Abstract
In vitro cultures play a promising role for production of pharmaceutically important plant secondary metabolites and the use of elicitation can mitigate the low productivity of active compounds. In the present study, the influence of cadmium chloride (CdCl2) elicitation on alkaloid yield was investigated in Rauvolfia serpentina. This heavy metal was employed to enhance the yield of reserpine and ajmalicine in leaf derived callus, leaves, stems and roots of in vitro grown cultures. Different concentrations [0.05 mM (C1), 0.10 mM (C2), 0.15 mM (C3) and 0.20 mM (C4)] of CdCl2 were added to the MS medium. The elicitor’s influence on callus biomass, biochemical attributes and the yield of alkaloids was monitored at regular intervals. The amendment of CdCl2 improved growth and maximum callus biomass (1.29 g fresh weight and 0.16 g dry weight) was noted at 0.15 mM (C3) after 6 days of elicitation. The addition of elicitor in medium caused cellular stress and to analyse the role of CdCl2 in plant defence responses various antioxidant enzymes, i.e., superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) activities were measured in treated and non-treated cultures. The antioxidant enzyme activity increased linearly with elevated levels of CdCl2 in medium; highest APX (0.88 EU min−1 mg−1protein), SOD (5.40 EU min−1 mg−1protein) and CAT (4.21 EU min−1 mg−1protein) activity were observed in leaves of in vitro regenerated plants at C4. The quantitative analyses of reserpine and ajmalicine were conducted in different elicitated tissues using high-performance thin-layer chromatography (HPTLC) method. The study reveals enriched level of reserpine and ajmalicine in cultivated tissues and the enhancement was noted up to C3 (0.15 mM) elicitor level. Reserpine yield was maximum (0.191 mg g−1 DW) in roots of in vitro regenerated plants. The accumulation of ajmalicine was, however, better in leaf derived callus at C3 (0.131 mg g−1 DW). Higher elicitor dose (0.20 mM) inhibited callus biomass growth and subsequent alkaloid accumulation. The present study indicates the use of CdCl2 as a propitious method in enhancing reserpine and ajmalicine yield in R. serpentina.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02339-6) contains supplementary material, which is available to authorized users.
Keywords: Plant secondary metabolites, R. serpentina, Elicitation, Reserpine, Ajmalicine, CdCl2, HPTLC
Introduction
Rauvolfia serpentina (L.) Benth ex Kurz, an endangered medicinal plant, is an evergreen perennial shrub belonging to the Apocynaceae family. Rauvolfia contains more than 80 species and occurs widely in countries like India, Indonesia, Burma and Bangladesh (Kumari et al. 2013). R. serpentina has been used as ayurvedic/folk medicine for centuries for the treatment of snake bites, malaria, dysentery, febrifuge and as a remedy for insanity (Chauhan et al. 2017). The current pharmacological investigation reports the plant to be actively potent against hysteria, epilepsy, gastrointestinal disorders and insomnia (Soni et al. 2016). The plant is efficient in treating cancer and can cure cardiovascular disorders (Lobay 2015). The immense therapeutic significance of R. serpentina is chiefly due to the presence of alkaloids of diverse nature (Poonam and Mishra 2013). The plant is a rich source of over 50 different alkaloids; the important indole alkaloids identified are reserpine, ajmalicine, ajmaline, serpentine, deserpidine, raubasine, yohimbine and isoajmaline (Verma and Verma 2010). Reserpine is the most prevalent and promising indole alkaloid in R. serpentina. Although the content varies in different tissues, maximum accumulation (0.03–0.15%) of reserpine was noted in dried root bark (Hareesh et al. 2010). Recent study indicated that the reserpine is the most widely FDA approved drug molecule used against hypertension (Singh et al. 2017). The antihypertensive mechanism is due to its binding to catecholamine storage vesicle, which obstructs normal catecholamine and serotonin accumulation, leading to the drop of catecholamine in nerve cells (Qu et al. 2009). Earlier report also demonstrated reserpine’s depressant action on central—and peripheral nervous system; it similarly activates central parasympathetic system, improvises neurotransmitters in adrenergic neurons, restricts normal functioning of autonomous nervous system (Nammi et al. 2005; Martins and Brijesh 2018). Ajmalicine is another important alkaloid present in R. serpentina has applications in treating various circulatory disorders, it extricates smooth cerebral blood flow, thus has important role in averting strokes (Srivastava et al. 2006; Thakore et al. 2017). Maximum content of ajmalicine is noted in leaves and stem of the plant.
Since the news of medicinal significance of these alkaloids surfaced in literature, a gradual depletion of natural reserves is reported. Due to over exploitation and to meet the growing demand of products across globe, R. serpentina has been kept in “red” listed plants in India and International Union for the Conservation of Nature and Natural Resources, IUCN placed the plant under ‘threatened’ category (Susila et al. 2013). To exterminate this issue and to conserve R. serpentina natural population, mass cultivation of plants coupled with alkaloids production are essential by adopting diverse strategies and modern techniques. Plant tissue culture has emerged in this direction as a propitious technique for mass propagation of plants including genetically engineered clones, conservation of germplasm and for controlled synthesis of secondary compounds (Naik and Al-Khayri 2016).
Plant secondary metabolites are organic compounds, synthesized by plants to expedite adaptation process in response to biotic and abiotic challenges and stresses (Yang et al. 2018). These metabolites are exclusive sources of pharmaceutically important medicinal compounds, fragrances, food flavours and colours etc. (Hussain et al. 2012). In plants, the yield of these secondary metabolites could be very low to high, depending upon the developmental stage and physiological state of the plant (Ramakrishna and Ravishankar 2011). An attractive alternative route to avoid extraction from entire plant material is the production of secondary metabolites using in vitro grown tissues/cultures (Efferth 2019). One of the most efficient biotechnological strategies used for improving these secondary metabolites is elicitation (Ali et al. 2019). Recent researches confirmed that the employment of elicitors modify cell metabolism in cultures while enriching bioactive compounds (Murthy et al. 2014). Elicitors are the compounds, on incorporation even in trace amounts in medium, trigger stress responses in tissues, improving the biosynthesis of target secondary metabolites (Patel and Krishnamurthy 2013; Maqsood and Mujib 2017). The elicitors are categorised into two—biotic and abiotic on the basis of nature and their origin. The substances of biological origin are referred to as biotic elicitors which include chitin, cellulose, pectin and other polysaccharides derived from cell wall of micro-organisms and plants. Abiotic elicitors on the other, are compounds of non-biological origin and primarily contain physical factors like drought, light, thermal stress etc. and chemical stresses (Naik and Al-Khayri 2016). The practice of adding these biotic and abiotic elicitors in culture gradually became a booming strategy in stimulating target product synthesis by slashing time required to get enhanced levels of products (Zafar et al. 2017). The growing use of chemical elicitors especially metals in pharmaceutical sectors and several agro-industries made these compounds most important, predominant abiotic stress agents (Cai et al. 2013). The present work was undertaken to study the impact of CdCl2 on reserpine and ajmalicine yield in callus, leaf, stem and roots of in vitro regenerated R. serpentina. The associated biochemical alterations in response to CdCl2 induced stress in cultured tissues were also investigated.
Materials and methods
Plant material and culture establishment
Tender leaf and nodal explants of R. serpentina were obtained from herbal garden of Jamia Hamdard, New Delhi. The explants were thoroughly washed, followed by a treatment with 1.0% Tween-20 solution for about 20 min. The detergent was washed off under running tap water for another 20 min. Furthermore, the explants were sterilized with 0.1% HgCl2 for 2–3 min and rinsed thrice with sterilized double distilled water to remove the traces of HgCl2. The disinfected leaves and nodal explants were carefully sliced into 0.5–1.0 cm sized pieces and cultured on MS (Murashige and Skoog 1962), added with sucrose (3.0% w/v), agar (0.8% w/v) and augmented with different concentrations of plant growth regulators (PGRs). The pH of the medium was maintained at 5.7 before autoclaving at 121 °C for 15 min. All the cultures were incubated in culture room at 24 ± 2 °C under a 12-h photoperiod with cool white fluorescent lamps (100 μmol m−2 s−1 Photon flux density, PFD).
