Abstract
Over 1,000 clinical studies with probiotics, registered at ClinicalTrials.gov and/or the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization, have addressed over 700 different diseases and conditions. The average size of a clinical trial with probiotics (74 participants) is comparable to the overall average of all studies in ClincialTrials.gov. Lactobacillus rhamnosus GG (LGG) and Bifidobacterium animalis ssp. lactis BB12 are the probiotic strains studied most. The exact composition of the product which is used, including the dosage, is not always indicated in the registry. The majority of probiotics studies at ClinicalTrials.gov is registered in the USA or Europe (56%). The data from ICTRP show the rapid expansion of clinical studies with probiotics in Asia, notably Iran and China.
Keywords: Prebiotics, Probiotics, Bacteria, Microorganism, Digestive system, Immunology, Immune system, Microbiota, Clinical trials
Prebiotics; Probiotics; Bacteria; Microorganism; Digestive system; Immunology; Immune system; Microbiota, Clinical trials.
1. Introduction
Probiotics are defined as live microorganisms which when administered in adequate amounts confer a health benefit on the (human) host (FAO/WHO, 2002). This definition of 2002 was confirmed by a panel of experts in 2014 (Hill et al., 2014) apart from a minor grammatical change. Among other characteristics, to designate a microbe as probiotic, clinical benefits must be established. This means appropriately designed and sized clinical studies in the relevant target population need to be performed, and the outcomes published (Gibson et al., 2011; EFSA NDA Panel, 2016). Within the field of clinical research publication bias has long been of concern; intervention studies with positive results are published, while null and negative studies are less likely to be published (Johnson and Dickersin, 2007; Brassington, 2017). The latter research can provide multiple insights if their information is made publicly available through publication, especially where unexpected side effects or secondary effects could be observed. To address this issue, a registry was created where clinical trials would be announced before they commence. This followed the US Congress with the FDA Modernization Act of 1997 which required the National Institutes of Health (NIH) to operate a public information source of clinical trials (Public Law, 1997). In February 2000, the U.S. National Library of Medicine within the NIH made ClinicalTrials.gov available to the public via the internet. Since then, many other clinical trial registries have been founded: The World Health Organization (WHO) has made an effort to consolidate all primary registries into one platform: International Clinical Trials Registry Platform (ICTRP) (World Health Organization). Furthermore, since 2005 the International Committee of Medical Journal Editors decided that trials will only be considered for publication if they have been included in a clinical trial registry (de Angelis et al., 2004). Finally, the 2008 revised Declaration of Helsinki states that "Every clinical trial must be registered in a publicly accessible database before recruitment of the first subject” (World Medical Association, 2008). Although the registries were primarily set up to record planned trials on drugs, diagnostics, devices and therapy protocols, over the years also dietary intervention studies, including probiotics, have increasingly been registered. This addresses the critique that probiotic clinical trials are heterogeneous, biased and with low quality study results, in addition to poor clinical trial reporting in publications (Suez et al., 2019).
The aim of the present study was to investigate what clinical studies with probiotics have been registered and where, and if the design, characteristics and quality of the studies, as can be derived from the online registrations, is comparable to the overall registrations in ClinicalTrials.gov. An analysis was made of the diseases and conditions which were investigated as well as a geographical comparison of registered probiotic trials between the 2 major databases: ClinicalTrials.gov and the ICTRP.
2. Methods
As a basis for this study, clinical studies with probiotics were analyzed (primary analysis by TMGD, data checked by GTR) from ClinicalTrials.gov (https://clinicaltrials.gov/) as well as from the ICTRP (https://www.who.int/ictrp/en/) up to August 1, 2019. From the retrieved studies, the date of registration as well as the starting and end date of the study were noted.
The parameters which were collected and analyzed include the number of participants in the study, their age category, gender, health status, the underlying disease or condition (e.g. digestive system disease), nature of the study (intervention, observational), product (species, strain), dosage, route of administration, frequency, duration, primary outcome measurement (health effect), duration of the intervention/study, start and end date of inclusion, sponsors, and location (country, city). The current functionality of the ClinicalTrials.gov database allows the user to download this information directly from the website.
