Summary
Immune cold tumor characterized by low immunogenicity, insufficient and exhausted tumor-infiltrating lymphocytes, and immunosuppressive microenvironment is the main bottleneck responsible for low patient response rate of immune checkpoint blockade. Here, we developed biosynthetic functional vesicles (BFVs) to convert immune cold into hot through overcoming hypoxia, inducing immunogenic cell death, and immune checkpoint inhibition. The BFVs present PD1 and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on the surface, whereas load catalase into their inner core. The TRAIL can specifically induce immunogenic death of cancer cells to initiate immune response, which is further synergistically strengthened by blocking PD1/PDL1 checkpoint signal through ectogenic PD1 proteins on BFVs. The catalase can produce O2 to overcome tumor hypoxia, in turn to increase infiltration of effector T cells while deplete immunosuppressive cells in tumor. The BFVs elicit robust and systematic antitumor immunity, as demonstrated by significant regression of tumor growth, prevention of abscopal tumors, and excellent inhibition of lung metastasis.
Subject Areas: Immunity, Immune Response, Cell Biology
Graphical Abstract

Highlights
-
•
BFVs integrated PD1, TRAIL, and Catalase to convert immune cold tumor into hot
-
•
TRAIL induces cancer cell immunogenic death, ectogenic PD1 blocks checkpoint signal
-
•
Catalase reduces TME hypoxia to enhance effector T cell infiltration and activation
-
•
BFVs boost systematic antitumor immunity and achieve long-term immune memory
Immunity; Immune Response; Cell Biology
Introduction
Immune checkpoint blockade (ICB) therapy has shown great therapeutic potential in various cancer types, including melanoma, renal carcinoma, head and neck squamous cell carcinomas, non-small cell lung cancer, urothelial carcinoma, and bladder cancer (Tang et al., 2018; Tumeh et al., 2014). In particular, ICB therapy by monoclonal antibodies specific for the immune checkpoint proteins of programmed cell death receptor-1 (PD1) or its predominant ligand (PDL1) has achieved tremendous success in clinic (Boussiotis, 2016; Zou et al., 2016). However, only a small portion of patients benefit from current ICB therapies (Robert et al., 2015; Topalian et al., 2012), mainly ascribed to many tumors inherently featured as immune cold with low level and exhausted tumor-infiltrating lymphocytes (TILs), insufficient tumor antigen burden, and immunosuppressive microenvironment caused by tumor hypoxia, which does not respond well to ICB therapies (Galon and Bruni, 2019; Huang et al., 2019). Although combination of ICB with various other therapeutic modalities (i.e., radiotherapy [Chao et al., 2018], chemotherapy [Wang et al., 2018], phototherapy [He et al., 2016], and other immunotherapies [Wolchok et al., 2013]) has achieved a synergistic effect to some degree, the clinical outcome is still not satisfactory as these therapeutic modalities are also very difficult to convert immune cold into hot as well as they have their own intrinsic defects, such as radiation of radiotherapy, severe systemic toxicity of chemotherapy, shallow tissue penetration of phototherapy and serious cumulative immune-related toxicities of combinational immunotherapies, all of which inevitably hinder their further clinical efficacy (Yue et al., 2019). Therefore, more rational synergistic approaches that can robustly convert the immune cold tumor into hot are extremely on demand to improve the therapeutic potential of ICB.
Up to now, several strategies have been developed to amplify immunomodulatory effects (Chen et al., 2019; Chiang et al., 2018; Galstyan et al., 2019; Mi et al., 2018; Ruan et al., 2019; Wang et al., 2016, 2019). Among them, biosynthetic functional vesicles (BFVs) with surface presenting PD1 proteins hold great promise to improve cancer immunotherapy efficiency, owing to their unique advantages including excellent biocompatibility and high fidelity of surface markers (Liu et al., 2019; Zhang et al., 2018a, 2018b, 2018c). However, other typical immunosuppressive features involved in immune cold tumor, for example, low tumor associated antigen (TAA) burden that inhibits the recognition ability or activation of cytotoxic T cells in tumor, still extensively exist to significantly impair the immunotherapy effect. Thus, in addition to PDL1 blockade, the BFVs that can simultaneously induce immunogenic cell death (ICD) of cancer cells to increase tumor immunogenicity (such as TAA burden) are extremely needed to improve the ICB efficacy. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising biotherapeutic candidate that can specifically induce apoptosis of cancer cells without causing toxicity to normal cells (von Karstedt et al., 2017). Therefore, TRAIL might be a potential ICD inducer and could seamlessly cooperate with ectogenic PD1 when both of them are presented on BFVs.
Although the cooperation of TRAIL and PD1 can synergistically improve the tumor immunogenicity, the dynamic immune evasion mechanisms due to the complicated tumor microenvironment (TME) of solid cold tumor still often lead to therapeutic failure (Binnewies et al., 2018; Kim et al., 2018). Typically, solid tumors are characterized by a highly hypoxic TME, which contributes to immune resistance and immune suppression/tolerance through multi-pathways, including recruitment of immunosuppressive cells (i.e., myeloid-derived suppressor cells [MDSCs], tumor-associated macrophages [TAMs], and T-regulatory [Treg] cells), making cancer cells resistant to cytotoxic T lymphocyte (CTL)-mediated lysis, desensitization of chemokine receptors, and reduction of proinflammatory cytokines (Noman et al., 2015). Therefore, alleviating the hypoxia of TME would be a powerful strategy to reverse immune cold into hot for improving immunotherapy outcomes. Although respiratory hyperoxia has been applied to decrease the intra-tumoral hypoxia for reinforcing immunotherapy effect (Hatfield et al., 2015), this protocol also produced excessive amounts of reactive O2 species (ROS) that might overwhelm natural antioxidant defenses to destroy normal cellular structures (e.g., inducing hyperoxic acute lung injury [Kallet and Matthay, 2013]). Compared with such systemic O2 supply strategy, in situ intra-tumoral generation of O2 will be a more efficient, favorable, and safe approach for alleviating hypoxia but has rarely been reported to modulate the immune responses.
