Summary
Muscles preferentially utilize glycolytic or oxidative metabolism depending on the intensity of physical activity. Transcripts required for carbohydrate and lipid metabolism undergo circadian oscillations of expression in muscles, and both exercise capacity and the metabolic response to exercise are influenced by time of day. The circadian repressors CRY1 and CRY2 repress peroxisome proliferator-activated receptor delta (PPARδ), a major driver of oxidative metabolism and exercise endurance. CRY-deficient mice exhibit enhanced PPARδ activation and greater maximum speed when running on a treadmill but no increase in exercise endurance. Here we demonstrate that CRYs limit hypoxia-responsive transcription via repression of HIF1α-BMAL1 heterodimers. Furthermore, CRY2 appeared to be more effective than CRY1 in the reduction of HIF1α protein steady-state levels in primary myotubes and quadriceps in vivo. Finally, CRY-deficient myotubes exhibit metabolic alterations consistent with cryptochrome-dependent suppression of HIF1α, which likely contributes to circadian modulation of muscle metabolism.
Subject Areas: Cell Biology, Chronobiology
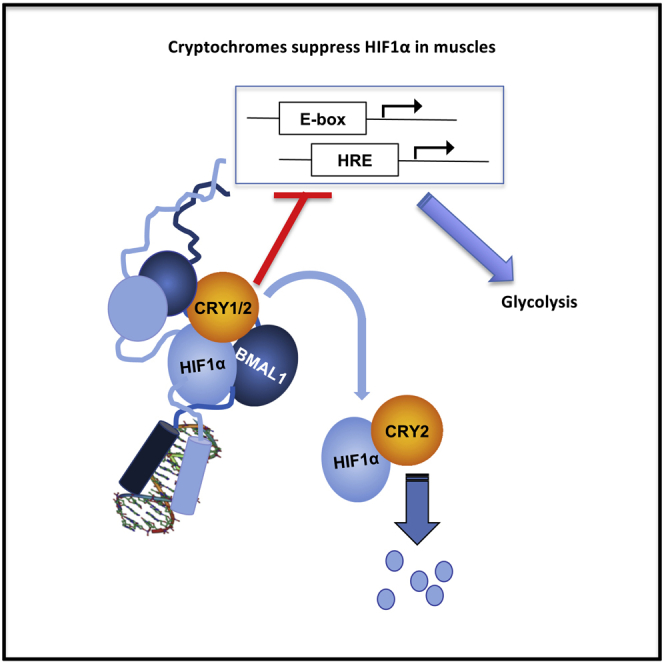
Graphical Abstract
Highlights
-
•
CRY2 plays a unique role in regulating HIF1α protein accumulation in muscle
-
•
HIF1α and BMAL1 heterodimers are transcriptionally active
-
•
CRY1/2 represses transcription driven by HIF1α/BMAL1 heterodimers
-
•
Cryptochromes influence skeletal muscle substrate preference and utilization
Cell Biology; Chronobiology
Introduction
Tissue hypoxia results from an imbalance between local oxygen consumption and the ability of associated vasculature to replenish oxygen. Hypoxia has long been studied in the context of cancer, with the observation that cancerous cells residing deep within solid tumors are exposed to an oxygen-poor local microenvironment. Cancer cells preferentially produce energy through glycolysis rather than oxidative phosphorylation, which allows them to survive in hypoxic environments and provides additional growth advantages that are as yet incompletely understood (Liberti and Locasale, 2016). In normal physiology, the hypoxia signaling network is an adaptive response designed to accommodate the immediate metabolic demands of a tissue and facilitate long-term adaptation to inadequate oxygen levels (Hoppeler and Vogt, 2001). This is particularly important in skeletal muscle, which must adapt to rapid and dramatic fluctuations in mean oxygen tension during strenuous exercise. Activation of hypoxic signaling in muscles stimulates both an immediate shift to glycolytic flux and long-term adaptations such as increased vascularization (Lindholm and Rundqvist, 2016). Mice with genetically attenuated hypoxia signaling in skeletal muscles exhibit enhanced reliance on oxidative phosphorylation for energy production. When sedentary, they have greater low-intensity exercise endurance. However, they are more susceptible to muscle damage; after training, they exhibit lower endurance capacity than wild-type (WT) controls (Mason et al., 2004).
Much of the hypoxic signaling cascade in mammals is activated via a group of transcription factors known as hypoxia-inducible factors (HIFs), which are part of the larger bHLH-PAS domain family of DNA-binding proteins (Dengler et al., 2014). These proteins are canonically composed of two subunits that heterodimerize and activate transcription of target genes via hypoxia response elements (HREs). This heterodimer pair is composed of the oxygen-sensitive HIF1α and the constitutively expressed HIF1β subunits. Regulation of hypoxic signaling involves the integration of numerous metabolic signals, including components of the MAPK pathway, citric acid cycle intermediates, and reactive oxygen species (ROS) (Bardos and Ashcroft, 2005). Most directly implicated in regulation of HIF1α/HIF1β signaling, however, are the class of enzymes known as prolyl hydroxylases (PHDs), which in mammals are encoded by the genes EGLN1-3. When oxygen is readily available, HIF1α is rapidly hydroxylated by PHDs on two prolines within its oxygen-dependent degradation domain, leading to its association with the von Hippel-Lindau (vHL) E3 ubiquitin ligase and subsequent degradation by the proteasome (Ivan and Kaelin, 2017). These PHD enzymes are 2-oxoglutarate (also known as alpha ketoglutarate, αKG)-dependent dioxygenases that rely on molecular oxygen, αKG, vitamin C, and iron as cofactors for their function. As such, PHD enzymes are inhibited under conditions of hypoxia or upon exposure to iron chelators or small molecules that displace αKG (Tian et al., 2011; Yuan et al., 2003). This leads to stabilization of HIF1α, which heterodimerizes with HIF1β to activate target genes containing one or more HRE sequences within their promoters (Ivan and Kaelin, 2017).
Like HIFs, the circadian clock is highly regulated and sensitive to modulation by metabolic signals. In mammals, circadian oscillators are composed of a transcription/translation feedback loop, in which the bHLH-PAS transcription factors CLOCK and BMAL1 activate the transcription of numerous clock-controlled genes, including their own repressors, period (PER1/2/3) and cryptochrome (CRY1/2) (Takahashi, 2017). HIF1α, HIF1β (also known as ARNT), CLOCK, and BMAL1 all belong to the bHLH-PAS family (Wu and Rastinejad, 2017) and can form heterodimeric pairs via their highly conserved bHLH domains (Fribourgh and Partch, 2017). HIF1α and BMAL1 can directly interact in yeast two-hybrid assays (Hogenesch et al., 1998) and can associate in mammalian cells (Wu et al., 2017). Furthermore, genetic deletion of Bmal1 prevents hypoxia-induced recruitment of HIF1α to a significant fraction of its binding sites in chromatin (Wu et al., 2017). Although these prior findings support a role for BMAL1-HIF1α heterodimers in a subset of hypoxia responses, the extent of this interaction under normal physiological conditions and its implications for circadian and hypoxia-responsive transcriptional networks are not well understood.
It was recently demonstrated that HIF1α is involved in the regulation of circadian clock. Mice subjected to mildly reduced atmospheric oxygen were reported to exhibit HIF1α-dependent accelerated adaptation to a change in lighting conditions that mimics jet lag (Adamovich et al., 2017). In addition, chromatin immunoprecipitation studies revealed HIF1α occupancy on the promoters of numerous core clock genes such as PER1, PER2, and CRY1 (Peek et al., 2017; Wu et al., 2017). Conversely, the core circadian clock modulates HIF1α. In mouse liver, Hif1α mRNA may be expressed in a circadian manner (Wu et al., 2017), although this has not been observed consistently (Koike et al., 2012; Panda et al., 2002; Ueda et al., 2002). Mouse fibroblasts deficient in CRYs or PERs exhibit elevated levels of HIF1α protein accumulation upon exposure to hypoxia mimetics, with the opposite phenotype observed in cells lacking BMAL1 (Peek et al., 2017; Wu et al., 2017; Dimova et al., 2019), which has been attributed to BMAL1-driven transcription of Hif1α mRNA. Further suggesting direct cross talk between hypoxia responsive and circadian transcriptional networks, studies utilizing luciferase reporters driven by canonical hypoxia (HRE) and circadian (E-Box) elements have demonstrated that overexpressed BMAL1/HIF1α heterodimeric complexes can activate both pathways (Peek et al., 2017).