Scientific publications originating from clinical trials with probiotics were searched for based on their ClinicalTrials.gov identifier (NCT number). The PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) was used for that purpose and the search based on the NCT number. A sample of 100 randomly selected (completed) studies with probiotics and 100 random other studies from ClinicalTrials.gov were analyzed.
Statistical significance of differences between groups was calculated by Chi-square using the Analysis Toolpack add-in of Excel 2016 (Microsoft Corporation, Redmond, Washington, United States).
3. Results and discussion
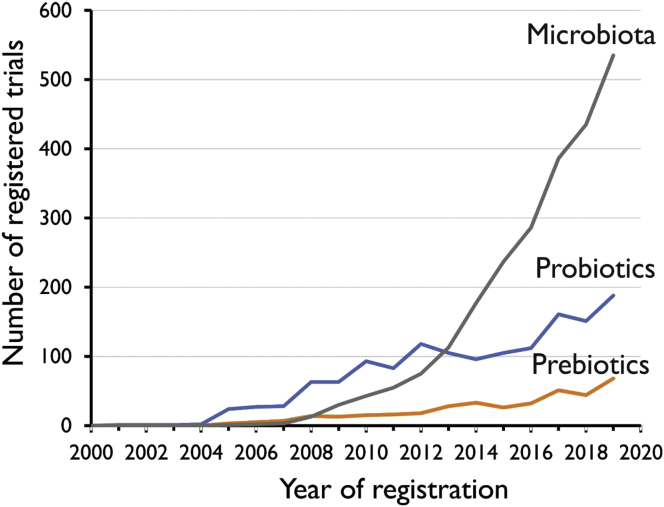
On August 1, 2019, the ClinicalTrials.gov database contained 1341 studies which could be retrieved using the search term “probiotics”. For comparison, during this same period, “microbiota” yielded 2151 studies and “prebiotics” 342 studies. As shwn in Figure 1, registrations of clinical studies with probiotics registered in ClinicalTrials.gov has been stable with around 100 studies annually since 2010 with a tendency to increase in numbers over the most recent years.
Figure 1.
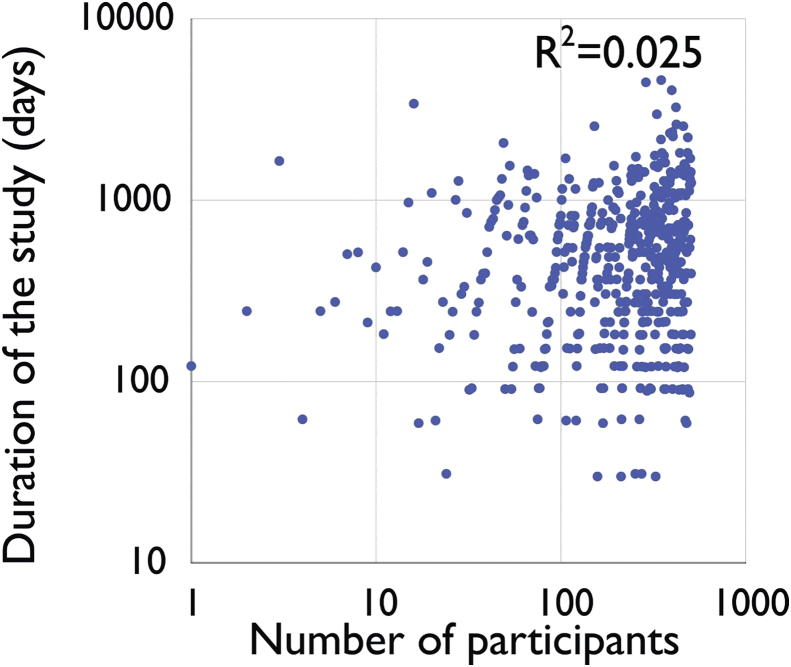
Lack of correlation between the number of participants in clinical studies on probiotics and duration of the study.