To overcome the low immune responses of ICB therapy in cold tumors, we herein proposed a proof-of-concept strategy by designing BFVs with surface presenting PD1 and TRAIL while encapsulating catalase (CAT) into the inner core to thoroughly convert tumor from immune cold into hot milieu for eliciting robust and systematic antitumor immunity (Figure 1A). We hypothesized that the TRAIL component on BFVs could initiate the immune responses by specifically inducing immunogenic cancer cell death, which could be further strengthened by collaborating with presented ectogenic PD1 proteins as the checkpoint blockade to reactivate the anergic tumor-specific CTLs; in addition, the CAT could catalyze highly abundant H2O2 in TME for in situ generation of O2, thus to ameliorate tumor hypoxia for systematically reversing the un-favorable environment of ICB therapy to ensure the trafficking and killing activities of CTLs in tumors. The comprehensive immuno-modulating ability and robust antitumor immunity of our BFVs have been clearly demonstrated in immune cold tumor models (4T1 breast tumor models [Sagiv-Barfi et al., 2015], including both subcutaneous and orthotopic models), through significant primary tumor growth regression, excellent metastasis prevention, obvious distal and immune-memory effects, and long-term survival benefits. Taken together, the here reported BFVs can elicit potent and durable immune responses by turning immune cold tumors into hot, and in principle it might be an universal immunotherapy paradigm for various other solid tumors and very promising for future translation.
Figure 1.
BFVs Synergistically Improve Immunotherapy by Converting Immune Cold into Hot
(A) Schematic illustration of the mechanism of BFVs to generate robust antitumor immune responses. TRAIL can specifically induce immunogenic cancer cell death (ICD) to initiate immune responses. Ectogenic PD1 proteins on BFVs can block the PD-1/PDL1 immune checkpoint to further synergistically strengthen the immune responses. CAT can alleviate tumor hypoxia to enhance intra-tumoral infiltration of effector T cells and weaken immunosuppression.
(B) Confocal images of HEK293 FT cells stably expressing mouse PD1 and mouse TRAIL on cell membranes, respectively. DiO and DiI were used to stain cell membrane (scale bar: 10 μm).
(C) The representative flow cytometric analysis of PD1-expressing (gated on mCherry+) and TRAIL-expressing (gated on EGFP+) HEK293 FT stable cells.
(D) Western blot assay to confirm the expression of mouse PD1 (indicated as PD1+) and mouse TRAIL (indicated as TRAIL+) in 293FT cells. Actin was used as the loading control.
(E) Confocal images to demonstrate the co-existence of PD1-mCherry and TRAIL-EGFP on the single vesicle by the overlap of red/green fluorescence (scale bar: 10 μm).
(F) The transmission electron microscopy image of BFVs (scale bar: 50 nm).
(G) The size distribution of BFVs through dynamic light scattering analysis.
(H) Coimmunoprecipitation (coIP) and western blot to examine the right outside-out orientation of PD1 on the BFVs. Anti-PD1 antibody could pull down BFVs, whereas IgG as a control could not bind to PD1 proteins on BFVs.
Results
Fabrication of BFVs
To bio-synthesize the functional vesicles (as depicted in Figure S1), we first established stable HEK293 FT cells expressing the proteins of mouse PD1 fused with mCherry (PD1-mCherry) and TRAIL fused with EGFP (TRAIL-EGFP) on the cell membrane, respectively, by using lentiviral vector for corresponding gene transfection (Figure S2). As shown in Figure 1B, the PD1 fusing proteins and the TRAIL fusing proteins were nicely expressed on the plasma membrane of transfected cells through confocal laser scanning microcopy (CLSM) observation, evidenced by their co-localization with cell membrane-staining dyes. Flow cytometry analysis further proved that nearly 90% of the genetically engineered cells were PD1 or TRAIL positive (Figure 1C), and the stable over-expression of the PD1 or TRAIL proteins can be further confirmed by western blot (WB) analysis (Figures 1D, S3A, and S3B). Collectively, these results clearly confirm the successful display of PD1 or TRAIL proteins on the membrane of HEK293 FT cells.
Then, the cell membranes of engineered HEK293 FT cells were extracted, and the BFVs were obtained by co-extrusion of PD1 anchoring membranes, TRAIL anchoring membranes, and commercially available CAT through 1, 0.4, 0.2, and 0.1 μm pore-sized polycarbonate membrane filters. To vividly observe the co-existence of PD1 and TRAIL on BFVs, the rough products extruded through 1-μm filters were imaged by CLSM (Figure 1E). As expected, the vesicles presented both green fluorescence of TRAIL-EGFP and red fluorescence of PD1-mCherry, demonstrating the successful fusion of both cell membranes. Next, the morphology and size of BFVs were characterized by transmission electron microscopy and dynamic light scattering analyses, which showed a uniform vesicular morphology with average diameter around 100 nm (Figures 1F, 1G, S4A and S4B). The zeta potential of the BFVs was determined as −24.9 ± 1.1 mV (Figure S4C). The immunoprecipitation assay showed that the anti-PD1 antibody could pull down the majority of BFVs, demonstrating the PD1 protein had a right outside-out orientation on most of the BFVs (Figures 1H and S3C).
In addition, we fabricated BFVs with larger size by extruding cell membranes (pre-labeled with DiO) and CAT (pre-labeled with Cy5) through 10-μm filters, to intuitively demonstrate the CAT can be encapsulated inside the inner core of BFVs by using CLSM. As shown in Figure S5A, the physical mixture of CAT and cell membranes (without co-extrusion) that exhibited only DiO green fluorescence, implying the free CAT unloaded in BFVs, could be completely removed by repeated washing in the process of producing BFVs. However, BFVs/CAT obtained by co-extrusion of BFVs and CAT showed dual and merged fluorescence signals, demonstrating the co-existence of cell membranes and CAT. Furthermore, 3D reconstruction of z stack images (Figure S5A) and plot profile (Figure S5B) along a single merged fluorescence vesicle revealed that the CAT was successfully encapsulated inside the BFV. Furthermore, we also compared the enzyme activity of the final product from physical mixing or from the co-extrusion procedure through detecting the H2O2 scavenge ability by using TiOSO4 as a color probe (from colorless to yellow after encountering H2O2 with maximum absorbance at 450 nm), to verify the encapsulation of CAT inside BFVs. As shown in Figure S5C, the product from physical mixture has negligible H2O2 scavenge ability, as demonstrated by nearly the same absorbance as control group. In sharp contrast, the TiOSO4 absorbance in BFVs/CAT group was much lower. These results demonstrated the successful encapsulation of CAT in the BFVs by our co-extrusion procedure.
The TRAIL and PD-1 contents in BFVs were determined by ELISA (Figure S6), and the ratio to total proteins in BFVs was calculated to be 1.02% and 5.50%, respectively. To estimate the loading content of CAT, it was labeled with Cy5 prior to loading into BFVs; then, the CAT content was analyzed by fluorescence measurement (Figure S6), and the ratio to total proteins in BFVs was calculated to be 35.07%.