Here, we report that primary myotubes from mice lacking Cry1 and/or Cry2 exhibit increased induction of hypoxia target genes in response to limited oxygen or to small molecules that mimic the effects of hypoxia, compared with WT controls. Intriguingly, Cry2−/− cells uniquely display enhanced accumulation of HIF1α protein in the absence of changes in Hif1α mRNA. We find that overexpressed HIF1α interacts with BMAL1, and this requires several amino acids that are conserved between BMAL1 and ARNT (also known as HIF1β). Both CRY1 and CRY2 can interact with and repress BMAL1/HIF1α heterodimers. Furthermore, we report interaction of endogenous BMAL1 and endogenous HIF1α in primary cultured fibroblasts. Together, these data support a model in which CRY1 and CRY2 suppress HIF1α-mediated gene expression, through a combination of transcriptional repression by CRY1 and/or CRY2 and post-translational regulation uniquely involving CRY2. Finally, we demonstrate that CRY2-deficient primary myotubes are more glycolytic than WT controls under normoxic conditions and are less responsive to hypoxia. Together, these findings reveal that the circadian repressors CRY1 and CRY2 play an unexpectedly direct role in suppressing hypoxia signaling.
Results
Cryptochromes Suppress HIF1α Protein Levels and Hypoxia-Induced Transcription
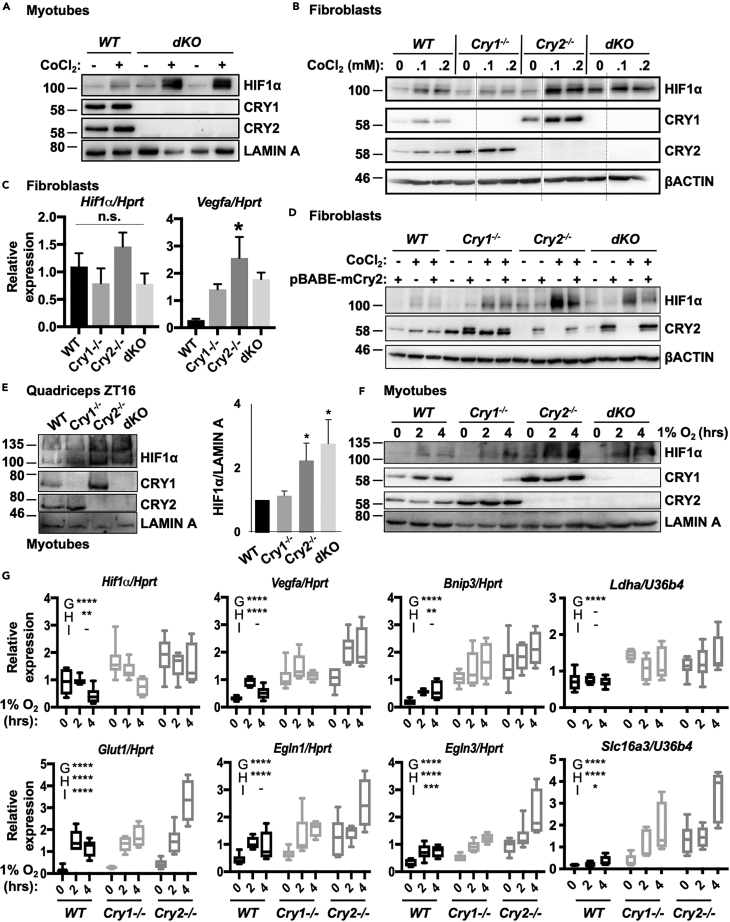
We previously demonstrated that quadriceps muscles from mice lacking both CRY1 and CRY2 exhibit increased activation of hypoxia-regulated genes in response to exercise (Jordan et al., 2017). In addition, the core clock regulates Hif1α transcription (Peek et al., 2017; Wu et al., 2017) and the circadian transcriptional activator BMAL1 is required for the recruitment of HIF1α to a subset of its target genes in response to hypoxia (Wu et al., 2017). We measured HIF1α protein in WT and CRY-deficient primary myotubes upon exposure to cobalt chloride (CoCl2), which inhibits both hydroxylation of HIF1α and its interaction with the vHL factor (Yuan et al., 2003) and found that its accumulation is greatly enhanced in myotubes lacking both CRY1 and CRY2 (Figure 1A). Both Cry1−/−;Cry2−/− (dKO) and Cry2−/− fibroblasts exposed to CoCl2 also express greater levels of HIF1α protein (Figures 1B, S1A, and S1B), whereas Cry1−/− fibroblasts exhibit lower levels of HIF1α accumulation in response to CoCl2. Prior reports attributed increased HIF1α in cells that lack both Cry1 and 2 (Peek et al., 2017) or both Per1 and 2 (Wu et al., 2017) to enhanced Hif1α transcription due to loss of the repressive arm of the circadian clock (Dimova et al., 2019). However, we find that whereas loss of Cry2 phenocopies dKO cells in terms of increased HIF1α protein, loss of the circadian repressor Cry1 exhibits the opposite phenotype. Furthermore, we did not detect changes in Hif1α mRNA in CRY-deficient fibroblasts (Figure 1C). We acknowledge that this may be due to the specific cell type examined in this study, as prior reports utilizing mouse embryonic fibroblasts did detect changes in Hif1α mRNA in CRY-deficient cells (Wu et al., 2017; Dimova et al., 2019). Strikingly, all the Cry-deficient fibroblasts exhibited increased induction of the HIF1α target gene Vegfa, even though HIF1α protein levels were diminished in Cry1−/− cells. To determine whether deletion of Cry2 caused the observed enhanced HIF1α protein accumulation in Cry2−/− and dKO cells, we used a retrovirus to ectopically express CRY2 in fibroblasts of various Cry background genotypes (Figure 1D). Indeed, ectopic expression of Cry2 reduced HIF1α levels in Cry2−/− and dKO fibroblasts, but not in cells lacking Cry1, demonstrating that the elevation of HIF1α in Cry2-deficient cells is caused by the absence of Cry2. Of note, mutants lacking Cry1 express high basal levels of endogenous Cry2, which may explain the lack of observable effect of exogenous overexpression of mCRY2 in these cells. HIF1α protein is reported to exhibit daily fluctuations in kidney, brain, and quadriceps muscle from sedentary mice or rats, peaking in the early night (Adamovich et al., 2017; Sato et al., 2019). We measured HIF1α protein in quadriceps nuclear fractions prepared from WT, Cry1−/−, Cry2−/−, and dKO mice at zeitgeber time (ZT, hours after lights on) 16, when CRY2 protein expression is normally high. As in fibroblasts and myotubes, quadriceps nuclei from mice lacking Cry2 contain increased HIF1α (Figure 1E). Notably, reduced levels of endogenous BMAL1 were detected in quadriceps nuclear extracts from CRY1/2-deficient mice compared with WT littermates (Figure S1C), indicating that increased HIF1α abundance cannot be attributed to increased BMAL1.
Figure 1.
CRYs Suppress HIF1α
(A) Accumulation of HIF1α protein detected by immunoblot (IB) in unsynchronized primary myotubes (1°MTs) isolated from WT and dKO mice and treated with vehicle control or 100 μM CoCl2. Two technical replicates are shown for dKO 1°MTs.
(B) Accumulation of HIF1α protein detected by IB in ear fibroblasts (EFs) isolated from WT, Cry1−/−, Cry2−/−, and dKO mice and treated with vehicle control or 0–0.2 mM CoCl2. Faint vertical lines indicate where blot images were spliced to remove samples treated with 50 μM CoCl2.
(C) Expression of the indicated transcripts measured by quantitative PCR (qPCR) in fibroblasts of the indicated genotypes, normalized to Hprt.
(D) Accumulation of HIF1α protein detected by IB in EFs isolated from mice of the indicated genotype and infected with virus carrying either empty vector or pBABE-mCry2 plasmid, before treatment with vehicle control (−) or 100 μM CoCl2 (+). (Note: the faint CRY2 signal in lane 12 reflects a small amount of sample from lane 13 that spilled into the neighboring well).
(E) Left, accumulation of HIF1α protein detected by IB in nuclei of cells isolated from quadriceps muscles of mice of the indicated genotype at ZT16. Right, quantitation of three experiments performed as shown at left.
