Abstract
Background
Hidradenitis suppurativa (HS), also known as acne inversa, is a recurring, painful, chronic, and sometimes disfiguring inflammatory skin disease.
Objectives
Our objective was to report the baseline clinical characteristics, natural history, and associated outcomes of patients with HS from the ongoing, prospective, non-interventional UNITE registry that is collecting data regarding the natural history and associated outcomes of HS.
Methods
Patients with inflammatory HS lesions were enrolled, including adolescents (aged 12 to < 18 years) and adults (aged ≥ 18 years). None had participated in previous or current originator-adalimumab studies/registries. Patients received treatment consistent with site-specific, routine clinical practice. HS disease status was assessed by HS lesions and disease flare; treatment and outcomes data were collected at enrolment and every 6 months for ≤ 4 years.
Results
Enrolment (N = 594; 89.1% adults; 10.9% adolescents) occurred from 29 October 2013 to 29 December 2015 at 73 sites in 12 countries. At baseline, the majority were female (69.7%) and White (81.2%), had moderate-to-severe disease (Hurley stage II or III; 93.3%), and had undergone prior procedures/surgery for HS (68.7%). In total, 61.6% of adults and 49.2% of adolescents were obese; 40.2% of patients reported current tobacco use. Scarring due to lesions occurred in 91.2% of patients. The prevalence of comorbidities of interest was as follows: depression (13.3%), other psychiatric disorders (9.6%), inflammatory bowel disease (2.7%), diabetes (9.1%), and polycystic ovary syndrome (5.2%).
Conclusions
In this population from the UNITE HS registry, obesity and smoking were common, and disease burden was high, manifesting as multiple lesions, scarring, surgical history, and considerable comorbidities.
Electronic supplementary material
The online version of this article (10.1007/s40257-020-00504-4) contains supplementary material, which is available to authorized users.
Key Points
| This report of the baseline characteristics from UNITE, a non-interventional long-term hidradenitis suppurativa (HS) registry, provides the first geographically diverse, detailed clinical description of HS in an observed population across North America, Europe, and Australia. |
| Clinical aspects of the disease appeared similar between adults and adolescents, although adolescents were more likely to have been diagnosed earlier in the disease course. |
| Lesion count and scarring affected the groin/pelvic/pubic area most commonly and severely compared with the inframammary area, in contrast to previous reports. |
Introduction
Hidradenitis suppurativa (HS; or acne inversa) is a chronic, inflammatory skin disease that manifests as inflammatory nodules, abscesses, draining fistulas, and—in some cases—severe scarring [1]. The disease affects mainly intertriginous areas (axillae, inguinal, genital, and inframammary regions), which are subject to friction [1, 2].
HS occurs about three times more frequently in women than in men [3–6]. The estimated prevalence is 0.1% based on US claims data and approximately 1–4% in Europe [7, 8]. Clinical experience suggests that HS is under-recognised, especially among the non-dermatology community [9], which frequently results in substantial delay in diagnosis (≥ 7 years average) [10, 11]. Onset is generally post-pubertal (20–30 years of age) [4] but can rarely occur in prepubescence [5]. The disease is diagnosed by the presence of characteristic lesions in typical body sites, but there is no confirmatory diagnostic test [12]. Adults and adolescents share the clinical features of HS.
Although not fully understood, the pathogenesis of HS is thought to be a combination of genetic, environmental, and physiological factors, including auto-inflammatory mechanisms and microbiome alterations [11], and may also be related to follicular occlusion, inflammation, and abnormal immune response in the skin [1, 13, 14].
HS greatly impacts quality of life [12, 15–18], and disease burden can be substantial, especially as severity increases. The malodorous discharge from draining lesions can cause embarrassment, psychological stress, and social stigma, which may lead to social isolation [9]. Pain from active lesions can substantially impair quality of life and lead to work disability [16]. Symptoms can also be caused by irreversible scarring, which can limit physical activity and cause psychological impairment [19]. Noncutaneous comorbidities can also be significantly burdensome [20] and include depression, arthritis (including spondyloarthropathies), metabolic syndrome, polycystic ovary syndrome (PCOS), obesity, skin cancer, and inflammatory bowel disease [7, 11, 12, 21, 22].
Treatment for HS is typically individualised based on clinical presentation and disease impact [23]. Evidence-based treatments include anti-inflammatory antibiotics (topical clindamycin, then oral doxycycline or minocycline) and adalimumab (the only approved pharmacological treatment for HS). Less well-researched treatments include zinc gluconate, topical resorcinol, intralesional and systemic corticosteroids, acitretin, and infliximab. Other agents with limited levels of supporting evidence include hormone therapy, cyclosporine, dapsone, and others [24, 25]. Classical surgery and/or laser techniques can be combined with medical therapy and may be appropriate for locally recurring lesions and for severe disease [26]. Adjuvant measures include pain management, treatment of superinfections, weight loss, and tobacco abstinence [24].
Improved characterisation of the natural history, diagnostic patterns, and clinical characteristics of HS could inform the development of treatments and improve patient care. To that end, UNITE is the first geographically diverse, longitudinal, prospective, observational registry to collect long-term clinical and health-related quality-of-life data from patients with HS. The objective of this ongoing registry is to evaluate the natural history and risk of progression of HS, evolving clinical practice trends, and associated outcomes in patients with HS. We report UNITE’s baseline demographic and clinical characteristics for adult and adolescent patients with HS. Baseline patient-reported outcomes from UNITE, including comprehensive quality-of-life outcomes, are published separately [27].
Materials and Methods
Study Design
In this non-interventional registry, patient treatment is rendered per routine clinical practice at each site. The protocol does not mandate using specific medication(s) or treatment procedure(s). Data on HS treatment and associated clinical and patient-reported outcomes were collected at enrolment and approximately every 6 months thereafter for up to 4 years. As this registry is observational, no pre-defined hypotheses exist regarding the magnitude of any resulting differences.
Patients
Approximately 500–600 patients were to be enrolled from 75–100 sites across 12 countries. UNITE’s sample size was based on the size needed for a reliable assessment of the descriptive statistics. Adolescents (aged 12–18 years) and adults (aged ≥ 18 years) with a confirmed diagnosis of HS, presence of HS inflammatory nodules and/or abscesses, and no previous or current participation in an adalimumab clinical study or registry were enrolled. To minimise potential bias in outcome measurement due to varying levels of disease severity, the registry attempted to enrol a balanced proportion of adults across Hurley stages I, II, and III (mild, moderate, and severe disease, respectively); given the lower numbers of adolescents enrolled, balance across stages was not attempted.
