Abstract
Background & Aims
Enterochromaffin-like (ECL) cells in the stomach express gastrin/cholecystokinin 2 receptor CCK2R and are known to expand under hypergastrinemia, but whether this results from expansion of existing ECL cells or increased production from progenitors has not been clarified.
Methods
We used mice with green fluorescent protein fluorescent reporter expression in ECL cells (histidine decarboxylase [Hdc]-green fluorescent protein), as well as Cck2r- and Hdc-driven Tamoxifen inducible recombinase Cre (Cck2r-CreERT2, Hdc-CreERT2) mice combined with Rosa26Sor-tdTomato (R26-tdTomato) mice, and studied their expression and cell fate in the gastric corpus by using models of hypergastrinemia (gastrin infusion, omeprazole treatment).
Results
Hdc-GFP marked the majority of ECL cells, located in the lower third of the gastric glands. Hypergastrinemia led to expansion of ECL cells that was not restricted to the gland base, and promoted cellular proliferation (Ki67) in the gastric isthmus but not in basal ECL cells. Cck2r-CreERT2 mice marked most ECL cells, as well as scattered cell types located higher up in the glands, whose number was increased during hypergastrinemia. Cck2r-CreERT2+ isthmus progenitors, but not Hdc+ mature ECL cells, were the source of ECL cell hyperplasia during hypergastrinemia and could grow as 3-dimensional spheroids in vitro. Moreover, gastrin treatment in vitro promoted sphere formation from sorted Cck2r+Hdc- cells, and increased chromogranin A and phosphorylated- extracellular signal-regulated kinase expression in CCK2R-derived organoids. Gastrin activates extracellular signal-regulated kinase pathways in vivo and in vitro, and treatment with the Mitogen-activated protein kinase kinase 1 inhibitor U0126 blocked hypergastrinemia-mediated changes, including CCK2R-derived ECL cell hyperplasia in vivo as well as sphere formation and chromogranin A expression in vitro.
Conclusions
We show here that hypergastrinemia induces ECL cell hyperplasia that is derived primarily from CCK2R+ progenitors in the corpus. Gastrin-dependent function of CCK2R+ progenitors is regulated by the extracellular signal-regulated kinase pathway.
Keywords: Enterochromaffin-Like (ECL) Cells, Progenitor Cell, CCK2R or Gastrin Receptor, Gastrin, Stem Cell Niche, Histidine Decarboxylase (Hdc), Cholecystokin-2 Receptor (CCK2R), Chromogranin A (CgA), Proton Pump Inhibitors (PPI)
Abbreviations used in this paper: CreERT, Tamoxifen inducible recombinase Cre system; ECL, enterochromaffin-like; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; GFP, green fluorescent protein; HDC, histidine decarboxylase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; PBS, phosphate-buffered saline; PPI, proton pump inhibitor; CCK2R, cholecystokinin 2 receptor; CgA, chromogranin A; TAM, tamoxifen
Graphical abstract
Summary.
Histidine decarboxylase–positive enterochromaffin-like (ECL) cells are part of the cholecystokinin 2 receptor (Cck2r) lineage under hypergastrinemia. Mature ECL cells lack progenitor function, whereas Cck2r+ histidine decarboxylase–negative cells comprise progenitor cells. Hypergastrinemia induces ECL cell hyperplasia primarily through generation of new ECL cells from Cck2r+ progenitors via extracellular signal-regulated kinase/mitogen-activated protein kinase signaling.
Enterochromaffin-like (ECL) cells form the major endocrine cell population of the gastric oxyntic mucosa and represent a key regulatory cell for the secretion of gastric acid.1 ECL cells express the enzyme histidine decarboxylase (Hdc) and, in response to neural and hormonal factors, they release histamine, which stimulates parietal cells to secret HCl. Interest in ECL cells greatly increased in the 1980s with the development of powerful acid-suppressive medications, such as proton pump inhibitors (PPIs): treatment of rats with PPI promotes expansion of ECL cells, and these lesions occasionally progress to gastric carcinoid tumors, thus initially generating concerns regarding the safety of these medications.2 However, although such neoplastic lesions are not prevalent in PPI-treated patients, there continues to be a strong interest in investigating the mechanisms of ECL cell expansion in response to acid-suppressive medications.
Additional work identified gastrin as the likely mediator for the observed ECL cell hyperplasia, resulting in the so-called gastrin hypothesis. Gastrin, a peptide hormone secreted from antral gastrin-expressing endocrine cells (G cells), is known to primarily regulate acid secretion and proliferation of the oxyntic mucosa.3, 4, 5 Proposed simultaneously by several groups in 1990,6,7 the gastrin hypothesis posited that the development of ECL cell hyperplasia or carcinoid tumors was related to chronic increases in circulating amidated gastrin, which is increased in the setting of prolonged hypochlorhydria. Indeed, long-term hypergastrinemia can induce growth of the oxyntic mucosa, leading to increased stomach weight and mucosal thickness, although the effects appear most marked on ECL cells.8 This hypothesis was borne out by multiple studies, including correlations between the degree and duration of hypergastrinemia and ECL cell hyperplasia; and studies in which omeprazole-induced hypergastrinemia was reversed by antrectomy.9 In addition, the long-term effects of hypergastrinemia can be blocked by the use of gastrin-receptor antagonists.10 Interestingly, the use of gastrin-receptor antagonists does not reduce the number or density of ECL cells under homeostatic conditions, but can block the increases observed during hypergastrinemia.
Although the finding of ECL cell expansion under conditions of hypergastrinemia has been well accepted, the potential mechanism for such expansion has yet to be fully clarified. Initial studies largely focused on the effects of gastrin on the proliferation of mature ECL cells. Indeed, it has been reported that hypergastrinemia increases 3H-thymidine uptake by ECL cells, although the labeling rates typically were low and generally seen after several weeks of increased gastrin.11,12 Furthermore, multiple studies highlighted that exogenously administered gastrin stimulates cell division primarily in the proliferative zone situated in the gastric isthmus, while mature ECL cells normally reside near the crypt base. Thus, one hypothesis suggested that gastrin stimulates progenitor cells to expand and increasingly differentiate into ECL cells as well as other cell types.13 The existence of a common stem cell origin for the stomach epithelium, including endocrine cells, initially was supported by the observation that gastric glands are mostly clonal.14 Moreover, it recently was confirmed by lineage tracing studies, which showed that corpus stem cells can generate all epithelial lineages.15,16 However, the direct cellular origin leading to the generation of ECL cell hyperplasia in response to hypergastrinemia and other perturbations has yet to be shown.
Amidated gastrin mediates its effects on mucosal growth and acid secretion through binding to the gastrin/cholecystokinin-2 receptor (CCK2R). Although the receptor initially was cloned from a parietal cell library,17 it soon became clear that ECL cells in the oxyntic mucosa were the primary site of Cck2r expression.18 Indeed, targeted disruption or knockout of the Cck2r gene in mice leads to complete loss of mature ECL cells, with smaller ECL-like cells instead, which lack secretory vesicles and histamine.18, 19, 20 This suggests that CCK2R signaling is required for maturation of progenitors in ECL cell lineage. Studies using laser capture microdissection and in situ hybridization further showed that the gastrin/CCK2R also is expressed at lower levels in proliferating neck cells of the gastric oxyntic glands, consistent with progenitor cells.21,22 We previously reported using a Cck2r driven Tamoxifen inducible recombinase Cre system a Cck2r driven Tamoxifen inducible recombinase Cre system (Cck2r-CreERT) that Cck2r identifies stem cells in the gastric antrum,23 but the pattern of expression of Cck2r in the corpus glands in these mice has not been explored. Here, we show that CCK2R marks not only mature ECL cells but also isthmus progenitors, which are the source of the ECL cell hyperplasia observed with sustained hypergastrinemia. Furthermore, we provide evidence that gastrin-induced ECL cell expansion relies on the extracellular signal-regulated kinase (ERK) signaling pathway.