(F) Accumulation of HIF1α protein detected by IB in unsynchronized 1°MTs isolated from mice of the indicated genotype upon exposure to 1% O2 for 0–4 h.
(G) Expression of the indicated transcripts measured by qPCR in unsynchronized 1°MTs plated under parallel conditions as those in (F). For (C and E), data represent the mean + SD for 3 samples per condition. ∗p < 0.05 versus WT by one-way ANOVA with Dunn's multiple comparison test. In (G) data represent the range (min to max with mean ± SD shown in box) for 6 samples per condition, each measured in triplicate. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 for a main effect of genotype (G), hypoxia (H), or an interaction between the two, determined by two-way ANOVA. Results of post-hoc analysis are not shown to highlight the main effect results of two-way ANOVA. The position of molecular weight markers (in kDa) are shown to the left of all western blot images. Note that overexpressed epitope-tagged proteins are slightly larger than endogenous ones.
See also Figure S1.
In WT primary myotubes, exposure to 1% oxygen increased HIF1α protein and induced transcription of HIF target genes within 2 h, whereas Hif1α transcript itself was not significantly induced until much later (Figures S1D and S1E). Examination of WT, Cry1−/−, Cry2−/−, and dKO myotubes in the same protocol revealed more rapid accumulation of HIF1α protein in cells lacking Cry2 (Figure 1F). In myotubes, Hif1α mRNA was slightly elevated, and hypoxia target gene expression was basally elevated in all cryptochrome mutants, especially in those lacking Cry2, and was further induced by hypoxia (Figure 1G). Although we did not examine HIF2α specifically in this study, some studies suggest that Egln3 is primarily regulated by HIF2α (Dengler et al., 2014), and we found that this transcript is elevated in CRY-deficient cells. Ldha and Slc16a3 are thought to be primarily regulated by HIF1α (Rathmell and Chen, 2008), and their expression is increased in cells lacking cryptochromes. Basal Hif1α expression was unaffected by the absence of CRY1 and/or CRY2 in fibroblasts exposed to CoCl2, but the transcriptional response to hypoxia was accelerated in Cry2−/− and dKO cells (Figure S1F). These data indicate that both cryptochromes influence hypoxia target gene transcription, and cells lacking Cry2 mount an especially rapid transcriptional response, perhaps due to increased HIF1α protein.
Together, these results suggest that both CRY1 and CRY2 can suppress HIF1α-mediated transcription, whereas CRY2 may have a more dominant role in modulating HIF1α abundance in the cell types examined.
HIF1α Interaction with BMAL1 Facilitates Interaction with Circadian Proteins
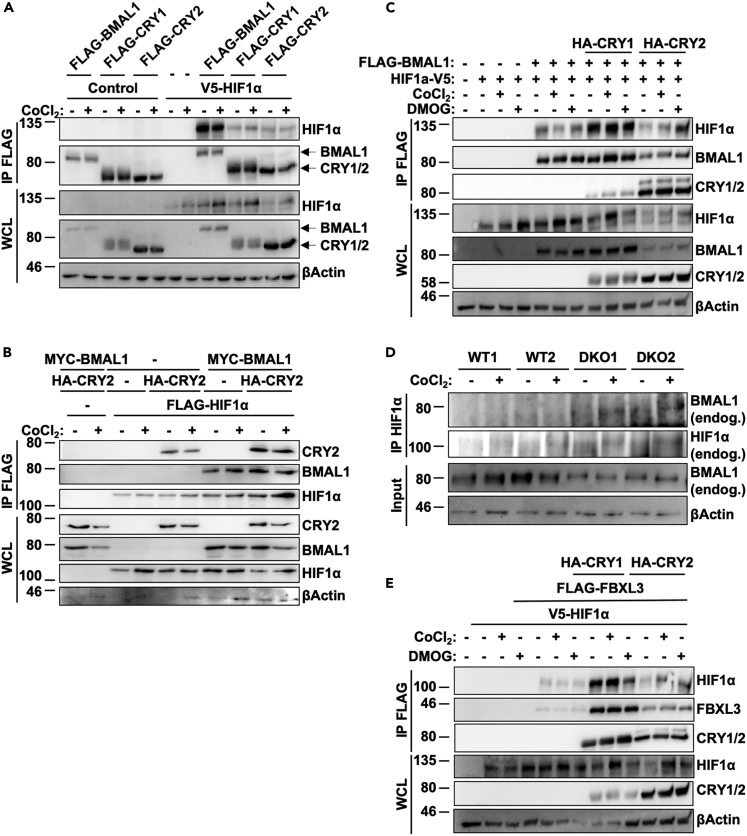
bHLH-PAS family proteins form heterodimeric complexes consisting of class I and class II subunits. Class I proteins are thought to be highly regulated and expressed in a tissue-specific manner, whereas class II subunit proteins are ubiquitously expressed. The generation of circadian rhythms in mammals is driven by the class I protein CLOCK interacting with its class II partner BMAL1 (Fribourgh and Partch, 2017). Multiple studies have indicated direct interaction between BMAL1 and the class I protein HIF1α (Hogenesch et al., 1998; Wu et al., 2017), and have implicated BMAL1 in modulation of HIF1α-driven gene transcription (Wu et al., 2017). Consistent with those prior reports, we found that HIF1α co-immunoprecipitated with overexpressed BMAL1, CRY1, or CRY2, with a clear preference for interaction with BMAL1 (Figure 2A). When HIF1α, BMAL1, and CRYs are co-expressed, the presence of BMAL1 enhances co-immunoprecipitation of CRY2 with HIF1α (Figure 2B). Notably, overexpression of CRY2 consistently reduces the steady-state levels of HIF1α protein present in cell lysates (Figures 2A–2C), whereas CRY1 does not appear to alter HIF1α protein levels as consistently, and enhances the co-immunoprecipitation of HIF1α with BMAL1 (Figure 2C). Consistent with these observations, we detected endogenous BMAL1 in HIF1α-containing complexes immunopurified from fibroblast lysates (Figure 2D), suggesting that these proteins interact in mammalian cells. Notably, more BMAL1 was detected in HIF1α-containing complexes precipitated from CRY-deficient cells despite reduced levels of BMAL1 protein present in the lysates.
Figure 2.
HIF1α Interacts with the Core Clock Machinery
(A–E) (A–C and E) Proteins detected by immunoblotting (IB) in whole-cell lysates (WCL) or following immunoprecipitation (IP) of the FLAG tag from lysates of HEK293T cells expressing the indicated plasmids and treated with vehicle control (−), 100 μM CoCl2, or 200 μM DMOG as indicated. (D) Endogenous BMAL1, HIF1α, and β-ACTIN detected in WCL or following IP of HIF1α from lysates of ear fibroblasts of the indicated genotypes treated with vehicle control (−) or 100 μM CoCl2.
We previously demonstrated a role for CRY2 as a co-factor in the SCFFBXL3-mediated degradation of the oncoprotein c-MYC (Huber et al., 2016). To investigate whether a similar mechanism could be involved in CRY2-mediated turnover of HIF1α, we examined whether HIF1α and FBXL3 can interact. We found that FBXL3 can bind HIF1α, and that this interaction is greatly enhanced in the presence of either CRY1 or CRY2. In addition, overexpression of CRY2 reduces both total HIF1α and FLAG-FBXL3 compared with CRY1 (Figure 2E), suggesting that CRY2 could play a more prominent role in promoting degradation of HIF1α-containing complexes compared with CRY1. Indeed, whereas HIF1α is increased in the presence of either BMAL1 alone or simultaneous overexpression of BMAL1/CRY1, the presence of CRY2 decreases both total HIF1α and BMAL1 (Figure 2C). Together, these results suggest a possible role for a FBXL3/CRY2 complex in directing HIF1α protein for degradation.
BMAL1-HIF1a Interaction Involves Their bHLH and PAS Domains
The two main distinguishing features of the bHLH-PAS proteins, the eponymous bHLH and PAS domains, serve distinct functions within these proteins. The bHLH region binds DNA, and the PAS fold is involved in heterodimerization between family members (Kewley et al., 2004). Although all bHLH-PAS proteins exhibit high levels of sequence conservation within their PAS domains, sequence alignments reveal a higher level of conservation among family members regulating the same pathway (Fribourgh and Partch, 2017). In addition, crystal structures of canonical BMAL1/CLOCK and HIF1α/HIF1β complexes exhibit distinct spatial arrangements of the PAS domains (Fribourgh and Partch, 2017). Taken together, these results suggest that subtle variations in sequence among various bHLH-PAS members allows for markedly divergent spatial organization of the complexes they form, and thus for highly specific regulation of downstream transcriptional networks. Given our data demonstrating interactions between BMAL1 and HIF1α, as well as previous reports of transcriptionally active BMAL1/HIF1α complexes (Peek et al., 2017; Wu et al., 2017), we investigated which region(s) of these proteins were required for heterodimerization.
