPS total pancreatectomy with splenectomy.
Inhibitory rate of drugs in the HDRA
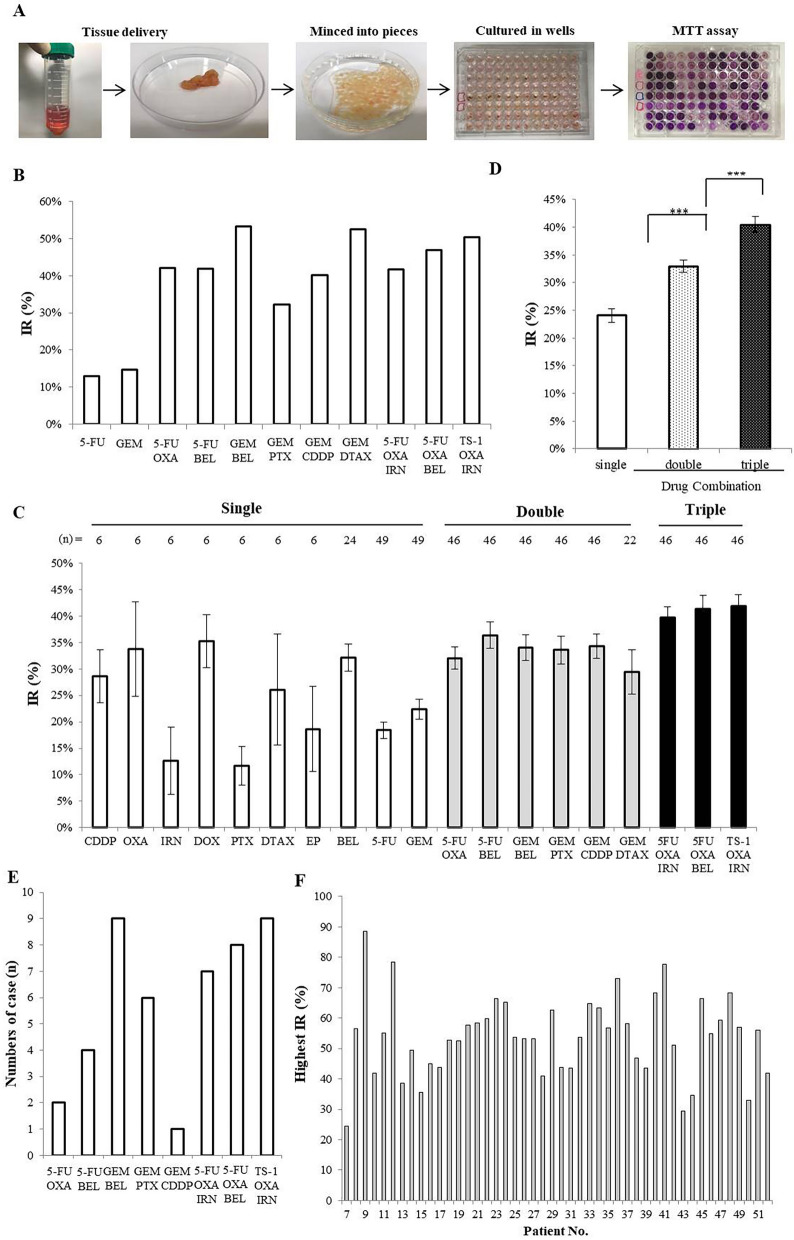
The inhibitory rate (IR) of various drug regimens in the HDRA was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. As shown in Fig. 1A, the tissue obtained through surgery was transferred to the laboratory, divided into small tumor pieces, and the pieces were placed in each well of 96 well plates. Phosphate buffered saline (PBS) was used in the control well, and drugs were added to the other wells. The plates were incubated for 72 h with the drugs and the inhibitory rate of each drug was determined with the MTT assay (Fig S1, Table S1). In the first six patients, we analyzed the IRs of nine single drugs (Table 2). Among the single drugs, OXA, DOX, and BEL showed superior inhibitory rates compared to the other drugs tested. With this initial experiment, we determined that it is possible to distinguish the efficacy drugs for pancreatic cancer in the HDRA. Subsequently, 46 patients were tested in the HDRA, using single clinically-approved drugs and combination regimens. As a representative example, the IR values for patient No. 27 are shown in Fig. 1B. The IRs for each drug or combinations demonstrated that the efficacy of the drug regimen improved in the order of a single drug (24.0%); a two-drug combination (33.0%); a three-drug combination (40.5%). The differences between IR values were relatively small between various triple-drug combination regimens, even though they comprised different drugs (Fig. 1C, D). The efficacy of these regimens did not differ significantly whether pre-operative chemotherapy was previously administered to the patient or not (Fig. S2). GEM/BEL in the two drug combination and TS-1/OXA/IRN in the three-drug combination were the most effective regimens (Fig. 1E). The highest IR values for each patient among 46 patients using a combination regimen are shown in Fig. 1F. The minimum and maximum values of the highest IR in the HDRA were 24.5% and 88.6%, respectively, for the present cohort of patients.
Figure 1.
