Abstract
Transcobalamin (TCN1) is a vitamin B12 (cobalamin)-binding protein that regulates cobalamin homeostasis. Recent studies and bioinformatic analyses have found that TCN1 is highly expressed in cancer tissues and is associated with tumour aggressiveness and poor prognosis. The present study aimed to detect TCN1 as a novel biomarker for prognosis and chemosensitivity of colon cancer. Next-generation sequencing showed that TCN1 was one of several upregulated mRNAs in colon cancer, which was verified by further bioinformatics analyses. Western blotting (n = 9) and quantitative real time polymerase chain reaction (qRT-PCR, n = 30) revealed that TCN1 was highly expressed in colon cancer tissues at both the protein and mRNA level. A total of 194 cases of colon cancer were examined by immunohistochemistry and revealed that TCN1 expression level was related to advanced stages (P < 0.005). Kaplan–Meier analysis verified that patients with lower TCN1 expression usually had longer overall survival (P = 0.008). In addition, TCN1 was highly expressed in pulmonary metastatic tumour tissues (n = 37, P = 0.025) and exhibited higher levels in right-sided colon cancer than in left-sided colon cancer (P = 0.029). TCN1 expression in specimens that had received neoadjuvant chemotherapy decreased compared with that in colonoscopy biopsy tissues (n = 42, P = 0.009). Further bioinformatics analyses verified that apoptosis pathways might have a role in high TCN1 expression. All the studies revealed that TCN1 expression in colon cancer was significantly associated with malignant biological behaviour. Therefore, TCN1 could be used as a novel biomarker for colon cancer aggressiveness and prognosis and might also be a potential biomarker for predicting neoadjuvant chemosensitivity.
Subject terms: Cancer, Biomarkers, Oncology
Introduction
According to the data from GLOBOCAN 2018, colorectal cancer (CRC) is one of the most common intestinal tumours and ranks as the fourth leading cause of morbidity and the second leading cause of cancer-related mortality worldwide1. Despite major developments in surgery and therapeutics, long-term survival remains far from satisfactory2,3, mainly because CRC is often detected at more advanced stages. Currently, diagnosis, relapse, and metastasis monitoring of CRC largely relies on colonoscopy and imaging data, which is usually delayed. Therefore, new sensitive biomarkers are urgently required to ensure early diagnosis and to predict progression and administer timely treatments.
Transcobalamin I (TCN1) is a type of vitamin B12 (cobalamin)-binding protein that transports cobalamin from the stomach to the intestines. It plays various roles in maintaining the basic function of cell proliferation and metabolism, especially in haematopoiesis and the nervous system4,5. Researchers have found that high levels of cobalamin and TCN1 in human serum are associated with leukaemia, hepatocellular carcinoma, and phyllodes of breast tumours 6–8. Overexpression of TCN1 in tumour tissues is associated with tumorigenesis and poor biological behaviour9. Chu et al. showed that TCN1 is an oncogene, ranking as one of the top six differentially expressed mRNAs in CRC10. Feodorova et al. revealed that NOTUM, TCN1, MACC1, YKL40, GPC3, AXIN2, and IL6 are significantly upregulated in CRC and have roles in tumour invasion and metastasis11. Our previous next-generation sequencing (NGS) results verified that TCN1 is one of the top five overexpressed mRNAs in CRC12. Taken together, these findings indicate that TCN1 is a CRC-related gene that requires further study. To our knowledge, the present study is the first to investigate the relationship between TCN1 expression and the clinical behaviour of colon cancer.
NGS, western blotting, and immunohistochemistry (IHC) revealed that TCN1 was highly expressed in colon cancer tissues compared with adjacent normal mucosal tissues at both the protein and mRNA level. IHC staining of tissue microarray (TMA) slices also verified that TCN1 was highly expressed in colon cancer tissues (73.20%, 142/194) and pulmonary metastatic tumours (83.78%, 31/37). We also found that the expression of TCN1 was higher in right-sided colon cancer than in left-sided. High levels of TCN1 expression were associated with advanced pathological features and poor prognosis. Furthermore, TCN1 expression levels were decreased after neoadjuvant chemotherapy, which provided evidence that TCN1 might have a role in predicting chemosensitivity. Thus, we can conclude that high expression of TCN1 is significantly correlated with advanced clinicopathological features and poor prognosis of colon cancer. It may be novel biomarker for progression and prognosis and might be a potential biomarker to predict neoadjuvant chemosensitivity of colon cancer.