Results
Hdc-GFP Marks ECL Cells in the Gastric Corpus, Which Are Increased by Hypergastrinemia
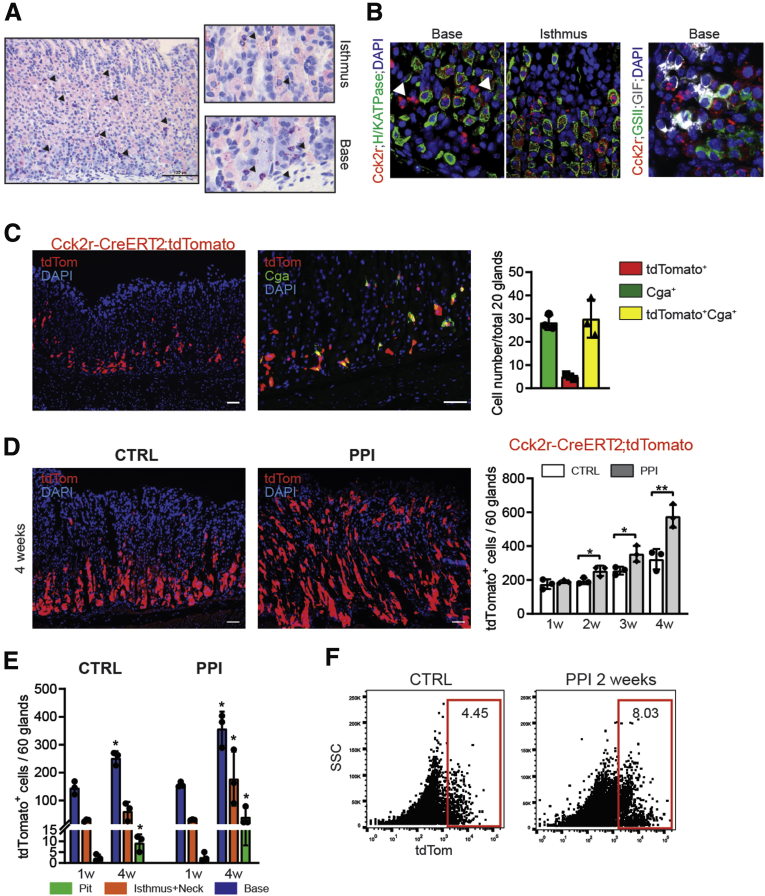
We previously described the generation of an Hdc-green fluorescent protein (GFP) bacterial artificial chromosome-transgenic reporter mouse, which labels endocrine-like cells at the base of the gastric oxyntic glands where ECL cells are known to reside.24 Immunofluorescence staining for chromogranin A (CgA), a marker of endocrine cells, showed that CgA co-localized with Hdc-GFP+ cells in the corpus (Figure 1A). In particular, more than 90% of the Hdc-GFP+ cells also were positive for CgA (Figure 1A), and there was no overlap between Hdc-GFP and markers of parietal cells (H+/K+-adenosine triphosphatase) or chief cells (gastric instrinsic factor) (Figure 1B), consistent with previous findings that in the noninflamed gastric mucosa Hdc-GFP is a highly specific marker of ECL cells.25
Figure 1.
Hdc-GFP marks ECL cells in the gastric corpus, which are increased by hypergastrinemia. (A) Immunofluorescence staining for CgA in the corpus of Hdc-GFP mice (N = 3) (original magnification, ×100). (B) Immunofluorescence staining for H+/K+-adenosine triphosphatase (ATPase) and GIF in the corpus of Hdc-GFP mice (original magnification, ×100). (C) Plasma gastrin levels in mice treated with a gastrin pump (left) and PPI (right) (N ≥ 3). (D and E) Quantification of Hdc-GFP+ cells in (D) gastrin pump and (E) PPI-treated mice (N ≥ 3) for 1–4 weeks (original magnification, ×100). Bars indicate means ± SD. ∗P < .05 and ∗∗P < .01 compared with control. DAPI, 4′,6-diamidino-2-phenylindole. Scale bars: 100 um.
We next induced hypergastrinemia in Hdc-GFP mice using previously reported protocols of prolonged gastrin infusion26 and treatment with omeprazole, a PPI.27 Both gastrin and omeprazole treatments increased plasma gastrin levels in a time-dependent manner, with significant increases by 2–4 weeks (Figure 1C). In parallel with the increase in circulating gastrin levels, both treatments led to expansion of Hdc-GFP+ ECL cells throughout the gastric corpus glands (Figure 1D and E). Taken together, Hdc-GFP efficiently marks gastric ECL cells, which expand in response to hypergastrinemia.
Hypergastrinemia-Induced Proliferation Is Observed Primarily in the Gastric Isthmus, and Rarely Observed in Hdc+ ECL Cells
Next, we aimed to determine whether the gastrin-dependent expansion of Hdc-GFP+ ECL cells arises from proliferation of existing mature ECL cells. To this end, we evaluated the number and location of Ki67+ proliferating cells and Hdc-GFP+ ECL cells in the oxyntic mucosa of mice treated with either gastrin infusion or omeprazole. Gastrin and PPI treatments increased the number of Ki67+ cells in a time-dependent manner (Figure 2A and B); in particular, the increase of Ki67+ cells occurred primarily near the gastric isthmus where corpus stem and progenitor cells are thought to reside.16,28 Although a small number of Ki67+ cells could be detected at the glands’ base, we did not observe any overlap between Hdc-GFP+ and Ki67+ cells (Figure 2C). In line with these observations, both gastrin and PPI treatments increased the number of Hdc-GFP+ cells, and this increase occurred primarily from the surface pit to isthmus/neck region, where under normal conditions ECL cells rarely reside (Figure 1D). These results strongly suggest that hypergastrinemia does not induce cell division or proliferation in mature ECL cells, but on the contrary, new ECL cells appear to arise from stem/progenitor cells in the proliferating isthmus and neck region.29
Figure 2.
Hypergastrinemia-induced proliferation was observed primarily in the gastric isthmus, and rarely was observed in HDC+ECL cells. (A and B) Representative images and quantification for Ki67 staining in the gastric corpus of Hdc-GFP mice with (A) 4 weeks of gastrin or (B) PPI treatment (N ≥ 3) (original magnification, ×100). (C) Enlargement of Ki67 staining at the level of the gland base, which shows no colocalization between Ki67+ and Hdc-GFP+ cells. (D) Total number of positive cells according to their specific localization within the gastric gland in gastrin- and PPI-treated groups (N ≥ 3) (original magnification, ×100). Bars indicate means ± SD. ∗P < .05 and ∗∗P < .01 compared with control. CTRL, control; DAPI, 4′,6-diamidino-2-phenylindole. Scale bars: 100 um.
Cck2r Is Expressed in ECL and Parietal Lineages, and Hypergastrinemia Promotes Differentiation Toward These Cell Types
In addition to expression of Hdc, ECL cells in the oxyntic mucosa are characterized by increased Cck2r expression.18 We previously reported that Cck2r expression marks antral stem cells, but detailed characterization of Cck2r-expressing cells in the corpus has not been performed.23 In situ hybridization for Cck2r in the oxyntic mucosa confirmed strong expression in ECL-like cells near the gland base (Figure 3A). In addition, a subset of parietal cells, as well as undifferentiated cells residing near the isthmus, were found to express Cck2r (Figure 3A and B). We did not observe apparent Cck2r expression in the neck region or in the chief cell lineage that express GIF and/or Glutamine Synthetase (Figure 3B). A similar expression pattern was observed from Cck2r-CreERT2 lineage tracing when we looked at the corpus glands of Cck2r-CreERT2;tdTomato mice 1 week after tamoxifen (TAM) induction (Figure 3C). Co-immunostaining confirmed a high degree of overlap between CgA+ and Cck2r-CreERT2;tdTomato-labeled cells (85.7%) (Figure 3C), indicating that mature ECL cells are marked efficiently by CCK2R.30 Notably, nearly half of tdTomato+ cells were negative for CgA, corroborating what was observed via in situ hybridization: Cck2r also is expressed in non-ECL cells, such as parietal cells and isthmus progenitor cells (Figure 3C).23
Figure 3.
Cck2r is expressed in ECL and parietal lineage, and hypergastrinemia promotes differentiation toward these cell types. (A) Cck2r expression in gastric corpus by in situ hybridization (original magnification, ×100). (B) Staining with H+/K+ adenosine triphosphatase (left side), GSII and GIF (right side) in corpus with Cck2r in situ hybridization (original magnification, ×200). (C) CgA staining in corpus of Cck2r-creERT2;tdTomato mice after 1 week of TAM induction (N = 3) (original magnification, ×100). (D) Representative pictures and quantification of Cck2r-creERT2;tdTomato+ traced cells in control and after PPI treatment (original magnification, ×100). (E) Number of tdTomato+ traced cells according to their specific localization within the gastric gland (N ≥ 3). (F) Fluorescence-activated cell sorter plot of Cck2r-CreERT2;tdTomato mice with 2 weeks of PPI treatment. Bars indicate means ± SD. ∗P < .05 and ∗∗P < .01 compared with the control. CTRL, control; DAPI, 4′,6-diamidino-2-phenylindole. Scale bars: 100 um.