Callus induction and direct shoot regeneration
For callus induction, leaf explant was inoculated on MS supplemented with 2.0 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.5 mg l−1of 6-benzylaminopurine (BAP). Whitish, yellow, friable callus was obtained after 3 weeks of culture. The induced callus was further sub cultured at regular interval of 3–4 weeks. The fresh and dry weight were taken at periodic intervals to analyse the callus biomass growth. For shoot regeneration (direct organogenesis), the nodal explants were cultured on MS fortified with 2.0 mg l−1 BAP. After every 3 weeks, the induced shoots were sub cultured onto fresh medium. Data for shoot induction and multiplication were recorded after 4 weeks of culture.
Root induction and acclimatization
Healthy plantlets were excised and cultured on MS amended with 1.0 mg l−1 NAA for rooting. The roots were formed within a few days and the plantlets with well-developed shoots and roots were transferred to pots containing soilrite and soil (1:1) for acclimatization. To maintain high humidity, the pots were covered with polythene. The acclimatized/hardened plants were then shifted to greenhouse.
Fresh weight and dry weight quantification
To determine the callus biomass growth with and without elicitor treatment, the fresh and dry weights of calli were measured. For monitoring the biomass increase, a known weight of 0.35 g of fresh callus mass was inoculated onto MS, amended with 1.0 mg l−1 2,4-D + 0.25 mg l−1 BAP. For quantification of fresh weight, both the elicitor treated (CdCl2 treatment) and non-treated calli were harvested from culture medium at a regular interval of 2 days (2, 4 and 6 days) and were finally weighed. For dry weight analysis, the calli were first air dried for some days and then weighed. All these experiments were conducted in three replicates and were repeated twice.
CdCl2 treatment
The leaf derived calli, stems, leaves and roots of in vitro regenerated plantlets were cultivated in CdCl2 amended medium at four different concentrations (0.05, 0.10, 0.15, 0.20 mM) and were designated as C1, C2, C3 and C4, respectively, and a control (C0) was kept for comparative analysis. As mentioned above, all the treatments were given in replicates of three and were cultured twice.
Biochemical analysis
Protein, proline and sugar estimation
The total protein contents of cultured tissues were estimated following the spectrometric method of Bradford (1976) for which 0.5 g of CdCl2 treated as well as non-treated (control) samples (calli, stems, leaves and roots) were ground in 1.5 ml (0.1 M) phosphate buffer (pH 7.0) in a chilled mortar and pestle, placed on ice and the homogenate was centrifuged at 5000 rpm for 10 min. The sample was again centrifuged with 0.5 ml trichloroacetic acid (TCA) at 4 °C for 10 min at 5000 rpm. The pellet was washed with chilled acetone after discarding the supernatant, and was dissolved in 1.0 ml of 0.1 M sodium hydroxide (NaOH). The optical density was taken at 595 nm and the protein content was measured using bovine serum albumin (BSA) as standard.
For extraction and estimation of free proline, 200 mg of treated and non-treated samples (calli, stems, leaves and roots) were homogenized in 5.0 ml of 3.0% aqueous sulfosalicylic acid and this homogenate was centrifuged for 10 min at 5000 rpm to eliminate the debris. About 1.0 ml of extract was mixed with1.0 ml of glacial acetic acid and 1.0 ml acid ninhydrin; and the reaction mixture was boiled for 1 h at 100 °C in a water bath. To abort the reaction, the mixture was placed on ice bath and each sample was extracted with 2.0 ml of toluene. The upper organic layer was read on a UV–Vis spectrophotometer at the wavelength of 520 nm. The free proline content was determined by the method of Bates et al. (1973) using L-proline as standard.
The total sugar content estimation was done following the method of Dey (1990) for which 500 mg of fresh calli, stems, leaves and roots were used twice with 90% ethanol. The final volume of the sample extract was made up to 25 ml with double distilled water. To 1.0 ml of aliquot, 1.0 ml of 5.0% phenol and 5.0 ml of concentrated analytical grade sulphuric acid were added. The optical density of the sample was read at 490 nm. A solution containing 1.5 ml 55% glycerol, 0.5 ml ninhydrin and 4.0 ml double distilled water was used as calibration standard.
Antioxidant enzyme activity
Superoxide dismutase (SOD), Catalase (CAT) and Ascorbate peroxidase (APX)
The SOD activity was measured as described by Dhindsa et al. (1981). With 2.0 ml of extraction solution [0.5 M sodium phosphate buffer, pH 7.3 + 3.0 mM ethylenediaminetetraacetic acid (EDTA) + 1.0% (w/v) polyvinylpyrollidone (PVP) + 1.0% (v/v) Tritonx100], the sample tissues were homogenized and centrifuged at 10,000 rpm at 4 °C. The SOD activity of the supernatant was assayed by its ability to prevent photo-chemical reduction. The reaction mixture contained 1.5 ml reaction buffer, 0.2 ml methionine, 0.1 ml of extract and same amount of 1.0 M NaCO3, 2.25 mM NBT solution, 3.0 mM EDTA, riboflavin and 1.0 ml of double distilled water. The whole reaction assay was put in test tubes and incubated at 20 °C for 10 min under light consisting of two 15 W florescent lamps. A 50% disappearance in colour was considered as one unit of enzyme activity expressed in enzyme unit (EU) mg−1 protein h−1.
CAT activity was measured following the procedure of Aebi (1984), 0.1 g of fresh samples/tissues were crushed and ground in 2.0 ml of extraction buffer (0.5 M Na-phosphate, pH 7.0, 3 mM EDTA, 1% PVP, 1% TritonX100). The sample mixture was centrifuged at 10,000 rpm at 4 °C for 20 min. In resulting supernatant, the CAT activity was determined by monitoring the rate of decay of H2O2 as assayed by the decrease of absorbance at 240 nm. A final volume of 2.0 ml of 0.5 M of phosphate buffer (Na-phosphate, pH 7.5), 0.1 ml of 3 mM EDTA, 0.1 ml extract and 0.1 ml H2O2 was run for a reaction, allowed to go on for 3 min. The amount of CAT required to decompose 1.0 µmol of H2O2/min was defined as one unit of CAT. The CAT activity (EU mg−1protein min−1 was used as unit) was assayed using the coefficient of absorbance of 0.036 mM−1 cm−1.
Following the method of Nakano and Asada (1981), the APX activity was estimated by monitoring the ascorbate oxidation rate at 290 nm. The assay mixture consisted of 1.0 ml 0.1 M sodium buffer, pH 7.2 + 0.1 ml 3 mM EDTA + 0.1 ml extract. The reaction was allowed to run for 3 min at 25 °C after adding ascorbate to the solution. At 290 nm, a decrease in absorbance was spectrophotometrically observed due to ascorbate oxidation. The enzyme activity was estimated using the absorption coefficient of 2.8 mM−1 cm−1 and the expression unit was taken as EU mg−1protein min−1. One unit of enzyme determines the least amount required to degrade 1.0 µmol of ascorbate per min.
Reserpine and ajmalicine quantification by HPTLC following CdCl2 elicitation
Preparation of extract
The extraction of reserpine and ajmalicine was done following previously described procedure (Kumar et al. 2010; Negi et al. 2014). Dried tissue (callus, stems, leaves and roots) of 0.1 g was pulverized in a mortar and pestle, 1.0 ml of ammonia was added to the crushed sample and left aside for 15 min. To this mixture, 10 ml of methanol was added and the solvent was allowed to dry under vacuum, 1.0 ml methanol was again added to the concentrated methanolic extract. The final extract was filtered through 0.45 µm syringe filter and processed for HPTLC.