3.1. Probiotic strains and description
Because of the great diversity of probiotic bacteria, it is of importance to define the probiotics used not only at the species level, but also the specific strain used. At the strain level, Lactobacillus rhamnosus GG (LGG) (Gorbach, 1996; Goldin et al., 1996) was the probiotic strain most frequently registered (146 studies) followed by Bifidobacterium animalis ssp. lactis BB12 (Jungersen et al., 2014) with 55 studies. VSL#3, a consortium of 3 different bifidobacteria, 4 lactobacilli, and 1 Streptococcus thermophilus strain (Mora et al., 2019), was the most registered multispecies preparation in 74 studies (see also below).
In many cases, the description of the probiotic preparation used in the clinical study was incomplete. The specific bacterial species and strains, and even the dosage used, was not always indicated. Complete strain identification was given in 49% of the studies. Of 852 study registrations which could be analyzed, in 321 studies (38%) either a single strain or combination of 2 strains was used. A smaller number of studies, 89 (10%), used a preparation of 3 or more strains.
Part of the definition of probiotics is that they are administered in adequate amounts (WHO/FAO Working Group report 2002, Hill et al., 2014). The range of dosages reported was 107 to 9 × 1011 CFU (colony forming units) per day, so an almost 100,000-fold range in dosage. In some studies, the dosage was not reported in CFU, as recommended, but in grams, number of drops, or not indicated at all. In 42% of the studies the dosage was reported correctly.
3.2. Participant characteristics
Studies with probiotics focused more on children and less on elderly >65 years of age as compared with all NCT studies included in ClinicalTrials.gov (Table 1).
Table 1.
Characteristics of clinical studies with probiotics registered in ClinicalTrials.gov.
| Probiotics |
All NCT Studies |
P value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| #number of studies | 1,348 | 315,440 | |||
| with children (birth-17) | 428 | 31.8 | 63,840 | 20.2 | <0.01 |
| adults (18–65) | 1,000 | 74.2 | 290,938 | 92.2 | <0.01 |
| older adults (>65) | 714 | 53.0 | 240,336 | 76.2 | <0.01 |
| female | 1,314 | 97.5 | 300,240 | 95,2 | |
| male | 1,183 | 87.8 | 284,370 | 90,2 | |
| healthy participants | 590 | 43.8 | 79,144 | 25.1 | <0.01 |
| interventional | 1,289 | 95.6 | 249,185 | 70.0 | <0.01 |
| observational | 59 | 4.4 | 64,853 | 20.6 | <0.01 |
| study results | 62 | 4.6 | 38,560 | 12.2 | <0.01 |
NCT is the identification code of a study registered at ClinicalTrials.gov The 3 different age categories add up to >100% because many studies include participants across multiple age categories.
Significance of differences between studies with probiotics and all NCT studies were calculated by Chi-square.
Most studies registered at ClinicalTrials.gov are performed with patients. Yet, 583 studies with probiotics (43% of total) also included healthy participants. This percentage is higher than the average of 25% of all registered clinical trials that accept healthy volunteers (Table 1). One reason could be that health claim applications for probiotics at regulatory authorities such as the European Food Safety Authority (EFSA) need to be supported by studies in healthy individuals (EFSA NDA panel, 2016). In this context, it then becomes interesting and useful to know which fraction of clinical trials with probiotics are sponsored by industry and fortunately the ClinicalTrials.gov database allows to filter studies by type of funding. For studies with probiotics, 33.6% are funded by industry. This is not significantly different from the 33.1% of all clinical trials which were funded by industry (p > 0.05 by Chi-square), but lower than clinical trials with biologicals (42% funded by industry).
A large variation was found in both the number of participants in clinical probiotics studies (ranging from 1 (one) to 500), as well as in the duration of the study (ranging from 30 days to over 10 years). While it would be reasonable to assume that studies with more participants would take longer to recruit and thus have a longer over all duration, there was no significant association between number of participants and duration of probiotic studies (R2 = 0.025; p > 0.05) (Figure 2).
Figure 2.
Figure 1. Development in the number of clinical trials over time (in ClinicalTrials.gov) on probiotics (blue line), prebiotics (orange line) or microbiota (grey line). The Y-axis shows the number of registered studies per calendar year.