Results
TCN1 is overexpressed in CRC tissues compared with adjacent mucosal tissues
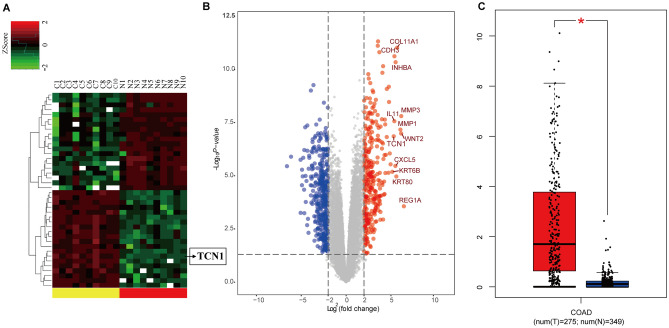
Differentially expressed mRNAs in CRC and paired adjacent normal mucosal tissues were screened by NGS (GSE104836)12. A total of 706 mRNAs (310 upregulated and 396 downregulated) were differentially expressed in CRC tissues (|log2FoldChange|> 2 and adjusted P < 0.05). A heat map and hierarchical clustering (|log2FoldChange|> 2 and adjusted P < 0.05) revealed the top 20 differentially expressed mRNAs (Fig. 1A), while a volcano plot (|log2FoldChange|> 2 and adjusted P < 0.05) indicated the differences in gene expression (Fig. 1B). The top five upregulated mRNAs were REG1B, TCN1, MMP7, CST1, and SLCO1B3, and the top five downregulated mRNAs were ADIPOQ, OTOP2, KRT24, RERGL, and CA7. TCN1 was ranked the second highest upregulated mRNA. Based on the COAD cohort in the GEPIA database, further bioinformatics analyses were performed and confirmed that TCN1 was significantly upregulated in CRC tissues (n = 275) compared with non-tumour tissues (n = 349) (P < 0.05, Fig. 1C).
Figure 1.
Next generation screening of TCN1 mRNA expression in 10 cases of CRC and adjacent normal mucosal tissues and database verification. (A) Heat map of the top 20 differentially expressed mRNAs. (B) Volcano plot of differential mRNA expression (|log2FoldChange|> 2 and adjusted P < 0.05). (C) COAD data set verification of TCN1 expression in CRC.
TCN1 was confirmed to be highly expressed in human colon cancer tissues
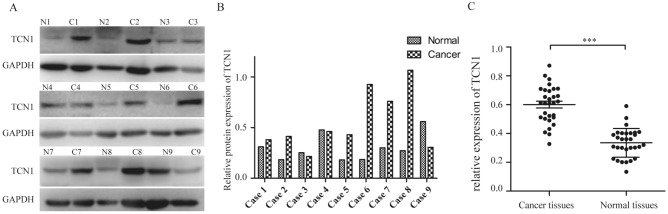
Western blot and qRT-PCR assays revealed that TCN1 was highly expressed in most of colon cancer tissues both at the protein (Fig. 2A, B) and mRNA level (Fig. 2C), but was poorly expressed in adjacent normal colon mucosal tissues. The relative mRNA expression level of TCN1 in cancer tissues was higher than in non-cancerous tissues (0.601 ± 0.024 vs. 0.335 ± 0.018, P < 0.001).
Figure 2.
TCN1 expression in colon cancer. (A, B) Western blot analysis of TCN1 in 9 cases of colon cancer. (C) qRT-PCR analysis of TCN1 mRNA in 30 cases of colon cancer versus normal tissues (***P < 0.001).
Increased TCN1 expression was associated with clinicopathological features and poor prognosis of colon cancer
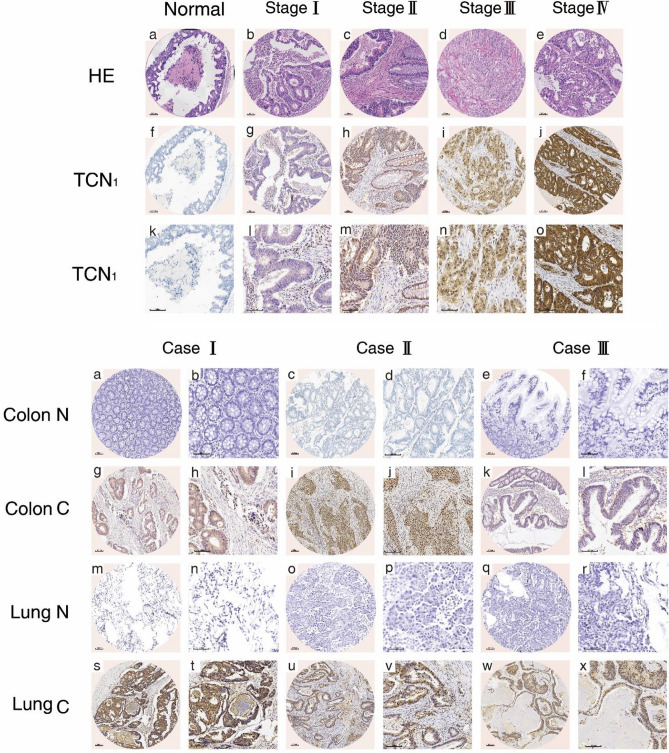
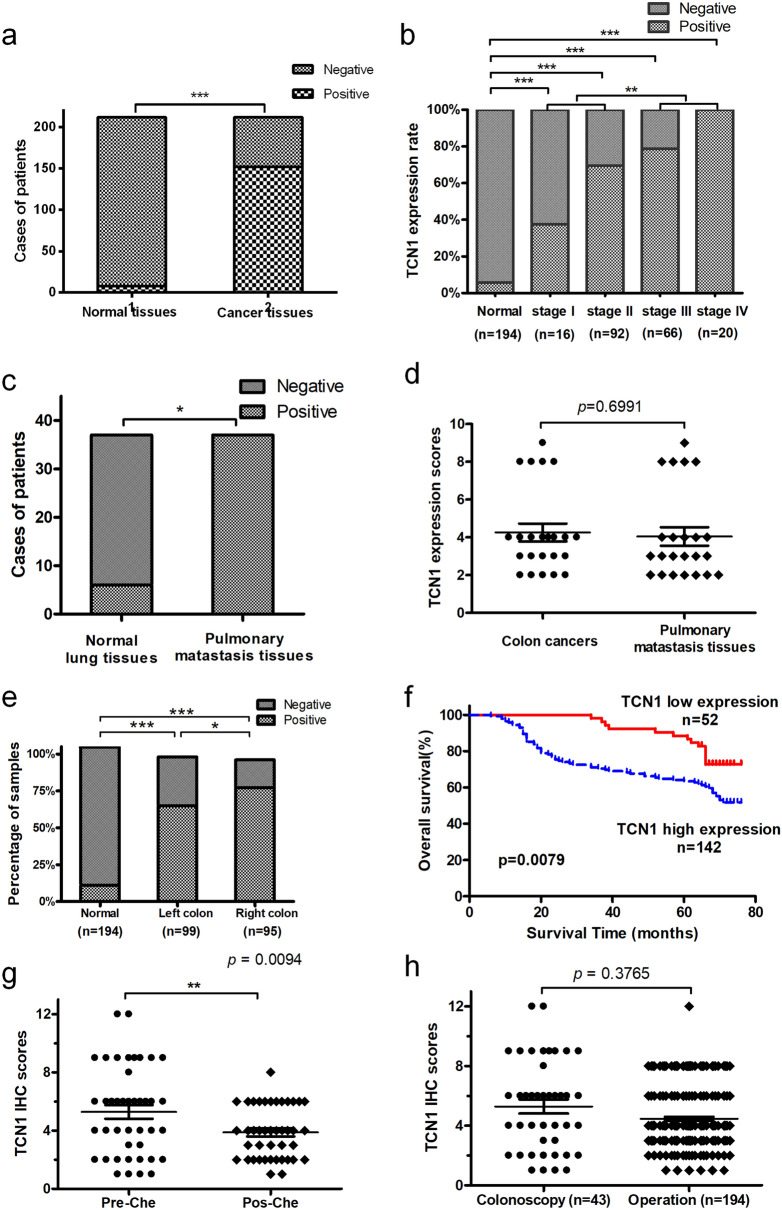