Baseline Characteristics
The last non-missing measurement collected at or before enrolment was used as the baseline for summary of demographics, disease characteristics, and disease activity. Adverse events were not prospectively collected since this is a non-drug, non-interventional disease registry. No routine laboratory results were collected.
The clinical course of HS was assessed by lesions located in the overall body areas and in the anatomic region within each body area, as defined in Online Resource 1. The following were assessed at enrolment: lesions and hypertrophic scarring, lesion type (abscess, inflammatory nodule, non-inflammatory nodule, draining fistula, and non-draining fistula), lesional drainage requiring use of pad(s), and frequency of HS flares. “Flare” was identified by the investigator and patient.
Statistical Analysis
All baseline characteristics were summarised by descriptive statistics. Means and standard deviations (SDs) were reported for continuous variables; count and proportion were reported for categorical variables. Analyses were conducted for all enrolled in the registry. When appropriate, results were also reported by age group (adolescents and adults) and/or by baseline Hurley stage. All statistical analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Enrolment started on 29 October 2013 and completed on 29 December 2015. As of 24 September 2018, most patients had participated in the registry for ≥ 3.5 years. A total of 594 patients were enrolled from 73 sites in 12 countries: USA (323; with an additional 10 in Puerto Rico), Canada (76), Netherlands (36), Germany (35), Spain (32), Italy (30), Hungary (14), Greece (11), Switzerland (11), Australia (8), France (5), and Czech Republic (3).
Patient Characteristics at Baseline
The majority of patients were adults (89.1%, N = 529), of whom 97.7% were aged < 65 years and 10.9% (N = 65) were adolescents (Table 1). The mean age was 36.1 years. Most patients were female (69.7%) and White (81.2%). Current or previous tobacco use was reported by 64.1% of adults and 6.1% of adolescents. Current tobacco use was reported by 40.2% of all patients and increased with increasing disease severity (32.5%, 39.8%, and 43.1% at Hurley stages I, II, and III, respectively).
Table 1.
Baseline demographics
| Characteristics | Adults N = 529 |
Adolescents N = 65 |
All patients N = 594 |
All patients, by Hurley stage | ||
|---|---|---|---|---|---|---|
| I (N = 40) | II (N = 394) | III (N = 160) | ||||
| Sex | ||||||
| Male | 166 (31.4) | 14 (21.5) | 180 (30.3) | 11 (27.5) | 105 (26.6) | 64 (40.0) |
| Female | 363 (68.6) | 51 (78.5) | 414 (69.7) | 29 (72.5) | 289 (73.4) | 96 (60.0) |
| Racea | ||||||
| White | 425 (80.3) | 53 (88.3) | 478 (81.2) | 31 (81.6) | 330 (84.4) | 117 (73.1) |
| Black | 88 (16.6) | 7 (11.7) | 95 (16.1) | 7 (18.4) | 52 (13.3) | 36 (22.5) |
| Asian | 11 (2.1) | 0 | 11 (1.9) | 0 | 7 (1.8) | 4 (2.5) |
| Native Hawaiian or Pacific Islander | 2 (0.4) | 0 | 2 (0.3) | 0 | 1 (0.3) | 1 (0.6) |
| American Indian or Alaskan Native | 2 (0.4) | 0 | 2 (0.3) | 0 | 1 (0.3) | 1 (0.6) |
| Multi-race | 1 (0.2) | 0 | 1 (0.2) | 0 | 0 | 1 (0.6) |
| Ethnicity | ||||||
| Hispanic or Latino | 65 (12.3) | 6 (9.2) | 71 (12.0) | 8 (20.0) | 46 (11.7) | 17 (10.6) |
| Age, years | 38.6 ± 12.57 | 15.6 ± 1.38 | 36.1 ± 13.88 | NC | NC | NC |
| Age categories, years | ||||||
| 12 to ≤ 17 | 0 | 65 (100) | 65 (10.9) | 12 (30.0) | 48 (12.2) | 5 (3.1) |
| 18 to < 40 | 296 (56.0) | 0 | 296 (49.8) | 14 (35.0) | 203 (51.5) | 79 (49.4) |
| 40 to ≤ 64 | 221 (41.8) | 0 | 221 (37.2) | 13 (32.5) | 134 (34.0) | 74 (46.3) |
| ≥ 65 | 12 (2.3) | 0 | 12 (2.0) | 1 (2.5) | 9 (2.3) | 2 (1.3) |
| Tobacco useb | ||||||
| User | 235 (44.6) | 3 (4.6) | 238 (40.2) | 13 (32.5) | 156 (39.8) | 69 (43.1) |
| Former user | 103 (19.5) | 1 (1.5) | 104 (17.6) | 7 (17.5) | 70 (17.9) | 27 (16.9) |
| Non-user | 189 (35.9) | 61 (93.8) | 250 (42.2) | 20 (50.0) | 166 (42.3) | 64 (40.0) |
| Alcoholc | ||||||
| User | 279 (54.1) | 3 (4.7) | 282 (48.6) | 15 (40.5) | 193 (50.0) | 74 (47.1) |
| Former user | 63 (12.2) | 0 | 63 (10.9) | 2 (5.4) | 37 (9.6) | 24 (15.3) |
| Non-user | 174 (33.7) | 61 (95.3) | 235 (40.5) | 20 (54.1) | 156 (40.4) | 59 (37.6) |
Data are presented as mean ± standard deviation or N (%) unless otherwise indicated
NC data not collected
aMissing data (two and three adolescents at Hurley stage I and II, respectively)
bTobacco use unknown (two adults at Hurley stage II)
cAlcohol use unknown (13 adults: two, eight, and three at Hurley stage I, II, and III, respectively; one adolescent at Hurley stage I)
The majority (83.7% of adults and 75.4% of adolescents) were overweight to obese, defined as body mass index (BMI) ≥ 25 kg/m2 for adults and ≥ 85th percentile for adolescents. Most adults (61.6%) and almost half of adolescents (49.2%) were obese (BMI ≥30 kg/m2 for adults and BMI ≥95th percentile for adolescents) (Table 2).