3.3. Study details and outcomes
The ClinicalTrials.Gov database allows the addition of study documents with the registration of the trial. These documents can include the study protocol, the statistical analysis plan as well as the informed consent form, and can be helpful to assess the quality of a study. Unfortunately, only in 2% of all registered trials, the study protocol and statistical analysis plan are added. Excerpts of the protocol and statistical analysis plan may be found in the registration, but the level of detail of that information is greatly variable (as indicated above for the description of the study product). Informed consent forms are included in 0.5% (n = 4) of all registered studies with probiotics. It should be mentioned that these numbers for probiotic studies do not differ from those of all studies registered at ClinicalTrials.Gov.
The outcome of clinical studies with probiotics, as with all clinical studies, is frequently meta-analyzed by institutes such as Cochrane Library (https://www-cochranelibrary-com). The Cochrane Library currently (August 2019) holds 50 reviews matching on probiotics in either Title, Abstract or Keyword. In 2015, a review was published on treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis (Singh et al., 2015). The paper concluded that “low quality evidence suggests that VSL#3 (a consortium of 11 different strains, taken in a daily dosage of 6 × 1011 CFU) may be more effective than placebo”. The authors indicate that “well-designed, adequately powered studies are needed to determine the optimal therapy for treatment and prevention of pouchitis”. Within this context it is encouraging to note that since the Cochrane publication, 7 new studies have been initiated on this issue using VSL#3, other probiotics, or other forms of microbiota management for that matter. This is a conservative estimate, as additional studies may not have been registered or have been registered in other databases.
3.4. Diseases and conditions
The average number of participants of clinical studies with probiotics did not differ from that of overall clinical studies. For gastrointestinal diseases and digestive system diseases, 128 studies with probiotics are registered with an average number of 74 participants. This is comparable to the average number of 71 participants in all clinical studies recorded in this registry. Over the years the average number of participants in studies with probiotics has not changed (based on a trend analysis from 2000 to 2017).
The ClinicalTrials.Gov database lists the date of registration of the study, as well as the date of inclusion of the first participant, and the end date of the study. Close analysis of the clinical studies with probiotics showed that only 32% of the studies were registered before the first participants were included. The majority of studies were registered prior to completion of the study. However, some studies were registered as late as 900 days after completion of the study. This is not in agreement with Good Clinical Practice because the primary and secondary outcome parameters should be predefined (and registered) before the actual start of the study, not in retrospect.
The diseases and conditions which are addressed in the studies registered at ClinicalTrials.gov are described by a set of keywords. For studies with probiotics, 713 different conditions are addressed. Of the completed studies, the most frequently studied conditions are digestive system diseases (253 studies) and gastrointestinal diseases (253 studies). Seven out of the top 10 studied conditions are gastrointestinal diseases and conditions. For the most recent studies with probiotics (listed as registered, but not yet recruiting) a shift can be noted towards non-gastrointestinal conditions. In 86 active but not recruiting studies, 198 conditions are addressed. The top 3 conditions are communicable diseases (15 studies), infection (15) and metabolic diseases (12). The latter indicates a potential shift in emphasis towards metabolic syndrome.
3.5. Geographical differences and trends between registration platforms
The ClinicalTrials.gov database is designed and operated by the U.S. National Library of Medicine, as part of NIH. It contains the data of 313,704 clinical studies in the USA as well as 208 other countries (assessed August, 2019). Of all registered clinical studies, 123,860 (39%) are registered in the USA. For studies on probiotics, the USA does not have such a dominant position with an 18% share. Most (38%) studies on probiotics registered on ClinicalTrials.gov take place in Europe (but see also below).
The World Health Organization (also) has set up a web based platform for registration of clinical trials: the ICTRP (World Health Organisation; https://www.who.int/ictrp/en/). This platform incorporates the national registry platforms of China, Cuba, Germany, India Iran, Japan, Republic of Korea, The Netherlands, Peru, Sri Lanka and Thailand, as well as the EU and Pan African registries, and ClinicalTrials.gov. In December 2017, ICTRP contained data of 625 clinical studies on probiotics. Of these 625 studies, 347 also have a ClinicalTrials.gov (i.e. NCT) number. The other probiotics trials are solely registered with ICTRP and originate mainly from Iran, Japan, Australia and New Zealand and China. The geographic location of probiotic trials from ICTRP are compared with ClinicalTrials.gov in Table 2. From these data, it can be concluded that the number of registered trials at ICTRP is growing at a faster pace than at ClinicalTrials.gov, and that China and Iran are the countries showing the largest increase in clinical studies on probiotics.