Preparation of standard stock solutions
Reserpine and ajmalicine 0.1 mg each was dissolved in 1.0 ml of methanol and a stock solution concentration of 0.1 mg ml−1 was prepared. A range of volumes (2, 4, 6, 8, 10 and 12 µl) from stock solution equivalent to 200, 400, 600, 800, 1000 and 1200 µg/ml were applied to thin-layer chromatography (TLC) plates. The standard curve, regression equation and correlation coefficient were obtained using WinCATS software.
HPTLC instrumentation and chromatographic conditions
For mobile phase, a freshly prepared mobile solution containing chloroform, toluene, ethylacetate and diethylamine in the ratio of 7:7:4:1 was used. For stationary phase, the coated TLC aluminium sheets of 10 × 10 cm with silica gel coating (60 F254, Merck) were used. Using automatic Linomat 5 (CAMAG) applicator, both the standard and the sample solutions were loaded on silica gel plates in the form of bands with 100 µl syringe. A constant 80 ml s−1 application rate was maintained using N2 gas and the samples were applied to the plate as 5.2 mm band. The loaded silica plates were air dried for 10–15 min and kept in a twin through chamber (CAMAG, 20 × 10 cm) filled with freshly prepared mobile solution. Once the solvent moved up to about 85 mm, the plates were removed from the chamber and again air dried for about 15–20 min. The developed silica plates were documented using CAMAG reproster under UV light. The reserpine and ajmalicine containing stationary phase was scanned in absorbance mode at 240 nm and 280 nm, respectively, using CAMAG TLC scanner V controlled by WinCATS software. The standard peaks of reserpine and ajmalicine were used as reference peaks and were compared to the sample peaks to identify the alkaloids in tissue samples.
Statistical analysis
The data on the effect of CdCl2 on callus biomass, differences in biochemical attributes, the activity of the antioxidant enzymes and the yield of the alkaloids in different tissues were analysed and expressed as mean ± standard error. Each set of experiment consisted of three replicates and each experiment was performed twice. The presented mean values were separated using Duncan’s Multiple Range Tests (DMRT) at p ≤ 0.05.
Results
Callus induction and direct shoot organogenesis
On 2 mg l−1 2, 4-D + 0.5 mg l−1 BAP amended MS medium, the leaf explants produced callus and the induced callus was compact (Fig. 1a), yellowish white in colour and exhibited intense growth. The callus induction started after 2 weeks of culture and the induced callus was further sub cultured on 1.0 mg l−1 2,4–D + 0.25 mg l−1 BAP, in which callus proliferated vigorously (Fig. 1b, c). For direct organogenesis, the nodal explants were cultured on 2.0 mg l−1BAP augmented MS medium. The initial bud break with subsequent shoot regeneration was observed after 3 weeks of culture (Fig. 1d).The regenerated shoots elongated and multiplied well on 1.0 mg l−1BAP supplemented MS medium (Fig. 1e, f).
Fig. 1.
a Callus induction from leaf explant, MS medium amended with 2.0 mg l−1 2, 4-D + 0.5 mg l−1 BAP, b, c proliferation of leaf derived callus, MS medium supplemented with 1.0 mg l−1 2, 4-D + 0.25 mg l−1 BAP, d direct organogenesis from nodal explant, MS medium augmented with 2.0 mg l−1 BAP, e, f shoot elongation and multiplication, MS medium contained 1.0 mg l−1 BAP, g rooting of the micro shoots in 1.0 mg l−1 NAA amended MS medium, h regenerated potted plants (bar a–c: 0.5 cm, d–f: 1.0 cm, g–h: 1.2 cm)
Rooting and acclimatization
Well-developed in vitro regenerated shoots were cultured on 1.0 mg l−1 NAA added MS medium for root induction. After 4 weeks of culture, long, yellowish roots with root hairs were formed in the cultured plantlets (Fig. 1g). NAA proved to be an efficient choice for root induction. Healthy plants with a few expanded leaves and well-developed roots were put in small pots containing soilrite and soil (Fig. 1h). After a couple of weeks, the plants were transferred to field soil, where they grew normally in natural outdoor conditions.
Biomass growth in response to elicitation
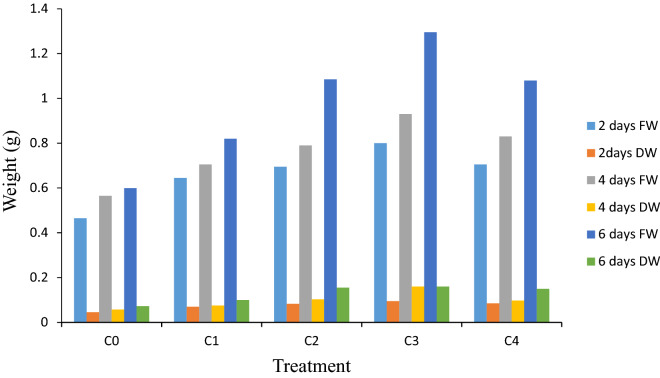
The increase in biomass growth is a sign of cell division with rapid callus proliferation. The leaf derived calli were subject to CdCl2 elicitation at various levels and the callus growth was monitored at regular interval of 2 days (2, 4 and 6 days). We observed that the inclusion of CdCl2 in medium resulted in faster growth of callus as compared to control (Fig. 2). The callus biomass increased up to C3 and a maximum fresh weight (1.29 g) and dry weight (0.16 g) were obtained at this elicitor treatment after 6 days of culture. Higher level of elicitation, i.e., C4 exhibited reduced callus biomass growth.
Fig. 2.
Callus biomass and growth under various CdCl2 concentrations. Initial 0.35 g of callus was inoculated on MS medium, supplemented with optimized 1.0 mg l−1 BAP + 0.5 mg l−1 IAA [C0: Control; C1: 0.05; C2: 0.10; C3: 0.15; C4: 0.20 mM]. Values are means ± standard errors of three replicates of two experiments; within each column mean followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT. FW: fresh weight, DW: dry weight
CdCl2 elicitation and its impact on biochemical attributes
The calli, stems, leaves and roots of regenerated plantlets were placed on four different concentrations (0.05, 0.10, 0.15, 0.20 mM) of CdCl2 along with a control (C0). The biochemical analyses of tested tissues showed alteration in biochemical profiles in response to CdCl2 elicitation. The protein, proline and sugar in different tissues were measured after 2, 4 and 6 days of culture, following CdCl2 treatments.
Protein, proline and sugar content
With the increase in elicitor dose and exposure time, the protein level increased up to C3 of almost all the cultures and the maximum protein was found after 6 days of elicitation. In callus, the minimum level of protein was observed in control, C0 (3.62 mg g−1 FW) and the highest level was at C3 (8.88 mg g−1 FW) at 6 days of elicitation (Table 1). In leaves, the protein level was noted highest at C3 (9.79 mg g−1 FW) after 6 days of treatment and at C0, the protein level was the least, i.e., 4.08 mg g−1 FW (Table 2). In stem, the protein level was low at control (3.40 mg g−1 FW), but attained its maximum level at C3 (7.89 mg g−1 FW) after 6 days of treatment (Table 3). In roots, the maximum protein was found at C3 (7.65 mg g−1 FW) after 6 days of treatment (Table 4).
Table 1.