The clinicopathological characteristics of the 194 colon cancer patients are presented in Table 1. IHC staining was performed on TMA slides, and immunoreactivity of TCN1 was observed primarily in the cytoplasm (Fig. 3). TCN1 expression was significantly higher in colon cancer tissues than in adjacent normal tissues (73.20% vs. 5.67%, P < 0.01, Fig. 4a). Furthermore, positive TCN1 expression was significantly correlated with T, N, M, TNM stage and age (Fig. 4b and Table1) and was higher in right-sided colon tumors than in left-sided tumors (Fig. 4e, Table 1). It was much higher in lung metastatic tissues (Fig. 4c) and the expression was consistent with that of the primer colon cancer tissues (Fig. 4d). Furthermore, Kaplan–Meier survival analysis demonstrated that higher TCN1 expression in patients represented significantly poorer OS (Fig. 4f). Univariate analysis showed that age, TNM staging and TCN1 expression were prognostic factors, while multivariate prognostic analysis showed that TCN1 expression level and M staging were independent prognostic factors (Table 2).
Table 1.
Relationship between TCN1 expression and clinicopathological parameters of 194 colonic cancer patients.
| Parameter | TCN1 expression (n) | χ2 value | P value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Gender | ||||
| Male | 25 | 72 | 0.105 | 0.746 |
| Female | 27 | 70 | ||
| Age | ||||
| ≤ 65 | 27 | 100 | 5.761 | 0.016 |
| > 65 | 25 | 42 | ||
| Tumor location | ||||
| Left sided | 33 | 65 | 4.763 | 0.029 |
| Right sided | 19 | 77 | ||
| T classification | ||||
| T1–T2 | 9 | 7 | 7.707 | 0.006 |
| T3–T4 | 43 | 135 | ||
| N classification | ||||
| N0 | 38 | 72 | 7.760 | 0.005 |
| N1–N2 | 14 | 70 | ||
| M classification | ||||
| M0 | 52 | 121 | 8.624 | 0.003 |
| M1 | 0 | 21 | ||
| TNM stage | ||||
| I–II | 38 | 70 | 8.722 | 0.003 |
| III–IV | 14 | 72 | ||
| Drinking history | ||||
| Yes | 14 | 39 | 0.006 | 0.940 |
| No | 38 | 103 | ||
| Smoking history | ||||
| Yes | 13 | 36 | 0.002 | 0.960 |
| No | 39 | 106 | ||
| Pathological type | ||||
| Adenocarcinoma | 40 | 114 | 0.262 | 0.609 |
| Mucinous adenocarcinoma | 12 | 28 | ||
| Lung metastatic and normal tissues | ||||
| Lung metastatic tissues | 0 | 37 | 4.811 | 0.025 |
| Lung normal tissues | 6 | 31 | ||
Figure 3.
Representative IHC staining of different tissues. (a) Representative haematoxylin–eosin (HE) and IHC staining images for distinct stages and staining degree of colon cancer. (b) Representative IHC images of paired colon cancer, pulmonary metastatic, and adjacent normal tissues.
Figure 4.