BFVs Block PDL1 on Cancer Cells to Increase Their Susceptibility to CTLs
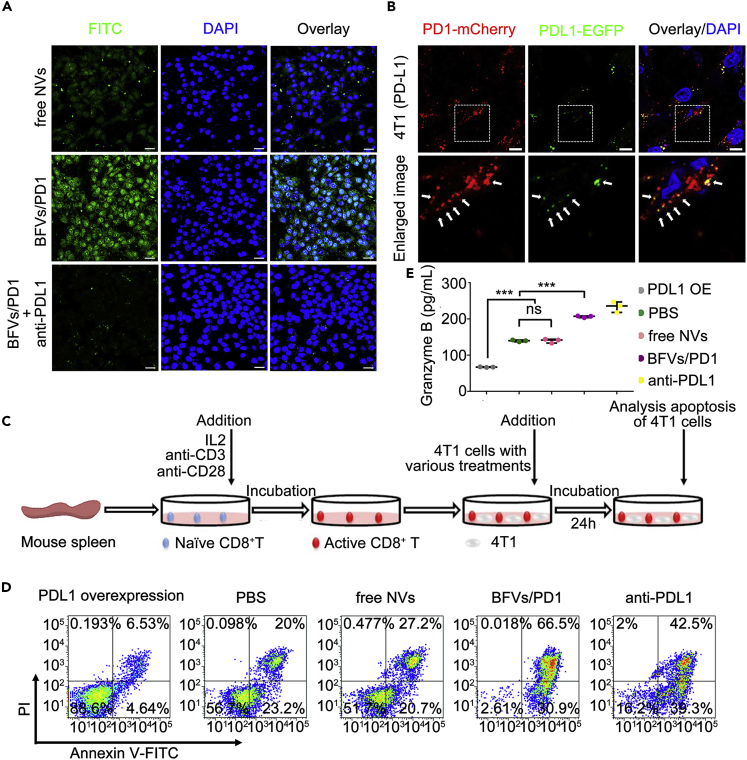
The interaction between PD1 on activated CTLs (CD8+ T cells) and its ligand PDL1 on cancer cells can exhaust CTLs and inhibit antitumor immune responses. Conversely, blockade of PD1/PDL1 axis could reverse this resistance and profoundly enhance therapeutic efficacy (Hirano et al., 2005). We next investigated whether our BFVs could act as a PDL1 checkpoint blockade. For this purpose, the 4T1 cells were incubated with FTIC-labeled PD1 presenting vesicles (BFVs/PD1) and imaged by CLSM. As shown in Figure 2A, BFVs/PD1 but not the control vesicles (without PD1 presenting, free NVs) could effectively bind to and be internalized by 4T1 cells after 4 h of incubation, but such binding and internalization could be blocked by PDL1 antibody pretreatment. To further intuitively demonstrate that the binding of BFVs/PD1 on 4T1 cells is mediated by the interaction between PD1 and PDL1, 4T1 cells were transfected with PDL1-EGFP plasmid to establish PDL1-EGFP expressing cells. Subsequently, the BFVs/PD1 with mCherry tag were incubated with these cells for 0.5 h at 4°C, then obvious co-localization with PDL1-EGFP on 4T1 cells was observed, in addition to some dissociative red fluorescence dots implying that BFVs/PD1 also might bind to endogenous PDL1 in 4T1 cells (Figure 2B). Similar results also could be observed in PDL1-EGFP-expressing H22 cells (Figure S7). Together, these results confirmed that the PD1-presenting BFVs could specifically interact with tumor cells through the binding between PD1 and PDL1.
Figure 2.
In Vitro Immune Checkpoint Inhibition of BFVs
(A) The CLSM images to present the internalization of FITC-labeled BFVs/PD1(50 μg/mL) by 4T1 breast cancer cells after 4 h of incubation (scale bar: 20 μm) at 37°C. free NVs: blank vesicles without presenting PD1 and TRAIL; BFVs/PD1: vesicles with surface presenting PD1 proteins; BFVs/PD1 + anti-PDL1: 4T1 cells pretreated with PDL1 antibody before BFVs/PD1 incubation.
(B) CLSM images to demonstrate the binding of BFVs/PD1-mCherry to the PDL1-EGFP over-expressed on 4T1 cell membrane (scale bar: 10 μm). 4T1 cells were transfected with PDL1-EGFP plasmid for 24 h, then incubated with BFVs/PD1-mCherry (50 μg/mL) for 0.5 h at 4°C. The lower panel is the enlarged image of the white dashed box on the upper panel.
(C) Representative procedure to schematically show the experimental design of the CTL cytotoxicity assay in vitro. Naive CD8+ T cells extracted from mouse spleen were activated by incubation with IL2, anti-CD3, and anti-CD28 antibodies, then they were added into 4T1 cells that previously underwent various treatments indicated as below: 4T1 cells over-expressing PDL1 (PDL1 OE), or normal 4T1 cells treated with PBS (PBS), free NVs, BFVs/PD1, or PDL1 antibody (anti-PDL1). After 24 h of co-incubation, the apoptosis ratio of 4T1 cells was measured.
(D) Apoptosis of the 4T1 cells treated as mentioned above by using Annexin V-FITC and PI cell apoptosis assay.
(E) The Granzyme B secreted in the suspensions of co-culture system after 24 h of treatment. Data are expressed as min to max, show all points (ns, no significance; ∗∗∗p < 0.001; n = 3).
We next attempted to explore whether the BFVs/PD1 could enhance the cytotoxicity of CTLs against cancer cells. As depicted in Figure 2C, naive mouse CD8+ T cells were first isolated from the spleen of BALB/c mice by magnetic bead sorting, followed by CD3/CD28 antibody stimulation to obtain activated CD8+ T cells. Subsequently, cancer cells with various treatments, such as blocking or overexpressing PDL1 on cell membranes, were added to the activated CD8+ T cells to check the cytotoxic activity by using Annexin V-FITC and PI cell apoptosis (shortened as APCA) assay. The PDL1 overexpressing 4T1 cells were less susceptible to the activated CD8+ T cells than the parental 4T1 cells; vice versa, when 4T1 cells were pretreated by BFVs/PD1 or PDL1 antibody, they became significantly more susceptible (Figures 2D and S8). These results indicated that blocking of PDL1 on cancer cells by BFVs/PD1 could effectively reverse the inhibition of T cell-mediated cytotoxic activity harnessed by cancer cells. As a main route of carrying out immunosurveillance, CTLs can secrete granzymes to induce cancer cell death (Cullen et al., 2010). As shown in Figure 2E, it was clearly demonstrated that blocking PD1/PDL1 interactions by BFVs/PD1 could obviously induce tumor cell death through the release of Granzymes B from the activated CD8+ T cells. These results strongly suggest that the BFVs/PD1 can block the PDL1 functions on cancer cells to re-strengthen T cell-mediated cytotoxic activity.