Comparison of inhibitory rates (IRs) of single and combination drug regimens. (A) The patient's tissue was excised, divided into fragments, and placed in culture medium, treated with drugs and tested with the MTT assay. Cell viability with various drugs was determined in the HDRA. (B) Example HDRA results from patient 27. (C) Comparison of IR average values for each drug regimen. (D) Comparison of IRs between single drug and combination regimens (Kruskal–Wallis test, *** p < 0.001). (E) The number of drug combinations with the highest IR among the cases tested in the HDRA. Compared to other regimens, GEM/DTAX was excluded due to the small number of assays. (F) Summary of the highest IR values for each patient. Results from patients 7 to 52 with combination regimens.
Table 2.
Inhibitory rate in the histoculture drug response assay (HDRA).
| No | Inhibitory rate (%) of drugs | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single regimens | Combination regimens | Avg | ||||||||||||||||||
| CD DP |
OXA | IRN | DOX | PTX | DT AX |
EP | BEL | 5FU | GEM | FOx | FOxIri | FBe | FBeOx | GBe | GT | GC | GD | TsOxIri | ||
| 5FU OXA |
5FU OXA IRN |
5FU BEL |
5FU BEL OXA |
GEM BEL |
GEM PTX |
GEM CDDP |
GEM DTAX |
TS-1 OXA IRN |
||||||||||||
| 1 | 27.8 | 46.9 | 10.8 | 37.2 | 22.9 | 22.9 | 7.4 | 16.0 | 29.1 | 24.6 | ||||||||||
| 2 | 10.2 | 65.5 | 10.4 | 41.8 | 4.3 | 74.6 | 4.5 | 4.5 | 52.1 | 29.8 | ||||||||||
| 3 | 21.5 | 41.3 | 2.8 | 44.4 | 8.9 | 3.0 | 7.0 | 10.7 | 5.3 | 16.1 | ||||||||||
| 4 | 45.3 | 19.9 | 43.5 | 22.4 | 6.1 | 4.9 | 42.1 | 28.4 | 12.6 | 25.0 | ||||||||||
| 5 | 28.2 | 3.3 | 2.4 | 18.2 | 5.0 | 25.3 | 4.8 | 3.1 | 4.9 | 10.6 | ||||||||||
| 6 | 38.7 | 26.2 | 5.7 | 47.7 | 23.2 | 25.9 | 46.1 | 18.3 | 27.5 | 28.8 | ||||||||||
| 7 | 16.4 | 9.6 | 3.4 | 10.5 | 24.5 | 18.4 | 5.9 | 5.5 | 8.9 | 11.5 | ||||||||||
| 8 | 56.5 | 53.6 | 20.0 | 46.3 | 12.3 | 46.5 | 17.8 | 19.8 | 49.2 | 35.8 | ||||||||||
| 9 | 21.2 | 18.3 | 55.1 | 51.0 | 48.7 | 88.6 | 22.3 | 45.7 | 50.7 | 45.0 | 45.2 | 44.7 | ||||||||
| 10 | 24.9 | 35.2 | 16.8 | 24.3 | 28.6 | 33.0 | 27.3 | 41.8 | 22.0 | 28.2 | ||||||||||
| 11 | 8.7 | 22.9 | 26.3 | 37.0 | 40.0 | 43.3 | 26.4 | 55.0 | 22.5 | 17.2 | 19.5 | 29.0 | ||||||||
| 12 | 21.0 | 55.8 | 55.5 | 55.5 | 30.7 | 78.4 | 67.4 | 67.4 | 65.5 | 72.2 | 60.5 | 57.3 | ||||||||
| 13 | 12.9 | 16.4 | 20.3 | 20.0 | 22.1 | 38.7 | 8.8 | 21.3 | 24.1 | 16.4 | 15.5 | 19.7 | ||||||||
| 14 | 26.1 | 37.1 | 27.9 | 27.5 | 49.5 | 46.9 | 31.1 | 40.5 | 42.9 | 41.0 | 40.1 | 37.3 | ||||||||
| 15 | 9.7 | 6.5 | 12.4 | 15.8 | 7.3 | 26.9 | 28.7 | 35.6 | 31.2 | 7.4 | 6.9 | 17.1 | ||||||||
| 16 | 14.3 | 21.3 | 17.4 | 36.8 | 16.5 | 31.9 | 16.8 | 22.7 | 43.4 | 24.9 | 44.9 | 26.4 | ||||||||
| 17 | 4.1 | 4.6 | 37.1 | 41.3 | 4.2 | 15.9 | 17.0 | 4.9 | 29.7 | 29.8 | 43.9 | 21.1 | ||||||||
| 18 | 18.4 | 12.5 | 20.9 | 20.3 | 28.3 | 52.7 | 33.3 | 11.7 | 41.6 | 4.8 | 48.5 | 26.6 | ||||||||
| 19 | 40.4 | 35.6 | 10.8 | 45.0 | 42.3 | 52.6 | 18.0 | 24.9 | 30.0 | 8.6 | 52.4 | 32.8 | ||||||||
| 20 | 15.1 | 18.3 | 40.9 | 47.7 | 25.9 | 43.4 | 18.6 | 36.7 | 46.4 | 20.8 | 57.7 | 33.8 | ||||||||
| 21 | 5.8 | 35.1 | 14.0 | 47.3 | 52.4 | 56.0 | 46.5 | 26.3 | 38.4 | 22.5 | 58.4 | 36.6 | ||||||||
| 22 | 40.6 | 17.8 | 49.3 | 51.7 | 59.5 | 44.9 | 35.6 | 24.3 | 21.7 | 13.0 | 59.8 | 38.0 | ||||||||