Increased TCN1 expression is associated with clinicopathological features, poor prognosis, and chemosensitivity of colon cancer. (a) TCN1 expression in colon cancer and adjacent normal mucosal tissues. (b) Expression status of distinct colon cancer clinical stage and adjacent normal mucosal tissues. (c) Expression of TCN1 in pulmonary metastatic tissues (PMT) and paired normal lung tissues (NLT). (d) Comparison of TCN1 expression in colon cancer and PMT. (e) Expression of TCN1 in left- and right-sided colon cancer. (f) Effect of TCN1 expression level on overall survival. (g) TCN1 expression level was decreased after platinum-containing chemotherapy (pre-chemical therapy vs post-chemical therapy). (h) TCN1 expression in colonoscopy biopsy tissues and specimens resected during surgery. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 2.
Univariate and multivariate analysis of different prognostic factors for overall survival of 194 colonic cancer patients.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender | 0.594 | 0.611–1.487 | 0.834 | |||
| Age | 0.969 | 0.608–1.545 | 0.895 | – | – | – |
| Cancer location | 0.800 | 0.513–1.247 | 0.324 | – | – | – |
| Pathology type | 1.202 | 0.674–2.142 | 0.534 | |||
| Smoking history | 0.923 | 0.550–1.548 | 0.762 | – | – | – |
| Drinking history | 0.860 | 0.517–1.430 | 0.561 | – | – | – |
| T classification | 1.179 | 0.513–2.712 | 0.699 | – | – | – |
| N classification | 1.725 | 1.105–2.692 | 0.016 | 2.155 | 0.847–5.479 | 0.107 |
| TNM Stage | 1.569 | 1.006–2.447 | 0.047 | 0.599 | 0.227–1.583 | 0.301 |
| M classification | 2.456 | 1.351–4.466 | 0.003 | 2.020 | 1.030–3.963 | 0.041 |
| Expression states | 2.189 | 1.206–3.973 | 0.010 | 1.855 | 1.003–3.432 | 0.047 |
TCN1 expression level decreases after chemotherapy
After treatment with platinum-containing neoadjuvant chemotherapy, TCN1 expression in tumour tissues was decreased(P = 0.009, Fig. 4g). There was no significant difference in TCN1 expression status between colonoscopy biopsy tissues and specimens removed during surgery (P = 0.3765, Fig. 4h).
GO (Gene Ontology) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis results of TCN1-related genes in CRC
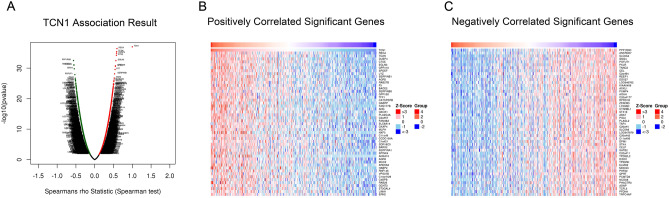
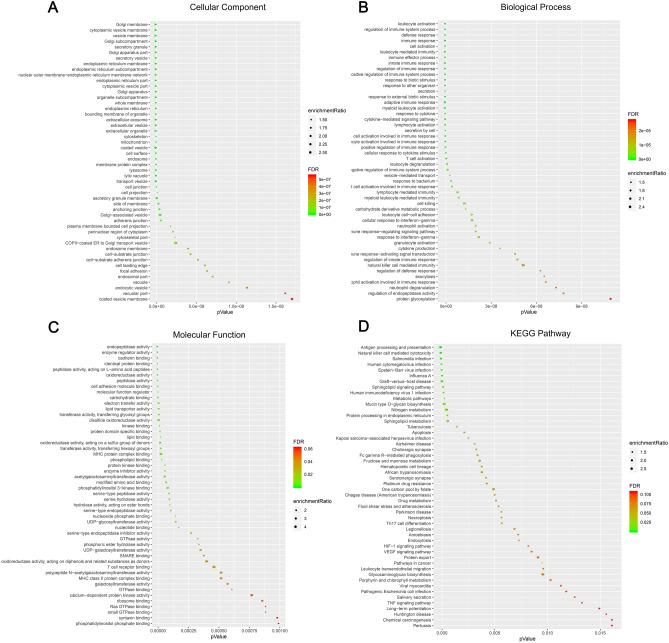
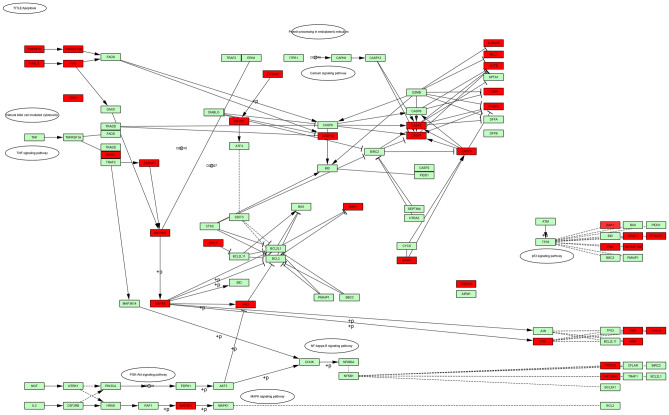
GO and KEGG analysis was performed, and a total of 5,857 differentially expressed genes (2,683 positively related and 3,174 negatively related, false discovery rate < 0.01) correlated with TCN1 were detected. Spearman’s test was conducted to analyse correlations between TCN1 and genes differentially expressed in CRC (red represents positively related genes and blue represents negatively related genes) (Fig. 5A). The top 50 genes that were positively and negatively correlated with TCN1 are shown in heat maps (Fig. 5B, C). Over-representation analysis (ORA) was conducted in the top 50 genes positively correlated with TCN1. GO analysis showed that the identified TCN1-related gene products were mainly expressed in the cell membrane, Golgi apparatus, endoplasmic reticulum, and vesicles, and are involved in cytokine production, protein glycosylation, apoptosis, exocytosis, and vesicle transport (Fig. 6A–C). KEGG pathway analysis showed that cancer-related signalling pathways were enriched, including immunity, apoptosis, platinum resistance, and metabolic, hypoxia-inducible factor-1A (HIF-1A) and vascular endothelial growth factor (VEGF) signalling pathways (Fig. 6D). Molecules (marked in red) connected with TCN1 are presented in an apoptotic pathway network in Fig. 7.