BFVs Induce ICD Responses in Cancer Cells
ICD plays an important role in initiating immune responses against tumor-associated antigens. Here, we further explore the potential of TRAIL as an ICD inducer, since it has been reported to selectively induce apoptosis by binding to the death receptors DR4 or DR5 in many cancer cell types while leaving normal cells unharmed (Jiang et al., 2016). Accordingly, we demonstrated that BFVs with surface presenting TRAIL-EGFP (BFVs/TRAIL) could co-localize with the DR5 on DR5-mCherry artificially over-expressed 4T1 cells or H22 cells (Figures 3A and S9). Meanwhile, TdT-mediated dUTP Nick-End Labeling (TUNEL) staining (Figure 3B) and APCA assay (Figures 3C and S10) demonstrated that the TRAIL on BFVs played a crucial role in inducing apoptosis of cancer cells (4T1 and H22) but not normal cells (NIH 3T3), whereas the PD1 proteins on BFVs had negligible effect. Calreticulin (CRT) is considered as one of the danger-associated molecular patterns (DAMPs) involved in ICD (Obeid et al., 2007). To confirm whether TRAIL on BFVs could promote ICD, the CRT expression of 4T1 cells with indicated treatments was analyzed by CLSM. As shown in Figure 3D, the CRT signals (green) dramatically increased in BFVs/PD1-TRAIL-treated group, compared with PBS or BFVs/PD1-treated groups.
Figure 3.
In Vitro ICD Responses of BFVs
(A) CLSM images to demonstrate the binding of BFVs/TRAIL-EGFP to the DR5-mCherry over-expressed on the 4T1 cells (scale bar: 10 μm). The 4T1 cells were transfected with DR5-mCherry plasmid for 24 h, then incubated with BFVs/TRAIL-EGFP (50 μg/mL) for 0.5 h at 4°C. The lower panel is the enlarged image of the white dashed box on the upper panel.
(B) TUNEL staining (green) and bright-field (BL) images of 4T1 cells with indicated treatments.
(C) Apoptosis of 4T1 cells with indicated treatments by flow cytometry analysis using Annexin V-FITC and PI staining, H2O2-treated cells were used as the positive control. The statistical analysis data are expressed as min to max, show all points (ns, no significance; ∗∗∗p < 0.001; n = 3).
(D) The CLSM examination of CRT expression on the surface of 4T1 breast cancer cells (scale bar: 50 μm).
(E) Representative procedure to schematically show the experimental design of DC maturation and T cell activation assay in vitro. First, the tumor cells were treated as indicated to obtain supernatant. Then the supernatant was incubated with immature DCs for 48 h to trigger maturation. Finally, the matured DCs were co-cultured with CD8+ T cells (pre-labeled with CFSE) for 72 h to trigger activation.
(F) The percentage of CD80+ CD86+ DCs through flow cytometry analysis after indicated treatments in the in vitro co-culture system, LPS-treated DC cells were used as the positive control.
(G) TNF-α, IFN-γ, and IL12-P70 levels in the supernatant of co-culture system after 2 days' treatment for DC maturation. Data are expressed as min to max, show all points (ns, no significance; ∗∗∗p < 0.001; n = 3).
(H) The flow cytometry analysis of CD8+ T cells labeled with CFSE 3 days after the co-culture.
(I) Mean fluorescence intensity (MFI) of CFSE labeled T cells. Data are expressed as min to max, show all points (ns, no significance; ∗∗∗p < 0.001; n = 3).
The release of DAMPs together with tumor-associated antigens (TAAs) could promote the maturation of DCs and further enable the ability to prime T cell activation (Yue et al., 2019). To verify this, we carried out a series of experiments as illustrated in Figure 3E. The supernatant of 4T1 cells after above treatments were added into immature DCs collected from bone marrow cells after stimulating with granulocyte macrophage colony stimulating factor (GM-CSF). After 48 h of incubation, the DC maturation was evaluated by dual staining for CD80 and CD86, the typical markers on the surface of maturated DCs. It showed that the supernatant of cancer cells treated with BFVs/PD1-TRAIL could significantly improve the degree of DC maturation, as demonstrated by the higher percentage of CD80/CD86-positive DC cells (Figure 3F). Consistently, the levels of DC-secreted immune cytokines, such as TNF-α, IFN-γ, and IL12-P70 in supernatant also significantly increased after the BFVs/PD1-TRAIL treatment (Figure 3G). Afterward, the matured DCs were incubated with carboxyfluorescein succinimidyl ester (CSFE)-labeled CD8+ T cells to explore their ability to prime T cell activation. The FACS analysis demonstrated that the matured DCs triggered by BFVs/PD1-TRAIL-treated cancer cells could significantly improve T cell proliferation (Figures 3H, 3I, and S11), compared with other groups without TRAIL component. Taken together, these results suggest that the TRAIL rather than PD1 on BFVs is able to induce ICD responses in cancer cells to effectively mobilize host adaptive immunity, and it is an important aspect for changing the immune cold state of tumor.
BFVs Relieve Tumor Hypoxia to Enhance Intra-tumoral Infiltration of Effector T Cells and Deplete Immunosuppressive Cells
It has been reported that CAT could decompose H2O2 into O2 (Figure 4A) (Chao et al., 2018); therefore, we wonder whether our BFVs with CAT in the inner core could in situ generate O2 in the tumor microenvironment (with relatively high level of H2O2) to relieve tumor hypoxia. We first evaluated the ability of CAT in BFVs to generate O2 in the presence of H2O2. As shown in Figure 4B, H2O2 and BFVs/PD1-TRAIL (without CAT loading) had negligible effect on O2 generation, whereas the BFVs/PD1-TRAIL-CAT (with CAT loading) yielded significantly higher amounts of O2 with H2O2 incubation. Afterward, we further explored the intracellular catalytic activity of BFVs/PD1-TRAIL-CAT by detecting H2O2 and O2 levels in cells with different treatments under hypoxic condition. As expected, the cells treated with BFVs/PD1-TRAIL-CAT exhibited much weaker fluorescence of H2O2 and hypoxia indicators as compared with other control groups, suggesting the loaded CAT kept excellent enzyme activity to catalyze H2O2 into O2 after internalization by cells (Figure 4C). For investigating the ability of relieving tumor hypoxia by BFVs/PD1-TRAIL-CAT in vivo, the oxygen saturation of tumors after local injection of BFVs/PD1-TRAIL-CAT was determined by photoacoustic imaging (PAI). Compared with the PBS group, the vascular saturated O2 (sO2) levels in the BFVs/PD1-TRAIL-CAT or free CAT-treated tumors were significantly higher (Figure 4D). To further confirm the relief of tumor hypoxia, the Hypoxyprobe-1 Plus Kits were used to assess the oxygenation status of tumor tissues. As shown in Figure 4E, the tumor treated with free CAT or BFVs/PD1-TRAIL-CAT showed dramatically decreased green fluorescence signal of hypoxia indicator. Concomitantly, the intra-tumoral H2O2 concentration in free CAT or BFVs/PD1-TRAIL-CAT-treated tumors was significantly lower than in the PBS-treated group (Figure 4F).