Table 2.
Registration of clinical trials with probiotics in international databases.
| Country | Registry | ICTR |
CTG |
||
|---|---|---|---|---|---|
| 12/2017 |
3/2019 |
12/2017 |
3/2019 |
||
| 625 | 1440 | 1067 | 1260 | ||
| China | ChiCTR | 24 | 311 | 33 | 46 |
| Netherlands | NTR | 14 | 15 | 19 | 20 |
| Germany | DRKS | 3 | 31 | 30 | 36 |
| Japan | JPRN | 38 | 53 | 4 | 4 |
| Korea | CRiS | 7 | 27 | 15 | 18 |
| Iran | IRCT | 62 | 248 | 6 | 6 |
| Brazil | ReBec | 3 | 35 | 28 | 33 |
| Cuba | PRCEC | 1 | 1 | - | - |
| Australia/NZl | ACTRN | 35 | 84 | 5 | 6 |
| NCT # | NCT | 347 | 677 | 1067 | 1260 |
Data in the table reflect the number of clinical trials identified by the keyword “probiotics” in the International Clinical Trials Registry Platform (ICTR) and ClinicalTrials.gov (CTG) on December 2017 and March 2019. Chinese Clinical Trial Registry (ChiCTR), The Netherlands National Trial Register (NTR), German Clinical Trials Register (DRKS), Japan Primary Registries Network (JPRN), Clinical Research Information Service (CRiS), Republic of Korea, Iranian Registry of Clinical Trials (IRCT), Brazilian Clinical Trials Registry (ReBec), Cuban Public Registry of Clinical Trials (RPCEC), Australian New Zealand Clinical Trials Registry (ANZCTR). NCT is the identification code of a study registered at clinicaltrials.gov.
3.6. Microbiota, probiotics and prebiotics
As already indicated above, in addition to the 1,341 studies on probiotics, ClinicalTrials.gov lists 2,151 studies on microbiota. Microbiota is the term which became popular a decade ago and which is used to describe the microbes that collectively inhabit a given ecosystem, such as the human gastrointestinal tract. The number of clinical studies retrieved using this search term shows a steep increase since 2010 and surpasses the number of studies on probiotics since 2013 (Figure 2). This interest in studying associations between clinical conditions c.q. clinical interventions with (changes in) composition of the gut microbiota can be a major route to the discovery of novel bacterial species with probiotic characteristics. At this moment, and not surprising, 24% of the studies are observational. Yet the importance of gut microbiota (both composition and function) for functionality of the human immune system has become evident in studies on the variable success of checkpoint inhibition therapy for cancer (Rijkers et al., 2019). Moreover, probiotics, in particular Lactobacillus reuteri, has been shown to alleviate the occurrence of colitis, a negative side-effect of checkpoint inhibition therapy (colitis) (Wang et al., 2019).
The number of clinical studies with prebiotics is slowly increasing with currently approximately 50 registered studies per year (Figure 2).
The outcome of planned, recruiting, and ongoing clinical trials with fecal microbiota transplants, as well as with probiotics, will hopefully lead to a higher success rate of checkpoint inhibition immunotherapy for cancer. It could also lead to novel applications of probiotics in modulation of the functionality of the (mucosal) immune system for prevention and treatment of disease.
4. Conclusions
A search for probiotics in ClinicalTrials.gov yielded 1341 studies, searching the ICTRP database yielded an additional 278 studies, totaling 1619. While the registration of probiotic studies can be improved, they do not seem to be grossly different from the studies registered in general in terms of size, inclusion of children and elderly, and publication in the scientific literature. Lactobacillus rhamnosus GG (LGG) and Bifidobacterium animalis ssp. lactis BB12 are the probiotic strains studied most. The majority of probiotics studies at ClinicalTrials.gov is registered in the USA or Europe (56%). The data from ICTRP show the rapid expansion of clinical studies with probiotics in Asia, notably Iran and China. Current studies on gut microbiota (including fecal microbiota transplantation) can lead to the discovery of new bacterial strains with probiotic properties. Ongoing and planned studies are not restricted to gastrointestinal diseases but also target neurological and neurodegenerative diseases as well as autoimmune diseases.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare the following conflict of interests: G. Rijkers and A. Ouwehand are members of the Scientific Advisory Board of the International Probiotics Association (http://internationalprobiotics.org/). A. Ouwehand is an employee of DuPont Nutrition and BioSciences, which manufactures and markets probiotics. Other members of this Board provided input for the opinions given in this paper.
