Abstract
We describe a dementia patient with comorbid recurrent cellulitis and lymphedema in the left lower limb who was treated successfully for recurrent cellulitis by lymphaticovenular anastomosis (LVA). The patient, an 83-year-old woman, suffered from recurrent cellulitis three times a year on average for 15 years. Compression therapy was impossible because of dementia. After LVA, there has been no recurrence of cellulitis for 2 years.
It is difficult to administer decongestive lymphatic therapy in some patients, such as patients with dementia. LVA is a promising treatment for recurrent cellulitis in a dementia patient with lymphedema.
Keywords: Lymphedema, Cellulitis, Lymphaticovenular anastomosis, Dementia
Recurrent cellulitis is one of the complications of lymphedema. Although supporting evidence is lacking, compression therapy to prevent recurrence of cellulitis is currently recommended on the basis of expert consensus for patients with chronic lower limb edema.1 However, it is difficult to administer decongestive lymphatic therapy in patients with dementia. A systematic review of the literature revealed that successful treatment for the reduction of cellulitis can be achieved by surgical treatment options for lymphedema, including lymphaticovenular anastomosis (LVA), suggesting LVA to be promising for the treatment of recurrent cellulitis secondary to lymphedema.2
We describe a patient with comorbid recurrent cellulitis and lymphedema in the left lower limb who was successfully treated for recurrent cellulitis by LVA. Written consent to publish the case details and images described in this case report was obtained from the patient and the patient's son.
Case report
An 83-year-old woman was referred with complaints of delayed healing of an ulcer in the left foot that was caused by necrotizing fasciitis after recurrent cellulitis (three times a year on average) during the preceding 15 years (Fig 1). The patient had a history of cervical cancer that had been totally resected, including pelvic lymph nodes, 22 years earlier. The patient had no diabetes mellitus or lower limb ischemia but had been diagnosed with severe dementia with a score of 5 on the revised Hasegawa Dementia Scale.3 The patient had no history of kidney or heart failure, hypoproteinemia, liver cirrhosis, deep venous thrombosis, chronic venous obstruction, drug-induced edema, thyroid dermopathy, or other endocrine cause of edema. The left lower extremity was the only area in which the edema existed. Cervical cancer was suspected as the cause of lymphedema.
Fig 1.
An 83-year-old woman presented with complaints of delayed healing of an ulcer in the left foot caused by necrotizing fasciitis after recurrent cellulitis (three times a year on average) during the preceding 15 years. She had a history of cervical cancer treated by resection 22 years earlier. The patient had no diabetes mellitus or lower limb ischemia but had been diagnosed with severe dementia (revised Hasegawa Dementia Scale score of 5). a, Status showing necrotizing fasciitis after recurrent cellulitis. b, At 1 month later. c, Wound healing established with conservative treatment for 6 months.
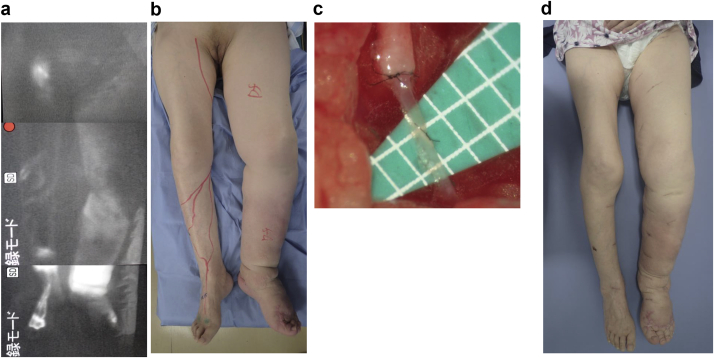
Indocyanine green (ICG) lymphography was conducted by injecting ICG subcutaneously into the lateral border of the Achilles tendon and the first web space of both feet.4 Lymphatic images were then obtained using an infrared camera system. ICG lymphography revealed dermal backflow of lymph in the whole left lower extremity (Fig 2, a and b). We made a diagnosis of left lower extremity lymphedema, International Society of Lymphology stage III,5 on the basis of these findings. The lower extremity lymphedema index was calculated as follows: (the sum of the squares of the circumferences at five areas in the lower extremity)/(the body mass index) = lower extremity lymphedema index.6 It was 182 on the right lower extremity and 313 on the left lower extremity before treatment (Fig 2, b). Compression therapy using a compression garment was attempted for the edema in the left lower extremity 10 years earlier, but the patient discontinued after several months. The dementia had started 5 years earlier and had been worsening. Currently, compression therapy is impossible because she is uncooperative and removes it herself.