Biochemical attributes (mg g−1 FW) of leaf derived callus under various levels of CdCl2 treatment
| Treatment | 2 Days | 4 Days | 6 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Proline | Sugar | Protein | Proline | Sugar | Protein | Proline | Sugar | |
| C0 | 3.62 ± 0.04d | 9.57 ± 0.06d | 12.87 ± 0.06e | 5.06 ± 0.05d | 12.18 ± 0.07d | 14.92 ± 0.06d | 6.97 ± 0.04d | 13.90 ± 0.06c | 18.16 ± 0.03d |
| C1 | 4.97 ± 0.05c | 11.13 ± 0.04c | 15.92 ± 0.05d | 5.37 ± 0.04c | 14.17 ± 0.05c | 19.13 ± 0.05c | 7.73 ± 0.06c | 16.30 ± 0.04b | 21.76 ± 0.03c |
| C2 | 5.27 ± 0.06b | 13.92 ± 0.05b | 17.90 ± 0.06c | 6.55 ± 0.04b | 15.94 ± 0.03bc | 19.99 ± 0.09c | 8.26 ± 0.05b | 18.17 ± 0.03a | 26.87 ± 0.04b |
| C3 | 5.66 ± 0.04a | 16.24 ± 0.04a | 21.07 ± 0.06a | 7.15 ± 0.03a | 17.29 ± 0.06a | 25.16 ± 0.06a | 8.88 ± 0.03a | 19.87 ± 0.05a | 31.46 ± 0.06a |
| C4 | 4.94 ± 0.02c | 15.36 ± 0.06a | 19.68 ± 0.04b | 6.77 ± 0.05b | 16.18 ± 0.05ab | 23.28 ± 0.05b | 8.03 ± 0.05bc | 16.95 ± 0.04b | 27.97 ± 0.04b |
Different CdCl2elicitor levels used: Control (C0), 0.05 mM (C1), 0.1 mM (C2), 0.15 mM (C3), 0.2 mM (C4). MS medium was supplemented with 1.0 mg l−12, 4-D + 0.25 mg l−1BAP. Values are means ± standard errors of three replicates of two experiments. Within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Table 2.
Biochemical attributes (mg l−1 FW) of leaves of in vitro regenerated plant under various levels of CdCl2 treatment
| Treatment | 2 Days | 4 Days | 6 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Proline | Sugar | Protein | Proline | Sugar | Protein | Proline | Sugar | |
| C0 | 4.08 ± 0.04d | 9.94 ± 0.05e | 14.20 ± 0.11e | 5.87 ± 0.06d | 13.26 ± 0.07d | 16.37 ± 0.06e | 7.82 ± 0.05d | 14.81 ± 0.02e | 19.22 ± 0.07e |
| C1 | 5.26 ± 0.05c | 12.69 ± 0.09d | 16.88 ± 0.05d | 6.83 ± 0.08c | 15.23 ± 0.05c | 20.10 ± 0.08d | 8.86 ± 0.06c | 17.19 ± 0.08d | 22.30 ± 0.09d |
| C2 | 6.08 ± 0.06b | 14.93 ± 0.06c | 19.29 ± 0.13c | 7.22 ± 0.07bc | 16.89 ± 0.10b | 22.42 ± 0.05c | 9.11 ± 0.08bc | 19.51 ± 0.07b | 28.10 ± 0.08c |
| C3 | 6.78 ± 0.04a | 17.59 ± 0.03a | 23.22 ± 0.05a | 7.95 ± 0.03a | 19.06 ± 0.07a | 27.01 ± 0.14a | 9.79 ± 0.07a | 21.30 ± 0.10a | 33.32 ± 0.09a |
| C4 | 5.84 ± 0.08b | 16.92 ± 0.08b | 21.34 ± 0.09b | 7.27 ± 0.06ab | 17.30 ± 0.12b | 25.10 ± 0.12b | 8.90 ± 0.07b | 18.92 ± 0.06c | 30.23 ± 0.07b |
Different CdCl2elicitor levels used: Control (C0), 0.05 mM (C1), 0.1 mM (C2), 0.15 mM (C3), 0.2 mM (C4). MS medium was supplemented with 1.0 mg l−1 BAP. Values are means ± standard errors of three replicates of two experiments. Within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Table 3.
Biochemical attributes (mg g−1 FW) of stem of in vitro regenerated plant under various levels of CdCl2 treatment
| Treatment | 2 Days | 4 Days | 6 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Proline | Sugar | Protein | Proline | Sugar | Protein | Proline | Sugar | |
| C0 | 3.40 ± 0.07d | 9.22 ± 0.14e | 11.90 ± 0.12e | 4.60 ± 0.05d | 10.94 ± 0.07d | 14.16 ± 0.09e | 6.30 ± 0.02d | 13.23 ± 0.06e | 17.55 ± 0.09e |
| C1 | 4.22 ± 0.09c | 10.38 ± 0.09d | 14.78 ± 0.02d | 5.24 ± 0.06c | 13.27 ± 0.04c | 18.89 ± 0.11d | 7.27 ± 0.10bc | 15.35 ± 0.10d | 21.22 ± 0.07d |
| C2 | 4.86 ± 0.08b | 12.14 ± 0.04c | 17.46 ± 0.08c | 6.18 ± 0.07b | 15.35 ± 0.05b | 19.84 ± 0.07c | 7.54 ± 0.17ab | 17.08 ± 0.10b | 25.90 ± 0.12c |
| C3 | 5.39 ± 0.12a | 15.18 ± 0.06a | 21.02 ± 0.07a | 6.60 ± 0.09a | 16.40 ± 0.06a | 25.24 ± 0.07a | 7.89 ± 0.08a | 18.52 ± 0.09a | 31.68 ± 0.10a |
| C4 | 4.63 ± 0.06b | 14.84 ± 0.07b | 18.47 ± 0.08b | 6.13 ± 0.07b | 15.45 ± 0.07b | 23.23 ± 0.09b | 7.08 ± 0.09c | 16.00 ± 0.02c | 27.01 ± 0.08b |
Different CdCl2 elicitor levels used: Control (C0), 0.05 mM (C1), 0.1 mM (C2), 0.15 mM (C3), 0.2 mM (C4). MS medium was supplemented with 1.0 mg l−1 BAP. Values are means ± standard errors of three replicates of two experiments. Within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Table 4.
Biochemical attributes (mg g−1 FW) of roots of in vitro regenerated plant under various levels of CdCl2 treatment
| Treatment | 2 Days | 4 Days | 6 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Proline | Sugar | Protein | Proline | Sugar | Protein | Proline | Sugar | |
| C0 | 3.11 ± 0.04d | 8.54 ± 0.08e | 11.25 ± 0.07e | 4.12 ± 0.03c | 10.79 ± 0.13e | 13.98 ± 0.09e | 5.95 ± 0.10c | 13.03 ± 0.09d | 16.94 ± 0.06e |
| C1 | 4.09 ± 0.06c | 9.84 ± 0.07d | 13.90 ± 0.12d | 4.46 ± 0.09c | 13.07 ± 0.08d | 18.24 ± 0.07d | 6.32 ± 0.09bc | 15.02 ± 0.09c | 20.52 ± 0.03d |
| C2 | 4.43 ± 0.08bc | 11.22 ± 0.06c | 15.61 ± 0.08c | 5.39 ± 0.05b | 14.55 ± 0.09c | 19.33 ± 0.10c | 7.34 ± 0.08a | 17.11 ± 0.06b | 25.04 ± 0.05c |
| C3 | 5.38 ± 0.09a | 13.83 ± 0.05a | 20.18 ± 0.02a | 6.00 ± 0.16a | 16.73 ± 0.11a | 24.11 ± 0.05a | 7.65 ± 0.07a | 18.45 ± 0.12a | 30.95 ± 0.04a |
| C4 | 4.85 ± 0.07b | 12.69 ± 0.14b | 16.96 ± 0.03b | 5.88 ± 0.09a | 15.15 ± 0.07b | 22.81 ± 0.04b | 6.69 ± 0.03b | 15.23 ± 0.07c | 26.21 ± 0.03b |
Different CdCl2 elicitor levels used: Control (C0), 0.05 mM (C1), 0.1 mM (C2), 0.15 mM (C3), 0.2 mM (C4). MS medium was supplemented with 1.0 mg l−1 NAA. Values are means ± standard errors of three replicates of two experiments. Within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
With increasing CdCl2 levels and duration, the accumulation of proline increased. In callus, the maximum amount of proline (19.87 mg g−1 FW) was found in 6 days of elicitation at C3. In leaves, the highest proline level was at C3 (21.30 mg g−1 FW) and the lowest at C0 (9.94 mg g−1 FW). A similar pattern of enhanced proline level was found in stem with highest level at C3 (18.52 mg g−1 FW) and a minimum at C0 (9.22 mg g−1 FW). In root, the maximum proline level was noted at C3 (18.45 mg g−1 FW) after 6 days of treatment and at C0 the level was very low (8.54 mg g−1 FW).