| 23 | 4.6 | 40.8 | 37.4 | 38.4 | 64.1 | 9.2 | 65.3 | 60.8 | 64.0 | 61.0 | 66.4 | 46.6 | ||||||||
| 24 | 25.3 | 32.2 | 35.1 | 53.4 | 55.6 | 59.0 | 65.3 | 28.6 | 59.5 | 50.8 | 60.1 | 47.7 | ||||||||
| 25 | 7.8 | 9.1 | 19.7 | 53.6 | 47.9 | 14.7 | 26.9 | 11.7 | 17.8 | 17.6 | 49.1 | 25.1 | ||||||||
| 26 | 11.4 | 19.7 | 10.6 | 6.4 | 3.5 | 11.4 | 42.9 | 20.6 | 30.1 | 53.3 | 26.2 | 21.5 | ||||||||
| 27 | 12.9 | 14.7 | 42.2 | 41.7 | 41.9 | 47.0 | 53.3 | 32.3 | 40.1 | 52.5 | 50.4 | 39.0 | ||||||||
| 28 | 10.9 | 8.9 | 31.1 | 41.0 | 23.5 | 19.8 | 8.3 | 16.0 | 12.7 | 21.2 | 31.8 | 20.5 | ||||||||
| 29 | 28.3 | 7.2 | 20.0 | 30.2 | 32.3 | 44.6 | 49.3 | 23.7 | 62.6 | 35.4 | – | 43.5 | 34.3 | |||||||
| 30 | 15.2 | 8.2 | 9.7 | 30.4 | 37.5 | 21.2 | 34.7 | 8.5 | 29.7 | 27.9 | – | 43.7 | 24.2 | |||||||
| 31 | 13.4 | 11.6 | 11.8 | 20.0 | 43.1 | 24.6 | 24.7 | 15.9 | 15.3 | 11.1 | – | 43.6 | 21.4 | |||||||
| 32 | 46.0 | 7.7 | 9.7 | 35.2 | 50.4 | 45.8 | 39.6 | 37.8 | 53.6 | 25.4 | – | 20.1 | 33.8 | |||||||
| 33 | 45.6 | 15.1 | 12.3 | 16.8 | 24.0 | 44.7 | 44.9 | 42.4 | 58.7 | 64.7 | – | 41.2 | 37.3 | |||||||
| 34 | 18.2 | 18.7 | 43.0 | 31.7 | 46.9 | 46.4 | 48.7 | 63.3 | 59.5 | 40.7 | – | 43.3 | 41.9 | |||||||
| 35 | 38.3 | 22.7 | 14.9 | 41.1 | 56.8 | 24.6 | 27.5 | 33.3 | 55.6 | 25.1 | – | 51.0 | 35.6 | |||||||
| 36 | 20.5 | 25.6 | 13.4 | 34.9 | 39.3 | 46.9 | 54.7 | 50.4 | 73.0 | 58.1 | – | 39.9 | 41.5 | |||||||
| 37 | 15.0 | 9.9 | 20.6 | 11.4 | 33.7 | 24.8 | 32.6 | 28.7 | 58.1 | 32.3 | – | 35.1 | 27.5 | |||||||
| 38 | 46.1 | 21.4 | 33.1 | 26.5 | 27.6 | 36.3 | 22.9 | 46.9 | 31.3 | 40.4 | – | 28.3 | 32.8 | |||||||
| 39 | 37.0 | 18.3 | 11.2 | 23.1 | 41.0 | 31.2 | 40.5 | 43.6 | 21.5 | 10.8 | – | 38.4 | 28.8 | |||||||
| 40 | 34.9 | 35.5 | 52.9 | 60.5 | 68.2 | 50.3 | 65.5 | 34.3 | 43.5 | 41.3 | 62.3 | 49.9 | ||||||||
| 41 | 40.5 | 40.4 | 38.1 | 48.8 | 52.0 | 77.7 | 52.1 | 55.5 | 40.5 | 53.0 | 60.8 | 50.9 | ||||||||
| 42 | 38.8 | 19.8 | 30.6 | 46.5 | 42.7 | 51.0 | 43.3 | 48.0 | 12.5 | 36.0 | 43.3 | 37.5 | ||||||||
| 43 | 10.6 | 16.0 | 7.9 | 20.9 | 29.4 | 25.5 | 28.3 | 13.4 | 11.9 | 13.2 | 27.0 | 18.6 | ||||||||
| 44 | 28.7 | 13.3 | 28.3 | 28.8 | 34.6 | 25.8 | 28.4 | 33.9 | 11.0 | 32.3 | 31.9 | 27.0 | ||||||||
| 45 | 41.2 | 40.9 | 16.7 | 46.9 | 66.2 | 61.6 | 66.5 | 35.3 | 46.8 | 33.9 | 53.2 | 46.3 | ||||||||
| 46 | 53.2 | 27.8 | 24.3 | 37.2 | 46.6 | 29.3 | 54.8 | 39.1 | 30.8 | 40.9 | 41.0 | 38.6 | ||||||||
| 47 | 36.4 | 11.7 | 15.1 | 59.3 | 39.6 | 50.4 | 55.9 | 58.1 | 22.0 | 51.1 | 55.3 | 41.4 | ||||||||
| 48 | 18.6 | 43.7 | 22.4 | 52.9 | 60.2 | 46.2 | 60.2 | 5.9 | 45.4 | 45.7 | 68.4 | 42.7 | ||||||||
| 49 | 44.4 | 30.1 | 31.7 | 39.5 | 34.3 | 41.1 | 42.7 | 57.1 | 32.4 | 9.8 | 42.9 | 36.9 | ||||||||
| 50 | 22.0 | 10.0 | 16.0 | 9.0 | 21.0 | 28.0 | 33.0 | 19.0 | 10.0 | 13.0 | 17.0 | 18.0 | ||||||||
| 51 | 44.0 | 41.0 | 16.0 | 41.0 | 42.0 | 56.0 | 46.0 | 33.0 | 17.0 | 45.0 | 41.0 | 38.4 | ||||||||
| 52 | 35.0 | 13.0 | 38.0 | 21.0 | 28.0 | 35.0 | 42.0 | 42.0 | 19.0 | 31.0 | 36.0 | 30.9 | ||||||||
| Avg | 28.6 | 33.8 | 12.6 | 35.3 | 11.7 | 26.1 | 18.6 | 32.2 | 18.4 | 22.4 | 32.1 | 39.7 | 36.4 | 41.3 | 34.1 | 33.6 | 34.4 | 29.4 | 41.9 | 32.1 |
The highest inhibitory rate was indicated in bold for each patient.