Figure 5.
Differentially expressed genes correlated with TCN1 in CRC based on the LinkedOmics database. (A) Hierarchical clustering is presented to show the differentially expressed genes correlated with TCN1. (B, C) The top 50 genes positively and negatively correlated with TCN1 are presented in heat maps. Red and blue represent genes positively and negatively correlated with TCN1, respectively.
Figure 6.
Genes positively correlated with TCN1. Genes positively correlated with TCN1 as represented by over-representation analysis (ORA) and classified by (A) cellular components, (B) biological processes, (C) molecular functions, and (D) KEGG pathway analysis.
Figure 7.
Apoptotic signalling pathways related to TCN1. Red represents the pathways correlated with apoptotic pathways.
Discussion
TCN1 is a vitamin B12-binding protein that is present in the cytoplasm and performs functions with TCN2 and intrinsic factors in the processes of cobalamin transport, metabolism, and homeostasis13,14. Studies have shown aberrant serum levels of vitamin B12 and TCN1 in some solid tumours15–19. Upregulation of TCN1 is associated with tumourigenesis and progress20–25. Chu’s meta-analysis of gene expression data based on microarrays demonstrated that TCN1 act as an oncogene and is overexpressed in CRC10. We hypothesized that TCN1 might have a role in colon cancer. The NGS results in CRC revealed that TCN1 ranked as the second most upregulated mRNA12. Bioinformatic analysis based on the COAD data set verified that TCN1 was indeed overexpressed in CRC. qRT-PCR and western blot assays also verified that TCN1 was highly expressed at both the mRNA and protein level in CRC tissues. All these results provided more evidence that TCN1 has a role in the tumourigenesis of CRC10,11, and suggested that TCN1 could be a potential novel biomarker.
IHC in TMAs proved that TCN1 was highly expressed in colon cancer tissues and demonstrated that high expression levels were correlated with advanced pathological features. Pulmonary metastatic tumour tissue from colon cancer also showed high expression of TCN1 and the expression level was in accordance with that in the primary colon cancer tissues. Thus, it could be inferred that TCN1 performed roles in colon cancer progression and metastasis. Lee et al. found that overexpression of TCN1 in rectal cancer was related to advanced pre-/post-treatment tumour status, nodal status, vascular invasion, and tumour regression grade21. Wu et al. also reported that TCN1 was highly expressed in pancreatic cancer26 and Chu et al. found it had a role in tumour invasion and metastasis, which were all consistent with our findings.
Patients with high expression of TCN1 showed shorter OS and poor prognosis. Previous studies demonstrated that high expression of TCN1 was related to poor prognosis of hypopharyngeal squamous cell and rectal cancer20,21. Our findings were concordant with these reports. Univariate analysis proved that TNM stage and TCN1 expression were prognostic factors, while multivariate analysis showed that TCN1 expression and M classification were independent prognostic factors. Further, we compared the expression levels in right- and left-sided colon cancer. Interestingly, TCN1 expression levels were much higher in right-sided than in left-sided colon cancer and the survival time was accordingly shorter, which was paralleled with the literature27. This provided further evidence that TCN1 expression was associated with colon cancer behaviour and prognosis.
We found that there was no significant difference in TCN1 expression between colonoscopy biopsy tissues and surgical specimens, which suggested that biopsy tissues obtained by colonoscopy might represent surgical specimens for the detection of TCN1 expression, and thus would make detection easier. Nevertheless, only a small cohort from a single centre was examined, prospective experiments with a larger sample size should performed to confirm the results. We also discovered that the expression level of TCN1 decreased after administration of platinum-containing chemotherapy. It could be inferred that the chemoradiotherapy will affect TCN1 expression through some mechanism. Zhang et al. screened differentially expressed genes from the Gene Expression Omnibus database and revealed that aberrant expression of TCN1 and TGFBI was regulated by DNA methylation28. Lee et al. performed in vitro assays and verified that downregulation of TCN1 led to an obvious reduction in the proliferation of FaDu cells. Knockdown of TCN1 significantly sensitized FaDu cells to cisplatin treatment, thus suggesting that it might function through an apoptosis pathway21. Researchers also proved that TCN1 expression levels were associated with neoadjuvant chemotherapy sensitivity in hypopharyngeal squamous cell cancer and chemoradiotherapy sensitivity in rectal cancer20,21, but the underlying mechanism remains unclear.