Fig 2.
a, Indocyanine green (ICG) lymphography revealed signs of dermal backflow of lymph in the left lower leg and thigh. b, International Society of Lymphology stage III lymphedema in left lower limb. Lower extremity lymphedema index was 182 on the right and 313 on the left. c, Lymphatic vessels and veins anastomosed end to end; six lymphaticovenular anastomoses (LVAs) were performed in total (three in the lower leg region and three in the thigh region). d, Compression therapy was impossible even after LVA. So far, there has been no recurrence of cellulitis for 2 years. The lower extremity lymphedema index was 189 on the right and 257 on the left at 2 years after LVA.
LVA was planned to reduce the risk of cellulitis after wound healing was established by conservative treatment. Written informed consent was provided by the patient's son. LVA was performed under general anesthesia. LVAs were performed along the great saphenous vein based on our experience of LVA under a surgical microscopic view. After 1- to 5-cm skin incisions were made, the collecting lymphatics and subcutaneous veins were dissected and identified. The veins and lymphatic vessels were anastomosed in an end-to-end manner using 11-0 or 12-0 nylon suture thread under the microscope (Fig 2, c). Multiple LVAs were performed because a positive correlation between the number of LVAs and therapeutic efficacy has been reported.7 Patency of each anastomosis was confirmed by lymph flow into the veins by massaging the peripheral region after anastomosis. In total, six LVAs were established (three in the lower leg area and three in the thigh area). All the LVAs were performed in an end-to-end manner between distal lymphatics and proximal veins because we believe end-to-end anastomosis is the most precise and longest lasting.
Compression therapy was impossible even after LVA. So far, as of this writing, there has been no recurrence of cellulitis for 2 years. The lower extremity lymphedema index was 189 on the right lower extremity and 257 on the left lower extremity at 2 years after LVA (Fig 2, d).
Discussion
The lymphedematous limb is immunocompromised. The intensive defense provided by acquired immunity for the protection of living organisms against infection requires functional lymphatic organs, including lymph nodes and lymphatic conduits. The lymphatic vessels play an important role in the trafficking of leukocytes and soluble antigens from the peripheral tissue to the lymph nodes, where acquired immunity priming takes place according to the type of antigens and immune cells transported.7,8 The main immune-related role of the lymphatics is performed in the lymphatic endothelium. Lymphatic endothelial cells secrete chemokines that help dendritic cells (DCs), T cells,9 and neutrophils10, 11, 12 enter and crawl through lymphatics.10 Lymph nodes provide a specialized microenvironment for the gathering of lymphocytes and antigen-presenting cells, including DCs. DCs exist in most peripheral tissues and are the most well studied type of cell. Lymph nodes organize cell trafficking from two sources: the lymphatic vessels, which transport interstitial fluid including DCs derived exclusively from adjacent tissues; and the blood vessels, through which the lymphocytes (including B cells, monocyte-derived DCs, and naive T cells) enter the lymph nodes.8 If the route from lymphatics is disturbed, the immune system would be isolated from an inflammatory process occurring in the afferent tissue and remain unengaged, thereby leading to immune ignorance.12 Lymphedema is a disease in which the lymphatic transport is disturbed or lymph nodes are lost. It is impossible to activate acquired immunity in lymphedema (Fig 3, left). The severe cellulitis that frequently occurs in association with lymphedema is assumed to be caused by impaired adaptive immunity. LVA may be considered to create a bypass through blood circulation for DCs to the lymph nodes by which DCs can transmit antigen information to T cells from the viewpoint of acquired immunity (Fig 3, right). A systematic review of the literature indicates that LVA reduces cellulitis and suggests that LVA is promising for the management of cellulitis secondary to lymphedema.2,13
Fig 3.
Left, Lymphedema is a state in which the lymphatic conduits are impaired or lymph nodes are lost; expression of acquired immunity is impossible in the presence of lymphedema. Right, From the viewpoint of acquired immunity, lymphaticovenular anastomosis (LVA) is considered to create a bypass to the lymph nodes through which dendritic cells (DCs) can transmit antigen information to T cells from the blood circulation. LVA creates a bypass between lymphatics and veins, which makes it possible for DCs to recirculate through blood vessels. APC, Antigen-presenting cell.
Based on current expert consensus, compression therapy to prevent the recurrence of cellulitis in lower limb edema is also recommended.1 However, it is difficult to administer decongestive lymphatic therapy in some patients, such as those with dementia, as in this report. An estimated 47 million people worldwide were reported to be living with dementia as of 2015, and this number is projected to triple by 2050.14 Cancer therapy is the most common cause of secondary lymphedema in industrialized countries. Lymphedema of the upper limb develops in approximately 30% of patients who have undergone breast cancer surgery.15 Furthermore, lymphedema develops in 10% to 30% of patients with gynecologic cancer.16, 17, 18 Recurrent cellulitis in dementia patients with lymphedema is considered a dire problem in need of a solution. Although dementia is often accompanied by older age, LVA is recommended even for older patients.19 In addition, LVA could be used in other conditions, such as for patients who live alone or have inadequate help to comply with usual lymphedema care.