In control animals, the number of tdTomato+ cells, including ECL and parietal cells, gradually increased over time, and after 4 weeks of TAM administration the majority of traced cells were observed in the lower half of glands. This suggests that Cck2r+ progenitor cells divide and supply daughter cells for a few cycles, and then such clones migrate from the isthmus region toward the lower part of the gland along with the bidirectional normal cellular flow in these glands.31 Interestingly, when we treated these mice with PPI to induce hypergastrinemia, the number of tdTomato+ cells dramatically increased in a time-dependent manner (Figure 3D) and could be observed throughout the corpus glands (Figure 3E). Increased tdTomato+ labeling after PPI treatment (2 weeks) also was confirmed by flow cytometry analysis (Figure 3F). Importantly, we did not observe fully traced glands by Cck2r-CreERT2;tdTomato+ cells even after 4 weeks of PPI treatment, suggesting that Cck2r + isthmal cells are short-lived progenitors, rather than long-lived multipotent stem cells. Taken together, CCK2R marks ECL and parietal lineages as well as isthmal progenitor cells, which expand and supply daughter cells in response to hypergastrinemia.
Hdc+ ECL Cells Are Part of the Expanded Cck2r Lineage
To investigate the contribution of Cck2r+ progenitor cells to the increase of Hdc-GFP+ cells after hypergastrinemia, we generated Cck2r-CreERT2;tdTomato;Hdc-GFP mice and treated them with PPI. Although at 1 week we did not observe differences in the number of tdTomato+Hdc-GFP+ cells between the 2 experimental groups (control vs PPI), starting at week 2 the number of double-positive cells increased significantly (Figure 4A and B). This effect was even stronger after 3 and 4 weeks of PPI treatment. The biggest expansion of tdTomato+Hdc-GFP+ cells took place in the isthmus region, followed by the bottom third of the corpus gland (Figure 4C). In contrast, Hdc-CreERT2;tdTomato mice, which specifically mark the mature ECL cell population, did not show any increase in lineage tracing after TAM induction between 1 and 4 weeks (Figure 4D and E). These data indicate that Hdc-labeled mature ECL cells, generated from Cck2r + progenitors in the isthmus, account for most of the increases in ECL cells after hypergastrinemia.
Figure 4.
Hdc+ECL cells are part of the expanded Cck2r lineage. (A and B) Representative (A) pictures and (B) quantification of tdTom+/GFP+(Cck2r+/Hdc+) cells in Cck2r-CreERT2;tdTomato;Hdc-GFP mice after 1–4 weeks of PPI treatment after TAM induction (original magnification, ×100). (C) Number of double-positive cells based on their position within the corpus gland. (D and E) Quantification and representative images of Hdc-CreERT;tdTomato linage tracing in the gastric corpus at 1–4 weeks after TAM induction (original magnification, ×100). Bars indicate means ± SD. ∗P < .05 and ∗∗P < .01 compared with control. DAPI, 4′,6-diamidino-2-phenylindole. Scale bars: 100 um.
Cck2r+ Progenitor Cells, but Not Mature ECL Cells, Can Form Organoids In Vitro
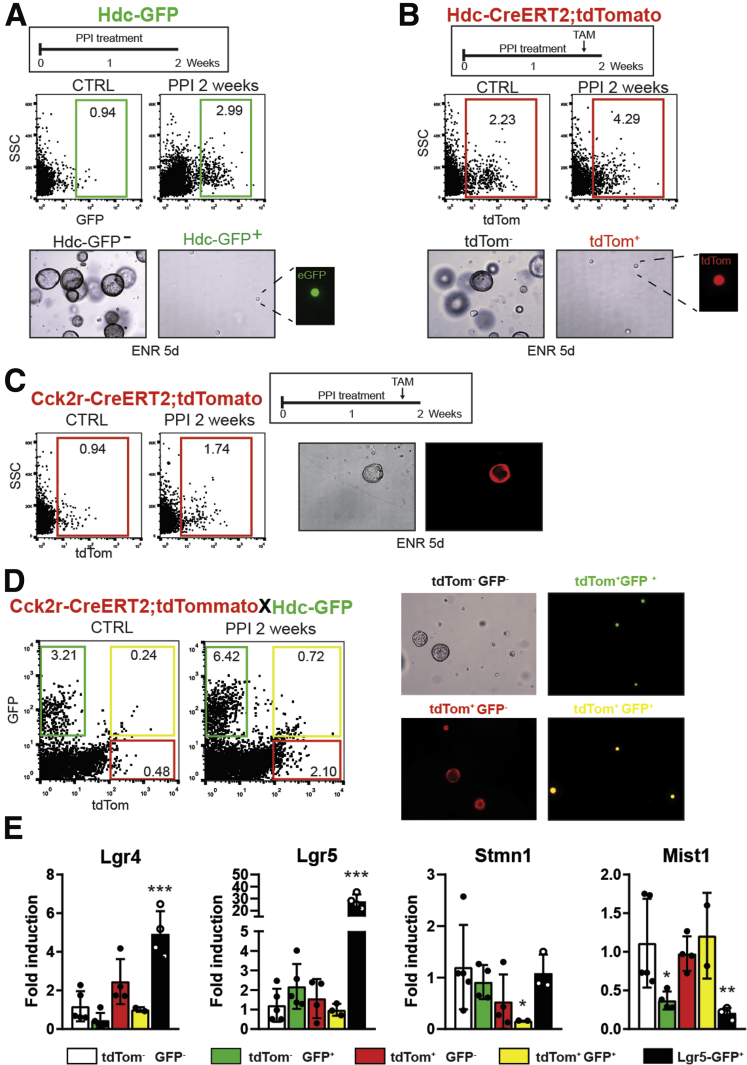
To further test the progenitor potential of corpus cells, we investigated the ability of corpus Hdc-GFP+ and Cck2r + cell populations to form spheroids in vitro using fluorescence-activated cell sorting and 3-dimensional culture. Sorted Hdc-GFP+ cells did not show any organoid formation, even after 2 weeks of PPI treatment before the cell isolation (Figure 5A). Analogous results were obtained when using Hdc-CreERT2;tdTomato mice (Figure 5B). These data further showed that PPI treatment increased the number of Hdc+ ECL cells compared with control animals (2.99% vs 0.94% of live cells in Hdc-GFP and 4.29% vs 2.23% in Hdc-CreERT2;tdTomato mice, respectively) (Figure 5A and B). When we performed the same experiment using Cck2r-CreERT2;tdTomato mice and sorting for tdTomato+ cells, we observed stable organoid formation by the sorted Cck2r-CreERT2;tdTomato+ cells in Epidermal Growth Factor/Noggin/R-spondin1 media (Figure 5C). In line with our in vivo observations, 2 weeks of PPI treatment followed by TAM induction increased the total number of Cck2r-CreERT2;tdTomato+ cells compared with control (1.74% vs 0.94%) (Figure 5C).
Figure 5.
Cck2r+progenitor cells, but not mature ECL cells, can form organoids in vitro. (A) Top: Fluorescence-activated cell sorter (FACS) plot for Hdc-GFP cells sorted from the corpus in control and 2-week PPI-treated mice (N = 3). Bottom: Representative pictures of cultured cells in vitro (original magnification, ×100). (B) Top: FACS plot for Hdc-CreERT2;tdTomato cells sorted from the corpus in control and 2-week PPI-treated mice (N = 3). Bottom: Representative pictures of cultured cells in vitro (original magnification, ×100). (C) Left: FACS plot for Cck2r-CreERT2;tdTomato cells sorted from the corpus in control and 2-week PPI-treated mice (N = 3). Right: Representative picture of Cck2r+-derived organoids after 5 days of culture (original magnification, ×100). (D) FACS plot of the 4 different populations sorted from Cck2r-CreERT2;tdTomato;Hdc-GFP mice and representative pictures of their in vitro culture (original magnification, ×100). (E) Quantitative PCR analysis of stem/progenitor markers in individually sorted populations from Cck2r-CreERT2;tdTomato;Hdc-GFP mice after 36 hours of TAM induction; Lgr5+ cells were isolated from the small intestine of Lgr5-eGFP-DTR mice and used as comparison (N ≥ 3 per group). Bars indicate means ± SD. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 compared with control. CTRL, control; SSC, side scatter.