To reveal the potential downstream mechanism of TCN1 in CRC, genes related to TCN1 were selected to determine the way it functions. Because TCN1 might have a role as an oncogene10, genes positively correlated with TCN1 were finally chosen for GO and KEGG analysis. As expected, the result proved that TCN1 is associated with many cancer-related genes and pathways, which provided evidence that TCN1 might affect tumourigenesis and progress by affecting transcription. Apoptotic pathways were enriched in the KEGG analysis and verified that lysosomes and apoptotic proteins of caspase 3 were located in a prominent location in the pathway network, suggesting that TCN1 may partially function through the apoptotic pathway. In the meantime, we found that TCN1 was significantly associated with immune response pathways, which provided a new avenue for our future study. Nevertheless, an experiment has not undertaken to verify this inference, which is the limitation of our study. We are focusing on this area and will explore the underlying mechanism in our future work.
To our knowledge, this is the first report to discuss the relationship between expression of TCN1 and colon cancer behaviour. The research proves that TCN1 is associated with prognosis and presents clinic evidence for previous research10,11. It also provides a new area for future study, especially the underlying mechanism of tumour invasion and the mechanism of predicting chemosensitivity.
Taken together, we conclude that TCN1 expression is significantly overexpressed in colon cancer and is correlated with advanced pathological features. TCN1 may be a predictor of prognosis and a potential biomarker for predicting chemosensitivity. However, further in vivo and in vitro experiments should be performed to explore the underlying mechanism.
Methods
Ethics statement
This study was carried out in accordance with the guidelines of the Fourth Hospital of Hebei Medical University and Hebei Provincial Tumour Hospital. The human samples were provided by volunteer donors who gave their informed consent, and the study was approved by the Ethical Committee of the Fourth Hospital of Hebei Medical University (ID 2017MEC115).
Tissue collection
All fresh specimens were collected from January 2018 to June 2019 at Hebei Medical University Fourth Affiliated Hospital. Resected tissues of colon cancer and matched adjacent non-tumour colon mucosal tissues (n = 39) were snap-frozen in liquid nitrogen and stored at − 80 °C for qRT-PCR assay and western blotting. None of the patients were treated by radiotherapy or chemotherapy before surgery.
RNA extraction, quantitative real-time PCR, and next-generation sequencing
Total RNA was extracted from tissues using the Trizol (Life Technologies, Massachusetts, USA) reagent12,29. cDNA was synthesized from the total RNA using the GoScript Reverse Transcription System (Promega, Wisconsin, USA) according to the manufacturer's instruction. A SYBR Green PCR kit (Promega, Wisconsin, USA) was used in the amplification process with a 7,500 Real-time PCR system (Applied Biosystems, Massachusetts, USA). The primers (HQP017978, Eockville, MD, USA) were purchased from GeneCopoeia. GAPDH was used as an internal control. The qRT-PCR results were analyzed with the 2−ΔΔCt method. All the reactions were conducted in triplicate and the values are shown as means ± SD. mRNA data were selected from our earlier NGS results11, which had been deposited in GEO database (GSE104836, https://www.ncbi.nlm.nih.gov/ geo/query/acc.cgi?acc = GSE104836). The differential expression of the genes was assessed by limma package30 (https://bioconductor.org/ packages/release/bioc/html/limma.html) in R Software using RNA-seq read counts. |log2FoldChange|> 2 and adjusted P < 0.05 as cut-off criteria for screening the differentially expressed genes (DEGs). A heatmap and volcano plot were drawn using pheatmap and ggplot2 packages in R software to indicate the DEGs.
Databases verification
The relative mRNA expression level of TCN1 in a colon adenocarcinoma (COAD) data set were analysed based on an online database: Gene Expression Profiling Interactive Analysis (GEPIA, https://gepia.cancer-pku.cn/)31. The database is based on The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx). ANOVA was used for the comparison of tumour tissues with paired normal tissues, with the following thresholds: |log2FC|= 1 and P = 0.05.
Western blot analysis
The tumor tissues and their paired adjacent normal colon mucosa were collected. Total proteins of each group were extracted with RIPA buffer containing protein inhibitors (Solarbio, Beijing, China) and quantified by BCA kit (Thermo Fisher Science). The protein cleavage products were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane (Solarbio, Beijing, China). Sealed with 5% skim milk at room temperature for 1 h, and incubated overnight with rabbit anti-human TCN1 monoclonal (a6414 ABclonal, Wuhan, China) at 4 °C. Then washed with Tris buffer containing Tween (TBST) for 3 times, and incubated with HRP labelled goat anti-rabbit second antibody (AS014, ABclonal, Wuhan, China) for 1 h at room temperature. After washing with TBST for 3 times, the signal was detected by chemiluminescence. GAPDH (ab9485, Bioworld, St Louis, MN, USA) was used as a protein loading control. The intensity of protein fragments was quantified with Image J software.
Tissue microarray (TMA)
TMA slices were obtained from the Department of Pathology, Hebei Medical University Fourth Affiliated Hospital. The TMA, which included 194 cases of colon cancer and paired adjacent normal mucosal tissue, 37 cases of pulmonary metastatic tumours from colon cancer and adjacent normal lung tissues, and 42 cases of colonoscopy biopsy and paired post-chemotherapy colon cancer specimens (these patients received platinum-containing neoadjuvant chemotherapy), was constructed from formalin-fixed, paraffin-embedded tissue blocks. Enrolled patients were followed up by the Department of Follow-up Centre, Hebei Medical University Fourth Affiliated Hospital, and all clinical data were collected. The final follow-up date was 20 May 2019 and the follow-up endpoint was overall survival (range 5.2–76.3 months) with a median follow-up period of 67.5 months.