The sugar level increased up to C3 in all cultures and had a gradual decline thereafter. The maximum sugar content in callus was noted at C3 (31.46 mg g−1 FW) and a minimum at C0 (12.87 mg g−1 FW). In leaves, the sugar content was highest at C3 (33.32 mg g−1 FW); in stem, the maximum sugar content was at C3 (31.68 mg g−1 FW) after 6 days of elicitation. Similarly, the sugar level had the maximum accumulation at C3 (30.95 mg g−1 FW) in roots.
Enzymatic activities
The augmentation of metal elicitor (abiotic stress) promotes excessive production of reactive oxygen species (ROS) in tissues, which cause oxidative damage, cellular injury to the cells and untimely cell death. To combat the rising level of ROS and metal induced oxidative damage, the antioxidant enzyme activities shoot up in plants. Increased activities of enzymes like APX, SOD and CAT are part of antioxidant defence system in response to metal ions. In the present investigation, the alteration in enzyme activity in different cultivating tissues in response to CdCl2 elicitation was studied.
APX, SOD and CAT activity
The APX, SOD and CAT activity of callus are presented in Online Resource ESM_1pdf. The APX activity in callus increased significantly from C0 to C4 with highest (0.85 EU min−1 mg−1 protein) at C4 after 6 days of elicitation. In leaves, similar trend of enzyme activity (0.88 EU min−1 mg−1 protein at C4) was observed (Online Resource ESM_1pdf). In stem, three enzyme activities are summarized and presented in Online Resource ESM_2pdf, i.e., APX activity was high at C4 (0.80 EU min−1 mg−1 protein) after 6 days of treatment, so was in roots, i.e., 0.76 EU min−1 mg−1 protein.
A linear increase in SOD activity of callus was observed from C0 to C4 and the maximum activity was noted to be 5.25 EU min−1 mg−1 protein at C4 after 6 days of treatment. In leaves, the maximum SOD activity was at C4 (5.40 EU min−1 mg−1 protein) and in stem, SOD activity increased linearly form a minimum of 2.38EU min−1 mg−1 protein at C0 to a maximum of 4.74 EU min−1 mg−1 protein at C4 after 6 days of elicitor treatment. Same was true in roots, i.e., highest being at C4, 4.50 EU min−1 mg−1 protein (Online Resource ESM_2pdf).
In callus, the CAT activity was highest, i.e., 4.05 EU min−1 mg−1 protein at C4 after 6 days of elicitation. In leaves, an increasing trend was noted from a minimum of 2.01 EU min−1 mg−1 protein at C0 to a maximum of 4.21EU min−1 mg−1 protein at C4 after 6 days of elicitation. Likewise in stem, the highest CAT activity (3.63 EU min−1 mg−1 protein) was observed at C4 (Online Resource ESM_2pdf). A similar trend was observed in roots, where the CAT activity increased from C0 (1.37 EU min−1 mg−1 protein) to C4 (3.51 EU min−1 mg−1 protein) after 6 days of elicitation (Online Resource ESM_2pdf).
Reserpine and ajmalicine yield in CdCl2 elicitated tissues: various doses and exposure time
Elicitation is an efficient approach to enrich plant secondary metabolites in low yielding cultures. These are signalling compounds employed to activate genes of plant defence system in enhancing accumulation of plant secondary metabolites. CdCl2 was used as an abiotic (heavy metal) elicitor and the yield of reserpine and ajmalicine was measured in both the elicitated and non-elicitated in vitro cultures (callus, leaf, stem and root). The mobile phase exhibited a single spot with Rf 0.15 for reserpine and 0.45 for ajmalicine. A good linearity was observed in calibration curve with a regression value of 0.9966 for reserpine and 0.9968 for ajmalicine. The tested concentrations of CdCl2 significantly increased reserpine accumulation in callus, leaves, stems and roots of in vitro raised plants of R. serpentina (Fig. 3a–d. The HPTLC chromatogram of all peaks of the above samples is presented in Fig. 3e–h. In callus, the maximum amount of reserpine was found in C3 (0.136 mg g−1 DW) after 4 days of treatment (Online Resource ESM_3pdf) and at higher dose, the reserpine yield declined. In leaf, C3 treatment had the highest amount of reserpine (0.133 mg g−1 DW) after 4 days of elicitation; and the minimum was in C0 (0.069 mg g−1 DW) (Online Resource ESM_3pdf). In stem (Online Resource ESM_3pdf), the maximum reserpine was noted at C3 (0.113 mg g−1 DW). It is evident that the reserpine content in roots was highest at C3 (0.191 mg g−1 DW) after 4 days of elicitor treatment. The roots of in vitro regenerated R. serpentina had the highest reserpine content in control as well as elicitated cultures as compared to the other viz. callus, leaf and stem tissues.
Fig. 3.
Reserpine peak/level of CdCl2elicitated: a callus, b leaves, c stems and d roots of in vitro regenerated plants at C3 after 4 days of elicitation, and HPTLC chromatogram of all peaks d callus, e leaves, f stem and g roots of in vitro regenerated R. serpentina treated with CdCl2
The amendment of CdCl2 improved ajmalicine yield as well in all the tested tissues viz. callus, leaves, stems and roots (Online Resource ESM_2pdf). The HPTLC chromatogram of the same is presented in Fig. 4e–h. The callus (leaf derived) had the highest ajmalicine level (0.131 mg g−1 DW) as compared to other tissues, i.e., leaves, stems and roots (Fig. 5a–d). This enhancement was noted at C3 after 4 days of incubation. In leaves, the ajmalicine content was highest (0.106 mg g−1 DW) at C3 after 4 days of elicitation (Figs. 4b, 5b). In stem, the ajmalicine level was high at C3 (0.088 mg g−1 DW) and was the least in C0 (0.044 mg g−1 DW). Likewise in roots, the alkaloid level was maximum at C3 (0.056 mg g−1 DW). In all the cases, however, ajmalicine accumulation enhanced with increasing CdCl2 concentrations up to C3, further rise in elicitor dosage declined alkaloid yield.
Fig. 4.
Ajmalicine peak/level of CdCl2 elicitated: a callus, b leaves, c stems and d roots of in vitro regenerated R. serpentina at C3 after 4 days of elicitation. HPTLC chromatogram of all peaks d callus, e leaves, f stems and g roots of in vitro regenerated R. serpentina, treated with CdCl2
Fig. 5.