To define the regions required for interaction between HIF1α and BMAL1, we generated a series of truncation mutants for each protein and tested their ability to interact in co-immunoprecipitation assays (Figures S2A–S2D). The strongest interactions occur with full-length proteins, whereas a truncated BMAL1 containing the bHLH and PAS-A domains robustly interacts with both CLOCK and HIF1α (Figures S2A and S2B). Similarly, a truncated version of HIF1α containing only the bHLH and PAS domains interacts robustly with both BMAL1 and ARNT (Figures S2C and S2D). These data suggest a conserved mode of interaction between various bHLH-PAS proteins, involving primarily the amino-terminal bHLH and PAS domains.
BMAL1 Enhances CRY2 Interaction with HIF1α
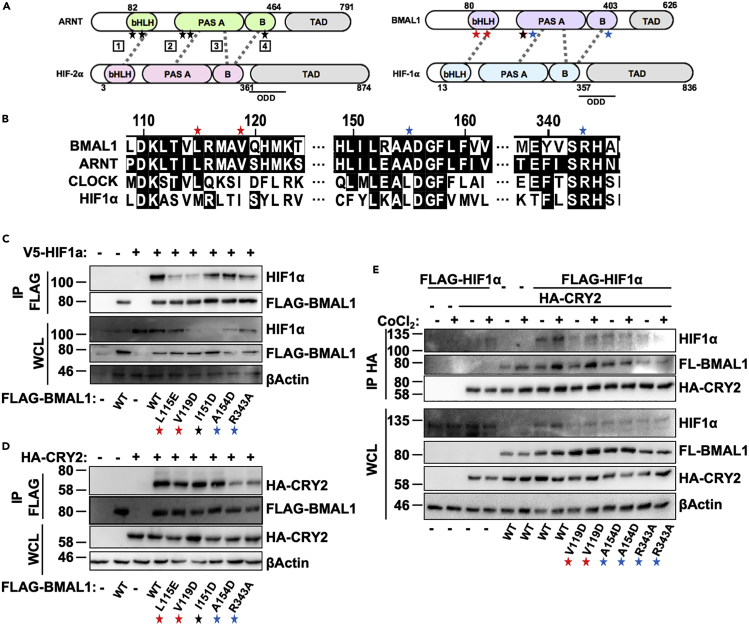
A 2015 study investigating the quaternary structure of various bHLH-PAS heterodimers revealed significant differences in the architecture of HIF2α-HIF1β and BMAL1-CLOCK complexes, despite the high level of sequence conservation in the bHLH and PAS domains (Wu et al., 2015). HIF2α and HIF1β form an asymmetric structure that involves contacts between the bHLH and PASA/B regions of each protein at six distinct interfaces. Of these six interfaces, mutations introduced at interfaces 1–4 had the greatest impact on heterodimer stability, suggesting that these regions are vital for HIF2α/HIF1β interaction. Importantly, mutations affecting the interaction between HIF2α and HIF1β also destabilized HIF1α/HIF1β heterodimer formation, confirming that these two heterodimers share a similar architecture. Of the six interfaces formed between HIF2α/HIF1β, three are conserved in BMAL1/CLOCK heterodimers, suggesting some shared modes of interaction, despite an overall dissimilar three-dimensional architecture. We generated a series of point mutations in BMAL1 analogous to those in HIF1β (ARNT) that disrupt interaction between HIF1α/HIF1β (Figures 3A and 3B). Of the five mutations examined, L115E and V119D consistently decrease interaction between BMAL1 and HIF1α (Figure 3C). Two mutations located closer to the C terminus (A154D, R343A) did not significantly disrupt interaction between BMAL1 and HIF1α, but did impair the ability of BMAL1 to pull down CRY2 (Figure 3D), suggesting that BMAL1 interacts with HIF1α and CRY2 independently. In contrast, none of the mutants examined impaired the ability of BMAL1 to interact with CRY1 in cells (Figure S2E), indicating that the given point mutations specifically disrupt BMAL1/CRY2 binding, rather than globally disrupting the protein structure of BMAL1, although the altered interaction likely depends on conformational rearrangement. We used point mutations in BMAL1 that independently disrupt interactions with HIF1α or CRY2 to investigate whether the interaction between HIF1α and CRY2 involves the formation of a trimeric complex with BMAL1. While full-length BMAL1 increased the ability of CRY2 to pull down HIF1α, this effect was abrogated by mutations in BMAL1 that decreased either BMAL1/HIF1α or BMAL1/CRY2 interaction (Figure 3E). These results indicate that maximal interaction between HIF1α and CRY2 requires BMAL1.
Figure 3.
HIF1α Interacts with Clock Proteins via Unique Domains
(A) Schematic diagram depicting five residues in the ARNT protein described in Wu et al. (2015) as critical for interaction between ARNT and HIF2α, as well as the location of the corresponding residues in BMAL1 (also known as ARNTL). Gray dashed lines depict interactions between interfaces of each heterodimer pair. Red stars indicate residues found to be critical for interaction between BMAL1 and HIF1α, whereas blue stars indicate residues found to be critical for BMAL1/CRY2 interaction.
(B) Sequence conservation between bHLH-PAS family proteins in the 115–343 amino acid region where mutations were introduced.
(C–E) Proteins detected by immunoblotting (IB) following immunoprecipitation (IP) of the FLAG (C and D) or hemagglutinin (HA) (E) tags from lysates of HEK293T cells expressing the indicated plasmids and treated with either vehicle control (−) or 100 μM CoCl2 (+).
See also Figure S2.
Cryptochromes Can Repress Transcriptional Activity of HIF1α-Containing Heterodimers
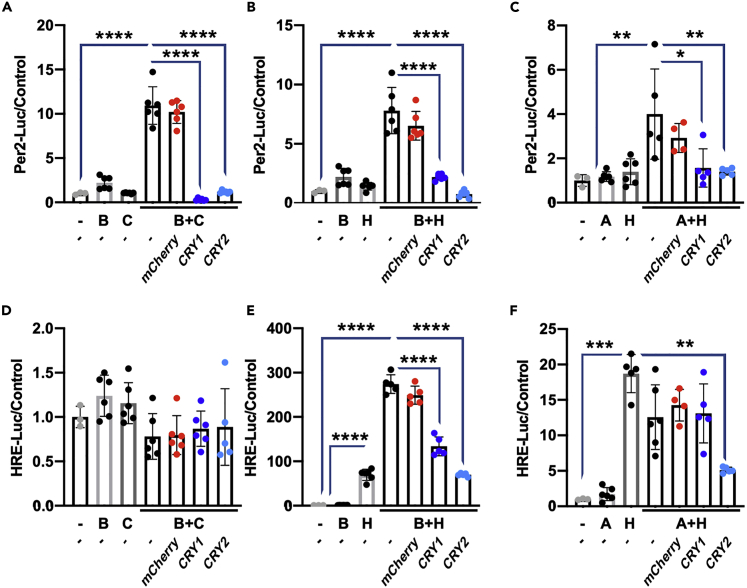
Consistent with earlier reports, we find that HIF1α and BMAL1 can interact. Furthermore, we find that cryptochromes can associate with HIF1α/BMAL1 heterodimers. HIF1α/BMAL1 has been reported to recognize both canonical circadian (E-Box) and hypoxia (HRE) promoter elements (Peek et al., 2017), and deletion of BMAL1 prevents recruitment of HIF1α to a subset of HREs in response to hypoxia (Wu et al., 2017). It is well established that cryptochromes repress BMAL1/CLOCK-driven transcription (Takahashi, 2017), and this can be observed in controlled assays using the E-Box-containing Per2:Luciferase reporter (Kriebs et al., 2017). Less is known about the ability of CRYs to influence BMAL1/HIF1α-driven transcription. Because we found that deletion of cryptochromes influences the transcriptional response to hypoxia, we examined whether cryptochromes can repress HIF1α-dependent activation of luciferase reporters driven by circadian elements and/or HREs. We found that Per2-Luciferase can be induced by either BMAL1/CLOCK or BMAL1/HIF1α complexes, and that each is repressed by CRYs to a similar extent (Figures 4A and 4B). Importantly, neither BMAL1 nor HIF1α alone activates Per2-Luciferase. Overexpression of ARNT with HIF1α results in a slight activation of Per2-Luciferase that is also suppressed by overexpression of CRYs (Figure 4C). Conversely, HRE:luciferase is not activated by BMAL1/CLOCK (Figure 4D). However, it is induced by HIF1α alone (Figure 4E). BMAL1 does not activate HRE-Luciferase alone, but co-expression of BMAL1 with HIF1α robustly enhances the activation of HRE-Luciferase and both CRY1 and CRY2 suppress the additional activation conferred by BMAL1 (Figure 4E). These results suggest that repression of HIF1α by cryptochromes occurs primarily via BMAL1. Surprisingly, whereas expression of HIF1α alone activated the HRE:Luciferase promoter, simultaneous overexpression of ARNT did not further increase HRE:Luciferase in U2OS cells under the conditions used. It is possible that ARNT competes with endogenous BMAL1 for interaction with HIF1α; because BMAL1 contains a transcriptional activation domain (TAD) that ARNT does not, dimerization of BMAL1/HIF1α would be expected to exhibit greater activation of this promoter, and indeed this has also been observed in C2C12 cells (Peek et al., 2017). Overexpression of CRY2 decreases ARNT + HIF1α-dependent HRE-Luciferase expression (Figure 4F), possibly by impacting HIF1α protein abundance. Furthermore, others have shown that the bHLH-PAS family of transcription factors exhibit promiscuous dimerization patterns with proteins other than their canonical binding partners (Wu et al., 2016). Further investigation will be required to fully understand the role of cryptochromes in repressing the activity of these complexes.