Haematoxylin–eosin (HE) staining and immunohistochemistry (IHC)
HE staining was performed for tumour diagnosis, and TCN1 expression was detected using IHC in tumour and adjacent normal tissues. Continuous sections with a thickness of 4 μm were performed for HE and IHC staining on Envision. Anti-TCN1 monoclonal antibody was purchased from AB-clonal (1:60, a6614, ABclonal, Wuhan, China). All slides were interpreted by two pathologists independently who were blinded to the clinical information. The IHC staining score included the proportion of positively stained tumour cells and the staining intensity. The proportion of positively stained tumour cells was scored as follows: 0 (no tumour cells stained), 1 (< 25% tumour cells stained), 2 (25–50% tumour cells stained), 3 (50–75% tumour cells stained), and 4 (75–100% tumour cells stained). The grading of staining intensity was evaluated by the following criteria: 3 (brown, strong staining), 2 (yellow brown, moderate staining), 1(light yellow, weak staining), and 0 (no staining). The final total staining score was calculated by multiplying the proportion of stained tumour cells and the staining intensity score. TCN1 expression was scored and a score of ≤ 3 indicated negative TCN1 expression, while a score of > 3 was considered as positive TCN1 expression. A score of ≤ 6 was designated as low expression and a score of > 6 as high expression. The pathological diagnosis was made in accordance with the histological classification of tumours developed by the World Health Organization.
GO and KEGG enrichment analysis of TCN1-related genes in CRC based on the LinkedOmics database
The LinkedOmics database (https://linkedomics.org), which has been used to analyse TCGA cancer-associated datasets27, was selected to identify the differentially expressed genes related to TCN1. The database included 379 CRC cohorts. Results from LinkedOmics were assigned and ranked. GO functional enrichment analysis and KEGG pathway analysis were performed by gene set enrichment analysis (GSEA). Spearman’s test was conduct to perform statistical analyses. All the results were graphically showed in heat maps and volcano plots. Linkfinder-related modules were selected to show the results of differentially expressed genes and the network analyses.
Statistical analysis
Statistical analysis was performed using the software package SPSS 21.0 (Chicago, IL, USA) and figures were constructed with Prism5.0 (GraphPad, Lnc., La Jolla, CA, USA) and R (version: 3.6.1). Correlations between TCN1 expression and clinicopathological features were determined using chi-squared tests or Fisher’s exact tests, as appropriate. The endpoints were defined as patient death or the final follow-up date. Overall survival (OS) was defined as the time between surgery time and death or the last follow-up visit. Kaplan–Meier survival analysis was conducted to plot survival curves and log-rank tests were used to evaluate the prognostic differences between subgroups. Cox multivariate regression analysis was conducted after prognostic significance at the univariate level was determined. A P value of < 0.05 was considered statistically significant.
Ethics approval
We confirm that all the methods had been carried out in accordance with the relevant guidelines and regulations of the Declaration of Helsinki.
Acknowledgements
Our paper was supported by the grants from the Cancer Research Project of the National Cancer Centre in 2017 (NCC2017A23), the Health and Family Planning Commission of Hebei Province (20150362), and a government-funded project of the Outstanding Talents Training Program in Clinical Medicine (2014-36100603). We thank H. Nikki March, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
G.W. and L.Z. conceived the study, G.L. and F.L. wrote the manuscript, G.L., Y.W., L.Z., X.Z., J.Z., and Y.S. collected clinical data and conducted experiments, M.Y, Y.L., and H.Y. performed the pathological assays, and Y.G. analysed the data and prepared all the figures. All authors reviewed the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Heinemann V, et al. FOLFIRI plus cetuximab versus folfiri plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 3.Boyle T, Fritschi L, Platell C, Heyworth J. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br. J. Cancer. 2013;109:814–822. doi: 10.1038/bjc.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray JG, Blom HJ. Vitamin B12 insufficiency and the risk of fetal neural tube defects. QJM Int. J. Med. 2003;96:289–295. doi: 10.1093/qjmed/hcg043. [DOI] [PubMed] [Google Scholar]
- 5.Magnus EM. Cobalamin and unsaturated transcobalamin values in pernicious anaemia: relation to treatment. Scand. J. Haematol. 1986;36:457–465. doi: 10.1111/j.1600-0609.1986.tb02281.x. [DOI] [PubMed] [Google Scholar]
- 6.Chong LY, et al. Keratin 15, transcobalamin I and homeobox gene Hox-B13 expression in breast phyllodes tumors: novel markers in biological classification. Breast Cancer Res. Tr. 2012;132:143–151. doi: 10.1007/s10549-011-1555-6. [DOI] [PubMed] [Google Scholar]