Table 2.
Baseline body weight and body mass index
| Characteristics | Adults N = 529 |
Adolescents N = 65 |
All patients N = 594 |
|
|---|---|---|---|---|
| Body weight, kg | ||||
| Male |
(N=166) 100.7 ± 26.00 |
(N = 14) 87.9 ± 21.45 |
(N = 180) 99.7 ± 25.86 |
|
| Female |
(N = 361) 93.7 ± 23.96 |
(N = 51) 75.9 ± 20.68 |
(N = 412) 91.5 ± 24.28 |
|
| BMI, kg/m2 |
(N = 526) 33.2 ± 8.10 |
(N = 64) 28.1 ± 6.43 |
(N = 590) 32.6 ± 8.08 |
|
| BMI kg/m2 categories | Adults (N = 526) | |||
| < 25 | Normal weight | 86 (16.3) | ||
| 25 to < 30 | Overweight | 116 (22.1) | ||
| 30 to < 40 | Obese | 219 (41.6) | ||
| ≥ 40 | Morbidly obese | 105 (20.0) | ||
| BMI percentile categories | Adolescents (N = 61) | |||
| < 5th | Underweight | 0 | ||
| 5th to < 85th | Normal weight | 15 (24.6) | ||
| 85th to < 95th | Overweight | 16 (26.2) | ||
| ≥ 95th | Obese | 30 (49.2) | ||
Data are presented as mean ± standard deviation or N (%) unless otherwise indicated
BMI body mass index
Disease Characteristics at Baseline
The majority of patients had Hurley stage II disease (65.4% of adults and 73.8% of adolescents; Table 3). In addition, a higher proportion of adults (94.7%) than adolescents (81.5%) had Hurley stage II or III disease. The median/mean time between onset of HS symptoms and diagnosis was 4.1/8.3 years for adults and 1.3/2.5 years for adolescents (Table 3). Approximately one-third of patients reported a family history of HS (30.1% adults, 35.4% adolescents).
Table 3.
Baseline characteristics associated with hidradenitis suppurativa
| Characteristics | Adults N = 529 |
Adolescents N = 65 |
All patients N = 594 |
|---|---|---|---|
| Hurley stagea | |||
| I | 28 (5.3) | 12 (18.5) | 40 (6.7) |
| II | 346 (65.4) | 48 (73.8) | 394 (66.3) |
| III | 155 (29.3) | 5 (7.7) | 160 (26.9) |
| ISH4 severity scoreb [43] | |||
| ≤ 3 (mild) | 121 (22.9) | 22 (34.4) | 143 (24.1) |
| 4–10 (moderate) | 147 (27.8) | 20 (31.3) | 167 (28.1) |
| ≥ 11 (severe) | 261 (49.3) | 22 (34.4) | 283 (47.6) |
| Age at start of HS symptoms, years |
(N = 528) 24.5 ± 12.3 |
(N = 65) 12.1 ± 3.4 |
(N = 593) 23.2 ± 12.3 |
| Years from HS symptom onset to diagnosis | (N = 527) | (N = 65) | (N = 592) |
| Mean ± SD | 8.3 ± 10.5 | 2.5 ± 3.3 | 7.7 ± 1.2 |
| Median (1st, 3rd quartiles) | 4.1 (0.6, 12.2) | 1.3 (0.3, 3.0) | 3.7 (0.5, 10.8) |
| Family history of HS | 159 (30.1) | 23 (35.4) | 182 (30.6) |
| HS lesion count | (N = 529) | (N = 64) | (N = 593) |
| Abscesses | 3.7 ± 8.8 | 2.6 ± 7.7 | 3.6 ± 8.8 |
| Draining fistulas | 2.6 ± 6.1 | 0.9 ± 2.0 | 2.4 ± 5.8 |
| Non-draining fistulas | 6.7 ± 22.6 | 1.2 ± 2.6 | 6.1 ± 21.5 |
| Inflammatory nodules | 7.1 ± 11.0 | 5.4 ± 7.4 | 6.9 ± 10.7 |
| Non-inflammatory nodules | 6.9 ± 17.5 | 6.4 ± 24.9 | 6.9 ± 18.4 |
| Used pads for lesion drainagec | – | – | 230 (38.7) |
| Mammary | – | – | 34 (14.8) |
| Pelvicd | – | – | 87 (37.8) |
| Axilla | – | – | 97 (42.2) |
| Buttock | – | – | 32 (13.9) |
| Number of HS flarese per patient during previous 6 months |
(N = 529) 5.8 ± 9.5 |
(N = 64) 4.6 ± 5.6 |
(N = 593) 5.7 ± 9.1 |
Data are presented as mean ± standard deviation or N (%) unless otherwise indicated
HS hidradenitis suppurativa, ISH4 International Hidradenitis Suppurativa Severity Score System, SD standard deviation
aWorst Hurley stage of all regions. Hurley stage I: Localised formation of single or multiple abscesses without sinus tracts and scarring. Hurley stage II: Recurrent abscesses with sinus tract formation and scarring; single or multiple lesions. Hurley stage III: Diffuse involvement with interconnected tracts and abscesses
bData missing for one adolescent
cPatients could have reported using pads in multiple areas. Data were not analysed for adults versus adolescents because the number of adolescent patients was too small to provide meaningful information
dIncludes groin
e“Flare” was as reported by patient or investigator and was not pre-defined in the protocol
Patients had substantial disease burden based on mean ± SD lesion counts and number of flares (Table 3). Among lesions, nodules were most frequent overall (6.9 ± 10.7 inflammatory and 6.9 ± 18.4 non-inflammatory). Flares during the 6 months before baseline were 5.8 ± 9.5 for adults (approximately one flare/month) and 4.6 ± 5.6 for adolescents. More than one-third of patients (38.7%) used pads for lesional drainage.