CDDP cisplatin, OXA oxaliplatin, IRN irinotecan, DOX Doxorubicin, PTX paclitaxel, DTAX docetaxel, EP epirubicin, BEL belotecan, 5-FU 5-fluorouracil, GEM gemcitabine, TS-1 tegafur, gimeracil.
Comparison of IR with tumour stage or CA 19-9 vales
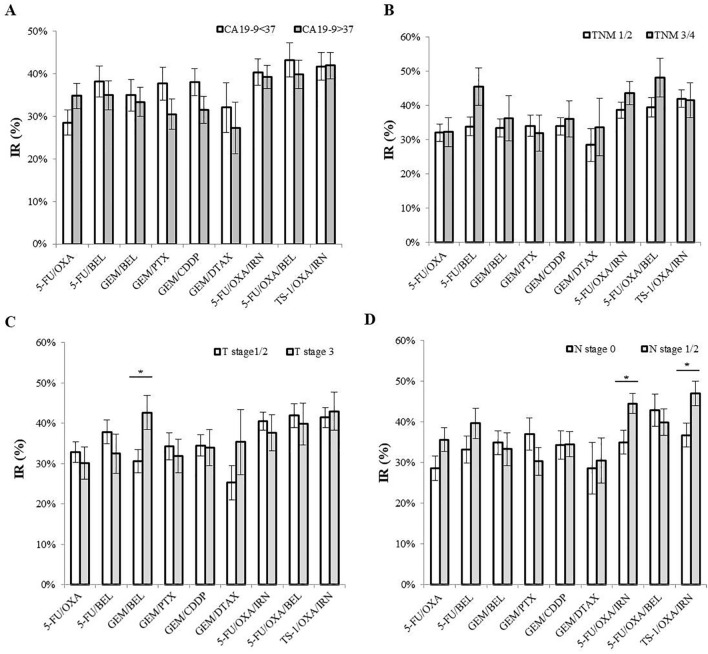
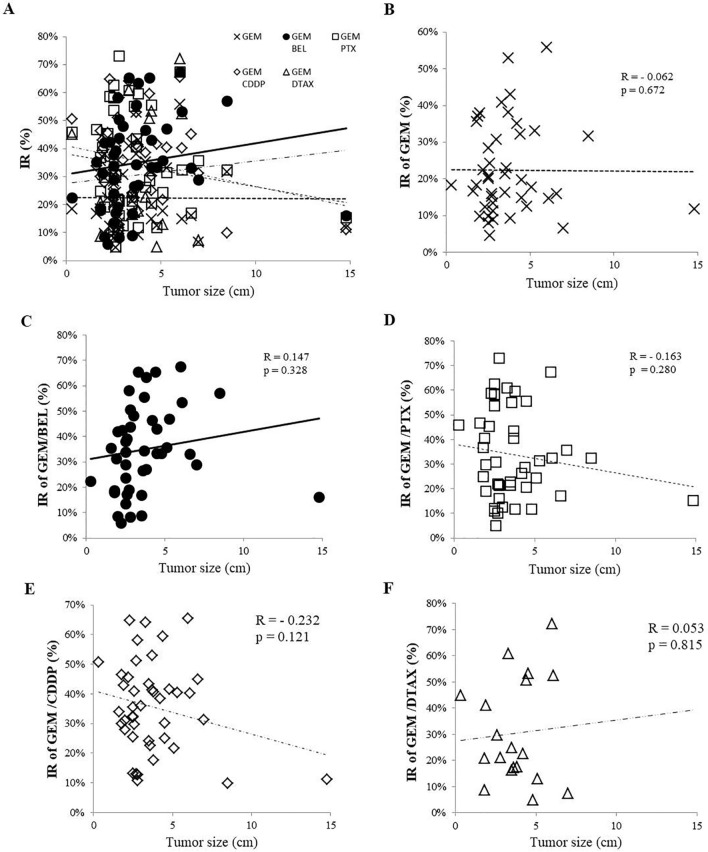
We have observed that when CA19-9 values are 37 or higher, the IR of the drugs tends to be relatively low, and when the TNM stage is increased, the IR tends to increase, but there is no statistically-significant difference for these comparisons (Fig. 2A, B). However, when the T-stage and the N-stage were analyzed separately, there were significant differences. GEM/BEL was more effective at T-stage 3 and 5-FU/OXA/IRN and TS-1/OXA/IRN were more effective at N-stage 1/2. GEM/BEL and GEM/DTAX showed positive correlation with increasing tumour size, and GEM/PTX and GEM/CDDP showed negative correlation. GEM alone did not correlate with tumour stage (Fig. 3).