Ajmalicine content (mg g−1 dry weight) in CdCl2 treated tissues: a callus, b leaves, c stems and d roots of in vitro regenerated R. serpentina (C0: Control; C1: 0.05; C2: 0.10; C3: 0.15; C4: 0.20 mM). Values are means ± standard errors of three replicates of two experiments; within each column means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Discussion
In the present study, the enrichment of reserpine and ajmalicine in in vitro grown cultures of R. serpentina was investigated following CdCl2 elicitation. As a first step, we started our experiment with induction of callus from leaves as an explant in R. serpentina. Young tender leaves are often the prime choice for callus induction, because the meristematic tissues divide fast under PGRs’ regulation (Singh et al. 2015). The auxins and cytokinins applied in specific, precise concentration and ratio exercise their response by amending the tissue’s endogenous levels of PGRs, which ultimately leads to callus formation (Su et al. 2011). Here, the successful callus induction was achieved on MS amended with 2.0 mg l−1 2, 4-D and 0.5 mg l−1 BAP after 4 weeks of culture. The induced callus, after exposed to high level of PGRs, was subject to the same PGRs at lower concentrations, promoted callus proliferation and the observation was analogous to the findings of Ikeuchi et al. (2013). It was noticed that the 2, 4-D along with BAP was a dynamic PGR combination for callus induction, growth and proliferation in R. serpentina and this observation falls in line with similar other previous reports (Haensch 2007; Sundarasekar et al. 2012). The addition of PGRs, particularly the cytokinins in medium stimulate shoot induction as the cytokinins possess proliferative mechanism in triggering shoot meristem activity thereby play an important key role in plant development (Victório et al. 2012). The cytokinins, especially BAP is known to enhance shoot proliferation rates in different species (Ashraf et al. 2014; Mujib et al. 2017). In this study, 2.0 mg l−1 BAP was figured out to be the most appropriate concentration for shoot bud induction from nodal explant. This strong response of BAP could be ascribed to better ligand-receptor binding ability as compared to the other PGRs in evoking shoot formation and proliferation (Javed et al. 2013). Various other workers reported similar promotive response of BAP in shoot bud induction in other plant genera including Rauvolfia (Goel et al. 2007; Ranjusha et al. 2012). Adventitious rooting efficiency generally varies with the use of explant sources, choice of PGR and also with the type of species. Rooting occurs more efficiently in tender plant parts than the mature ones as the young part possess active meristemic tissues (Javed and Anis 2015). In our study, 1.0 mg l−1 NAA augmented MS was quite effective for root induction in in in vitro regenerated shoots. The effectiveness of NAA in rooting has earlier been described in several previous reports (Yan et al. 2014). The rooted plantlets were shifted to pots containing soilrite and soil as a planting substrate and later transferred to field with good survival rate.
In the present study, the effect of CdCl2, an abiotic elicitor on leaf derived callus biomass was evaluated at varying concentrations. Of the various elicitor concentrations tested, C3 (0.15 mM) was noticed to be more effective in promoting growth compared to C1, C2 and C4. Higher elicitor dose (C4) was deleterious for callus biomass growth and this decline may be due to excess availability of Cd2+ ions that disrupted cell division in arresting cell growth (Gill and Tuteja 2011). Similar growth inhibiting response due to over metal ion toxicity on cultural growth was reported in several other investigations (Muszynska et al. 2018; Morkunas et al. 2018).
The addition of abiotic elicitors alters the synthesis of biochemical attributes like protein, proline and sugar as was monitored in our present investigation. In the present study, the differences in biochemical profile as well as the antioxidant enzyme activity were analysed in treated and non-treated callus, leaves, stems and roots of in vitro regenerated plants of R. serpentina. One of the several biochemical compounds that get accumulated during stress is protein and the enhanced levels help adapting various stress response conditions (Wani et al. 2016). In our study, protein levels improved with increasing CdCl2 concentrations and similar observation on increased protein level was reported in other plant genera on exposure to adverse stress situations (Lakra et al. 2016). Likewise, plants also accumulate large quantities of proline in response to metal stress (Liang et al. 2013). Proline has been observed to alleviate heavy metal stress in various ways; it acts as heavy metal chelators by formating phytochelatins, which decreases heavy metal toxicity like Cd (Hayat et al. 2012). Proline regulates redox potentials by acting as an energy sink, protects macromolecules against denaturation (Meena et al. 2019). Proline at the same time acts as a special osmolyte and an appropriate solute in plant cells, which facilitates cells in maintaining turgidity under water deficit conditions (Bidabadi et al. 2012). Beside acting as osmolyte, proline stabilizes sub-cellular structures like proteins and membranes, scavenges free radicals and maintains cellular redox potential under stress conditions (Ashraf and Foolad 2007). Islam et al. (2009) reported similar increase of proline in tobacco cells in response to cadmium stress in protecting plant cells. The soluble sugar level significantly changed with increasing CdCl2 treatments in all cultures especially after 4 days of elicitation. Tran et al. (2007) reported that the sugar signalling connect into an intricate network with the stress pathways to harmonize metabolic plant responses. Sugars are also known to have the ability to feed NADPH- producing metabolic pathways like oxidative pentose-phosphate, which has a direct role in ROS scavenging (Bolouri-Moghaddam and Ende 2012). Under stress conditions, sugars adequately protect cellular membranes obligatory for cell survival (Garvey et al. 2013). The sugar fluctuation under abiotic stress is due to differential CO2 assimilation with specific gene expression related enzyme activity (Rosa et al. 2009; Hausler et al. 2014).
In vitro culture exposed to abiotic stress causes the accumulation of reactive oxygen species (ROS) due to the activation of specific ion channels and kinase cascades. A genetic machinery reprogramming makes adequate defence responses in increasing plant tolerance to biological damage provoked by the stress (Rejeb et al. 2014). Plants possess an inherent mechanism for ROS eradication, which includes antioxidant enzyme activities like CAT, APX and SOD (Malar et al. 2014). In R. serpentina in vitro cultures, exposure of CdCl2 promoted SOD as an act of defence activity in response to CdCl2-linked excessive production of ROS like superoxide anion (O−), singlet oxygen (O2), hydrogen peroxide (H2O2) and hydroxyl radical (.OH). Increased SOD activity in relation to various abiotic elicitors was investigated in several other plant genera (Mishra et al. 2014; Fatima et al. 2015). Enhanced levels of SOD produces increased H2O2 content which is quite toxic and essential to eliminate. CAT and APX are the other categories of antioxidant enzymes detoxify H2O2 into water and oxygen thereby the redox state is retained in cells (You and Chan 2015). Here, in Rauvolfia, the activities of CAT and APX were enhanced in cultures following CdCl2 exposure. Increased CAT and APX activities in mitigating abiotic stresses were earlier reported in other plant genera (Zahid and Mujib 2012; Feng-tao et al. 2013).
In the present study, the in vitro cultures of R. serpentina were subject to CdCl2 elicitation with the aim to scale up the biosynthesis of reserpine and ajmalicine. CdCl2 is an affirmed potent elicitor promotes tissues growth and subsequently triggers the accumulation of secondary metabolites in numerous plant cultures (Sivanandhan et al. 2012). The growing conditions of cultures, however, strongly influence the levels of plant secondary metabolites in which the regulation of metabolic pathways is important for enrichment of natural products (Ramakrishna and Ravishankar 2011). On CdCl2 elicitated treatments, increased yield of reserpine and ajmalicine was noted with elevated elicitor levels, highest being at C3 (0.15 mM). Highest reserpine was observed in elicitated in vitro root cultures, whereas maximum amount of ajmalicine was noted in leaf derived callus. This enriched level of alkaoids may be due to up- regulation of genes, encoding tryptophan decarboxylase (TDC), an enzyme catalyse the initial step of biosynthetic pathway in reserpine and ajmalicine synthesis. Our observations fall in consonance with previous report of Pitta-Alvarez et al. (2000) in which two tropane alkaloids, scopolamine and hyoscyamine were noted to be accumulated in hairy root cultures of Brugmansia candida in response to CdCl2 treatment. Similar result of reserpine enhancement against AlCl3 elicitation was reported recently by Zafar et al. (2017). Thus, considering the increased callus biomass growth in conjunction with improved reserpine and ajmalicine accumulation in present study, elicitation is undeniably a potential approach for persistent, large scale synthesis of these important alkaloids.