We next used Cck2r-CreERT2;tdTomato;Hdc-GFP mice to test the spheroid-forming capacity of the different corpus cell populations during homeostasis as well as after 2 weeks of PPI treatment. We harvested the corpus at 36 hours after TAM and sorted 4 different cell populations: tdTom-/GFP-(Cck2r-/Hdc-), tdTom+/GFP-(Cck2r+/Hdc-), tdTom-/GFP+ (Cck2r-/Hdc+), and tdTom+/GFP+(Cck2r +/Hdc+) (Figure 5D). As noted earlier, we observed an increase in Cck2r+/Hdc-, Cck2r -/Hdc+, and Cck2r+/Hdc+ cells with 2 weeks of PPI treatment compared with control (tdTom+/GFP-: 2.1% vs 0.48%; tdTom-/GFP+: 6.42% vs 3.21%; tdTom+/GFP+: 0.72% vs 0.24%). Only Cck2r+Hdc- (tdTom+eGFP-) progenitor cells, but not Cck2r+/Hdc+ (tdTom+eGFP+) and Hdc+ (eGFP+) ECL cells, were able to form organoids (Figure 5D) in Epidermal Growth Factor/Noggin/R-spondin1 medium. These results further suggest that Hdc+ cells are postmitotic differentiated cells with no progenitor capacity.
To further characterize the progenitor potential among these corpus cell populations, we investigated the gene expression of markers previously proposed to identify stem cells within the stomach, including Lgr5, Mist1, and Stmn1, in each individual sorted population. The results (Figure 5E) indicated that these particular stem cell markers were not enriched in Cck2r+ cells, suggesting that Cck2r+ progenitors may be distinct from the more stem-like populations. In contrast, we noticed an enrichment, although not significant (P = .07), of Lgr4 expression in Cck2r+ cells compared with the other groups. It should be noted that Lgr4 has been shown to characterize proliferating progenitor cells in different organs,32 suggesting that Cck2r+/Hdc-GFP- cells likely are proliferative progenitors, consistent with their ability to form organoids in vitro.
Hypergastrinemia Promotes Expansion and Differentiation of CCK2R+ Cells In Vitro Through the ERK Pathway
To investigate the effects of hypergastrinemia on corpus organoid growth, we cultured sorted cells with various concentrations of gastrin for 1 week. We found that gastrin promoted sphere formation from the Cck2r+Hdc- progenitor cells in a dose-dependent manner, with significant increases found at 100 nmol/L and 1 umol/L concentrations, whereas such effects by gastrin were not evident with Cck2r -Hdc- cells, which include Cck2r- stem/progenitor cells (Figure 6A and B). Importantly, sorted Hdc-GFP+ as well as Hdc-CreERT2;tdTomato+ positive cells did not form organoids in the presence of high concentrations of gastrin in vitro (Figure 6C). This also was confirmed using Cck2r-CreERT2;tdTomato;Hdc-GFP mice, in which neither tdTom-eGFP+ (Cck2r-Hdc+) or tdTom+eGFP+ (Cck2r+Hdc+) cells could form organoids in the presence of high concentrations of gastrin in vitro (Figure 6D); further suggesting that Hdc marks differentiated ECL cells with limited or no progenitor capacity. Intriguingly, gastrin treatment increased CgA protein expression in Cck2r+Hdc- cell–derived organoids, compared with untreated controls (Figure 6E and F). These results suggest that gastrin directly stimulates Cck2r+ progenitors, promoting their proliferation and differentiation to CgA+ ECL cells, as shown in vivo.
Figure 6.
Hypergastrinemia promotes expansion and differentiation of Cck2r+cells in vitro through the ERK pathway. (A and B) Representative images and quantification of the number of tdTom-eGFP- (Cck2r-/Hdc-) and tdTom+eGFP- (Cck2r+/Hdc-) formed organoids after exposure to various gastrin concentrations (from 10 nmol/L to 1 umol/L) (original magnification, ×100). (C) Representative images of single sorted Hdc-GFP+ and Hdc-CreERT2;tdTom+ cells after culture in the presence of 80 nmol/L gastrin. (D) Representative images of single sorted HDC+ cell populations from Cck2r-CreERT2;tdTomato;Hdc-GFP mice after culture in the presence of 80 nmol/L gastrin. (E) CgA protein expression in tdTom-eGFP- and tdTom+eGFP- formed organoids with or without gastrin (100 nmol/L) by Western Blot. (F and G) CgA staining and protein expression in the aforementioned organoids with gastrin (100 nmol/L) plus U0126 treatment (20 umol/L) (original magnification, ×400). (H) Representative images and quantification of the sphere number of tdTom-eGFP- and tdTom+eGFP formed organoids plus gastrin (100 nmol/L) and U0126 (20 umol/L) (original magnification, ×100). Bars indicate means ± SD. ∗P < .05 and ∗∗P < .01 compared with control. CTRL, control; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pERK, phosphorylated extracellular signal-regulated kinase.
Previous studies have shown that the epidermal growth factor receptor (EGFR)/ERK pathway is activated in response to gastrin/CCK2R signaling in gastric epithelial cells.33,34 Thus, we tested if the gastrin-dependent growth of Cck2r+Hdc- corpus progenitors in 3-dimensional culture is mediated by the ERK pathway. First, we confirmed that gastrin treatment induces phosphorylation of ERK along with up-regulation of CgA in Cck2r+Hdc- cell–derived organoids, although such effects were absent in Cck2r-Hdc- cell–derived organoids (Figure 6G). Moreover, simultaneous treatment with U0126, a specific Mitogen-activated protein kinase kinase 1 (MEK1) inhibitor,35,36 blocked the up-regulation of CgA and growth-promoting effects induced by gastrin (Figure 6G and H). These data suggest that gastrin can stimulate Cck2r+ progenitor cell growth through ERK signaling, promoting their organoid-forming capacity as well as their differentiation toward CgA-expressing ECL cells.
MEK1 Inhibitor Inhibits Hypergastrinemia-Induced ECL Cell Expansion In Vivo
To test the effects by ERK inhibition in the setting of hypergastrinemia in vivo, we administered U0126 together with a PPI for 4 weeks as previously described.37,38 Under normal conditions, we found no effect on the number of Hdc-GFP+ ECL cells after U0126 treatment alone (Figure 7A and B). However, in mice treated with PPI, the increase in the number of Hdc-GFP+ cells was blocked by U0126 treatment (Figure 7A and B). Western blot analysis confirmed that PPI treatment induced phosphorylation of ERK in the mouse stomach, which was attenuated by U0126 treatment (Figure 7C). Similarly, Cck2r-derived lineage tracing, which is increased during PPI treatment, was blocked by U0126 administration, whereas U0126 treatment under normal conditions did not affect Cck2r-derived lineage tracing (Figure 7D–F). Given that EGFR is expressed selectively in the corpus isthmus and the lower gastric pits (Figure 7G), gastrin may activate ERK signal specifically in these EGFR+ cells near the isthmus. Taken together, these data further show that hypergastrinemia-induced expansion of ECL cells occurs primarily through activation of ERK/MAPK signaling in Cck2r+ progenitor cells. Moreover, U0126 can be used to efficiently block this ECL cell hyperplasia.
Figure 7.
The MEK1 inhibitor inhibits hypergastrinemia-induced ECL cells expansion in vivo. (A and B) Representative pictures and quantification of Hdc+ cells in corpus of Hdc-GFP mice with or without PPI plus U0126 treatment for 4 weeks (N ≥ 3) (original magnification, ×100). (C) WB analysis of p-ERK in the gastric corpus after treatment with PPI and/or U0126 in Hdc-GFP mice. (D and E) Representative images and quantification of toTomato+ cells in corpus of Cck2r -creERT2;tdTomato mice with or without PPI plus U0126 treatment for 4 weeks after TAM (N ≥ 3) (original magnification, ×100). (F) WB analysis of p-ERK in the gastric corpus after treatment with PPI and/or U0126 in Cck2r -CreERT2;tdTomato mice. (G) Immunohistochemistry for EGFR in the gastric corpus. Bars indicate means ± SD. ∗P < .05 compared with control. Scale bars: 100 um CTRL, control; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pERK, phosphorylated extracellular signal-regulated kinase; VC, vehicle; WB, Western blot.