Figure 4.
BFVs Alleviate Tumor Hypoxia to Enhance Intra-tumoral Infiltration of Effector T Cells and Relieve Immunosuppressive Tumor Microenvironment
(A) The schematic illustration of relieving tumor hypoxia through decomposing H2O2 into O2 by CAT.
(B) Oxygen generation in H2O2 solutions (0.5 mM) with BFVs/PD1-TRAIL (without CAT) or BFVs/PD1-TRAIL-CAT (with CAT).
(C) Fluorescence microscopy images of 4T1 cells treated with BFVs/PD1-TRAIL and BFVs/PD1-TRAIL-CAT, whereas the cells treated with PBS were used as the control. The 4T1 cells were stained with Hydrogen Peroxide Assay Kit (green) or oxygen indicator of [Ru(dpp)3]Cl2 (red) (scale bar: 50 μm). The statistical analysis data are expressed as min to max, show all points (∗∗∗p < 0.001; n = 3).
(D) Photoacoustic imaging to show the vascular saturated O2 (sO2) levels of tumor after i.t. injection of PBS, CAT, and BFVs/PD1-TRAIL-CAT at different time points. The black-white scale bar shows the ultrasound imaging signals and the blue-red scale bar shows the photoacoustic signals.
(E) Immunofluorescence staining of the tumor slices by using Hypoxyprobe-1 Plus Kits (green), scale bar: 50 μm.
(F) Intra-tumoral H2O2 concentration in the mice after 4 h of i.t. injection with PBS, CAT, and BFVs/PD1-TRAIL-CAT (∗∗p < 0.01; n = 3).
(G) The flow cytometry analysis of the apoptosis and exhausting of CD8+ T cells incubated under normoxic and hypoxic conditions by using APCA assay and TIGIT-PerCP-eFluor 710 staining (gated on CD8+), respectively. The statistical analysis data are expressed as min to max, show all points (∗∗∗p < 0.001; n = 3).
(H–J) The flow cytometry analysis of the frequency of effector T cells (h, gated on CD3+), Tregs (I, gated on CD4+), and MDSCs (J) inside the tumor treated with PBS, CAT, and BFVs/PD1-TRAIL-CAT for 2 days after i.t. injection.
Emerging evidences demonstrated that tumor hypoxia could impair the antitumor immune responses by impeding killing function of CTLs, while promoting local immune suppression (Noman et al., 2015). Then, the influence of O2 on the activation of CD8+ T cells was first studied in vitro. After 48 h of culture, the primary CD8+ T cells were admitted to APCA assay. The ratio of apoptotic CD8+ T cells was significantly higher under hypoxic condition, compared with normoxic condition (Figures 4G, S12A, and S12B); the expression of TIGIT on CD8+ T cells, a T cell exhausting marker (Joller et al., 2011), was also significantly up-regulated under hypoxic environment (Figures 4G, S12C, and S12D). Therefore, we anticipated the relief of hypoxia in TME might reverse the inhibition of antitumor immunity of CD8+ effector T cells to some degree. To further explore the effects of relieving hypoxia on immune responses, we analyzed the infiltrated immune cell populations in tumors with different treatments after intra-tumoral (i.t.) injection by FACS analysis. The results showed that our BFVs/PD1-TRAIL-CAT not only could promote the intra-tumoral infiltration of CD8+ T cells (Figures 4H and S13 and Tables S1 and S2) but also could deplete the immunosuppressive cells like Treg cells (Figures 4I and S14 and Table S3) and MDSCs (Figures 4J and S15 and Table S4). These results suggest that BFVs can remodel hypoxia TME and reverse the hypoxia-associated immunosuppression, which is crucial for converting immune cold tumor into hot.
In Vivo Antitumor Effect of BFVs
To evaluate the in vivo therapeutic effect of BFVs, a bilateral 4T1 tumor model was first established by subcutaneously (s.c.) injecting 1 × 106 and 5 × 105 4T1 tumor cells into the left and right flanks of mice as primary and abscopal tumors, respectively. The primary tumor received various treatments by i.t. injection at days 1, 4, 7, and 10 with the equivalent dosage (Figure 5A), while leaving the abscopal tumors untreated. Antitumor growth effects against the primary and abscopal tumors are summarized in Figures 5B and 5C. The primary tumors in the group of anti-PDL1, BFVs/PD1, BFVs/TRAIL, and BFVs/PD1-TRAIL treatment displayed slower tumor growth compared with the mice treated with PBS and blank vesicles without any functional components (free NVs). Notably, our BFVs containing PD1, TRAIL, and CAT (BFVs/PD1-TRAIL-CAT) exerted the highest antitumor efficacy among all groups, suggesting their synergistic effects. For abscopal tumors without direct treatment, the therapeutic outcomes are consistent with the primary tumors, with the highest therapeutic efficacy in BFVs/PD1-TRAIL-CAT-treated mice, and 70% of mice in this group survived for over 60 days, whereas all the mice in other groups survived no longer than 40 days, which might be attributed to the systematic activation of antitumor immunity. To verify this explanation, we analyzed the infiltration of TILs and the pro-inflammatory cytokines in abscopal tumors of the treated mice. As expected, BFVs/PD1-TRAIL-CAT-treated mice showed significantly promoted CD8+ T cell infiltration (Figure 5D), as well as the highest level of TNF-α, IFN-γ, and IL12-P70 compared with other groups (Figure 5E), implying the cooperation of PD1, TRAIL, and CAT to deeply convert immune cold into hot, which in turn boosts the efficacy of immunotherapy.
Figure 5.
Bilateral 4T1 Tumor Model Was Established to Evaluate the Abscopal Effect of BFVs
(A) Treatment schedule for BFVs-mediated immunotherapy. Tumors on the left were designated as the primary tumor for direct treatment with i.t. injection, and those on the right side were designated as distant tumor without treatment.
(B) Growth curves of the primary tumors and abscopal tumors after various treatments (PBS; free NVs; BFVs/PD1; BFVs/TRAIL; anti-PDL1, BFVs/PD1-TRAIL; BFVs/PD1-TRAIL-CAT). Average tumor volume of the treated mice in different groups (n = 8–10) (error bar, mean ± SD).
(C) Survival curves for the mice that received different treatments as indicated above (n = 10; ∗∗∗p<0.001).