Conclusion
CdCl2 was used as abiotic elicitor and its effect on callus biomass growth, biochemical attributes and alkaloid yield (reserpine and ajmalicine) was investigated. This study demonstrates that CdCl2 elicitor when employed in low doses (up to C3) enriched reserpine and ajmalicine yield in in vitro cultures of R. serpentina. The addition of this compound in medium enhanced different studied antioxidant enzyme activities, confirming cellular stress in elicitated tissues. Hence, the present investigation with CdCl2 indicates the expediency of elicitation as an effective approach for enhancing the yield of secondary metabolites.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors greatly acknowledge Department of Science and Technology (DST) for the financial support. The authors are also thankful to Department of Botany and Central Instrumental Facility (CIF), Jamia Hamdard, for providing laboratory, instruments and other facilities.
Author contributions
NZ performed all the experimental works; Other scientists (MA, DT, BZ, MQM, JM, RS) involved in this study helped in designing experiments, preparing tables, figures and photoplates; and AM supervised and edited manuscript for final submission.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest authors certify.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ali M, Mujib A, Gulzar B, Zafar N. Essential oil yield estimation by Gas chromatography–mass spectrometry (GC–MS) after Methyl jasmonate (MeJA) elicitation in in vitro cultivated tissues of Coriandrum sativum L. 3 Biotech. 2019;9:41. doi: 10.1007/s13205-019-1936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59:206–216. [Google Scholar]
- Ashraf MF, Aziz MA, Kemat N, Ismail I. Effect of cytokinin types, concentrations and their interactions on in vitro shoot regeneration of Chlorophytum borivilianum Sant. and Fernandez. Electro J Biotechnol. 2014;17(6):275–279. [Google Scholar]
- Bates L, Waldren PP, Teare JD. Rapid determination of free proline of water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bidabadi SS, Meon S, Wahab Z, Subramaniam S, Mahmood M. In vitro selection and characterization of water stress tolerant lines among ethyl methanesulphonate (EMS) induced variants of banana (Musa spp., with AAA genome) Aust J Crop Sci. 2012;6:567–575. [Google Scholar]
- Bolouri-Moghaddam MR, Van den Ende W. Sugars and plant innate immunity. J Exp Bot. 2012;63:3989–3998. doi: 10.1093/jxb/ers129. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein, utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–541. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai Z, Kastell A, Speiser C, Smetanska I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl Biochem Biotechnol. 2013;171:330–340. doi: 10.1007/s12010-013-0354-4. [DOI] [PubMed] [Google Scholar]
- Chauhan S, Kaur A, Pareek RK. Pharmacobotanical and pharmacological evaluation of ayurvedic crude drug: Rauwolfia serpentina (Apocynaceae) Int J Green Pharm. 2017;11(4):S686. [Google Scholar]
- Dey PM. Methods in plant biochemistry. Carbohydarates. 2. London: Academic Press; 1990. [Google Scholar]
- Dhindsa RH, Plumb-Dhindsa R, Thorpe TA. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot. 1981;32:93–101. [Google Scholar]
- Efferth T. Biotechnology applications of plant callus cultures. Engineering. 2019;5(1):50–59. [Google Scholar]
- Fatima S, Mujib A, Tonk D. NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. Plant Cell Tissue Org Cult. 2015;121:445–458. [Google Scholar]
- Feng-tao LI, Jian-min QI, Gao-yang Z, Li-hui L, Ping-ping F, Fen TA, Jian-tang XU. Effect of cadmium stress on the growth antioxidative enzymes and lipid peroxidation in two kenaf (Hibiscus cannabinus L.) plant seedlings. J Inte Agr. 2013;12:610–620. [Google Scholar]
- Garvey CJ, Lenne T, Koster KL, Kent B, Bryant G. Phospholipid membrane protection by sugar molecules during dehydration-insights into molecular mechanisms using scattering techniques. Int J Mol Sci. 2013;14(4):8148–8163. doi: 10.3390/ijms14048148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Cadmium stress tolerance in crop plants. Plant Signal Behav. 2011;6(2):215–222. doi: 10.4161/psb.6.2.14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel MK, Kukreja AK, Khanuja SPS. Cost effective approaches for in vitro mass propagation of Rauvolfia serpentina Benth. Ex. Kurz. Asian J Plant Sci. 2007;6(6):957–961. [Google Scholar]
- Haensch KT. Influence of 2, 4-D and BAP on callus growth and the subsequent regeneration of somatic embryos in long-term cultures of Pelargonium x domesticum cv. Madame Layal Electron J Biotechnol. 2007;10(1):1–6. [Google Scholar]
- Hareesh KV, Nirmala SS, Rajendra CE. Reserpine content of Rauwolfia serpentina in response to geographical variation. Int J Pharm Biosci. 2010;1(4):429–434. [Google Scholar]
- Hausler RE, Luisa H, Jessica S, Ulf-Ingo F. How sugars might coordinate chloroplast and nuclear gene expression during acclimation to high light intensities. Mol Plant. 2014;7(7):1121–1137. doi: 10.1093/mp/ssu064. [DOI] [PubMed] [Google Scholar]
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments. Plant Signal Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M. Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci. 2012;4(1):10–20. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell. 2013;25(9):3159–3173. doi: 10.1105/tpc.113.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y. Exogenous proline and glycine betaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol. 2009;166:1587–1597. doi: 10.1016/j.jplph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Javed SB, Anis M. Cobalt induced augmentation of in vitro morphogenic potential in Erythrina variegata L.: a multipurpose tree legume. Plant Cell Tissue Org Cult. 2015;120:463–474. [Google Scholar]
- Javed SB, Anis M, Khan PR, Aref IM. In vitro regeneration and multiplication for mass propagation of Acacia ehrenbergiana Hayne: a potential reclaimant of denude arid lands. Agro For Syst. 2013;87:621–629. [Google Scholar]
- Kumar VH, Shashidhara S, Anitha S, Rajesh MS. Quantitative detection of reserpine in Rauwolfia serpentina using HPTLC. Int J Pharm Sci. 2010;2(4):87–89. [Google Scholar]
- Kumari R, Rathib B, Ranic A, Bhatnagar S. Rauvolfia serpentina L. Benth. ex Kurz.: phytochemical, pharmacological and therapeutic aspects. Int J Pharm Sci Rev Res. 2013;23(2):348–355. [Google Scholar]
- Lakra N, Tomar PC, Mishra SN. Growth response modulation by putrescine in Indian mustard Brassica Juncea L. under multiple stress. Ind J Exp Biol. 2016;54:262–270. [PubMed] [Google Scholar]
- Liang X, Zhang L, Natrajan SK, Becker DF. Proline mechanisms of stress survival. Antioxidant Redox Signal. 2013;19(9):998–1011. doi: 10.1089/ars.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobay D. Rauwolfia in the treatment of hypertension. J Integr Med. 2015;14(3):40–46. [PMC free article] [PubMed] [Google Scholar]
- Malar S, Vikram SS, Favas PJC, Perumal V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)] Bot Stud. 2014;55(54):1–11. doi: 10.1186/s40529-014-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqsood M, Mujib A. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Braz J Pharm. 2017;408(5):549–556. [Google Scholar]
- Martins J, Brijesh S. Phytochemistry and pharmacology of anti-depressant medicinal plants: a review. Biomed Pharmacother. 2018;104:343–365. doi: 10.1016/j.biopha.2018.05.044. [DOI] [PubMed] [Google Scholar]
- Meena M, Divyansu K, Kumar S, Swapnil P, Zehra A, Shukla V, Yadav M, Upadhyay RS. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental condition. Heliyon. 2019;5(12):e02952. doi: 10.1016/j.heliyon.2019.e02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B, Sangwan RS, Mishra S, Jadaun JS, Sabir F, Sangwan NS. Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal) Protoplasma. 2014;251(5):1031–1045. doi: 10.1007/s00709-014-0613-4. [DOI] [PubMed] [Google Scholar]
- Morkunas I, Wozniak A, Mai VC, Rucinska-Sobkowiak R, Jeandet P. The role of heavy metals in plant response to biotic stress. Molecules. 2018;23(9):2320. doi: 10.3390/molecules23092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujib A, Tanu P, Ali M, Dipti T, Zafar N, Gulzar B. In vitro propagation of Althaea officinalis: the role of plant growth regulators in morphogenesis. BioTechnol. 2017;98(3):167–173. [Google Scholar]
- Murthy HN, Lee EJ, Paek KY. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Org Cult. 2014;118(1):1–16. [Google Scholar]
- Muszynska E, Hanus-Fajerska E, Kozminska A. Differential tolerance to lead and cadmium of micropropagated Gypsophila fastigiata ecotype. Water Air Soil Pollut. 2018;229:42. doi: 10.1007/s11270-018-3702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik PM, Al-Khayri JM. Impact of abiotic elicitors on in vitro production of plant secondary metabolites: a review. J Adv Res Biotechnol. 2016;1(2):1–7. [Google Scholar]
- Naik PM, Al-Khayri JM (2016) Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants, abiotic and biotic stress in plants. In: Shanker AK, Shanker C (eds) IntechOpen, 10.5772/61442.