Additional information
No additional information is available for this paper.
References
- de Angelis C., Drazen J.M., Frizelle F.A., Haug C., Hoey J., Horton R., Kotzin S., Laine C., Marusic A., Overbeke A.J., Schroeder T.V., Sox H.C., Van Der Weyden M.B. Clinical trial registration: a statement from the international committee of medical journal editors. N. Engl. J. Med. 2004;351:1250–1251. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- Brassington I. The ethics of reporting all the results of clinical trials. Br. Med. Bull. 2017;121(1):19–29. doi: 10.1093/bmb/ldw058. [DOI] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. EFSA J. 2016;14(1):4369. 2016. 23. [Google Scholar]
- FAO/WHO . 2002. Guidelines for the Evaluation of Probiotics in Food.https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf [Google Scholar]
- Gibson G.R., Brummer R.J., Isolauri E., Lochs H., Morelli L., Ockhuizen T., Rowland I.R., Schrezenmeir J., Stanton C., Verbeke K. The design of probiotic studies to substantiate health claims. Gut Microb. 2011;2(5):299–305. doi: 10.4161/gmic.2.5.18002. [DOI] [PubMed] [Google Scholar]
- Goldin B.R., Gualtieri L.J., Moore R.P. The effect of Lactobacillus GG on the initiation and promotion of DMH-induced intestinal tumors in the rat. Nutr. Canc. 1996;25:197–204. doi: 10.1080/01635589609514442. [DOI] [PubMed] [Google Scholar]
- Gorbach S.L. The discovery of Lactobacillus GG. Nutr. Today. 1996;31:2S–5S. [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotics. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Johnson R.T., Dickersin K. Publication bias against negative results from clinical trials: three of the seven deadly sins. Nat. Clin. Pract. Neurol. 2007 Nov;3(11):590–591. doi: 10.1038/ncpneuro0618. [DOI] [PubMed] [Google Scholar]
- Jungersen M., Wind A., Johansen E., Christensen J.E., Stuer-Lauridsen B., Eskesen D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12(®) Microorganisms. 2014;2(2):92–110. doi: 10.3390/microorganisms2020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora D., Filardi R., Arioli S., Boeren S., Aalvink S., de Vos W.M. Development of omics-based protocols for the microbiological characterization of multi-strain formulations marketed as probiotics: the case of VSL#3. Microb. Biotechnol. 2019 doi: 10.1111/1751-7915.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Law 105–115: Food and Drug Administration Modernization Act of 1997. 1997. https://www.gpo.gov/fdsys/pkg/PLAW-105publ115/pdf/PLAW-105publ115.pdf Adopted November 21, 1997.
- Rijkers G., Andriessen Q., van Overveld F.J. Death and the Miser: microbiota regulate the outcome of checkpoint inhibition immunotherapy. Expet Rev. Anticancer Ther. 2019 doi: 10.1080/14737140.2019.1677158. [DOI] [PubMed] [Google Scholar]
- Singh S., Stroud A.M., Holubar S.D., Sandborn W.J., Pardi D.S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst. Rev. 2015;(11) doi: 10.1002/14651858.CD001176.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019 May;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- Wang T., Zheng N., Luo Q., Jiang L., He B., Yuan X., Shen L. Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front. Immunol. 2019;10:1235. doi: 10.3389/fimmu.2019.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. International clinical trials registry platform. "https://www.who.int/ictrp/about/en/Accessed August 2019.
- World Medical Association . 2008. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subject . 59nd WMA General Assembly.https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/doh-oct2008/ Seoul, Korea. [Google Scholar]