Figure 2.
IR according to (A) concentration of CA19-9, (B) TNM stage, (C) T stage, and (D) N stage (n = 46; Kruskal–Wallis test, * p < 0.05).
Figure 3.
IRs of GEM, alone, and GEM-based combination regimens according to tumour size. (A) IRs of 5 regimens. (B) IR of GEM. (C) IR of GEM/BEL. (D) IR of GEM/PTX. (E) IR of GEM/CDDP. (F) IR of GEM/DTAX [n = 43 (exception, GEM/DTAX n = 19); Pearson correlation analysis].
IR of drug regimens based on 5-FU and GEM
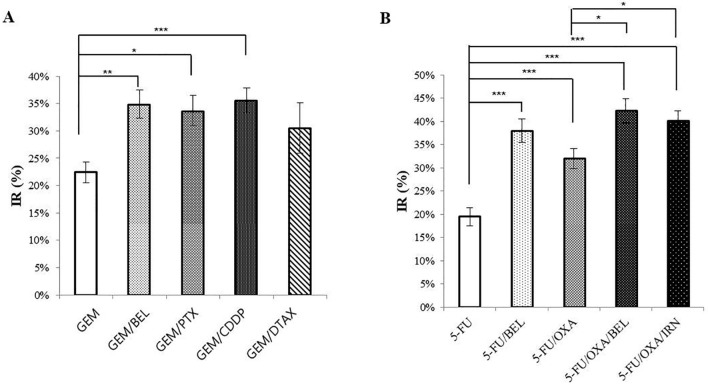
We compared combination regimens containing 5-FU or GEM, which are first-line chemotherapy in pancreatic cancer patients (Fig. 4). When combined with GEM, the efficacy of PTX, CDDP, and BEL was significantly increased. The IR of DTAX, when combined with GEM, was also increased, but the increase was not statistically significant. Next, 5-FU combined with BEL (p < 0.001) was more effective than combined with OXA (p < 0.005), and combining 5-FU/OXA and BEL (p < 0.05) was more effective than combining with IRN. In addition, the combination of 5-FU and BEL showed better efficacy than the combination of GEM with BEL (Fig. S3).
Figure 4.
IRs of various combination drug regimens based on GEM and 5-FU. (A) Comparison of IR among GEM-based combination regimens [n = 43 (exception, GEM/DTAX n = 19); Kruskal–Wallis test,* p < 0.05, ** p < 0.005, *** p < 0.001]. (B) Comparison of IRs among 5-FU-based combination regimens (n = 43; Kruskal–Wallis test,* p < 0.05, ** p < 0.005, *** p < 0.001).
Discussion
The HDRA was developed in 1987 using sponge gel-supported three-dimensional histoculture of tumour fragments from cancer patients21, which has many advantages including culture of intact tumour fragments which maintain native cancer-cell stroma-cell interaction allowing an in vivo-like environment much superior to 2D culture25. These 3D tumour fragments are also more realistic than “organoids” which are grown from “stem cells” which are not true tumour fragments and require long time periods to establish and do not contain stromal cells26,27. Patient-derived orthotopic xenografts that take 2–4 months to obtain drug response data, are therefore useful to select second-line and higher therapy28. The HDRA yield results in 3-days with an over 90% success rate and has been validated by numerous clinical trials22,29–40. For example, drug response in the HDRA was correlated to recurrence-free survival for stomach and colon cancer22,31,33. The responses of breast cancer to several drugs in the HDRA were correlated to recurrence-free survival34,35. Lung cancer response to nine drugs in the HDRA was correlated to the presence or absence of smoking history36,37. The HDRA has been used as a platform for the validation of drugs developed for cholangio-carcinoma38. Also, drug response in the HDRA of head and neck cancer was correlated to patient survival29,39.
Drug combinations that are efficacious in other tumour types were investigated in the present study for their efficacy on pancreatic cancer in the HDRA41,42. Given the enormous cost and R&D time required to develop an effective drug, it is necessary to interrogate existing drugs and their combinations as therapy for refractory tumours such as pancreatic cancer.