(D) Immunofluorescence staining of the tumor slices to show the infiltrated CD4+ T cells (green) and CD8+ T cells (pink) (scale bar: 100 μm). The lower panel is the enlarged image of the white dashed box on the upper panel (scale bar: 50 μm).
(E) The ELISA analysis of cytokine levels (IFN-γ, TNF-α, IL12-P70) in abscopal tumor from mice isolated at day 15 after i.t. injection for primary tumor. The statistical analysis data are expressed as min to max, show all points (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; n = 3).
Furthermore, we employed the orthotopic 4T1 tumor model, as it is more clinically relevant, to investigate the therapeutic effect of BFVs with systematic administration. The experimental protocol is shown in Figure 6A, wherein the orthotopic tumor model was established by injecting 5 × 105 4T1 cells into abdominal mammary fat pads of female BALB/c mice. Before therapeutic assessment, in vivo distribution of ICG-labeled vesicles was analyzed by NIR II fluorescence imaging system. It revealed that vesicles containing PD1 proteins (BFVs/PD1) had excellent tumor accumulation even after 48 h of injection, enabling their durable therapeutic effect (Figure S16). In addition, the tumor accumulation of BFVs/PD1 was significantly higher than the control vesicles (without PD1 presenting), ascribing to the targeting ability of PD1 to PDL1 on cancer cells rather than the difference of Pharmacokinetics (Figure S17). In contrast, free ICG, which was easily cleared out from the body, had negligible fluorescence signal in major organs including tumor tissue compared with BFVs (Figure S16). With regard to antitumor efficacy (Figures 6B–6D, S18, and S19), the overall trends of tumor growth inhibition on the orthotopic tumor model were approximately the same as those on the subcutaneously xenografted tumor model, highlighting the distinctive antitumor ability of BFVs/PD1-TRAIL-CAT owing to the synergistic contributions from TRAIL, PD1, and CAT to convert immune cold into hot. Histological examinations of the tumor tissues stained with H&E, TUNEL, and Ki67 also confirmed that our BFVs/PD1-TRAIL-CAT induced the most extensive level of tumor cell necrosis and apoptosis (Figures 6E and S20).
Figure 6.
Orthotopic 4T1 Tumor Model Was Established to Evaluate the Antitumor Effect of BFVs
(A) Treatment schedule for the antitumor evaluation of BFVs on orthotopic 4T1 tumor model by i.v. injection.
(B) Average tumor volume of 4T1 tumors with indicated treatments. The statistical analysis data are expressed as min to max, show all points (∗∗∗p < 0.001; n = 9–10).
(C) Average tumor weight of the mice that received indicated treatments at the day 23. The statistical analysis data are expressed as min to max, show all points (∗p < 0.05, ∗∗∗p < 0.001; n = 9–10).
(D) Photographs of tumor excised from sacrificed mice of each group at the day 23.
(E) Left panel: H&E and Ki67 antigen immunohistochemistry staining images of tumor slices in different groups. Right panel: TUNEL and anti-CRT immunofluorescence staining images of tumor slices in different group. 1: PBS; 2: free NVs; 3: BFVs/PD1; 4: BFVs/TRAIL; 5: BFVs/PD1-TRAIL; 6: BFVs/PD1-TRAIL-CAT.
(F) Immunofluorescence staining of the tumor slices to analyze the infiltrated CD3+ T cells (red), CD4+ T cells (green), and CD8+ T cells (pink) (scale bar: 100 μm). The lower panel is the enlarged image of the white dashed box on the upper panel (scale bar: 50 μm).
(G) Cytokines levels in blood isolated from differently treated mice by ELISA analysis. The statistical analysis data are expressed as min to max, show all points (∗∗p < 0.01, ∗∗∗p < 0.001; n = 3).
(H) Representative plot analysis of T cells in blood isolated from differently treated mice by flow cytometry (gated on CD3+).
We next further explored the mechanisms underlying the remarkable antitumor efficiency of our BFVs-mediated synergistic therapy. First, immunofluorescent examination of tumor sections proved that the BFVs/PD1-TRAIL-CAT significantly increased CRT expression compared with other groups (Figure 6E). Since the CRT together with other DAMPs would promote the DC maturation for antigen presenting to T lymphocytes in draining lymph nodes (LNs), we analyzed the levels of DC maturation in draining LNs by FACS after 3 days of the last injection (Figure S21). As expected, the BFVs/PD1-TRAIL-CAT therapy triggered the highest level of DC maturation (CD11c+CD80+CD86+) among all groups. Meanwhile, the intra-tumoral infiltration of T lymphocytes and immune-related cytokines were examined by multispectral immunofluorescence staining (Figure 6F) and ELISA (Figure 6G), respectively. It showed that the BFVs/PD1-TRAIL-CAT treatment triggered a significantly higher degree of CD8+ T cell inflltration in tumors than other treatments. The cytokine secretion results also supported that BFVs/PD1-TRAIL-CAT treatment could elicit robust antitumor immune activity in tumor tissues, with the significantly increased secretion of pro-inflammation cytokines (TNF-α, IFN-γ, IL12-P70, Granzyme B) and decreased secretion of anti-inflammatory cytokines (TGF-β1, IL10). We also analyzed, in addition to tumor tissues, the frequency of T lymphocytes in peripheral blood by using FACS, and the result showed the percentage of CD8+ T cells in BFVs/PD1-TRAIL-CAT-treated groups was the highest compared with other groups, indicating the enhanced systemic immune responses after BFVs treatment (Figures 6H and S22). Taken together, these data suggest that the BFVs integrated functional components of TRAIL, PD1, and CAT could significantly boost systematic antitumor immune responses through converting immune cold into hot by simultaneously overcoming hypoxia, increasing immunogenic cell death, and checkpoint inhibition.
As the distinct characteristics of adaptive immune responses, immune-memory effects can provide long-term prevention against tumor recurrence and distant metastasis. Thus, we further assessed the immune-memory effects of our BFVs by i.v. infusion of 4T1 cells into the mice after the aforementioned treatments at day 30, whereas the residual tumor mass of each group was surgically resected from each mouse at the day 23. The animal experimental design is shown in Figure 7A. From photographs of the lung and H&E images (Figures 7B–7D), the PBS and free NVs-treated mice displayed large amounts of lung metastasis, whereas other control vesicle (BFVs/PD1, BFVs/TRAIL and BFVs/PD1-TRAIL)-treated mice showed certain degree of lung metastasis inhibition; as a remarkable contrast, the BFVs/PD1-TRAIL-CAT-treated mice showed almost no detectable metastasis, confirming the excellent immune memory effects after converting immune cold into hot.
Figure 7.
Long-Term Immune-Memory Effect of BFVs
(A) Schematic illustration of BFV treatment for lung metastasis inhibition of 4T1 tumor in BALB/c mice.
