Abstract
OBJECTIVE
We sought to determine the prevalence of diabetes and associated cardiovascular outcomes in a contemporary cohort of young individuals presenting with their first myocardial infarction (MI) at age ≤50 years.
RESEARCH DESIGN AND METHODS
We retrospectively analyzed records of patients presenting with a first type 1 MI at age ≤50 years from 2000 to 2016. Diabetes was defined as a hemoglobin A1c ≥6.5% (48 mmol/mol) or a documented diagnosis of or treatment for diabetes. Vital status was ascertained for all patients, and cause of death was adjudicated.
RESULTS
Among 2,097 young patients who had a type 1 MI (mean age 44.0 ± 5.1 years, 19.3% female, 73% white), diabetes was present in 416 (20%), of whom 172 (41%) were receiving insulin. Over a median follow-up of 11.2 years (interquartile range 7.3–14.2 years), diabetes was associated with a higher all-cause mortality (hazard ratio 2.30; P < 0.001) and cardiovascular mortality (2.68; P < 0.001). These associations persisted after adjusting for baseline covariates (all-cause mortality: 1.65; P = 0.008; cardiovascular mortality: 2.10; P = 0.004).
CONCLUSIONS
Diabetes was present in 20% of patients who presented with their first MI at age ≤50 years and was associated with worse long-term all-cause and cardiovascular mortality. These findings highlight the need for implementing more aggressive therapies aimed at preventing future adverse cardiovascular events in this population.
Introduction
In 2015, an estimated 30.3 million people in the U.S. (9.4% of the U.S. population) had diabetes (1). Importantly, 18.9 million were <65 years of age. While the prevalence of diabetes has been increasing in all age-groups (2), the relative rate of increase has been greatest in adolescents and young adults (3,4). A study using a Markov-like computer model to simulate the life course of a hypothetical cohort of adolescents/young adults in the U.S. found that those with type 2 diabetes lose ∼15 years from average remaining life expectancy (5).
Cardiovascular disease, including coronary artery disease (CAD), is a well-known complication of hyperglycemia (6). Prior research has suggested that the hazard of developing a myocardial infarction (MI) may be more than three times higher in patients with early-onset type 2 diabetes (diagnosed at age 18–44 years) compared with usual-onset type 2 diabetes (diagnosed at age ≥45 years) (7). Yet, little is known about how often diabetes is present in individuals who have an MI at a young age and whether the presence of diabetes is associated with worse outcomes in young patients post-MI. A study from 1996 found that 12% of the patients aged ≤40 years presenting with acute MI had diabetes (8). Given the paucity of data on the frequency and prognostic implications of diabetes among those who experience an MI at a young age in the current era, we sought to determine the prevalence of diabetes and associated cardiovascular outcomes in a more contemporary cohort of young individuals presenting with their first MI at age ≤50 years.
Research Design and Methods
Study Group
The design of the YOUNG-MI registry has been previously described (9). This is a retrospective cohort study from two large academic medical centers (Brigham and Women’s Hospital and Massachusetts General Hospital) that included patients who experienced an MI at age ≤50 years between 2000 and 2016. The presence and type of MI were separately adjudicated by two physicians. In cases of discrepancy that could not be resolved, the final determination was performed by an adjudication committee. The Third Universal Definition of MI was used to define a type 1 MI as a spontaneous MI related to atherosclerotic plaque rupture, ulceration, assuring, erosion, or dissection (10). For the present analysis, only patients with type 1 MI were included. Individuals with known CAD (defined as a prior MI or revascularization) were excluded from this analysis. The YOUNG-MI registry has been approved by the institutional review board at Partners HealthCare (Boston, MA).
Diabetes
The presence of diabetes on index admission was determined through a detailed review of medical records and was defined as having a hemoglobin A1c ≥6.5% (48 mmol/mol) or a documented diagnosis of or treatment for diabetes. Electronic medical records were reviewed for clinic notes before admission, admission history and physical examination, and discharge summaries to determine the presence and type of diabetes as well as the presence or absence of insulin therapy. In addition, we evaluated all available hemoglobin A1c data. The hemoglobin A1c within 90 days of the admission for MI was used as the index hemoglobin A1c.
Risk Factors
A detailed review of the electronic medical record was conducted to determine the presence of cardiovascular risk factors during or before the index admission. For each risk factor, we also determined whether it was known before admission or diagnosed during that hospitalization. The definitions of all risk factors have been previously described in detail (9). Briefly, hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or diagnosis/treatment of hypertension. Dyslipidemia was defined as total cholesterol ≥240 mg/dL, serum triglycerides ≥150 mg/dL, HDL cholesterol <40 mg/dL in men or <50 mg/dL in women, or diagnosis/treatment of dyslipidemia. Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) between 60 and 15 mL/min/m2 before admission or a diagnosis of chronic kidney disease. Peripheral vascular disease (PVD) was defined as prior peripheral arterial revascularization or a diagnosis of peripheral artery disease or limb claudication. Smoking was defined as current (tobacco products used within the past month), former, or never. Family history of premature CAD was defined as MI or coronary revascularization occurring before age 55 years for male family members and before age 65 years for female family members.
To adjust for comorbidities, we calculated the Charlson comorbidity index, a method of predicting the risk of mortality on the basis of comorbidities, for each patient as determined by ICD-9 diagnosis and billing codes associated with the index hospitalization (11). For patients within the sample who were not coded for MI, we added an additional ICD-9 code of 410 (acute MI) before calculating the Charlson comorbidity index. Additionally, because lower socioeconomic status is known to be associated with all-cause death, household income for each patient was estimated on the basis of household zip codes in conjunction with the 2015 inflation-adjusted median household income data provided by the U.S. Census Bureau (12).