We used HDRA to rapidly determine the response of existing drugs and their combinations, and to show novel combination therapy, that included first-line pancreatic cancer drugs was more effective than monotherapy for pancreatic cancer. In the early phase in our experiments, we used nine single drugs that have been used in many cancers for a long time. Through this, it was confirmed that the HDRA assay can be useful for in pancreatic cancer. We tested several combination drug regimens based on 5-FU, GEM, and TS-1. The concentration of each drug was based on published HDRA papers with various tumour types22,29–39. The concentrations were adjusted in consideration of the characteristics of pancreatic cancer. We then verified the efficacy of the combination regimens (5-FU/OXA, 5-FU/OXA/IRN, GEM/PTX) currently used in patients with pancreatic cancer and then identified other new effective combinations. BEL- or TS-1-based combination regimens that are actively used on other cancers than pancreatic cancer. The HDRA experiments in the present study showed these and other novel combination regimen were effective in pancreatic cancer.
The HDRAs may be particularly useful for pancreatic cancer, which has a large amount of extracellular matrix (ECM) components that have a significant effect on the resistance to anticancer drugs. The HDRA is advantageous because the response to an anticancer drug can be obtained intuitively and quickly since the cancer and the various stromal cells, and extracellular matrix components in the tumour are not disrupted.
The present study identified three interesting points; First, combination regimens showed higher drug efficacy and less variation than a single drug. Drug combinations are likely more effective due to the complexity and heterogeneity of the tumour microenvironment43,44. Of the initial drug combinations we tested, 5-FU/BEL/OXA and TS-1/OXA/IRN regimens were the most effective. However, these have rarely been used to treat pancreatic cancer, but since they are specific for different targets, they will likely be effective in new drug combinations for this disease (Table S2). Based on additional research demonstrating why these combinations are more effective, new clinical trials may be justified.
Second, we correlated the efficacy of some drug combinations to the degree of tumour progression. As a tumour progresses, its microenvironment changes, and quantitative and qualitative changes in the drug target occur45,46. Therefore, the same drug can have varying efficacy, depending on the patients. Our results showed that GEM/BEL, 5-FU/OXA/IRN, and TS-1/OXA/IRN were more effective against advanced tumours. This may be due to changes in the tumour microenvironment or target genes or ability of the drug to reach into the tumour. The goal is to enable the selection of more effective drugs, especially combinations in the HDRA.
Third, better combination regimens containing 5-FU or GEM were designed. Even though there are many drugs available to patients, there is a lack of evidence on which combination of drugs will ultimately be more effective for each patient. Usually, drug combinations are designed based on the results of large clinical trials, but this strategy is very costly and time-consuming and does not consider the individuality of the patient. In our results, GEM/CDDP and 5-FU/BEL were found to be very effective combinations. Effective drug combinations in the HDRA can be rapidly identified, shorten the time to the next clinical trial and increase the success rate of the clinical trial.
Although the HDRA requires a relatively large amount of tumour tissue to evaluate drug efficacy, it can be overcome by obtaining sufficient tumour specimens after surgery. Especially, the HDRA can be a useful platform to rapidly identify drug responsiveness for each patient. Future clinical trials will determine the clinical efficacy of the novel drug combinations identified in the HDRA of pancreatic cancer in the present study.
We investigated the utility of the HDRA as a platform for identifying candidate effective drug regimens, especially combination therapy in pancreatic cancer. HDRA allowed us to identify the most effective candidate drug regimen for each patient and provided information that guided the selection of effective drug combination regimens. Further research is needed to design drug combinations that are optimal for each pancreatic cancer patient.
Materials and methods
All experiments and methods were performed in accordance with relevant guidelines and regulations.
Study design
Fifty-two patients who underwent surgery for pancreatic cancer at the Asan Medical Center between 2015 and 2017 were included in the study. Surgical specimens were obtained from the patents after informed consent with approval by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2015-0480). Surgical specimens were transported in Hanks' balanced salt solution (HBSS; Gibco) to the MetaBio Laboratory, MetaBio Co., Inc., where the assay was conducted.
Drug concentration for HDRA
Information about the drugs used in the study are summarized based on the KEGG (Kyoto Encyclopedia of Genes and Genomes) Drug Database (https://www.genome.jp/kegg/drug/, Table S2). Each anticancer drug used was purchased from a pharmaceutical company. Each drug was dissolved in 0.5% dimethyl sulfoxide (DMSO) or phosphate-buffered saline (PBS). The concentrations for each drug used in the study were as follows: 5-fluorouracil: 50 ug/ml; gemcitabine: 50 μg/ml; cisplatinum: 5 μg/ml; oxaliplatinum: 20 μg/ml; irinotecan: 40 μg/ml; doxorubicin: 6 μg/ml; paclitaxel: 5 μg/ml; docetaxel: 75 μg/ml; epirubicin: 10 μg/ml; belotecan: 20 μg/ml; and TS-1(tegafur/gimeracil): 500 μg/ml. Drug concentrations were calculated from pharmacodynamic parameters and clinical doses in the published papers and also modified from growth-inhibition studies using pancreatic cancer cell lines22,33,47–49.