(B) Photographs of the lungs excised from sacrificed mice of each group.
(C) Statistical analysis of lung metastasis nodules (∗p < 0.05, ∗∗p < 0.01; n = 3).
(D) H&E staining images of lung metastasis slices in different groups.
(E) Flow cytometry analysis of the TCM and TEM in peripheral blood isolated from sacrificed mice after various treatments by staining CD44-PE-Cy7 and CD62L-PerCP-Cy5.5.
(F–I) The statistical analysis for the percentage of the TCM and TEM in CD8+ or CD4+ T cells, data are expressed as min to max, show all points (∗∗p < 0.01, ∗∗∗p < 0.001; n = 3).
To further identify the possible mechanisms of the long-term immune memory post synergistic immunotherapy of BFVs, we analyzed the central memory T cells (TCM) and effector memory T cells (TEM) in peripheral blood of mice after various treatments. Compared with TCM only providing immunities in the condition of expansion, differentiation, and migration, TEM can induce immediate protections upon secondary challenge (Ko and Formenti, 2018; McGranahan et al., 2016). As shown in Figures 7E–7I, S23, and S24, BFVs/PD1-TRAIL-CAT exhibited the highest frequency of CD8+ TEM and CD4+ TEM along with the lowest frequency of CD8+ TCM and CD4+ TCM in comparison with other groups, demonstrating the ability of our BFVs/PD1-TRAIL-CAT to shift systemic CD8+ and CD4+ TCM cells toward a TEM phenotype in the process of converting primary tumor from immune cold into hot, which is responsible for generating more-potent antitumor immune memory responses to prevent recurrence.
Discussion
With a review of the recent developments of tumor immunotherapy, the combination paradigms of conventional therapeutic modalities with ICB have shown great potency to augment cancer immunotherapy efficacy, but their effectiveness largely depends on the baseline immune responses. In such a case, most of solid tumors featured as immune cold lacking pre-existing adaptive immunity do not respond well to the current combinational therapeutic strategies (Galon and Bruni, 2019). Another concern with the present approaches would be the concurrent increase in undesired side effects (Whiteside et al., 2016). The main mechanisms involved in cold tumor include lack of TAA burden, deficient and exhausted TILs, and hypoxic TME-driven immunosuppressive environment. Conventional therapeutic modalities (e.g., radiotherapy, chemotherapy) can augment the antigenicity of tumor cells through increasing the release of TAAs, whereas these released TAAs are often poorly processed, presented, and recognized by immune cells owing to the extensive existing immune-suppression mechanisms; thus, the immune responses in such case are generally very limited (McGranahan et al., 2016). Meanwhile, like a double-edged sword, radiotherapy or chemotherapy can also subvert tumor immunosurveillance through recruiting myeloid cell types with distinct roles in T cell suppression and other side effects on normal tissue (Ko and Formenti, 2018). To restore the exhausted TILs, monoclonal antibody such as checkpoint inhibitor has gained considerable success in a subset of patients, but it also suffers from inefficient delivery into targeted site and severe immune-related adverse events (Naidoo et al., 2015). With respect to remodeling TME, respiratory hyperoxia (60% oxygen) has been verified to decrease intra-tumoral hypoxia for promoting antitumor immune response, whereas this systemic O2 supply approach might cause acute lung injury (Kallet and Matthay, 2013). Thus, how to simultaneously, safely, and effectively overcome these obstacles (converting tumor from immune cold into hot) is the key challenge for boosting robust immune responses to fight against cold tumors.
Compared with the existing therapeutic approaches that failed to turn cold tumor into hot milieu, our BFVs integrating PD1, TRAIL, and CAT showed several distinct merits and could efficiently convert cold tumor into hot. In our design, the TRAIL could selectively elicit ICD of cancer cells, whereas it had limited toxicity on normal cells; the CAT in BFVs not only could in situ generate O2 by virtue of the high levels of H2O2 in cancer cells without producing pulmonary O2 toxicity but also could reduce the malignant biological roles caused by H2O2 including metastasis of cancer cells, angiogenesis, and resistance to therapies (López-Lázaro, 2007). Furthermore, all components in our BFVs belong to biomolecules whose biological functions are nicely preserved in TME and have excellent biocompatibility and biodegradability without any noticeable toxicities (Figures S25–S29). Therefore, our BFVs integrating PD1, TRAIL, and CAT could effectively convert immune cold into hot through simultaneously overcoming hypoxia, inducing ICD and immune checkpoint inhibition. As a result, our BFVs not only boosted robust and systemic antitumor immune responses that simultaneously inhibited primary and abscopal tumor growth but also achieved long-term immune memory effects to prevent tumor recurrence/metastasis. In principle, our reported BFVs also could be suitable for the immunotherapy of other solid tumors. Thus, our approach might be particularly meaningful and readily adapted to a broad diversity of immune cold tumors that do not respond well to ICB therapies.
Limitations of the Study
The BFVs proposed here have comprehensive immuno-modulating ability to elicit robust and systematic antitumor immunity, but their antitumor efficiency might be heavily affected by the heterogeneity of cancer cells, tumor microenvironment, and patients' baseline immunity, which are very difficult to precisely measure and predict. Furthermore, the exact components of naturally obtained cell membranes are very difficult to accurately determine and quantify, which might be an obstacle for pharmaceutical translation, owing to difficulty in getting the same quality BFVs from batch to batch, and also lack of precise quality control strategy for large-scale production. These limitations should be further resolved for future translational applications.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Xiaolong Liu (xiaoloong.liu@gmail.com).
Materials Availability
This study did not generate new unique materials.
Data and Code Availability
All data produced or analyzed for this study are included in the published article and its supplementary information files.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 61727823, 81671813, 81601538), the joint research projects of Health and Education Commission of Fujian Province (Grant No. 2019-WJ-20).
Author Contributions
M.W., D.Zheng., and D.Zhang. contributed equally to this work; M.W., J.Liu., and X.Liu. designed the project; M.W., D.Zheng., D.Zhang., P.Y., L.P., F.C., Z.L., Z.C., J.Li., Z.W., and X.Lin. conducted the experiments; M.W., D.Zheng., and D.Zhang. analyzed and interpreted the data; X.Liu. and J.Liu. supervised the overall research; M.W., D.Zheng., and X.Liu. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101341.
Contributor Information
Jingfeng Liu, Email: drjingfeng@126.com.
Xiaolong Liu, Email: xiaoloong.liu@gmail.com.