- 7.Kudo H, et al. Distribution of vitamin B12 R-binder in carcinomas of the digestive tract. J. Clin. Pathol. 1988;41:320–323. doi: 10.1136/jcp.41.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adrouny A, Seraydarian A, Levine AM, Hungerford GF, Carmel R. Cyclic eosinophilic leukocytosis in eosinophilic Leukemia with observations on transcobalamin I and eosinophils. Cancer Am. Cancer Soc. 1984;54:1374–1378. doi: 10.1002/1097-0142(19841001)54:7<1374::aid-cncr2820540724>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Martinelli M, et al. A Candidate gene study of one-carbon metabolism pathway genes and colorectal cancer risk. Br. J. Nutr. 2013;109:984–989. doi: 10.1017/S0007114512002796. [DOI] [PubMed] [Google Scholar]
- 10.Chu C, et al. Gene expression profiling of colorectal tumors and normal mucosa by microarrays meta-analysis using prediction analysis of microarray, artificial neural network, classification, and regression trees. Dis. Markers. 2014;2014:1–11. doi: 10.1155/2014/634123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feodorova Y, et al. Novel insights into transcriptional dysregulation in colorectal cancer. Neoplasma. 2018;65:415–424. doi: 10.4149/neo_2018_170707N467. [DOI] [PubMed] [Google Scholar]
- 12.Li M, et al. Differentially expressed lncRNAs and mRNAs identified by NGS analysis in colorectal cancer patients. Cancer Med. 2018;7:4650–4664. doi: 10.1002/cam4.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oner V, et al. Response to a letter to the editor entitled "Low serum B12 level does not mean vit. B12 deficiency-problems related to the diagnosis of vitamin B12 deficiency". Curr. Eye Res. 2014;39:427–428. doi: 10.3109/02713683.2013.833249. [DOI] [PubMed] [Google Scholar]
- 14.Ozsoylu S. Vit B12 treatment. Eur. J. Pediatr. 2012;171:737–739. doi: 10.1007/s00431-011-1637-9. [DOI] [PubMed] [Google Scholar]
- 15.Carmel R. Extreme elevation of serum transcobalamin I in patients with metastatic cancer. N. Engl. J. Med. 1975;292:282–284. doi: 10.1056/NEJM197502062920603. [DOI] [PubMed] [Google Scholar]
- 16.Carmel R, Eisenberg L. Serum vitamin B12 and transcobalamin abnormalities in patients with cancer. Cancer Am. Cancer Soc. 1977;40:1348–1353. doi: 10.1002/1097-0142(197709)40:3<1348::aid-cncr2820400352>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari A, Torrezan GT, Carraro DM, Aguiar JS. Association of folate and vitamins involved in the 1-carbon cycle with polymorphisms in the methylenetetrahydrofolate reductase gene (MTHFR) and global DNA methylation in patients with colorectal cancer. Nutrients. 2019;11:1368. doi: 10.3390/nu11061368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranti EH, et al. Low vitamin B12 increases risk of gastric cancer: a prospective study of one-carbon metabolism nutrients and risk of upper gastrointestinal tract cancer. Int. J. Cancer. 2017;141:1120–1129. doi: 10.1002/ijc.30809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Wei Y, Song A, Li Y. Association study between genome-wide significant variants of vitamin B12 metabolism and gastric cancer in a Han Chinese population. IUBMB Life. 2016;68:303–310. doi: 10.1002/iub.1485. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Transcobalamin I: a novel prognostic biomarker of neoadjuvant chemotherapy in locally advanced hypopharyngeal squamous cell cancers. Onco Targets Ther. 2018;11:4253–4261. doi: 10.2147/OTT.S166514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, et al. Overexpression of transcobalamin 1 is an independent negative prognosticator in rectal cancers receiving concurrent chemoradiotherapy. J. Cancer. 2017;8:1330–1337. doi: 10.7150/jca.18274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa K, et al. Distribution of vitamin B12 R-binder in lung tumors. Implications for cell differentiation. Pathol. Res. Pract. 1989;184:234–241. doi: 10.1016/S0344-0338(89)80125-6. [DOI] [PubMed] [Google Scholar]
- 23.Waibel R, et al. New derivatives of vitamin B12 show preferential targeting of tumors. Cancer Res. 2008;68:2904–2911. doi: 10.1158/0008-5472.CAN-07-6771. [DOI] [PubMed] [Google Scholar]
- 24.Chong LY, et al. Keratin 15, transcobalamin I and homeobox gene Hox-B13 expression in breast phyllodes tumors: novel markers in biological classification. Breast Cancer Res. Treat. 2012;132:143–151. doi: 10.1007/s10549-011-1555-6. [DOI] [PubMed] [Google Scholar]
- 25.Alkushi A, et al. Immunoprofile of cervical and endometrial adenocarcinomas using a tissue microarray. Virchows Arch. 2003;442:271–277. doi: 10.1007/s00428-002-0752-4. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, et al. Orchestrating a biomarker panel with lncRNAs and mRNAs for predicting survival in pancreatic ductal adenocarcinoma. J. Cell Biochem. 2018;119:7696–7706. doi: 10.1002/jcb.27119. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, et al. Expression and gene regulation network of RBM8A in hepatocellular carcinoma based on data mining. Aging (Albany NY). 2019;11:423–447. doi: 10.18632/aging.101749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Dong S, Feng J. Epigenetic profiling and mRNA expression reveal candidate genes as biomarkers for colorectal cancer. J. Cell. Biochem. 2019;120:10767–10776. doi: 10.1002/jcb.28368. [DOI] [PubMed] [Google Scholar]
- 29.Li J, et al. The lncRNA FEZF1-AS1 promotes the progression of colorectal cancer through regulating OTX1 and targeting miR-30a-5p. Oncol. Res. 2020;28:51–63. doi: 10.3727/096504019X15619783964700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie ME, et al. Limma powers differential expression analyses for rna-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Z, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]