Discussion
ECL cells are known to expand under hypergastrinemia, but where new ECL cells are generated from has remained an unsolved question. Indeed, several lineage tracing studies failed to identify the direct precursor or cell-of-origin of ECL cells.39,40 Although we found in our study that mature ECL cells in the gastric corpus express CCK2R, we discovered that CCK2R also labeled isthmal progenitor cells, which in response to hypergastrinemia supply new ECL cells. Therefore, our data answer the long-standing question as to the cellular source of gastrin-dependent ECL cell expansion, that is, whether hypergastrinemia-induced ECL cell hyperplasia occurs primarily through generation of new ECL cells from Cck2r+ progenitor cells rather than cell division or proliferation of mature ECL cells.
Previous studies have suggested that most, if not all, gastric glands are monoclonal, thus epithelial cells, including endocrine lineage, likely arise from a common stem cell.14,41 Studies using electron microscopy showed the existence of granule-free, undifferentiated stem cells in the isthmus that first give rise to progenitors such as preparietal cells or preneck cells, then differentiated mature cell types.42,43 In this series of studies, ECL cells appeared to first arise from undifferentiated isthmus progenitors, then migrate mostly downward while a few migrated upward from the isthmus, in a similar manner to the migration of the parietal cell lineage.44 Although several candidate multipotent stem cells that have been identified recently in the gastric corpus are a likely source for both ECL cells and parietal cells,15,16 lineage-committed progenitors that give rise to these cell types have not been well studied. Our group previously identified Tff2-expressing progenitors that give rise to parietal, neck, and chief lineage.28 In our current study, we identify the unique Cck2r+ corpus progenitors that specifically supply and differentiate into ECL and parietal cells. Cck2r+ progenitors do not express the typical proposed markers of adult gastric stem cells (ie, Mist1, Stmn1), but appears to be enriched for Lgr4 expression, a marker of proliferative progenitor cells in various organs.32 In addition to ECL cell hyperplasia, it was reported that PPI treatment and subsequent hypergastrinemia also induces hyperplasia of parietal cells, particularly immature parietal cell types.45 Although it remains unclear whether CCK2R marks 2 unipotent progenitors (preparietal and pre-ECL cells) or 1 bipotent progenitor that gives rise to 2 lineages, gastrin/CCK2R signal appears to be central to proliferation and differentiation in these lineages.
Hypergastrinemia-induced proliferation was observed primarily in the gastric isthmus, and rarely observed in Hdc+ ECL cells at the base. Over time, the proliferative zone marked by Ki67 in the isthmus expanded and we observed a few proliferating cells in the basal third of the gland, consistent with the migration of gastric progenitors from the isthmus region as previously proposed.29 Thus, the combination of increased isthmus proliferation, along with a more diffuse expansion of ECL cell markers, strongly suggested the origins of this gastrin-dependent expansion of ECL cells in progenitor cells, rather than mature ECL cells. It was suggested that gastrin might stimulate progenitor cells that proceed to increasingly differentiate into gastric ECL cells as well as other cell types.13 Our current study confirmed this hypothesis of a progenitor origin of ECL cells by analysis of lineage tracing in Cck2r-CreERT2 and Hdc-CreERT2 mice.
In addition, we identified the ERK pathway as a key regulator for proliferation and differentiation in the Cck2r+ cell lineage. Previous studies using ERK kinase inhibitors have shown that the ERK1/2 pathway activated by CCK2R may control cell proliferation, migration, and regulation of gastrin-sensitive gene transcription.46,47 In gastric epithelial cells expressing the CCK2R, gastrin can transactivate the EGFR/MEK1/ERK pathway and induce CgA up-regulation and proliferation.23,48, 49, 50 More recently, it was found that EGFR is central to the regulation of mitogen-activated protein kinase (MAPK)-dependent cell proliferation in the gastric corpus of hypergastrinemic mice.51 Because EGFR is expressed specifically in the isthmus and surface pit region in the mouse corpus, gastrin’s effects on proliferation and differentiation likely would be mediated through transactivation of EGFR in Cck2r+ progenitors, as previously suggested. Nevertheless, multiple signaling cascades can be activated by EGFR, such as Janus kinase/signal transducer and activator of transcription, Phosphoinositide 3-kinase/Protein Kinase B/mammalian target of rapamycin, and protein kinase C/nuclear factor-κB signaling; further studies are needed to address the exact role of EGFR and other signaling pathways in gastrin-mediated effects. Interestingly, it was reported that in the small intestine, differentiation into endocrine lineage is enhanced when the EGFR/MAPK pathway is inhibited,52 unlike our findings with respect to ECL cell differentiation in the stomach. On the other hand, differentiation into the endocrine-like tuft cell lineage is enhanced by activation of EGFR/ERK signaling both in the stomach and intestine.52, 53, 54 Therefore, it appears that regulatory mechanisms of cellular differentiation and proliferation are likely to be cell type–dependent and/or organ-dependent.
It should be noted that gastrin has opposing effects on the proximal and distal stomach. In hypergastrinemic mice, proliferation and tumorigenesis are promoted in the proximal stomach, whereas they are inhibited in the distal stomach.55 In addition, knockout of gastrin accelerates tumor development in the distal stomach.56 These observations, together with the existence of Cck2r-expressing stem/progenitors in both sites,23 suggest that the effects of amidated gastrin on Cck2r+ progenitor cells may vary depending on their location. Gastrin stimulates proliferation in Cck2r+ progenitors in the proximal stomach, but may suppress their mitotic activity in the distal stomach.
The functional activity of ECL cells, including CgA synthesis and histamine secretion, is controlled mainly by the hormone gastrin, which exerts its effects through binding to and activation of CCK2R.3 Previous studies also have shown that gastrin acts on CCK2R to control the synthesis of ECL cell histamine, accelerating the expression of Hdc and CgA at both the transcription and the translation/post-translation levels.57,58 Therefore, earlier and current observations suggest that CCK2R signaling is integral to both the origins and the maturation of ECL cells.
In conclusion, hypergastrinemia leads to ECL cell hyperplasia primarily through expansion of new ECL cells from Cck2r+ progenitor cells in corpus via ERK signaling. We speculate that such Cck2r-labeled progenitor cells also may represent the origin of neuroendocrine tumors observed in hypergastrinemia and other conditions. The ability of MEK inhibitors to block the growth and progression of such tumors may be worth exploring in future studies.
Materials and Methods
All authors had access to the study data and reviewed and approved the final manuscript.
Animal Models
All animal studies were conducted in compliance with the National Institutes of Health guidelines for animal research and approved by the Institutional Animal Care and Use Committee of Columbia University.
Bacterial artificial chromosome transgenic Hdc-GFP, Hdc-CreERT2;tdTomato, and Cck2r-CreERT2;tdTomato mice were described previously.23,24 For lineage tracing experiments, mice received 3 mg TAM in 200 μL corn oil by oral gavage. Hypergastrinemia was induced in vivo via gastrin (240 nmol/kg/day) applied in subcutaneous osmotic pumps or by daily administration of omeprazole via oral gavage (400 μmol/kg/day). U0126 (25 μmol/kg) was dissolved in dimethyl sulfoxide and injected intraperitoneally twice a week. All control groups received respective vehicle. All experiments were repeated at least twice, with 3 biological replicates if not otherwise indicated.
Tissue Collection and Histologic Analysis
The stomach and proximal duodenum were removed and incised along the greater curvature. Linear gastric strips from the lesser curvature were fixed overnight in 4% phosphate- paraformaldehyde dissolved in phosphate-buffered saline (PBS) and embedded into Optimal Cutting Temperature compound. A small piece of the gastric tissue was snap-frozen in dry ice and stored at –80°C for RNA and protein analysis. For immunofluorescence staining, slides were washed with 1% Triton (ThermoFisher, Waltham, MA) X-100 in PBS, rinsed, and blocked for 30 minutes with 5% bovine serum albumin. Primary antibodies and uorophore-conjugated secondary antibodies were diluted in 2% bovine serum albumin and incubated overnight at 4°C. A list of antibodies is provided in Supplementary Table 1. The number of positive cells in the corpus glands was counted using the Nikon TE2000 microscope (Nikon, Inc, Melville, NY), the total number of positive cells is expressed as the average on a total of 60 glands.
Gastrin Quantification
Blood samples collected from cardiac puncture were used for this experiment. The plasma gastrin concentration was determined using the gastrin enzyme immunoassay kit (RayBiotech, Peachtree Corners, GA) according to the manufacturer’s instruction.
Gene Expression Analysis
RNA of individual sorted cell populations was extracted using the RNeasy Micro Kit (Qiagen, Hilden, Germany) after complementary DNA generation using qScript complementary DNA SuperMix (Quantabio, Beverly, MA). Quantitative PCR results were calculated using the delta delta Ct method using double-negative cells as control.