Figure 4.
CRYs Repress BMAL1-Containing Heterodimers
(A–F) Luciferase activity in U2OS cells expressing Per2:Luciferase (A–C) or HRE:Luciferase (D–F). Activation of the reporter is achieved by transfection of the indicated plasmids (B, BMAL1; C, CLOCK; H, HIF1α). mCherry was used as a negative control, and luminescence was normalized to Renilla luciferase activity. Data represent the mean + SD superimposed with 3–11 individual replicates per condition from a representative of at least three experiments. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p < 0.0001 by one-way ANOVA.
Deletion of Cryptochromes Enhances Glycolysis in Myotubes
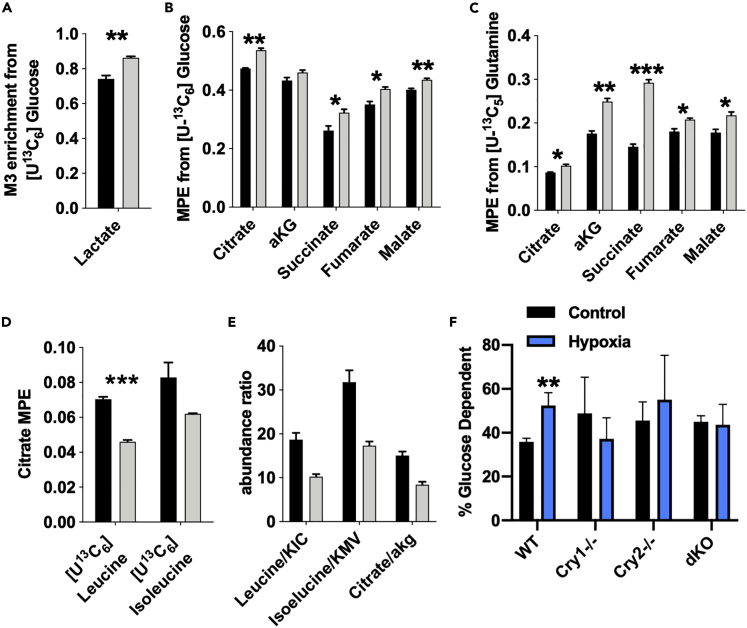
Metabolic tracing analysis conducted in WT and dKO myotubes revealed that cells lacking cryptochromes exhibited increased enrichment in lactate and Krebs cycle intermediates from [U-13C6]glucose (Figures 5A and 5B); dKO cells also had higher enrichment in Krebs cycle intermediates from [U-13C5]glutamine compared with WT cells (Figure 5C). In contrast, dKO cells exhibit reduced catabolism of the branched-chain amino acids (BCAAs) leucine and isoleucine (Figure 5D). Consistent with these trends, we observed a significant decrease in the ratio of the abundance of BCAAs to their respective branched keto acids and citrate/αKG (Figure 5E), suggesting decreased branched-chain keto acid dehydrogenase (BCKDH) activity and increased reductive carboxylation.
Figure 5.
CRYs alter Muscle Metabolic Profile
(A–E) Stable isotope tracing analysis and metabolite abundance performed in primary myotubes (1°MTs) from mice of the indicated genotype. Fractional enrichment (A) or mole percent enrichment (MPE) (B–D) in metabolites following addition of indicated tracer for 24 h. For (A–E), data represent the mean ± SEM of 3 replicates per condition, which are representative of 3 independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 from two-tailed Student's t test.
(F) Glucose dependency measured in unsynchronized 1°MTs isolated from mice of the indicated genotype and treated with either vehicle control (black) or 100 μM CoCl2 (blue). ∗∗p ≤ 0.01 versus control by t test using two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli, with Q = 5%. Data in (F) represent the mean + SEM of the results from 4 independent experiments.
See also Figure S3.
Studies utilizing PEO1 ovarian cancer cells demonstrated that depletion of HIF1α decreases glucose dependency in a mitochondrial substrate flux assay and overexpression of HIF1α increases glucose usage (Kitajima et al., 2017). We used a similar strategy to measure glucose dependency in myotubes to determine whether Cry1/2 deletion impacts cellular metabolism in a manner consistent with elevated endogenous HIF1α activity. As expected, WT myotubes exhibited increased glucose dependency upon treatment with the hypoxia mimetic CoCl2 (Figure 5F). In contrast, Cry1−/−, Cry2−/−, and dKO myotubes tend to have increased basal reliance on glucose, with hypoxia treatment failing to increase glucose dependency. No significant differences in glutamine or fatty acid dependency were observed for any of the conditions examined (Figures S3A and S3B). These metabolic analyses in Cry1/2-deficient myotubes suggest that the loss of cryptochromes alters skeletal muscle metabolism in a manner consistent with enhanced HIF1α activity. Furthermore, our findings support prior studies suggesting that hypoxia and HIF1α strongly influence Krebs cycle and amino acid metabolism. Consistent with these trends, the most significantly altered metabolites observed in a targeted analysis of homogenized quadriceps muscles of dKO compared with WT mice were glutamine and serine (Figure S3C).
Discussion
Studies in rats have indicated not only that levels of oxygen in the blood fluctuate in a circadian manner but also that the relative oxygenation of tissues such as the kidneys exhibits cyclic fluctuations with peak levels during the active phase. Moreover, rhythms in oxygen concentration can synchronize the circadian clocks of cultured cells in a HIF1α-dependent manner (Adamovich et al., 2017). These results indicate that daily fluctuations in oxygen consumption and behavior, likely driven by the master pacemaker in the suprachiasmatic nucleus, can contribute to indirectly resetting peripheral tissue clocks via HIF1α. Moreover, HIF1α protein levels (but not Hif1α mRNA) exhibit daily rhythms in metabolically active tissues such as the kidney and brain (Adamovich et al., 2017). This is important, as it indicates that HIF1α not only influences the timing of peripheral clocks but also is reciprocally regulated at the level of protein accumulation by circadian clocks. Indeed, reports have indicated that maximal transcriptional activation of hypoxia pathways is gated by circadian timing (Peek et al., 2017; Wu et al., 2017). Together, these results suggest a system in which circadian/hypoxia pathway cross talk allows peripheral tissues to both anticipate repetitive behaviors such as feeding and locomotor activity, as well as to adapt to metabolic challenges such that fuel utilization is coordinated with substrate availability (Schroder and Esser, 2013). This is particularly important in the context of skeletal muscle, which has evolved to maximize metabolic flexibility to adapt to daily fluctuations in fuel source, availability, and demand (Dyar et al., 2014). Indeed, our finding of enhanced basal reliance on glucose in cells lacking the circadian repressors CRY1 and CRY2 combined with the decreased glucose reliance in BMAL1-deficient cells (Dyar et al., 2014) indicates that muscle clocks modulate substrate selection across the day-night cycle.