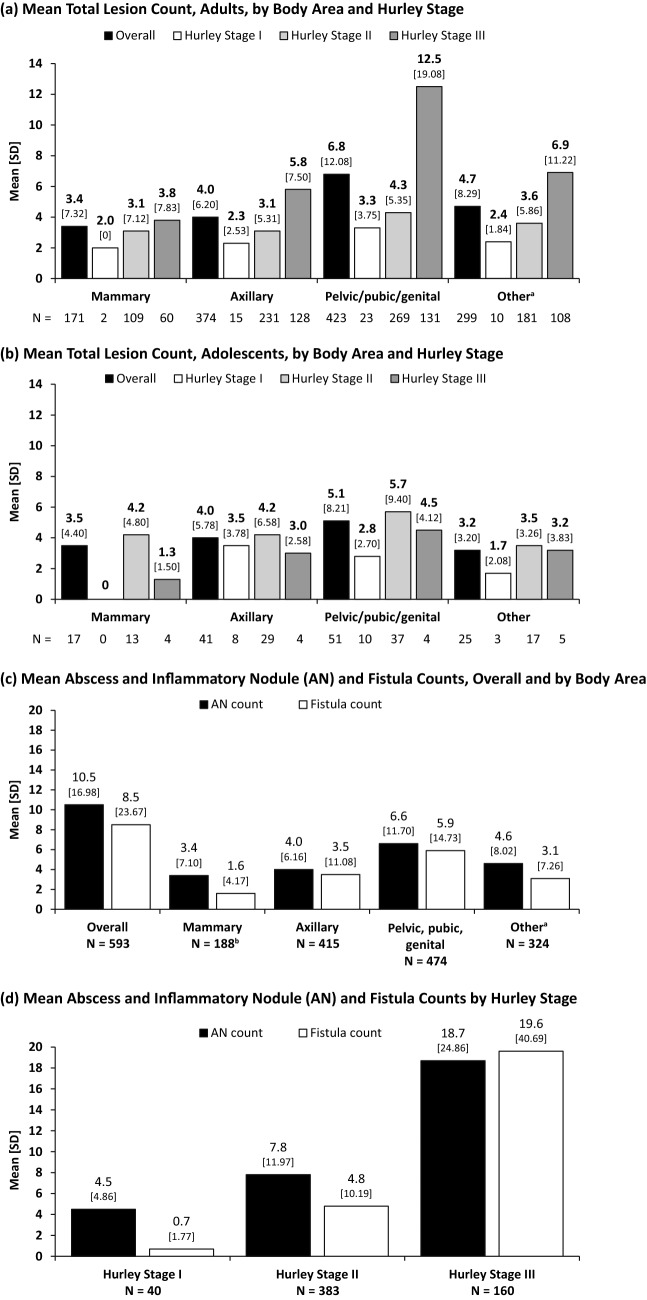
Mean total lesion count was highest in the pelvic/pubic/genital region compared with other body areas (Fig. 1). Mean total lesion count was highest in each body area at Hurley stage III for adults (Fig. 1a) but Hurley stage II for adolescents (Fig. 1b). The pelvic/pubic/genital region had the highest total mean ± SD abscess and inflammatory nodule (AN) count (6.6 ± 11.7) and total fistula (draining and non-draining) count (5.9 ± 14.7) compared with other body areas (Fig. 1c). Patients at Hurley stage III had the highest AN and fistula counts (18.7 ± 24.9 and 19.6 ± 40.7, respectively; Fig. 1d).
Fig. 1.
Hidradenitis suppurative lesion count by body area and Hurley stage. Mean total lesion count, by body area and Hurley stage: a adults and b adolescents. Mean abscess and inflammatory nodule and fistula counts, c overall and by body area and d by Hurley stage. aThe “other” category includes abdomen, back, chest excluding infra- and intermammary areas, face including ears and behind ears, genitals, left and right upper and lower extremities, neck, pubic region, scalp; bN = 187 for fistulas in the mammary area. Lesions include a, b abscess, inflammatory and non-inflammatory nodules, draining and non-draining fistulas, hypertrophic scar caused by HS. c, d “Fistulas” includes fistulas and non-draining fistulas; “N” represents the number of patients in each Hurley stage who had any lesion present in the corresponding body area; used as denominator when calculating mean lesion count. SD is indicated under each data label appearing above each bar of the graphs. AN abscesses and inflammatory nodule, HS hidradenitis suppurativa, SD standard deviation
Scarring due to HS was reported by 91.2% of patients overall and was more common in adults than in adolescents (92.8% and 78.5%, respectively). Scarring increased with Hurley stage (4.7%, 97.2%, and 100% for Hurley stage I, II, and III, respectively; Table 4). The most common areas affected by scarring were pelvic/pubic/genital, followed by axilla (69.4% and 60.8% of patients, respectively).
Table 4.
Patients with scarring due to hidradenitis suppurativa for enrolled patients (N = 594)
| Scarring area | Any scarring | By Hurley stage | ||
|---|---|---|---|---|
| I (N = 43) | II (N = 392) | III (N = 159) | ||
| Entire body | ||||
| Overall, N = 594 | 542 (91.2) | 2 (4.7) | 381 (97.2) | 159 (100.0) |
| Adults, N = 529 | 491 (92.8) | – | – | – |
| Adolescents, N = 65 | 51 (78.5) | – | – | – |
| Body area | Any scarring | Body area with scarring | |||
|---|---|---|---|---|---|
| 1–25% | 26–50% | 51–75% | 76–100% | ||
| Mammarya | 152 (25.6) | 106 (69.7) | 29 (19.1) | 11 (7.2) | 6 (3.9) |
| Pelvic/pubic/genitalb | 412 (69.4) | 220 (53.4) | 106 (25.7) | 46 (11.2) | 40 (9.7) |
| Axillac | 361 (60.8) | 195 (54.0) | 86 (23.8) | 37 (10.2) | 43 (11.9) |
| Other body aread | |||||
| Abdomen | 81 (13.6) | 60 (74.1) | 16 (19.8) | 4 (4.9) | 1 (1.2) |
| Back | 36 (6.1) | 27 (75.0) | 4 (11.1) | 3 (8.3) | 2 (5.6) |
| Cheste | 14 (2.4) | 9 (64.3) | 3 (21.4) | 2 (14.3) | 0 |
| Facef | 31 (5.2) | 21 (67.7) | 6 (19.4) | 2 (6.5) | 2 (6.5) |
| Upper extremity, right | 20 (3.4) | 16 (80.0) | 3 (15.0) | 1 (5.0) | 0 |
| Upper extremity, left | 17 (2.9) | 15 (88.2) | 1 (5.9) | 1 (5.9) | 0 |
| Lower extremity, right | 36 (6.1) | 29 (80.0) | 4 (11.1) | 2 (5.6) | 1 (2.8) |
| Lower extremity, left | 37 (6.2) | 30 (81.1) | 2 (5.4) | 3 (8.1) | 2 (5.4) |
| Neck | 36 (6.1) | 24 (66.7) | 9 (25.0) | 2 (5.6) | 1 (2.8) |
| Scalp | 12 (2.0) | 7 (58.3) | 5 (41.7) | 0 | 0 |
Data are presented as N (%) unless otherwise indicated
aIncludes intermammary and left and right inframammary areas
bIncludes buttocks, inguinal fold, perianal, perineal, pubic, genital areas
cIncludes left and right axilla areas
dLists anatomic regions that are included in the body area labelled “Other”
eExcluding mammary, infra- and intermammary areas
fIncluding ears and behind ears
Comorbidities at Baseline
The most common comorbidities were hypertension (14.3% of all patients), depression (13.3%), and other cognitive/psychiatric disorders, including anxiety (9.6%) (Table 5). Notable skin manifestations included pilonidal cyst (7.1% of patients), contracture, or strictures due to HS scarring (4.5%), severe acne (4.2%), and acne conglobata (2.7%). Metabolic conditions included diabetes mellitus (9.1%) and hyperlipidaemia (5.9%). Inflammatory bowel disease was reported in 2.7% of all patients, and 5.2% had PCOS.