HDRA with the MTT end point
The method of Hoffman and colleagues was utilized employing the MTT end point, which has been validated in clinical trials22,29,37. Collagen sponge gels manufactured from pig skin were purchased from Health Design Inc. (Rochester, NY)21,22. The cancerous portion of the specimens was scissors-minced into 12 pieces approximately 1 mm in diameter, which were then randomly placed on each piece of the prepared collagen sponge gel in 12 wells of a 96-well plate. The plates were incubated for 3 days at 37℃ with the 11 drugs and PBS as control) dissolved in RPMI 1,640 medium containing 20% fetal calf serum in a humidified atmosphere containing 95% air and 5% CO2. After histoculture, 900 μL of Hank's Balanced Salt Solution containing 0.1 mg/μL collagenase (type I; Sigma, US) and 100 μL MTT (Sigma, US) solution were dissolved in 4 mg/μL PBS, and added to each culture well and incubated for another 2 h. After extraction with DMSO, the absorbance of the solution in each well was read at 540 nm. The absorbance/g of the histocultured tumour tissue was calculated from the mean absorbance generated by each culture well, and the tumour-tissue weight was determined prior to culture. The inhibition rate was calculated using the following formula:
Clinical data collection of patients
Medical records for each patient were retrospectively reviewed for surgery-, oncology-, and survival-related data. Age, sex, and BMI (kg/m2) were confirmed. Preoperative blood tests included WBC, Hb, albumin, total bilirubin, and CA 19-9. The surgical procedure was determined according to tumour location and extension. Either pylorus-preserving or classic pancreaticoduodenectomy was performed to resect tumours of the head or uncinate of the pancreas. Distal pancreatectomy with splenectomy was performed on lesions in the pancreatic body or tail. Total pancreatectomy was performed when the tumour extended to the head and tail. Oncology-related factors included tumour size, tumour differentiation, lymphovascular invasion, perineural invasion, stage (T, N, or M), and pathologic diagnosis.
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 (IBM, Armonk, NY). Data expressed as the mean ± standard error for continuous variables and as frequency for categorical variables. Student’s t-tests, chi-square tests or Kruskal–Wallis test were used to analyze differences between the values of continuous and categorical variables, respectively. Pearson correlation analysis was performed to evaluate the correlation between the responses of drug regimens. Pearson correlation coefficients were expressed as R. A p-value of < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (no. HI14C2640).
Abbreviations
- 5-FU
5-Fluorouracil
- GEM
Gemcitabine
- CDDP
Cisplatinum
- OXA
Oxaliplatinum
- IRN
Irinotecan
- DOX
Doxorubicine
- PTX
Paclitaxel
- DTAX
Docetaxel
- EP
Epirubicin
- BEL
Belotecan
- TS-1
Tegafur, gimeracil
Author contributions
Study conceptualization; S.C.K., R.M.H., E.J., Patient enroll and tissue evaluation; M.B.K., J.S.L., Y.P, W.L., J.K., K.B.S., Formal analysis; S.L., D.W.H., J.H.L., Figure/Table preparation; S.L., E.J., W.L., Writing for original draft; E.J., S.C.K., R.M.H.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Robert M. Hoffman, Email: all@anticancer.com
Song Cheol Kim, Email: drksc@amc.seoul.kr.
Supplementary information
is available for this paper at 10.1038/s41598-020-68703-x.
References
- 1.Oldfield LE, Connor AA, Gallinger S. Molecular events in the natural history of pancreatic cancer. Trends Cancer. 2017;3:336–346. doi: 10.1016/j.trecan.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Gillen S, Schuster T, MeyerZumBuschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahib L, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N. Engl. J. Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klaiber U, Hackert T, Neoptolemos JP. Adjuvant treatment for pancreatic cancer. Transl. Gastroenterol. Hepatol. 2019;4:27. doi: 10.21037/tgh.2019.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy T, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br. J. Cancer. 2009;100:246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groot VP, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 2018;267:936–945. doi: 10.1097/SLA.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 10.Groot VP, et al. Current strategies for detection and treatment of recurrence of pancreatic ductal adenocarcinoma after resection: A nationwide survey. Pancreas. 2017;46:e73–e75. doi: 10.1097/MPA.0000000000000899. [DOI] [PubMed] [Google Scholar]
- 11.Sohal DP, Walsh RM, Ramanathan RK, Khorana AA. Pancreatic adenocarcinoma: Treating a systemic disease with systemic therapy. J Natl Cancer Inst. 2014;106:dju011. doi: 10.1093/jnci/dju011. [DOI] [PubMed] [Google Scholar]
- 12.Moyer MT, Gaffney RR. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014;371:2140. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 13.Neoptolemos JP, et al. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018;15:333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 14.Chantrill LA, et al. Precision medicine for advanced pancreas cancer: The individualized molecular pancreatic cancer therapy (IMPaCT) trial. Clin. Cancer Res. 2015;21:2029–2037. doi: 10.1158/1078-0432.CCR-15-0426. [DOI] [PubMed] [Google Scholar]
- 15.Tesfaye AA, Kamgar M, Azmi A, Philip PA. The evolution into personalized therapies in pancreatic ductal adenocarcinoma: Challenges and opportunities. Expert Rev. Anticancer Ther. 2018;18:131–148. doi: 10.1080/14737140.2018.1417844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gkretsi V, Stylianou A, Papageorgis P, Polydorou C, Stylianopoulos T. Remodeling components of the tumor microenvironment to enhance cancer therapy. Front. Oncol. 2015;5:214. doi: 10.3389/fonc.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yosifov DY, Wolf C, Stilgenbauer S, Mertens D. From biology to therapy: The CLL success story. Hemasphere. 2019;3:e175. doi: 10.1097/HS9.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 20.Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: Selecting optimal treatments for individual patients. J. Clin. Oncol. 2015;33:1787–1796. doi: 10.1200/JCO.2014.60.0213. [DOI] [PubMed] [Google Scholar]
- 21.Vescio RA, et al. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc. Natl. Acad. Sci. USA. 1987;84:5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin. Cancer Res. 1995;1:305–311. [PubMed] [Google Scholar]
- 23.Whatcott CJ, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 25.25Hoffman, R. M. 3D Sponge-Matrix Histoculture: Methods and Protocols.