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nammi S, Boini KM, Koppula S, Sreemantula S. Reserpine-induced central effects: pharmacological evidence for the lack of central effects of reserpine methiodide. Can J Physiol Pharmacol. 2005;83:509–515. doi: 10.1139/y05-039. [DOI] [PubMed] [Google Scholar]
- Negi JS, Bisht VK, Bhandari AK, Bisht DS, Singh P, Singh N. Quantification of reserpine content and antibacterial activity of Rauvolfia serpentina (L.) Benth. ex Kurz. Afr J Microbiol Res. 2014;8(2):162–166. [Google Scholar]
- Patel H, Krishnamurthy R. Elicitors in plant tissue culture. J Pharmacogn Phytochem. 2013;2(2):60–65. [Google Scholar]
- Pitta-Alvarez SI, Spollansky TC, Giulietti AM. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzyme Microb Technol. 2000;26:252–258. doi: 10.1016/s0141-0229(99)00137-4. [DOI] [PubMed] [Google Scholar]
- Poonam AS, Mishra S. Physiological, biochemical and modern biotechnological approach to improvement of Rauvolfia serpentina. J Pharm Biol Sci. 2013;6(2):73–78. [Google Scholar]
- Qu L, Akbergenova Y, Hu Y, Schikorski T. Synapse-to-synapse variation in mean synaptic vesicle size and its relationship with synaptic morphology and function. J Comp Neurol. 2009;514(4):343–352. doi: 10.1002/cne.22007. [DOI] [PubMed] [Google Scholar]
- Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjusha AP, Radhamany PM, Gangaprasad A, Nair GM. In vitro shoot multiplication from shoot tip and nodal explants of Rauvolfia serpentina (L.) Benth. Ex. Kurz, an endangered medicinal plant. Int J Biol Pharm Allied Sci. 2012;1(8):1101–1108. [Google Scholar]
- Rejeb IB, Pastor V, Mauch-Mani B. Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants. 2014;3:458–475. doi: 10.3390/plants3040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Prado C, Podazza G, Interdonato R, González JA, Hilal M, Prado FE. Soluble sugars- metabolism, sensing and abiotic stress. Plant Signal Behav. 2009;4(5):388–393. doi: 10.4161/psb.4.5.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Joshi T, Kanojiya S, Tripathi V, Mishra DK. Callus culture and in vitro biosynthesis of echitamine from Alstonia scholaris (L.) R. Br. Plant Cell Tissue Org Cult. 2015;120:367–372. [Google Scholar]
- Singh M, Kaur R, Rajput R, Mathur G. Evaluating the therapeutic efficiency and drug targeting ability of alkaloids present in Rauwolfia serpentina. Int J Green Pharm. 2017;11(3):132–141. [Google Scholar]
- Sivanandhan G, Aruna M, Mayavana S, Rajesha M, Mariashibub TS, Manickavasagama M, Selvarajc N, Ganapathia A. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crops Prod. 2012;37(1):124–129. [Google Scholar]
- Soni R, Jaiswal S, Bara JK, Saksena P. The Use of Rauwolfia serpentina in hypertensive patients. J Biochem Biotechnol. 2016;2(5):28–32. [Google Scholar]
- Srivastava A, Tripathi AK, Pandey R, Verma RK, Gupta MM. Quantitative determination of reserpine, ajmaline, and ajmalicine in Rauvolfia serpentina by reversed-phase high-performance liquid chromatography. J Chromatogr Sci. 2006;44:557–560. doi: 10.1093/chromsci/44.9.557. [DOI] [PubMed] [Google Scholar]
- Su YH, Liu YB, Zhang XS. Auxin–cytokinin interaction regulates meristem development. Mol Plant. 2011;4(4):616–625. doi: 10.1093/mp/ssr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundarasekar J, Jeyanthi J, Anthony J, Murugaiyah V, Subramaniam S. Preliminary responses of 2, 4-D and BAP on callus initiation of an important medicinal-ornamental Hymenocallis littoralis plants. J Med Plants Res. 2012;6(11):2088–2093. [Google Scholar]
- Susila T, Reddy GS, Jyothsna D. Standardization of protocol for in vitro propagation of an endangered medicinal plant Rauwolfia serpentina Benth. J Med Plants Res. 2013;7(29):2150–2153. [Google Scholar]
- Thakore D, Srivastava AK, Sinha AK. Mass production of ajmalicine by bioreactor cultivation of hairy roots of Catharanthus roseus. Biochem Eng J. 2017;119:84–91. [Google Scholar]
- Tran LS, Nakashima K, Shinozaki K, Yamaguchi-Shinozaki K. Plant gene networks in osmotic stress response: from genes to regulatory networks. Methods Enzymol. 2007;428:109–128. doi: 10.1016/S0076-6879(07)28006-1. [DOI] [PubMed] [Google Scholar]
- Verma KC, Verma SK. Alkaloids analysis in root and leaf fractions of sarpaghanda (Rauwolfia serpentina) Agric Sci Dig. 2010;30(2):133–135. [Google Scholar]
- Victório CP, Luiz C, Lage S, Sato A. Tissue culture techniques in the proliferation of shoots and roots of Calendula officinalis. Rev Ciênc Agron. 2012;43(3):539–545. [Google Scholar]
- Wani SH, Kumar V, Shriram V, Sah SK. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016;4(3):162–176. [Google Scholar]
- Yan YH, Li J, Zhang XQ, Yang WY, Wan Y, Ma YM, Zhu YQ, Peng Y, Huang LK. Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. PLoS ONE. 2014;9(3):e90700. doi: 10.1371/journal.pone.0090700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wen KS, Ruan X, Zhao YX, Wei F, Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23(4):1–26. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Chan Z. ROS regulation during abiotic stress responses in crop plants. Front Plant Sci. 2015;6:1092. doi: 10.3389/fpls.2015.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar N, Mujib A, Ali M, Tonk D, Gulzar B. Aluminium chloride elicitation (amendment) improves callus biomass growth and reserpine yield in Rauvolfia serpentina leaf callus. Plant Cell Tissue Org Cult. 2017;130:357–368. [Google Scholar]
- Zahid SH, Mujib A. Accumulation of vincristine in calcium chloride elicitated Catharanthus roseus cultures. Nat Prod J. 2012;2(9):307–315. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.