Table 5.
Medical history of interest
| Body system | Condition/diagnosis | All patients N = 594 |
|---|---|---|
| Cardiovascular | Hypertension | 85 (14.3) |
| Anaemia | 19 (3.2) | |
| Peripheral vascular disease | 11 (1.9) | |
| Gastrointestinal | Gastroesophageal reflux disease | 31 (5.2) |
| Inflammatory bowel diseasea | 16 (2.7) | |
| Integument | Pilonidal cyst | 42 (7.1) |
| Contracture/strictures due to HS scarring | 27 (4.5) | |
| Psoriasis | 25 (4.2) | |
| Severe acne | 25 (4.2) | |
| Acne conglobata | 16 (2.7) | |
| Pyoderma gangrenosum | 6 (1.0) | |
| Dissecting folliculitis | 4 (0.7) | |
| Metabolic | Diabetes mellitus | 54 (9.1) |
| Hyperlipidaemiab | 35 (5.9) | |
| Irregular menstrual cycles | 32 (5.4) | |
| Polycystic ovary syndrome | 31 (5.2) | |
| Neurologic and psychiatric | Depression | 79 (13.3) |
| Other cognitive or psychiatric disorderc | 57 (9.6) | |
| Pulmonary | Asthma | 37 (6.2) |
Data are presented as N (%)
HS hidradenitis suppurativa
aIncluding Crohn’s disease and ulcerative colitis
bAlso referred to as dyslipidaemia or hypercholesterolemia
cExcludes depression; includes anxiety. Some patients reported more than one “other cognitive psychiatric disorder”; those who experienced multiple disorders were counted only once. This could result in under-representation of incident disorders as the reported number only reflects the proportion of patients experiencing any cognitive/psychiatric disorder(s) other than depression
Treatment Before Enrolment
The proportion of adults reporting a prior procedure/surgery for HS (72.4%) was higher than the proportion of adolescents (38.5%; Table 6). Similar proportions of adults and adolescents had used prior medication for HS (73.9% and 67.7%, respectively; Table 7). Overall, a majority (68.7%) had one or more prior procedure/surgery for HS; the most common was incision and drainage by a physician (33.8%). The proportion of patients reporting any prior HS procedure/surgery was higher at Hurley stage II or III (70.0%) than at Hurley stage I (50.0%; Table 6).
Table 6.
Prior procedure and surgery for hidradenitis suppurativa
| Adults N = 529 |
Adolescents N = 65 |
All patients N = 594 |
All patients by Hurley stage | |||
|---|---|---|---|---|---|---|
| I, N = 40 | II, N = 394 | III, N = 160 | ||||
| Any procedure | 383 (72.4) | 25 (38.5) | 408 (68.7) | 20 (50.0) | 267 (67.8) | 121 (75.6) |
| De-roofing | 40 (7.6) | 0 | 40 (6.7) | 0 | 23 (5.8) | 17 (10.6) |
| En bloc excision | 105 (19.8) | 2 (3.1) | 107 (18.0) | 2 (5.0) | 70 (17.8) | 35 (21.9) |
| Incision and drainage by patient | 71 (13.4) | 1 (1.5) | 72 (12.1) | 2 (5.0) | 58 (14.7) | 12 (7.5) |
| Incision and drainage by physician | 187 (35.3) | 14 (21.5) | 201 (33.8) | 9 (22.5) | 131 (33.2) | 61 (38.1) |
| Intralesional steroid injections | 91 (17.2) | 5 (7.7) | 96 (16.2) | 5 (12.5) | 61 (15.5) | 30 (18.8) |
| Laser treatment, CO2 | 9 (1.7) | 1 (1.5) | 10 (1.7) | 0 | 6 (1.5) | 4 (2.5) |
| Laser treatment, ND-YAG | 5 (0.9) | 0 | 5 (0.8) | 0 | 3 (0.8) | 2 (1.3) |
| Photodynamic therapy | 5 (0.9) | 0 | 5 (0.8) | 0 | 2 (0.5) | 3 (1.9) |
| External-beam radiation therapy | 1 (0.2) | 0 | 1 (0.2) | 0 | 0 | 1 (0.6) |
| Other surgery or procedures | 54 (10.2) | 2 (3.1) | 56 (9.4) | 4 (10.0) | 29 (7.4) | 23 (14.4) |
Data are presented as N (%)
ND-YAG neodymium-doped yttrium aluminum garnet
Table 7.