Training-induced increases in transcriptional networks that mediate a switch between oxidative and glycolytic metabolism contribute to enhanced exercise capacity (Hoppeler et al., 2003). During strenuous exercise, skeletal muscle regularly experiences dramatic fluctuations in the partial pressure of oxygen from 30 mmHg to less than 5 mmHg (Ortiz-Prado et al., 2019), and HIF1α is critical for the maintenance of ATP production in muscle (Seagroves et al., 2001). Among the mechanisms by which HIF1α impinges on cellular metabolism are induction of glucose transporters and glycolytic enzymes, induction of PDK1 to actively inhibit PDH conversion of pyruvate to acetyl-CoA, and induction of LDHA for utilization of pyruvate in glycolysis/lactate production (Kim et al., 2006). Thus, conversion from an oxidative to glycolytic phenotype by HIF1α is not merely a passive response to limiting oxygen supply, but an active shunting of resources away from mitochondrial utilization. Despite intense study of HIF1α-mediated glycolytic induction in the context of cancer, numerous studies have indicated that HIF1α functions in a normal physiological context to actively regulate glucose utilization within a fine range of oxygen concentrations. For instance, cells repress tricarboxylic acid (TCA) cycle components and induce glycolytic enzymes under conditions of 2% oxygen, which is not limiting for cellular respiration (Semenza, 2017). Furthermore, it has been demonstrated that HIF1α-null mouse embryonic fibroblasts (MEFs) actually produce more ATP at 1% O2 compared with WT cells, but ultimately undergo apoptosis due to excessive ROS production (Kim et al., 2006). Thus, rather than merely being a glycolytic “on/off” switch, HIF1α functions as a fulcrum with which to balance glucose flux through glycolysis or mitochondrial respiration to limit excessive ROS accumulation under conditions of either hyperoxia or hypoxia (Terraneo et al., 2014).
HIF1α thus influences exercise capacity via several overlapping pathways: by influencing levels of the glycolytic product and substrate for energy production, lactate (Myers and Ashley, 1997); by maintaining ATP production under low oxygen tension; and by facilitating long-term adaptation to exercise via activation of downstream genes such as VEGFA (Peek et al., 2017). Mice with muscle-specific deletion of HIF1α fail to induce glycolysis during exercise, and the compensatory increase in muscle oxidative phosphorylation leads to increased tissue damage (Mason et al., 2004). We have previously reported increased exercise capacity in mice lacking both CRY1/2. Although this is undoubtedly influenced by dKO mice having increased activation of exercise-adaptive pathways via PPARδ, dKO mice also exhibited elevated activation of hypoxia networks (Jordan et al., 2017). This, along with numerous publications demonstrating induction of HIF1α transcriptional targets in cells and tissues lacking the repressive arm of the circadian clock make it clear that these two pathways are vital for proper muscle function (Adamovich et al., 2017; Peek et al., 2017; Wu et al., 2017). Intriguingly, comprehensive transcriptional profiling of rodent skeletal muscles revealed striking high expression of Cry2 in the flexor digitorum brevis (FDB) muscle (Terry et al., 2018). As FDB is composed primarily of glycolytic type IIa/type IIx muscle fibers (Tarpey et al., 2018), this suggests that CRY2 could regulate hypoxic induction in fast-twitch fibers, thus influencing exercise capacity.
We found that cells lacking CRY2 have greatly increased accumulation of HIF1α protein both under conditions of artificial induction via the hypoxia mimetic CoCl2 and following prolonged hypoxic exposure. Other studies have noted that a lack of both CRY1 and CRY2 enhances HIF1α protein levels in both cells and tissues of mice (Adamovich et al., 2017; Peek et al., 2017; Wu et al., 2017; Dimova et al., 2019), whereas here we report individual and opposite effects of the individual cryptochromes on HIF1α steady-state protein levels. Increased HIF1α in dKO tissues does not appear, therefore, to arise due to a lack of the negative arm of the circadian clock increasing Hif1α transcription, but rather via CRY2-dependent regulation of HIF1α protein turnover. We find that whereas both CRY1 and CRY2 are capable of repressing transcription of HIF1α targets, transcription of Hif1α itself is largely unaffected by CRYs. In addition, induction of hypoxia target genes occurs on a far more rapid timescale than does induction of HIF1α itself in WT cells, which supports the finding that HIF1α is primarily regulated at the protein, rather than transcript, level (Bardos and Ashcroft, 2005). A recent study suggests that CRY1 decreases both HIF1α half-life and binding to its target gene promoters (Dimova et al., 2019). In our hands, CRY1 appeared to regulate basal transcription of hypoxia target genes, and to repress some HIF1α-containing heterodimeric complexes from activating an HRE promoter, but had limited impact on HIF1α protein accumulation. Different roles of CRY1 and CRY2 in modulating HIF1α protein levels in different cell types could contribute to the divergent observations that we make in muscles and primary muscle cell culture compared with the observations by Dimova and colleagues using fibroblasts. It is likely that regulation of the hypoxic gene network by CRYs occurs at multiple levels, with distinct HIF1α-containing complexes exhibiting unique regulation by CRY1 and/or CRY2 and other context-dependent factors.
Despite their high level of sequence conservation, CRY1 and CRY2 have divergent roles in repression of the core circadian clock, with single-knockout mutants displaying opposite phenotypes in terms of period length (Vitaterna et al., 1999). CRY1 interacts more strongly with the CLOCK-BMAL1 heterodimer than does CRY2 (Rosensweig et al., 2018) and is believed to be the more critical circadian repressor (Khan et al., 2012). CRY2 is a more efficient repressor of non-clock transcription factors that are targeted by cryptochromes, including nuclear hormone receptors (Kriebs et al., 2017) and c-MYC (Huber et al., 2016). We find that CRY2 also seems to be a stronger regulator of HIF1α than is CRY1, at least in muscles.
Using two complementary approaches, we found that cells lacking CRYs are more reliant on glucose, consistent with the concept that both CRY1 and CRY2 repress HIF1α-dependent transcriptional networks. This is in line with prior reports that loss of BMAL1 in skeletal muscle decreases glucose reliance and suggests that modulation of either the negative or the positive arms of the clock influence substrate preference (Harfmann et al., 2016). We also observed increased glutamine enrichment in TCA intermediates in dKO cells, consistent with a hypoxic metabolic phenotype (Vacanti et al., 2014). Although we did not find increased “glutamine dependency” when assessing oxygen consumption in the presence of the glutaminase inhibitor BPTES, this apparent discrepancy may be due to the cells' ability to compensate when the glutamine pathway is inhibited, as glutamine contributes significantly less to TCA cycle activity in primary myotubes compared with other cell types such as cancer cells (Vacanti et al., 2014). Although we found that dKO cells exhibit reduced incorporation of labeled BCAAs into citrate, they do not accumulate excess BCAAs but rather contain low levels of leucine and isoleucine relative to their respective ketoacids, KIC and KMV. This suggests that in dKO cells branched-chain amino acid tramsaminase (BCAT), which is responsible for conversion of BCAAs to ketoacids, is active but that dKO cells lack some downstream activity in this pathway, such as the BCKDH complex. Consistent with this model, some of us recently demonstrated that BCKDH activity is reduced under conditions of hypoxia (Wallace et al., 2018). Increased glucose flux in dKO cells combined with an inability to utilize BCAAs highlights the functional impact of CRY-mediated regulation of HIF1α.
Circadian regulation of the hypoxic response seems to reflect a complex interplay of transcriptional and post-translational regulation by CRYs. HIF1α protein turnover is driven by several pathways, the best characterized of which involves oxygen-dependent interaction with the vHL ubiquitin ligase (Ivan and Kaelin, 2017). HIF1α is also subject to autophagic degradation by both vHL-dependent and vHL-independent mechanisms (DePavia et al., 2016). Recently, it was demonstrated that CRYs are also susceptible to degradation via macroautophagy (Toledo et al., 2018). Further investigation will be required to understand how CRY2 impacts HIF1α protein levels.