Conclusions
It is difficult to administer decongestive lymphatic therapy in some patients, such as patients with dementia. LVA is a promising treatment for recurrent cellulitis in a dementia patient with lymphedema.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Webb E., Neeman T., Gaida J., Bowden F.J., Mumford V., Bissett B. Impact of compression therapy on cellulitis (ICTOC) in adults with chronic oedema: a randomised controlled trial protocol. BMJ Open. 2019;9:e029225. doi: 10.1136/bmjopen-2019-029225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharkey A.R., King S.W., Ramsden A.J., Furniss D. Do surgical interventions for limb lymphoedema reduce cellulitis attack frequency? Microsurgery. 2017;37:348–353. doi: 10.1002/micr.30115. [DOI] [PubMed] [Google Scholar]
- 3.Imai Y., Hasegawa K. The revised Hasegawa’s Dementia Scale (HDS-R)—evaluation of its usefulness as a screening test for dementia. J Hong Kong Coll Psych. 1994;4:20–24. [Google Scholar]
- 4.Mihara M., Hara H., Araki J., Kikuchi K., Narushima M., Yamamoto T. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One. 2012;7:e38182. doi: 10.1371/journal.pone.0038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Executive Committee The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology. 2016;49:170–184. [PubMed] [Google Scholar]
- 6.Yamamoto T., Yamamoto N., Hara H., Mihara M., Narushima M., Koshima I. Upper extremity lymphedema index: a simple method for severity evaluation of upper extremity lymphedema. Ann Plast Surg. 2013;70:47–49. doi: 10.1097/SAP.0b013e3182275d23. [DOI] [PubMed] [Google Scholar]
- 7.Mihara M., Hara H., Tange S., Zhou H.P., Kawahara M., Shimizu Y. Multisite lymphaticovenular bypass using supermicrosurgery technique for lymphedema management in lower lymphedema cases. Plast Reconstr Surg. 2016;138:262–272. doi: 10.1097/PRS.0000000000002254. [DOI] [PubMed] [Google Scholar]
- 8.Gwendalyn J.R., Stoyan I., Zinselmeyer B.H., Scallan J.P. The lymphatic system: integral roles in immunity. Annu Rev Immunol. 2017;35:31–52. doi: 10.1146/annurev-immunol-041015-055354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter M.C., Teijeira A., Halin C. T cell trafficking through lymphatic vessels. Front Immunol. 2016;7:613. doi: 10.3389/fimmu.2016.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo E., Teijeira A., Vaahtomeri K., Willrodt A.H., Bloch J.S., Nitschké M. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep. 2016;14:1723–1734. doi: 10.1016/j.celrep.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Beauvillain C., Cunin P., Doni A., Scotet M., Jaillon S., Loiry M.L. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117:1196–1204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 12.Lakkis F.G., Arakelov A., Konieczny B.T., Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M., Hara H., Furniss D., Narushima M., Iida T., Kikuchi K. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br J Surg. 2014;101:1391–1396. doi: 10.1002/bjs.9588. [DOI] [PubMed] [Google Scholar]
- 14.Baumgart M., Snyder H.M., Carrillo M.C., Fazio S., Kim H., Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 15.DiSipio T., Rye S., Newman B., Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 16.Ohba Y., Todo Y., Kobayashi N., Kaneuchi M., Watari H., Takeda M. Risk factors for lower-limb lymphedema after surgery for cervical cancer. Int J Clin Oncol. 2011;16:238–243. doi: 10.1007/s10147-010-0171-5. [DOI] [PubMed] [Google Scholar]
- 17.Tada H., Teramukai S., Fukushima M., Sasaki H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer. 2009;9:47. doi: 10.1186/1471-2407-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beesley V., Janda M., Eakin E., Obermair A., Battistutta D. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–2614. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida S., Koshima I., Imai H., Uchiki T., Sasaki A., Fujioka Y. Characteristics and outcomes of lymphaticovenular anastomosis in older patients with bilateral involvement versus younger patients with unilateral involvement in lower extremity lymphedema. J Vasc Surg Venous Lymphat Disord. 2020;8:646–657. doi: 10.1016/j.jvsv.2019.10.013. [DOI] [PubMed] [Google Scholar]