Prior medication for hidradenitis suppurativa
| Prior medication | Adults N = 529 |
Adolescents N = 65 |
All patients N = 594 |
|---|---|---|---|
| Any category of prior medication | 391 (73.9) | 44 (67.7) | 435 (73.2) |
| Retinoids | 98 (18.5) | 7 (10.8) | 105 (17.7) |
| Antibiotics | 365 (69.0) | 41 (63.1) | 406 (68.4) |
| Corticosteroids | 47 (8.9) | 5 (7.7) | 52 (8.8) |
| Opioids | 7 (1.3) | 1 (1.5) | 8 (1.3) |
| Biologics | 29 (5.5) | 0 | 29 (4.9) |
| Hormonal therapies | 25 (4.7) | 1 (1.5) | 26 (4.4) |
| Immunosuppressants | 6 (1.1) | 2 (3.1) | 8 (1.3) |
| Reasons for discontinuing prior HS medicationb | N = 411 | N = 47 | N = 458 |
| Treatment failure or inadequate response | 234 (56.9) | 20 (42.6) | 254 (55.5) |
| Treatment not tolerated | 64 (15.6) | 8 (17.0) | 72 (15.7) |
| Satisfactory remission | 66 (16.1) | 16 (34.0) | 82 (17.9) |
| Complete remission | 13 (3.2) | 3 (6.4) | 16 (3.5) |
| Othera | 34 (8.3) | 0 | 34 (7.4) |
Data are presented as N (%)
HS hidradenitis suppurativa
a“Other” mainly includes patients who were prescribed a short course of therapy
bFor patients with any previous treatment for hidradenitis suppurativa
The majority of patients (73.2%) had received prior medication for HS (Table 7). The most common medication category was antibiotics (68.4% overall; 69.0% adults, 63.1% adolescents), with retinoids (17.7%; topical vs. oral was not specified), corticosteroids (8.8%), hormonal therapies (4.4%), and immunosuppressants (1.3%) also reported. Only 5.5% of adults, and no adolescents, had been prescribed a biologic. Opioid use was reported by 1.3% of all patients. The main reason for discontinuation of prior medication for HS was inadequate response (55.5% overall; 56.9% adults, 42.6% adolescents).
Quality of Life
Patient quality of life was captured for 529 adults using the Dermatology Life Quality Index (DLQI) (mean score ± SD 12.6 ± 8.0) and for 65 adolescents using the Children’s DLQI (CDLQI) (mean score ± SD 6.9 ± 6.2). In adults, the mean ± SD DLQI score by Hurley stages I, II, and III was 8.8 ± 7.3 (N = 28), 11.6 ± 7.5 (N = 346), and 15.7 ± 8.2 (N = 155), respectively. In adolescents, the mean ± SD CDLQI score by Hurley stages I, II, and III was 5.5 ± 6.2 (N = 12), 6.6 ± 6.0 (N = 48), and 12.8 ± 6.2 (N = 5), respectively. A thorough description of additional quality-of-life and other patient-reported outcomes from UNITE are published separately [27].
Discussion
The ongoing UNITE registry is the first global, longitudinal, prospective, observational registry collecting long-term clinical and quality-of-life data for patients with HS. Baseline data confirm that the burden of HS is substantial, especially with more severe disease. Patients had multiple comorbidities and generally received multiple treatments before enrolment. The majority were female and White, and the mean age was 36.1 years, which is similar to that in other reports of HS populations [7, 22, 28, 29].
Results suggest that patients may have been at a more advanced disease stage by the time they saw a dermatologist; 93.3% were at Hurley stage II or III and—consistent with other reports [10, 11, 28, 29]—the average time from symptom start to disease diagnosis was 7.7 years overall (adults 8.4 years; adolescents 2.5 years). The shorter time for adolescents suggests a greater likelihood of timely diagnosis despite having, on average, less severe disease. The mean age at start of symptoms was fairly young (23.2 years overall; adults 24.5, adolescents 12.1).
A strong heritable component was observed for HS in UNITE; 30.6% reported a family history of HS (adolescents 35.4% vs. adults 30.1%), similar to other reported findings [28, 30]
The burden of disease at baseline in UNITE was evaluated by examining lesions, scarring, comorbidities, prior medical treatments, and quality of life. Although the axilla is reportedly the area most commonly affected with HS lesions [28, 29, 31], the highest mean number of lesions overall in UNITE was observed in the pelvic/pubic/genital region, followed by axillary and mammary regions. Scarring was also most common in the pelvic/pubic/genital region (69.4% of patients); 60.8% had axillary scarring. Over 90% of patients had some degree of scarring at baseline. Nearly 5% of those classified as having Hurley stage I disease had scarring at baseline; by Hurley stage definition, these patients were under-classified, suggesting that severity can be overlooked in HS. Scarring is one of the major components of disease burden. In a qualitative study, 80.9% of patients reported psychological symptoms and 38.1% reported physical symptoms or limitations due to scarring [19]. Thus, it is critical to manage HS aggressively across Hurley stages to minimise the potential for irreversible damage.
Smoking and obesity have been identified as major risk factors associated with HS and as factors that are likely to increase disease severity [7, 32]. Patients with HS are 13-fold more likely to be smokers and 4.4-fold more likely to be obese than are matched controls [7]. In UNITE, a substantial number of patients were current tobacco users at baseline, as also reported in other HS populations [7, 22, 28, 29]. Overall, the reported use at baseline increased as disease severity increased (measured by Hurley stage), a relationship also observed in another study [33]. Most UNITE patients were above normal weight, and the majority of those were obese, similar to baseline characteristics from a US-specific report of patients with HS [29]. However, two studies in France reported that just under half were overweight or obese, suggesting a potential geographical difference in obesity among patients with HS [7, 28]. In UNITE, a complete analysis of body weight or BMI by country of study site was not possible, as the majority of patients were enrolled in North America (USA and Canada).
The most common medical condition in UNITE was hypertension (14.3%); diabetes mellitus was reported by 9.1% of patients and hyperlipidaemia by 5.9%. These rates are lower than expected based on other reports [22, 34]. Approximately 2.7% of UNITE patients had a medical history of inflammatory bowel disease (Crohn’s disease or ulcerative colitis), similar to other published rates [35–37].
Given the variety of contributors to the disease burden in HS, as seen in this analysis and previous reports, a multidisciplinary and multimodal approach to treatment is necessary. Under-treatment of HS is a global problem, which is confirmed by this analysis. At baseline, aside from antibiotics, systemic treatments such as oral retinoids and immunosuppressants had been prescribed in a minority of patients (17.7% and 1.3%, respectively). During the majority of the study enrolment period (December 2013 to December 2015), no biologic treatment was approved for HS. As such, reports at baseline of any biologic use for HS was low (5.5% of adults and no adolescents) and may not approximate biologic use post-approval of adalimumab; this is a potential limitation of this study. Procedural and surgical treatment of lesions is an important part of the HS treatment paradigm, especially as disease severity increases [12, 26, 38], as was observed in UNITE; 50.0%, 67.8%, and 75.6% of patients with Hurley stages I, II, and III, respectively, reported prior procedures/surgeries. However, the high percentage of patients reporting a history of incision and drainage procedures, especially for patients with Hurley stage II or III disease (47.9% and 45.6%, respectively), suggests that disease in these patients is not adequately controlled; more aggressive surgical procedures such as de-roofing and en bloc excision were performed in far fewer of these patients (23.6% and 32.5% of patients with Hurley stage II and III disease, respectively).