In Vitro Culture System
Single-cell isolation and cultures were performed as described previously.23 Briefly, stomach corpus was harvested and chopped into 5-mm pieces. Afterward, tissue was washed with cold PBS and incubated on ice in 2.5 mmol/L EDTA in PBS for 60 minutes. The tissue fragments were suspended vigorously with a 10-mL pipette in cold PBS containing 10% fetal bovine serum, yielding supernatants enriched in crypts. The obtained crypt fractions were centrifuged at 900 rpm for 5 minutes at 4°C and then were passed through 100-μm filters (BD Biosciences, San Jose, CA). Crypts were dissociated with TrypLE express (Invitrogen, Carlsbad, CA) including 1 mg/mL DNase I (Thermo Fisher, Waltham, MA) for 10 minutes at 37°C. Dissociated cells were passed through a 40-μm cell strainer and washed with 2% fetal bovine serum/PBS. Viable epithelial cells were gated on the base of forward scatter, side scatter, a pulse-width parameter, and negative staining for 4′,6-diamidino-2-phenylindole. Sorted cells were collected, pelleted, and embedded in Matrigel (Corning, Corning, NY), followed by seeding on a 48-well plate (50–100 cells/well). Epidermal Growth Factor/Noggin/R-spondin1 culture medium containing advanced Dulbecco’s modified Eagle medium/F12 (Gibco, Thermo Fisher) medium plus penicillin/streptomycin, 50 μg/mL gentamicin, 10 mmol/L HEPES, GlutaMAX, N2, B27 (all from Invitrogen), 1 μmol/L N-acetylcysteine (Sigma-Aldrich, St. Louis, MO), 50 ng/mL epidermal growth factor, 100 ng/mL Noggin, R-spondin 1, and 100 ng/mL Wnt3A (all from Peprotech, Rocky Hill, NJ) were used if not otherwise indicated.
Y-27632, gastrin, and U0126 concentrations and time of administration are described in the legends of Figures 6 and 7. Culture medium was changed every 2 days. The number and size of organoids per well were counted on microscopic images via ImageJ software (National Institutes of Health, Bethesda, MD).
Western Blot Analysis
For Western blot analysis, whole protein lysates were prepared from organoids after the indicated treatment. The amount of loaded protein was adjusted to 30 μg/well and loaded onto 10% sodium dodecyl sulfate–polyacrylamide gels (Thermo Fisher), transferred to polyvinylidene difluoride membranes (Millipore Corp, Bedford, MA), blocked for 2 hours, and incubated with the primary antibody overnight at 4°C. The list of primary antibodies is provided in Supplementary Table 1. Membranes were incubated with horseradish peroxidase–conjugated monoclonal secondary antibody (Cell Signaling Technology, Danvers, MA) at room temperature for 1 hour. Immunoreactive protein bands were visualized with an enhanced chemiluminescence detection kit (Thermo Fisher).
In Situ Hybridization
Corpus tissues were fixed overnight in cold PBS with 4% formaldehyde in PBS. After fixation, tissues were dehydrated in increasing alcohol series ending in a 1-butanol step. Sample tissues were sectioned and mounted into 3-aminopropyltriethoxysilane–coated glasses. RNA probes were generated as described previously.23 The samples were evaluated by 2 pathologists in a blinded manner.
Statistical Analysis
Statistical analyses were performed using Excel (Microsoft Office 365, Microsoft, Redmond, WA), and graphs were plotted with GraphPad Prism (San Diego, CA) software. The differences between means were compared using the t test. All values were expressed as means ± SD.
Acknowledgments
The authors thank Bryana Belin and Madeline Strait for mouse colony maintenance and technical assistance; fluorescence-activated cell sorting analysis was performed in the Columbia Center for Translational Immunology Flow Cytometry Core (supported in part by the Office of the Director, National Institutes of Health, under awards S10OD020056 and S10RR027050). Images were collected and analyzed at the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, supported by National Institutes of Health grant P30 CA013696 (National Cancer Institute).
Footnotes
CRediT Authorship Contributions Weiwei Sheng (Data curation: Equal; Formal analysis: Equal; Investigation: Lead; Methodology: Equal; Writing – original draft: Equal); Ermanno Malagola (Data curation: Equal; Formal analysis: Equal; Writing – original draft: Equal); Henrik Nienhüser (Data curation: Equal; Methodology: Equal; Writing – original draft: Equal); Zhengyu Jiang (Data curation: Equal; Writing – review & editing: Equal); Woosook Kim (Conceptualization: Supporting; Formal analysis: Supporting; Supervision: Supporting); Leah Zamechek (Investigation: Equal; Methodology: Lead); Antonia Sepulveda (Conceptualization: Supporting; Supervision: Supporting); Masahiro Hata (Investigation: Supporting; Methodology: Supporting); Yoku Hayakawa (Data curation: Supporting; Investigation: Supporting; Supervision: Equal; Writing – review & editing: Supporting); Chun-Mei Zhao (Conceptualization: Supporting; Supervision: Supporting; Visualization: Supporting); Duan Chen (Conceptualization: Supporting; Visualization: Supporting); Timothy Cragin Wang, MD (Project administration: Lead; Supervision: Lead; Writing – review & editing: Supporting).
Conflicts of interest The authors disclose no conflicts.
Funding This research was supported by National Institutes of Health grant R37CA210088 (T.C.W.); the KAKENHI Grant-in-Aid for Scientific Research, 17K09347 and 17H05081, Project for Cancer Research and Therapeutic Evolution from Japan Agency for Medical Research and Development, the Takeda Science Foundation Visionary Research Grant, the Princess Takamatsu Cancer Research Fund, and the Advanced Research and Development Programs for Medical Innovation (Y.H.); and by the German Research Foundation grant NI1810/1-1 (H.N.).
Supplementary Material
Supplementary Table 1.
Reagents
| Reagent or resource | Vendor | Number |
|---|---|---|
| Antibodies | ||
| Anti-Ki67 antibody | Abcam (Cambridge, UK) | Cat: ab16667 |
| H+/K+ adenosine triphosphatase antibody (C-4) | Santa Cruz | Cat: sc-374094 |
| Anti-CgA antibody (ab15160) | Abcam | Cat: ab15160 |
| p44/42 MAPK (Erk1/2) | Cell Signaling | Cat: ab15160 |
| Phospho-p44/42 MAPK (Erk1/2) | Cell Signaling | Cat: 4695 |
| Glyceraldehyde-3-phosphate dehydrogenase | Proteintech (Rosemont, IL) | Cat: 60004-1-Ig |
| Alexa Fluor–594 goat anti-rabbit | Thermo Fisher Scientific | Cat:R37117 |
| Alexa Fluor–488 chicken anti-rabbit | Thermo Fisher Scientific | Cat:A-21441 |
| Alexa Fluor–555 goat anti-chicken | Thermo Fisher Scientific | Cat:A-21437 |
| Alexa Fluor–555 goat anti-mouse | Thermo Fisher Scientific | Cat:A-21422 |
| Chemicals, peptides, and recombinant proteins | ||
| Hank’s balanced salt solution | Gibco | 14175079 |
| DPBS, no calcium, no magnesium | Gibco | 14190250 |
| Advanced Dulbecco’s modified Eagle medium/F12 | Gibco | 12634010 |
| Fetal bovine serum | Gibco | 16140071 |
| DNase I | Thermo Fisher Scientific (Roche) | 10104159001 |
| DAPI solution | BD Pharmingen (Franklin Lakes, NJ) | 564907 |
| B-27 supplement (50×), serum free | Thermo Fisher Scientific | 17504-044 |
| N-2 supplement (100×) | Thermo Fisher Scientific | 17502-048 |
| Matrigel growth factor reduced basement membrane matrix | Corning | 356231 |
| Gastrin | Sigma-Aldrich | G9145 |
| RNAlater stabilization solution | Thermo Fisher Scientific | AM7020 |
| Mouse gastrin EIA | RayBiotech | EIAM-GAS-1 |
| U0126 | Cell Signaling | 9903S |
| Omeprazole | Sigma-Aldrich | O104 |
| HEPES | Thermo Fisher Scientific | 15630-080 |
| TrypLE Express Enzyme (1×), phenol red | Thermo Fisher Scientific | 12605-028 |
| TAM | Sigma-Aldrich | T5648-5G |
| GlutaMAX supplement | Thermo Fisher Scientific | 35050-079 |
| Penicillin-streptomycin, 10,000 U/mL | Thermo Fisher Scientific | 15140-122 |
| N-acetylcysteine | Sigma-Aldrich | A9165-5G |
| Y-27632 | Sigma-Aldrich | Y0503-1MG |
| Recombinant murine noggin | Peprotech | 250-38 |
| Recombinant human R-spondin-1 | Peprotech | 120-38 |
| Recombinant murine WNT-3a | Peprotech | 315-20B |
| Recombinant murine epidermal growth factor | Peprotech | 315-09 |
| UltraPure DNase/RNase-free distilled water | Thermo Fisher Scientific | 10977-023 |
| Hydrogen peroxide solution 30% (w/w) in H2O, contains stabilizer | Sigma-Aldrich | H1009-500ML |
| Falcon yellow nylon mesh cell strainer, 70 μ | BD Biosciences | 352350 |
| Falcon blue nylon mesh cell strainer, 40 μ | BD Biosciences | 352340 |
| Alzet pump model 2006, 0.15 μL/h, 6 wk | Alzet (Cupertino, CA) | 7223 |
| Alzet pump model 1002, 0.25 μL/h, 2 wk | Alzet | 4317 |
| Alzet pump model 1007D, 0.5 μL/h, 7 days | Alzet | 290 |
| 48-well uncoated glass dish | MatTek (Ashland, MA) | P48G-1.5-6-F Case |
| 35-mm collagen-coated dish | MatTek | P35GCOL-0-10-C Case |
DPBS, Dulbecco's Phosphate-Buffered Saline.