- 26.Choi SI, et al. Development of patient-derived preclinical platform for metastatic pancreatic cancer: PDOX and a subsequent organoid model system using percutaneous biopsy samples. Front. Oncol. 2019;9:875. doi: 10.3389/fonc.2019.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driehuis E, et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. USA. 2019 doi: 10.1073/pnas.1911273116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman RM. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 29.Singh B, et al. Prediction of survival in patients with head and neck cancer using the histoculture drug response assay. Head Neck. 2002;24:437–442. doi: 10.1002/hed.10066. [DOI] [PubMed] [Google Scholar]
- 30.Jung PS, et al. Progression-free survival is accurately predicted in patients treated with chemotherapy for epithelial ovarian cancer by the histoculture drug response assay in a prospective correlative clinical trial at a single institution. Anticancer Res. 2013;33:1029–1034. [PubMed] [Google Scholar]
- 31.Kubota T, et al. Potential of the histoculture drug-response assay to contribute to cancer patient survival. Clin. Cancer Res. 1995;1:1537–1543. [PubMed] [Google Scholar]
- 32.Robbins KT, Connors KM, Storniolo AM, Hanchett C, Hoffman RM. Sponge-gel-supported histoculture drug-response assay for head and neck cancer. Correlations with clinical response to cisplatin. Arch. Otolaryngol. Head Neck Surg. 1994;120:288–292. doi: 10.1001/archotol.1994.01880270036007. [DOI] [PubMed] [Google Scholar]
- 33.Yoon YS, et al. Applicability of histoculture drug response assays in colorectal cancer chemotherapy. Anticancer Res. 2012;32:3581–3586. [PubMed] [Google Scholar]
- 34.Kim KY, Chung BW, Yang I, Kim MB, Hoffman RM. Independence of cytotoxic drug sensitivity profiles and receptor subtype of invasive ductal breast carcinoma demonstrated by the histoculture drug response assay (HDRA) Anticancer Res. 2014;34:7197–7201. [PubMed] [Google Scholar]
- 35.Shinden Y, et al. Clinical significance of the histoculture drug response assay in breast cancer. Anticancer Res. 2016;36:6173–6178. doi: 10.21873/anticanres.11210. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimasu T, et al. Data acquisition for the histoculture drug response assay in lung cancer. J. Thorac. Cardiovasc. Surg. 2007;133:303–308. doi: 10.1016/j.jtcvs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimasu T, et al. Histoculture drug response assay for gefitinib in non-small-cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2009;57:138–143. doi: 10.1007/s11748-008-0332-x. [DOI] [PubMed] [Google Scholar]
- 38.Seubwai W, Wongkham C, Puapairoj A, Okada S, Wongkham S. 22-oxa-1,25-dihydroxyvitamin D3 efficiently inhibits tumor growth in inoculated mice and primary histoculture of cholangiocarcinoma. Cancer. 2010;116:5535–5543. doi: 10.1002/cncr.25478. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa Y, et al. Evaluation of optimal drug concentration in histoculture drug response assay in association with clinical efficacy for head and neck cancer. Oral Oncol. 2007;43:749–756. doi: 10.1016/j.oraloncology.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Lee SW, et al. In vitro chemosensitivity using the histoculture drug response assay in human epithelial ovarian cancer. Acta Med. Okayama. 2012;66:271–277. doi: 10.18926/AMO/48567. [DOI] [PubMed] [Google Scholar]
- 41.Veschi S, et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018;37:236. doi: 10.1186/s13046-018-0904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pecorelli N, et al. Postoperative outcomes and functional recovery after preoperative combination chemotherapy for pancreatic cancer: A propensity score-matched study. Front. Oncol. 2019;9:1299. doi: 10.3389/fonc.2019.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samadi AK, et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin. Cancer Biol. 2015;35(Suppl):S151–S184. doi: 10.1016/j.semcancer.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Block KI, et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2015;35(Suppl):S276–S304. doi: 10.1016/j.semcancer.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiao SL, Chu GC, Chung LW. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016;380:340–348. doi: 10.1016/j.canlet.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J. Urol. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 47.Ohie S, et al. Cisplatin sensitivity of ovarian cancer in the histoculture drug response assay correlates to clinical response to combination chemotherapy with cisplatin, doxorubicin and cyclophosphamide. Anticancer Res. 2000;20:2049–2054. [PubMed] [Google Scholar]
- 48.Yoon YS, et al. Development and applicability of integrative tumor response assays for metastatic colorectal cancer. Anticancer Res. 2017;37:1297–1303. doi: 10.21873/anticanres.11447. [DOI] [PubMed] [Google Scholar]
- 49.Wei B, et al. Combination of histoculture drug response assay and qPCR as an effective method to screen biomarkers for personalized chemotherapy in esophageal cancer. Oncol Lett. 2017;14:6915–6922. doi: 10.3892/ol.2017.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.