It has long been suspected that hypoxia and circadian networks interact (Hogenesch et al., 1998). Several reports indicate that BMAL1 can interact with HIF1α (Bersten et al., 2013; Hogenesch et al., 1998). We and others have shown that HIF1α can form transcriptionally active complexes with BMAL1 (Egg et al., 2013; Peek et al., 2017; Wu et al., 2017). However, the ability of circadian proteins and HIF1α to form functional heterodimers in a physiological context has been controversial (Cowden and Simon, 2002). Although HIF1α/BMAL1 heterodimers are unable to support normal vascularization in HIF1β-deficient mouse embryos (Cowden and Simon, 2002), it was recently demonstrated that muscle-specific deletion of HIF1β has no impact whatsoever on muscle vascularization (Badin et al., 2016). This finding suggests that an alternate partner, perhaps BMAL1, may cooperate with HIF1α to support muscle vascular remodeling. We found that the BMAL1-HIF1α complex directs transcription of a subset of circadian and hypoxia genes, rather than being functionally redundant to HIF1α/HIF1β. The roles of BMAL1 and/or CRYs in regulation of HIF1α-dependent physiology in vivo and how they are integrated with canonical ARNT/HIF1β signaling remain to be determined.
Although HIF1α has been most extensively studied in the context of aberrant cell metabolism and cancer progression, it is clearly indispensable for normal physiology. Indeed, knockout of HIF1α is associated with decreased exercise performance after training (Mason et al., 2004), perhaps due to a loss of regenerative capacity in response to tissue damage (Scheerer et al., 2013). A loss of HIF1α signaling can induce metabolic crisis due to ATP depletion, whereas overactivation of hypoxic pathways is detrimental for multiple metabolically active tissues (Asai et al., 2017; Mesarwi et al., 2016). Notably, a Per1/2 dKO model, which exhibits far greater induction of both Hif1α and target gene transcription than Cry1/2 dKO mice, was more susceptible to ischemic cell death in myocardial infarction (Wu et al., 2017).
In a broader physiological context, integration of circadian timing cues with hypoxic gene network activation may serve as a means for skeletal muscle to balance the expected substrate availability arising from predictable feeding/fasting cycles with the immediate need of this tissue to produce energy. Recently, several studies have addressed the impact of time of day on exercise, with HIF1α networks implicated as one of the major pathways gated by the clock (Ezagouri et al., 2019; Sato et al., 2019). That HIF1α has itself been demonstrated to alter circadian clock timing in peripheral tissues is intriguing (Adamovich et al., 2017), as it suggests a system through which exercise might increase the robustness of circadian clocks and thus, overall metabolic health. Future research will elucidate the role of circadian/hypoxia cross talk in the context of other oxygen-dependent physiology and pathology.
Limitations of the Study
Although we demonstrate that CRY2 suppresses the accumulation of HIF1α protein, we have not determined whether this involves modifying HIF1α stability, or defined the molecular mechanism by which this occurs. Furthermore, although our experiments indicate a more robust regulation of HIF1α accumulation by CRY2 than by CRY1, we cannot exclude the possibility that this is dependent on cell type or context, especially as others have demonstrated that CRY1 can suppress HIF1α accumulation (Dimova et al., 2019). Regarding the role of BMAL1 in promoting interaction between cryptochromes and HIF1α, we cannot conclude that BMAL1 is required for this interaction without examining whether deletion of BMAL1 abolishes the interactions of CRY1/2 with HIF1α. Finally, although we expect that CRY1 and CRY2 likely suppress HIF2α, as shown by others (Dimova et al., 2019), we did not study HIF2α here.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Katja Lamia (klamia@scripps.edu).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
This study did not generate datasets or code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was funded by NIH grants R01 DK097164 (to K.A.L.) and DK112927 (to K.A.L. and C.M.M.); R01CA234245 (to C. M.M.); 1S10OD16357, which funded the Seahorse Instrument at The Scripps Research Institute; and a Searle Scholars award to K.A.L. from the Kinship Foundation. We thank Enrique Saez, Luke Wiseman, Dennis Wolan, Drew Duglan, Marie Pariollaud, and Anne-Laure Huber for helpful discussions; sharing of technical expertise, equipment, and reagents; and/or critical reading of the manuscript, and T. Thomas for administrative assistance.
Author Contributions
Conceptualization, M.E.V. and K.A.L., Methodology, M.E.V., M.W., and M.K.H., Validation, M.E.V., M.W., M.K.H., K.A.L., and C.M.M., Formal Analysis, M.E.V., M.W., M.K.H., C.M.M., and K.A.L., Investigation, M.E.V., M.W., M.K.H., and A.B.C., Writing – Original Draft, M.E.V., Writing – Review and Editing, M.E.V., M.W., M.K.H., A.B.C., C.M.M., and K.A.L., Visualization, M.V., M.W., M.K.H., and K.A.L., Supervision, C.M.M. and K.A.L., Funding Acquisition, C.M.M. and K.A.L.
Declaration of Interests
Authors declare no conflict of interest.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101338.
Supplemental Information
References
- Adamovich Y., Ladeuix B., Golik M., Koeners M.P., Asher G. Rhythmic oxygen levels reset circadian clocks through HIF1alpha. Cell Metab. 2017;25:93–101. doi: 10.1016/j.cmet.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Asai Y., Yamada T., Tsukita S., Takahashi K., Maekawa M., Honma M., Ikeda M., Murakami K., Munakata Y., Shirai Y. Activation of the hypoxia inducible factor 1alpha subunit pathway in steatotic liver contributes to formation of cholesterol gallstones. Gastroenterology. 2017;152:1521–1535.e8. doi: 10.1053/j.gastro.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Badin P.M., Sopariwala D.H., Lorca S., Narkar V.A. Muscle Arnt/Hif1beta is dispensable in myofiber type determination, vascularization and insulin sensitivity. PLoS One. 2016;11:e0168457. doi: 10.1371/journal.pone.0168457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardos J.I., Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochim. Biophys. Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bersten D.C., Sullivan A.E., Peet D.J., Whitelaw M.L. bHLH-PAS proteins in cancer. Nat. Rev. Cancer. 2013;13:827–841. doi: 10.1038/nrc3621. [DOI] [PubMed] [Google Scholar]
- Cowden K.D., Simon M.C. The bHLH/PAS factor MOP3 does not participate in hypoxia responses. Biochem. Biophys. Res. Commun. 2002;290:1228–1236. doi: 10.1006/bbrc.2001.6309. [DOI] [PubMed] [Google Scholar]
- Dengler V.L., Galbraith M., Espinosa J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014;49:1–15. doi: 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePavia A., Jonasch E., Liu X.D. Autophagy degrades hypoxia inducible factors. Mol. Cell. Oncol. 2016;3:e1104428. doi: 10.1080/23723556.2015.1104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova E.Y., Jakupovic M., Kubaichuk K., Mennerich D., Chi T.F., Tamanini F., Oklejewicz M., Hanig J., Byts N., Makela K.A. The circadian clock protein CRY1 is a negative regulator of HIF-1alpha. iScience. 2019;13:284–304. doi: 10.1016/j.isci.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar K.A., Ciciliot S., Wright L.E., Bienso R.S., Tagliazucchi G.M., Patel V.R., Forcato M., Paz M.I., Gudiksen A., Solagna F. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egg M., Koblitz L., Hirayama J., Schwerte T., Folterbauer C., Kurz A., Fiechtner B., Most M., Salvenmoser W., Sassone-Corsi P. Linking oxygen to time: the bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol. Int. 2013;30:510–529. doi: 10.3109/07420528.2012.754447. [DOI] [PubMed] [Google Scholar]