References
- 1.Hakanson R., Chen D., Sundler F. The ECL cells. In: Johnson L.R., editor. Physiology of the gastrointestinal tract. 3rd ed. Raven Press; New York: 1994. pp. 1171–1184. [Google Scholar]
- 2.Hayakawa Y., Chang W., Jin G., Wang T.C. Gastrin and upper GI cancers. Curr Opin Pharmacol. 2016;31:31–37. doi: 10.1016/j.coph.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Dimaline R., Varro A. Novel roles of gastrin. J Physiol. 2014;592:2951–2958. doi: 10.1113/jphysiol.2014.272435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng R., Aihara E., Kenny S., Yang L., Li J., Varro A., Montrose M.H., Shroyer N.F., Wang T.C., Shivdasani R.A., Zavros Y. Indian Hedgehog mediates gastrin-induced proliferation in stomach of adult mice. Gastroenterology. 2014;147:655–666 e9. doi: 10.1053/j.gastro.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T.C., Dangler C.A., Chen D., Goldenring J.R., Koh T., Raychowdhury R., Coffey R.J., Ito S., Varro A., Dockray G.J., Fox J.G. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 6.Karnes W.E.J., Walsh J.H. The gastrin hypothesis. Implications for antisecretory drug selection. J Clin Gastroenterol. 1900;12(Suppl):S7–S12. [PubMed] [Google Scholar]
- 7.Hakanson R., Sundler F. Proposed mechanism of induction of gastric carcinoids: the gastrin hypothesis. Eur J Clin Invest. 1990;20(Suppl):S65–S71. doi: 10.1111/j.1365-2362.1990.tb01780.x. [DOI] [PubMed] [Google Scholar]
- 8.Koh T.J., Chen D. Gastrin as a growth factor in the gastrointestinal tract. Regul Pept. 2000;93:37–44. doi: 10.1016/s0167-0115(00)00176-2. [DOI] [PubMed] [Google Scholar]
- 9.Larsson H., Carlsson E., Hakanson R., Mattsson H., Nilsson G., Seensalu R., Wallmark B., Sundler F. Time-course of development and reversal of gastric endocrine cell hyperplasia after inhibition of acid secretion. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1988;95:1477–1486. doi: 10.1016/s0016-5085(88)80066-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen D., Zhao C.M., Norlen P., Bjorkqvist M., Ding X.Q., Kitano M., Hakanson R. Effect of cholecystokinin-2 receptor blockade on rat stomach ECL cells. A histochemical, electronic-microscopic and chemical study. Cell Tissue Res. 2000;299:81–95. doi: 10.1007/s004419900136. [DOI] [PubMed] [Google Scholar]
- 11.Ryberg B., Tielemans Y., Axelson J., Carlsson E., Hakanson R., Mattsson H., Sundler F., Willems G. Gastrin stimulates the self-replication rate of enterochromaffin-like cells in the rat stomach. Gastroenterology. 1990;99:935–942. doi: 10.1016/0016-5085(90)90610-d. [DOI] [PubMed] [Google Scholar]
- 12.Tielemans Y., Axelson J., Sundler F., Willems G., Hakanson R. Serum gastrin concentration affects the self-replication rate of enterochromaffin-like cells in the rat stomach. Gut. 1990;31:274–278. doi: 10.1136/gut.31.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh J.H. Role of gastrin as a trophic hormone. Digestion. 1990;47:11–16. doi: 10.1159/000200509. [DOI] [PubMed] [Google Scholar]
- 14.Thompson M., Flemming K.A., Evans D.J., Fundele R., Surani M.A., Wright N.A. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 1990;110:477–481. doi: 10.1242/dev.110.2.477. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka T., Fukuda A., Araki O., Ogawa S., Hanyu Y., Matsumoto Y., Yamaga Y., Nakanishi Y., Kawada K., Sakai Y., Chiba T., Seno H. Bmi1 marks gastric stem cells located in the isthmus in mice. J Pathol. 2019;248:179–190. doi: 10.1002/path.5244. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa Y., Ariyama H., Stancikova J., Sakitani K., Asfaha S., Renz B.W., Dubeykovskaya Z.A., Shibata W., Wang H., Westphalen C.B., Chen X., Takemoto Y., Kim W., Khurana S.S., Tailor Y., Nagar K., Tomita H., Hara A., Sepulveda A.R., Setlik W., Gershon M.D., Saha S., Ding L., Shen Z., Fox J.G., Friedman R.A., Konieczny S.F., Worthley D.L., Korinek V., Wang T.C. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopin A.S., Lee Y.M., McBridge E.W., Miller L.J., Lu M., Lin H.Y., Kolakowski L.F., Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci U S A. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakata H., Matsui T., Ito M., Taniguchi T., Naribayashi Y., Arima N., Nakamura A., Kinoshita Y., Chihara K., Hosoda D., Chiba T. Cloning and characterization of gastrin receptor from ECL carcinoid tumor of Mastomys Natalensis. Biochem Biophys Res Commun. 1992;187:1151–1157. doi: 10.1016/0006-291x(92)91317-j. [DOI] [PubMed] [Google Scholar]
- 19.Chen D., Zhao C.M., Al-Haider W., Hakanson R., Rehfeld J.F., Kopin A.S. Differentiation of gastric ECL cells is altered in CCK2 receptor-deficient mice. Gastroenterology. 2002;123:577–585. doi: 10.1053/gast.2002.34746. [DOI] [PubMed] [Google Scholar]
- 20.Langhans N., Rindi G., Chiu M., Rehfeld J.F., Ardman B., Beinborn M., Kopin A.S. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastric receptor-deficient mice. Gastroenterology. 1997;112:280–286. doi: 10.1016/s0016-5085(97)90000-7. [DOI] [PubMed] [Google Scholar]
- 21.Kazumori H., Ishihara S., Kawashima K., Fukuda R., Chiba T., Kinoshita Y. Analysis of gastric receptor gene expression in proliferating cells in the neck zone of gastric fundic glands using laser capture microdissection. FEBS Lett. 2001;489:208–214. doi: 10.1016/s0014-5793(01)02084-1. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima T., Konda Y., Izumi Y., Kanai M., Hayashi N., Chiba T., Takeuchi T. Gastrin stimulates the growth of gastric pit cell precursors by inducing its own receptors. Am J Physiol Gastrointest Liver Physiol. 2002;282:G359–G366. doi: 10.1152/ajpgi.00117.2001. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa Y., Jin G., Wang H., Chen X., Westphalen C.B., Asfaha S., Renz B.W., Ariyama H., Dubeykovskaya Z.A., Takemoto Y., Lee Y., Muley A., Tailor Y., Chen D., Muthupalani S., Fox J.G., Shulkes A., Worthley D.L., Takaishi S., Wang T.C. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–553. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X.D., Ai W., Asfaha S., Bhagat G., Friedman R.A., Jin G., Park H., Shykind B., Diacovo T.G., Falus A., Wang T.C. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker A.K., Park W.M., Chuang J.C., Perello M., Sakata I., Osborne-Lawrence S., Zigman J.M. Characterization of gastric and neuronal histaminergic populations using a transgenic mouse model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D., Zhao C.M., Dockray G.J., Varro A., Van Hoek A., Sinclair N.F., Wang T.C., Koh T.J. Glycine-extended gastrin synergizes with gastrin 17 to stimulate acid secretion in gastrin-deficient mice. Gastroenterology. 2000;119:756–765. doi: 10.1053/gast.2000.16480. [DOI] [PubMed] [Google Scholar]
- 27.Takaishi S., Cui G., Frederick D.M., Carlson J.E., Houghton J., Varro A., Dockray G.J., Ge Z., Whary M.T., Rogers A.B., Fox J.G., Wang T.C. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology. 2005;128:1965–1983. doi: 10.1053/j.gastro.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Quante M., Marrache F., Goldenring J.R., Wang T.C. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027.e2. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karam S.M., Straiton T., Hassan W.M., Leblond C.P. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322–336. doi: 10.1634/stemcells.21-3-322. [DOI] [PubMed] [Google Scholar]