Depression and anxiety are strongly associated with HS and are significant factors affecting quality of life for these patients [17, 18, 39, 40]. A retrospective case–control study reported that 58% of patients with HS had a psychiatric disorder [22]. Psychiatric comorbidities reported in UNITE at baseline included 13.3% with depression and 9.6% with other cognitive/psychiatric disorders, including anxiety. However, the patient-reported Hospital Anxiety and Depression Scale outcome from UNITE showed that 58% of patients reported anxiety and 30% reported depression at baseline. This suggests that psychological disorders may be under-diagnosed and/or under-reported in this population and confirms the need for adequate multidisciplinary care and screening for these comorbidities.
Quality of life at baseline in UNITE was significantly impaired in adults, and scores worsened markedly as disease severity increased. Additional quality-of-life patient-reported outcomes from UNITE are addressed in a separate publication [27].
Limitations of this analysis include the bias that can be present in observational studies, including methods of patient selection, selective recall, inconsistent reporting, measurement errors, and under-reporting, especially of information not apparent to the data collector, such as comorbidities and prior treatment [41]. Another potential limitation is the method of describing disease severity and the potential influence of disease severity on enrolment. Per the protocol, the original Hurley staging criteria [24] were used as the method for defining severity, which is not necessarily correlated to disease activity at any given time. A newer method of Hurley staging has since been published [42], in which Hurley IC and IIC are classified as “severe”, but this staging was not available at the time of data collection. In addition, while balancing of patients across Hurley stages was attempted, the vast majority enrolled had Hurley stage II and III disease; thus, their results may not be generalisable to the entire HS population. Although the majority of UNITE patients were enrolled in North American (68.9%) sites, these data contribute to the global characterisation of HS in patients in real-world clinical settings.
Conclusions
The UNITE baseline data confirm that overweight, obesity, and smoking are common risk factors in patients with HS. The disease burden is high, reflected by the extent of scarring, frequency of disease flares (once monthly on average) despite treatment, and the significant comorbidity burden. Our results also confirm that patients can have HS for years before receiving an accurate diagnosis (8 years average overall) and that those with more severe disease often resort to surgery. The impact on quality of life and the challenging therapeutic management of HS often necessitate a multidisciplinary approach to care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Marty M Okun of Fort HealthCare, Fort Atkinson, WI, USA, for contributions to the data adjudication process, which was funded by AbbVie, and Jody Bennett, employed by AbbVie, for medical writing support in the production of this publication.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymised, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: http://www.abbvie.com/data-and-information-sharing.
Compliance with Ethical Standards
Conflict of interest
EP Prens has received honoraria from AbbVie, Amgen, Celgene, Janssen, Galderma, Novartis, and Pfizer for participation as a speaker and on advisory boards and received investigator-initiated grants (paid to the Erasmus MC) from AbbVie, AstraZeneca, Janssen, and Pfizer. AM Lugo-Somolinos has received honoraria from AbbVie, Castle Creek, Celgene, Centocor, Janssen, Eli Lilly, Novartis, Pfizer, Regeneron, Scioderm, and Stiefel for participation as an investigator. AS Paller has received grants (to Northwestern University) from AbbVie, Anaptysbio, Eli Lilly, Galderma, Incyte, Leo, Janssen, Novartis, and Regeneron for investigator services, and honoraria from AbbVie, Amgen, Asana, Dermavant, Dermira, Eli Lilly, Forte, Galderma, Leo, Matrisys, Menlo, Mophosys/Galapagos, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, and UCB for consulting services. F Kerdel has received honoraria from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Leo, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, and Stiefel for participation as a speaker and grants from AbbVie, Amgen, AstraZeneca, Celgene, Dr. Reddy’s, Eli Lilly, Janssen, Novartis, Ortho Dermatologics, Pfizer, Regeneron, UCB, X-Biotech, and XOMA, for participation as an investigator. Y Duan, HD Teixeira, and M Longcore have received a salary as AbbVie employees and may have also received stocks and/or stock options. AB Kimball has received honoraria as a consultant and grants as an investigator from Janssen, AbbVie, Lilly, Novartis, Pfizer, and UCB and fellowship funding from Janssen and AbbVie.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee, institutional review board, and/or other local approval processes in the specific country or region of the study site and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Patients and/or their legal guardians voluntarily signed an informed consent, approved by an institutional review board or ethics committee as applicable, according to local law.
Funding
AbbVie Inc. funded this study and participated in the study design; study research; collection, analysis, and interpretation of data; and writing, reviewing, and approving of this publication. All authors had access to the data and participated in the development, review, and approval of and the decision to submit this publication. AbbVie Inc. funded Open Access of this article.