Supplemental Information
References
- Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis V.A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y., Xu L., Liang C., Feng L., Xu J., Dong Z., Tian L., Yi X., Yang K., Liu Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018;2:611. doi: 10.1038/s41551-018-0262-6. [DOI] [PubMed] [Google Scholar]
- Chen Q., Chen G., Chen J., Shen J., Zhang X., Wang J., Chan A., Gu Z. Bioresponsive protein complex of aPD1 and aCD47 antibodies for enhanced immunotherapy. Nano Lett. 2019;19:4879–4889. doi: 10.1021/acs.nanolett.9b00584. [DOI] [PubMed] [Google Scholar]
- Chiang C.-S., Lin Y.-J., Lee R., Lai Y.-H., Cheng H.-W., Hsieh C.-H., Shyu W.-C., Chen S.-Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018;13:746–754. doi: 10.1038/s41565-018-0146-7. [DOI] [PubMed] [Google Scholar]
- Cullen S., Brunet M., Martin S. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- Galstyan A., Markman J.L., Shatalova E.S., Chiechi A., Korman A.J., Patil R., Klymyshyn D., Tourtellotte W.G., Israel L.L., Braubach O. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 2019;10:3850. doi: 10.1038/s41467-019-11719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield S.M., Kjaergaard J., Lukashev D., Schreiber T.H., Belikoff B., Abbott R., Sethumadhavan S., Philbrook P., Ko K., Cannici R. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Hatfield. 2015;7:277ra230. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Duan X., Guo N., Chan C., Poon C., Weichselbaum R.R., Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016;7:12499. doi: 10.1038/ncomms12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano F., Kaneko K., Tamura H., Dong H., Wang S., Ichikawa M., Rietz C., Flies D.B., Lau J.S., Zhu G. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- Huang L., Li Y., Du Y., Zhang Y., Wang X., Ding Y., Yang X., Meng F., Tu J., Luo L. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019;10:4871. doi: 10.1038/s41467-019-12771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Fitch S., Wang C., Wilson C., Li J., Grant G.A., Yang F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. PNAS. 2016;113:13857–13862. doi: 10.1073/pnas.1615396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller N., Hafler J.P., Brynedal B., Kassam N., Spoerl S., Levin S.D., Sharpe A.H., Kuchroo V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallet R.H., Matthay M.A. Hyperoxic acute lung injury. Respir. Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.K., Herbst R.S., Chen L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol. 2018;39:624–631. doi: 10.1016/j.it.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E.C., Formenti S.C. Radiotherapy and checkpoint inhibitors: a winning new combination? Ther. Adv. Med. Oncol. 2018;10 doi: 10.1177/1758835918768240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu C., Zheng Z., Chen S., Pang X., Xiang X., Tang J., Ren E., Chen Y., You M. Vesicular antibodies: a bioactive multifunctional combination platform for targeted therapeutic delivery and cancer immunotherapy. Adv. Mater. 2019;31:1808294. doi: 10.1002/adma.201808294. [DOI] [PubMed] [Google Scholar]
- López-Lázaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252:1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- McGranahan N., Furness A.J., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K., Jamal-Hanjani M., Wilson G.A., Birkbak N.J., Hiley C.T. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y., Smith C.C., Yang F., Qi Y., Roche K.C., Serody J.S., Vincent B.G., Wang A.Z. A dual immunotherapy nanoparticle improves T-cell activation and cancer immunotherapy. Adv. Mater. 2018;30:1706098. doi: 10.1002/adma.201706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J., Page D., Li B., Connell L., Schindler K., Lacouture M., Postow M., Wolchok J. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman M.Z., Hasmim M., Messai Y., Terry S., Kieda C., Janji B., Chouaib S. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am. J. Physiol-cell Ph. 2015;309:C569–C579. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid M., Tesniere A., Ghiringhelli F., Fimia G.M., Apetoh L., Perfettini J.-L., Castedo M., Mignot G., Panaretakis T., Casares N. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- Ruan H., Hu Q., Wen D., Chen Q., Chen G., Lu Y., Wang J., Cheng H., Lu W., Gu Z. A dual-bioresponsive drug-delivery depot for combination of epigenetic modulation and immune checkpoint blockade. Adv. Mater. 2019;31:1806957. doi: 10.1002/adma.201806957. [DOI] [PubMed] [Google Scholar]
- Sagiv-Barfi I., Kohrt H.E.K., Czerwinski D.K., Ng P.P., Chang B.Y., Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. PNAS. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Yu J.X., Hubbard-Lucey V.M., Neftelinov S.T., Hodge J.P., Lin Y. The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug Discov. 2018;17:854–855. doi: 10.1038/nrd.2018.210. [DOI] [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Karstedt S., Montinaro A., Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer. 2017;17:352. doi: 10.1038/nrc.2017.28. [DOI] [PubMed] [Google Scholar]
- Wang C., Sun W., Wright G., Wang A.Z., Gu Z. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and anti-PD1 antibody. Adv. Mater. 2016;28:8912–8920. doi: 10.1002/adma.201506312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wang J., Zhang X., Yu S., Wen D., Hu Q., Ye Y., Bomba H., Hu X., Liu Z. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018;10:eaan3682. doi: 10.1126/scitranslmed.aan3682. [DOI] [PubMed] [Google Scholar]
- Wang D., Wang T., Yu H., Feng B., Zhou L., Zhou F., Hou B., Zhang H., Luo M., Li Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019;4:eaau6584. doi: 10.1126/sciimmunol.aau6584. [DOI] [PubMed] [Google Scholar]
- Whiteside T.L., Demaria S., Rodriguez-Ruiz M.E., Zarour H.M., Melero I. Emerging opportunities and challenges in cancer immunotherapy. Clin. Cancer Res. 2016;22:1845–1855. doi: 10.1158/1078-0432.CCR-16-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., Segal N.H., Ariyan C.E., Gordon R.-A., Reed K. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W., Chen L., Yu L., Zhou B., Yin H., Ren W., Liu C., Guo L., Zhang Y., Sun L. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat. Commun. 2019;10:2025. doi: 10.1038/s41467-019-09760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhang L., Qin Z., Hua S., Guo Z., Chu C., Lin H., Zhang Y., Li W., Zhang X. Genetically engineered liposome-like nanovesicles as active targeted transport platform. Adv. Mater. 2018;30:1705350. doi: 10.1002/adma.201705350. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang C., Wang J., Hu Q., Langworthy B., Ye Y., Sun W., Lin J., Wang T., Fine J. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv. Mater. 2018;30:1707112. doi: 10.1002/adma.201707112. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang J., Chen Z., Hu Q., Wang C., Yan J., Dotti G., Huang P., Gu Z. Engineering PD-1-presenting platelets for cancer immunotherapy. Nano Lett. 2018;18:5716–5725. doi: 10.1021/acs.nanolett.8b02321. [DOI] [PubMed] [Google Scholar]
- Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8:328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data produced or analyzed for this study are included in the published article and its supplementary information files.