- 30.Bakke I., Qvigstad G., Sandvik A.K., Waldum H.L. The CCK-2 receptor is located on the ECL cell, but not on the parietal cell. Scand J Gastroenterol. 2001;36:1128–1133. doi: 10.1080/00365520152584734. [DOI] [PubMed] [Google Scholar]
- 31.Hayakawa Y., Fox J.G., Wang T.C. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell Mol Gastroenterol Hepatol. 2017;4:89–94. doi: 10.1016/j.jcmgh.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker N., Tan S., Clevers H. Lgr proteins in epithelial stem cell biology. Development. 2013;140:2484–2494. doi: 10.1242/dev.083113. [DOI] [PubMed] [Google Scholar]
- 33.Cramer T., Juttner S., Plath T., Mergler S., Seufferlein T., Wang T.C., Merchant J., Hocker M. Gastrin transactivates the chromogranin A gene through MEK-1/ERK- and PKC-dependent phosphorylation of Sp1 and CREB. Cell Signal. 2008;20:60–72. doi: 10.1016/j.cellsig.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Hocker M., Henihan R.J., Rosewicz S., Riecken E.O., Zhang Z., Koh T.J., Wang T.C. Gastrin and phorbol 12-myristate 13-acetate regulate the human histidine decarboxylase promoter through Raf-dependent activation of extracellular signal-regulated kinase-related signaling pathways in gastric cancer cells. J Biol Chem. 1997;272:27015–27024. doi: 10.1074/jbc.272.43.27015. [DOI] [PubMed] [Google Scholar]
- 35.Cowley S., Paterson H., Kemp P., Marshall C.J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 36.Crews C.M., Alessandrini A., Erikson R.L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 37.Marampon F., Bossi G., Ciccarelli C., Di Rocco A., Sacchi A., Pestell R.G., Zani B.M. MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol Cancer Ther. 2009;8:543–551. doi: 10.1158/1535-7163.MCT-08-0570. [DOI] [PubMed] [Google Scholar]
- 38.Marampon F., Gravina G.L., Di Rocco A., Bonfili P., Di Staso M., Fardella C., Polidoro L., Ciccarelli C., Festuccia C., Popov V.M., Pestell R.G., Tombolini V., Zani B.M. MEK/ERK inhibitor U0126 increases the radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by downregulating growth and DNA repair signals. Mol Cancer Ther. 2011;10:159–168. doi: 10.1158/1535-7163.MCT-10-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H.J., Johnston B., Aiello D., Caffrey D.R., Giel-Moloney M., Rindi G., Leiter A.B. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology. 2014;146:754–764 e3. doi: 10.1053/j.gastro.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H.J., Kapoor A., Giel-Moloney M., Rindi G., Leiter A.B. Notch signaling differentially regulates the cell fate of early endocrine precursor cells and their maturing descendants in the mouse pancreas and intestine. Dev Biol. 2012;371:156–169. doi: 10.1016/j.ydbio.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomura S., Esumi H., Job C., Tan S.S. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124–135. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- 42.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec. 1993;236:333–340. doi: 10.1002/ar.1092360206. [DOI] [PubMed] [Google Scholar]
- 43.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 44.Karam S.M. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki J., Suzuki H., Minegishi Y., Sugai E., Tsugawa H., Yasui M., Hibi T. Acid suppression by proton pump inhibitors enhances aquaporin-4 and KCNQ1 expression in gastric fundic parietal cells in mouse. Dig Dis Sci. 2010;55:3339–3348. doi: 10.1007/s10620-010-1167-8. [DOI] [PubMed] [Google Scholar]
- 46.Daulhac L., Kowalski-Chauvel A., Pradayrol L., Vaysse N., Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- 47.Noble P.J., Wilde G., White M.R., Pennington S.R., Dockray G.J., Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol. 2003;284:G75–G84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- 48.Sinclair N.F., Ai W., Raychowdhury R., Bi M., Wang T.C., Koh T.J., McLaughlin J.T. Gastrin regulates the heparin-binding epidermal-like growth factor promoter via a PKC/EGFR-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2004;286:G992–G999. doi: 10.1152/ajpgi.00206.2002. [DOI] [PubMed] [Google Scholar]
- 49.Smith J.P., Nadella S., Osborne N. Gastrin and gastric cancer. Cell Mol Gastroenterol Hepatol. 2017;4:75–83. doi: 10.1016/j.jcmgh.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyazaki Y., Shinomura Y., Tsutsui S., Zushi S., Higashimoto Y., Kanayama S., Higashiyama S., Taniguchi N., Matsuzawa Y. Gastrin induces heparin-binding epidermal growth factor-like growth factor in rat gastric epithelial cells transfected with gastrin receptor. Gastroenterology. 1999;116:78–89. doi: 10.1016/s0016-5085(99)70231-3. [DOI] [PubMed] [Google Scholar]
- 51.Sierra J.C., Asim M., Verriere T.G., Piazuelo M.B., Suarez G., Romero-Gallo J., Delgado A.G., Wroblewski L.E., Barry D.P., Peek R.M., Jr., Gobert A.P., Wilson K.T. Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut. 2018;67:1247–1260. doi: 10.1136/gutjnl-2016-312888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basak O., Beumer J., Wiebrands K., Seno H., van Oudenaarden A., Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell. 2017;20:177–190 e4. doi: 10.1016/j.stem.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Okumura T., Ericksen R.E., Takaishi S., Wang S.S., Dubeykovskiy Z., Shibata W., Betz K.S., Muthupalani S., Rogers A.B., Fox J.G., Rustgi A.K., Wang T.C. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010;70:8435–8445. doi: 10.1158/0008-5472.CAN-10-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinoshita H., Hayakawa Y., Konishi M., Hata M., Tsuboi M., Hayata Y., Hikiba Y., Ihara S., Nakagawa H., Ikenoue T., Ushiku T., Fukayama M., Hirata Y., Koike K. Three types of metaplasia model through Kras activation, Pten deletion, or Cdh1 deletion in the gastric epithelium. J Pathol. 2019;247:35–47. doi: 10.1002/path.5163. [DOI] [PubMed] [Google Scholar]
- 55.Takaishi S., Tu S., Dubeykovskaya Z.A., Whary M.T., Muthupalani S., Rickman B.H., Rogers A.B., Lertkowit N., Varro A., Fox J.G., Wang T.C. Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol. 2009;175:365–375. doi: 10.2353/ajpath.2009.081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomita H., Takaishi S., Menheniott T.R., Yang X., Shibata W., Jin G., Betz K.S., Kawakami K., Minamoto T., Tomasetto C., Rio M.C., Lerkowit N., Varro A., Giraud A.S., Wang T.C. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140:879–891. doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindstrom E., Chen D., Norlen P., Andersson K., Hakanson R. Control of gastric acid secretion:the gastrin-ECL cell-parietal cell axis. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:505–514. doi: 10.1016/s1095-6433(00)00331-7. [DOI] [PubMed] [Google Scholar]
- 58.Chen D., Monstein H.J., Nylander A.G., Zhao C.M., Sundler F., Hakanson R. Acute responses of rat stomach enterochromaffin like cells to gastrin: secretory activation and adaptation. Gastroenterology. 1994;107:18–27. doi: 10.1016/0016-5085(94)90056-6. [DOI] [PubMed] [Google Scholar]