References
- 1.Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 2.van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: clinical presentations and phenotypes. J Am Acad Dermatol. 2015;73(5 Suppl 1):S23–S26. doi: 10.1016/j.jaad.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 3.Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosmatos I, Matcho A, Weinstein R, Montgomery MO, Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68(3):412–419. doi: 10.1016/j.jaad.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Palmer RA, Keefe M. Early-onset hidradenitis suppurativa. Clin Exp Dermatol. 2001;26(6):501–503. doi: 10.1046/j.1365-2230.2001.00876.x. [DOI] [PubMed] [Google Scholar]
- 6.Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa and associated factors: still unsolved problems. J Am Acad Dermatol. 2009;61(2):362–365. doi: 10.1016/j.jaad.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 7.Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case–control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35(2 Pt 1):191–194. doi: 10.1016/s0190-9622(96)90321-7. [DOI] [PubMed] [Google Scholar]
- 9.Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90(1062):216–221. doi: 10.1136/postgradmedj-2013-131994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunte DM, Boer J, Stratigos A, Szepietowski JC, Hamzavi I, Kim KH, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173(6):1546–1549. doi: 10.1111/bjd.14038. [DOI] [PubMed] [Google Scholar]
- 11.van der Zee HH, Laman JD, Boer J, Prens EP. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol. 2012;21(10):735–739. doi: 10.1111/j.1600-0625.2012.01552.x. [DOI] [PubMed] [Google Scholar]
- 12.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60(4):539–561. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ, Antonopoulou A, Petropoulou C, Mouktaroudi M, Spyridaki E, Baziaka F, et al. Altered innate and adaptive immune responses in patients with hidradenitis suppurativa. Br J Dermatol. 2007;156(1):51–56. doi: 10.1111/j.1365-2133.2006.07556.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheinfeld NS. A case of dissecting cellulitis and a review of the literature. Dermatol Online J. 2003;9(1):8. [PubMed] [Google Scholar]
- 15.Wolkenstein P, Loundou A, Barrau K, Auquier P, Revuz J. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56(4):621–623. doi: 10.1016/j.jaad.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 16.Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91(3):328–332. doi: 10.2340/00015555-1082. [DOI] [PubMed] [Google Scholar]
- 17.Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90(3):264–268. doi: 10.2340/00015555-0866. [DOI] [PubMed] [Google Scholar]
- 18.Onderdijk AJ, van der Zee HH, Esmann S, Lophaven S, Dufour DN, Jemec GB, et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2013;27(4):473–478. doi: 10.1111/j.1468-3083.2012.04468.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirby JS. Qualitative study shows disease damage matters to patients with hidradenitis suppurativa. J Am Acad Dermatol. 2016;74(6):1269–1270. doi: 10.1016/j.jaad.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menter A. Recognizing and managing comorbidities and complications in hidradenitis suppurativa. Semin Cutan Med Surg. 2014;33(3 Suppl):S54–S56. doi: 10.12788/j.sder.0093. [DOI] [PubMed] [Google Scholar]
- 21.Canoui-Poitrine F, Revuz JE, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61(1):51–57. doi: 10.1016/j.jaad.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Shlyankevich J, Chen AJ, Kim GE, Kimball AB. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case–control analysis. J Am Acad Dermatol. 2014;71(6):1144–1150. doi: 10.1016/j.jaad.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Deckers IE, Prens EP. An update on medical treatment options for hidradenitis suppurativa. Drugs. 2016;76(2):215–229. doi: 10.1007/s40265-015-0516-5. [DOI] [PubMed] [Google Scholar]
- 24.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhasz I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;30(29):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 25.Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17(3):343–351. doi: 10.1007/s11154-016-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg. 2012;38(4):517–536. doi: 10.1111/j.1524-4725.2011.02186.x. [DOI] [PubMed] [Google Scholar]
- 27.Kimball AB, Crowley JJ, Papp K, Calimlim B, Duan Y, Fleischer AB, et al. Baseline patient-reported outcomes from UNITE: an observational, international, multicentre registry to evaluate hidradenitis suppurativa in clinical practice. J Eur Acad Dermatol Venereol. 2019 doi: 10.1111/jdv.16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canoui-Poitrine F, Le Thuaut A, Revuz JE, Viallette C, Gabison G, Poli F, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Investig Dermatol. 2013;133(6):1506–1511. doi: 10.1038/jid.2012.472. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted county, Minnesota. J Investig Dermatol. 2013;133(1):97–103. doi: 10.1038/jid.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pink AE, Simpson MA, Desai N, Dafou D, Hills A, Mortimer P, et al. Mutations in the gamma-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa) J Investig Dermatol. 2012;132(10):2459–2461. doi: 10.1038/jid.2012.162. [DOI] [PubMed] [Google Scholar]
- 31.Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2009;23(9):985–998. doi: 10.1111/j.1468-3083.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 32.Konig A, Lehmann C, Rompel R, Happle R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198(3):261–264. doi: 10.1159/000018126. [DOI] [PubMed] [Google Scholar]
- 33.Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831–839. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- 34.Tzellos T, Zouboulis CC, Gulliver W, Cohen AD, Wolkenstein P, Jemec GB. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015;173(5):1142–1155. doi: 10.1111/bjd.14024. [DOI] [PubMed] [Google Scholar]
- 35.Egeberg A, Jemec GBE, Kimball AB, Bachelez H, Gislason GH, Thyssen JP, et al. Prevalence and risk of inflammatory bowel disease in patients with hidradenitis suppurativa. J Investig Dermatol. 2017;137(5):1060–1064. doi: 10.1016/j.jid.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 36.Shalom G, Freud T, Ben Yakov G, Khoury R, Dreiher J, Vardy DA, et al. Hidradenitis suppurativa and inflammatory bowel disease: a cross-sectional study of 3,207 patients. J Investig Dermatol. 2016;136(8):1716–1718. doi: 10.1016/j.jid.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Garg A, Hundal J, Strunk A. Overall and subgroup prevalence of crohn disease among patients with hidradenitis suppurativa: a population-based analysis in the United States. JAMA Dermatol. 2018;154(7):814–818. doi: 10.1001/jamadermatol.2018.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martorell A, Garcia FJ, Jimenez-Gallo D, Pascual JC, Pereyra-Rodriguez J, Salgado L, et al. Update on hidradenitis suppurative (part II): treatment. Actas Dermosifiliogr. 2015;106(9):716–724. doi: 10.1016/j.ad.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Kurek A, Johanne Peters EM, Sabat R, Sterry W, Schneider-Burrus S. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11(8):743–750. doi: 10.1111/ddg.12067. [DOI] [PubMed] [Google Scholar]
- 40.Shavit E, Dreiher J, Freud T, Halevy S, Vinker S, Cohen AD. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29(2):371–376. doi: 10.1111/jdv.12567. [DOI] [PubMed] [Google Scholar]
- 41.Hammer GP, du Prel JB, Blettner M. Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int. 2009;106(41):664–668. doi: 10.3238/arztebl.2009.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath B, Janse IC, Blok JL, Driessen RJ, Boer J, Mekkes JR, et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group. Acta Derm Venereol. 2017;97(3):412–413. doi: 10.2340/00015555-2513. [DOI] [PubMed] [Google Scholar]
- 43.Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Giamarellos-Bourboulis EJ, et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–1409. doi: 10.1111/bjd.15748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymised, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: http://www.abbvie.com/data-and-information-sharing.