- Ezagouri S., Zwighaft Z., Sobel J., Baillieul S., Doutreleau S., Ladeuix B., Golik M., Verges S., Asher G. Physiological and molecular dissection of daily variance in exercise capacity. Cell Metab. 2019;30:78–91.e4. doi: 10.1016/j.cmet.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Fribourgh J.L., Partch C.L. Assembly and function of bHLH-PAS complexes. Proc. Natl. Acad. Sci. U S A. 2017;114:5330–5332. doi: 10.1073/pnas.1705408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfmann B.D., Schroder E.A., Kachman M.T., Hodge B.A., Zhang X., Esser K.A. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skeletal Muscle. 2016;6:12. doi: 10.1186/s13395-016-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenesch J.B., Gu Y.Z., Jain S., Bradfield C.A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H., Vogt M. Muscle tissue adaptations to hypoxia. J. Exp. Biol. 2001;204:3133–3139. doi: 10.1242/jeb.204.18.3133. [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Vogt M., Weibel E.R., Fluck M. Response of skeletal muscle mitochondria to hypoxia. Exp. Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- Huber A.L., Papp S.J., Chan A.B., Henriksson E., Jordan S.D., Kriebs A., Nguyen M., Wallace M., Li Z., Metallo C.M. CRY2 and FBXL3 cooperatively degrade c-MYC. Mol. Cell. 2016;64:774–789. doi: 10.1016/j.molcel.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M., Kaelin W.G., Jr. The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol. Cell. 2017;66:772–779. doi: 10.1016/j.molcel.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S.D., Kriebs A., Vaughan M., Duglan D., Fan W., Henriksson E., Huber A.L., Papp S.J., Nguyen M., Afetian M. CRY1/2 selectively repress PPARdelta and limit exercise capacity. Cell Metab. 2017;26:243–255.e6. doi: 10.1016/j.cmet.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kewley R.J., Whitelaw M.L., Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Khan S.K., Xu H., Ukai-Tadenuma M., Burton B., Wang Y., Ueda H.R., Liu A.C. Identification of a novel cryptochrome differentiating domain required for feedback repression in circadian clock function. J. Biol. Chem. 2012;287:25917–25926. doi: 10.1074/jbc.M112.368001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kitajima S., Lee K.L., Hikasa H., Sun W., Huang R.Y., Yang H., Matsunaga S., Yamaguchi T., Araki M., Kato H. Hypoxia-inducible factor-1alpha promotes cell survival during ammonia stress response in ovarian cancer stem-like cells. Oncotarget. 2017;8:114481–114494. doi: 10.18632/oncotarget.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N., Yoo S.H., Huang H.C., Kumar V., Lee C., Kim T.K., Takahashi J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebs A., Jordan S.D., Soto E., Henriksson E., Sandate C.R., Vaughan M.E., Chan A.B., Duglan D., Papp S.J., Huber A.L. Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc. Natl. Acad. Sci. U S A. 2017;114:8776–8781. doi: 10.1073/pnas.1704955114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti M.V., Locasale J.W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm M.E., Rundqvist H. Skeletal muscle hypoxia-inducible factor-1 and exercise. Exp. Physiol. 2016;101:28–32. doi: 10.1113/EP085318. [DOI] [PubMed] [Google Scholar]
- Mason S.D., Howlett R.A., Kim M.J., Olfert I.M., Hogan M.C., McNulty W., Hickey R.P., Wagner P.D., Kahn C.R., Giordano F.J. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesarwi O.A., Shin M.K., Bevans-Fonti S., Schlesinger C., Shaw J., Polotsky V.Y. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PLoS One. 2016;11:e0168572. doi: 10.1371/journal.pone.0168572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Ashley E. Dangerous curves. A perspective on exercise, lactate, and the anaerobic threshold. Chest. 1997;111:787–795. doi: 10.1378/chest.111.3.787. [DOI] [PubMed] [Google Scholar]
- Ortiz-Prado E., Dunn J.F., Vasconez J., Castillo D., Viscor G. Partial pressure of oxygen in the human body: a general review. Am. J. Blood Res. 2019;9:1–14. [PMC free article] [PubMed] [Google Scholar]
- Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M., Schultz P.G., Kay S.A., Takahashi J.S., Hogenesch J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Peek C.B., Levine D.C., Cedernaes J., Taguchi A., Kobayashi Y., Tsai S.J., Bonar N.A., McNulty M.R., Ramsey K.M., Bass J. Circadian clock interaction with HIF1alpha mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017;25:86–92. doi: 10.1016/j.cmet.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell W.K., Chen S. VHL inactivation in renal cell carcinoma: implications for diagnosis, prognosis and treatment. Expert Rev. Anticancer Ther. 2008;8:63–73. doi: 10.1586/14737140.8.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosensweig C., Reynolds K.A., Gao P., Laothamatas I., Shan Y., Ranganathan R., Takahashi J.S., Green C.B. An evolutionary hotspot defines functional differences between CRYPTOCHROMES. Nat. Commun. 2018;9:1138. doi: 10.1038/s41467-018-03503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Basse A.L., Schonke M., Chen S., Samad M., Altintas A., Laker R.C., Dalbram E., Barres R., Baldi P. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab. 2019;30:92–110.e114. doi: 10.1016/j.cmet.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Scheerer N., Dehne N., Stockmann C., Swoboda S., Baba H.A., Neugebauer A., Johnson R.S., Fandrey J. Myeloid hypoxia-inducible factor-1alpha is essential for skeletal muscle regeneration in mice. J. Immunol. 2013;191:407–414. doi: 10.4049/jimmunol.1103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder E.A., Esser K.A. Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc. Sport Sci. Rev. 2013;41:224–229. doi: 10.1097/JES.0b013e3182a58a70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves T.N., Ryan H.E., Lu H., Wouters B.G., Knapp M., Thibault P., Laderoute K., Johnson R.S. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol. Cell Biol. 2001;21:3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36:252–259. doi: 10.15252/embj.201695204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey M.D., Amorese A.J., Balestrieri N.P., Ryan T.E., Schmidt C.A., McClung J.M., Spangenburg E.E. Characterization and utilization of the flexor digitorum brevis for assessing skeletal muscle function. Skeletal Muscle. 2018;8:14. doi: 10.1186/s13395-018-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraneo L., Virgili E., Caretti A., Bianciardi P., Samaja M. In vivo hyperoxia induces hypoxia-inducible factor-1alpha overexpression in LNCaP tumors without affecting the tumor growth rate. Int. J. Biochem. Cell Biol. 2014;51:65–74. doi: 10.1016/j.biocel.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Terry E.E., Zhang X., Hoffmann C., Hughes L.D., Lewis S.A., Li J., Wallace M.J., Riley L.A., Douglas C.M., Gutierrez-Monreal M.A. Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife. 2018;7 doi: 10.7554/eLife.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y.M., Yeoh K.K., Lee M.K., Eriksson T., Kessler B.M., Kramer H.B., Edelmann M.J., Willam C., Pugh C.W., Schofield C.J. Differential sensitivity of hypoxia inducible factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J. Biol. Chem. 2011;286:13041–13051. doi: 10.1074/jbc.M110.211110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo M., Batista-Gonzalez A., Merheb E., Aoun M.L., Tarabra E., Feng D., Sarparanta J., Merlo P., Botre F., Schwartz G.J. Autophagy regulates the liver clock and glucose metabolism by degrading CRY1. Cell Metab. 2018;28:268–281.e4. doi: 10.1016/j.cmet.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H.R., Chen W., Adachi A., Wakamatsu H., Hayashi S., Takasugi T., Nagano M., Nakahama K., Suzuki Y., Sugano S. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- Vacanti N.M., Divakaruni A.S., Green C.R., Parker S.J., Henry R.R., Ciaraldi T.P., Murphy A.N., Metallo C.M. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna M.H., Selby C.P., Todo T., Niwa H., Thompson C., Fruechte E.M., Hitomi K., Thresher R.J., Ishikawa T., Miyazaki J. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad. Sci. U S A. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M., Green C.R., Roberts L.S., Lee Y.M., McCarville J.L., Sanchez-Gurmaches J., Meurs N., Gengatharan J.M., Hover J.D., Phillips S.A. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 2018;14:1021–1031. doi: 10.1038/s41589-018-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Potluri N., Lu J., Kim Y., Rastinejad F. Structural integration in hypoxia-inducible factors. Nature. 2015;524:303–308. doi: 10.1038/nature14883. [DOI] [PubMed] [Google Scholar]
- Wu D., Rastinejad F. Structural characterization of mammalian bHLH-PAS transcription factors. Curr. Opin. Struct. Biol. 2017;43:1–9. doi: 10.1016/j.sbi.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Su X., Potluri N., Kim Y., Rastinejad F. NPAS1-ARNT and NPAS3-ARNT crystal structures implicate the bHLH-PAS family as multi-ligand binding transcription factors. Elife. 2016;5 doi: 10.7554/eLife.18790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Tang D., Liu N., Xiong W., Huang H., Li Y., Ma Z., Zhao H., Chen P., Qi X. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 2017;25:73–85. doi: 10.1016/j.cmet.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Hilliard G., Ferguson T., Millhorn D.E. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003;278:15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets or code.