Outcomes
The primary outcomes of interest were both all-cause mortality and cardiovascular mortality, including in-hospital deaths. Vital status was assessed through linkage with the Partners HealthCare electronic medical record system, the Social Security Administration Death Master File, and the National Death Index and was censored on the date of the latest query (28 September 2017). The cause of death was adjudicated independently by two cardiologists who were blind to both the existence and the type of diabetes. In cases of disagreement, consensus for the cause of death was reached by an adjudication committee. The cause of death was categorized into cardiovascular death (9,13), noncardiovascular death, or undetermined. If the cause of death was undetermined, patients were conservatively analyzed as having a noncardiovascular death.
In an exploratory secondary analysis, we also evaluated the incidence of heart failure admission 1 year post-MI. Ascertainment of this end point was by review of the longitudinal medical record. For an event to be classified as an admission for heart failure, discharge with a hospitalization diagnosis of heart failure was required. In addition, only events meeting the defined clinical criteria for the presence of symptoms, signs, and escalation of therapy for heart failure were classified as such (14,15).
Statistical Analysis
All analyses were performed using Stata 15.1 statistical software (StataCorp, College Station, TX). Categorical variables were reported as frequencies and proportions and compared using χ2 or Fisher’s exact tests, as appropriate. Continuous variables were reported as means or medians and compared using t tests or Mann-Whitney U tests, as appropriate. Kaplan-Meier survival estimates were calculated and compared using the log-rank test. Curves were constructed to illustrate time to death (all-cause or cardiovascular).
To study the baseline effect of diabetes on the primary outcomes of interest, individuals were stratified according to the presence of diabetes upon index admission. Univariable Cox proportional hazards modeling was then performed for survival free from both all-cause and cardiovascular death. Following this, multivariable adjustment was performed using variables that had significant univariable association or are known to be associated with either all-cause death or cardiovascular death. A sensitivity analysis of long-term outcomes excluding patients with newly diagnosed diabetes was also performed.
To examine the effect of insulin usage among patients with diabetes, Cox proportional hazards models were constructed for survival free from all-cause death. Multivariable risk adjustment was performed using those variables that had significant univariable association with the outcome in question. Additionally, to study the effect of diabetes requiring insulin on long-term outcomes, univariable and multivariable Cox proportional hazards modeling was then performed for survival free from both all-cause and cardiovascular death among patients without diabetes, patients with diabetes not requiring insulin, and patients with diabetes requiring insulin.
Finally, univariable Cox proportional hazards modeling was performed for survival free from heart failure admission 1 year post-MI on the basis of the presence of diabetes upon index admission as well as the absence of diabetes, presence of diabetes not requiring insulin, and presence of diabetes requiring insulin. Multivariable risk adjustment was performed using those variables that had significant univariable association with heart failure 1 year post-MI (sex, PVD, Charlson comorbidity index, creatinine, eGFR, and length of stay).
Results
Prevalence
Of 2,097 patients who experienced a type 1 MI, diabetes was present in 416 (20%) (Table 1). Of the 416 patients with diabetes, 244 (59%) were not receiving insulin, while the remaining 172 (41%) were receiving insulin (Table 2). Of the 172 patients receiving insulin, 39 (23%) had type 1 diabetes and 129 (75%) had type 2 diabetes. The type of diabetes could not be determined for four (2%) patients. Among patients with diabetes, the diagnosis of diabetes was present at the time of admission for 335 (81%) and newly diagnosed upon admission in 81 (19%).
Table 1.
Baseline characteristics: patients without diabetes and patients with diabetes
| Characteristic | Patients without diabetes (n = 1,681, 80%) | Patients with diabetes (n = 416, 20%) | P value |
|---|---|---|---|
| Demographics and MI type | |||
| Age at MI | 45.0 (41.0–48.0) | 45.0 (42.0–48.0) | 0.81 |
| Female | 308 (18.3) | 96 (23.1) | 0.028 |
| White | 1,265 (75.3) | 272 (65.4) | <0.001 |
| Income (thousands of dollars) | 72.8 (54.9–88.0) | 62.4 (50.9–80.3) | <0.001 |
| Insurance | 1,344 (89.7) | 328 (90.1) | 0.80 |
| ST-elevation MI | 909 (54.1) | 212 (51.0) | 0.26 |
| Risk factors | |||
| Hypertension | 726 (43.6) | 276 (67.3) | <0.001 |
| Hyperlipidemia | 1,523 (90.6) | 387 (93.0) | 0.12 |
| Obesity | 523 (31.1) | 227 (54.6) | <0.001 |
| BMI (kg/m2) | 29.2 (25.9–32.6) | 32.7 (28.4–36.6) | <0.001 |
| Current smoker | 863 (51.7) | 203 (49.9) | 0.51 |
| Alcohol use | 218 (13.1) | 51 (12.6) | 0.78 |
| Substance use | 197 (11.8) | 33 (8.1) | 0.03 |
| PVD | 26 (1.6) | 15 (3.7) | 0.006 |
| Premature CAD in first-degree relative | 480 (28.6) | 102 (24.5) | 0.098 |
| Angina | 1,483 (90.2) | 354 (87.0) | 0.056 |
| ASCVD score | 4.5 (2.6–7.0) | 9.7 (5.4–16.3) | <0.001 |
| Charlson comorbidity index | 1 (1–1) | 2 (2–3) | <0.001 |
| Laboratory values | |||
| Hemoglobin A1c (%) | 5.6 (5.4–5.8) | 9.1 (7.3–10.9) | <0.001 |
| Hemoglobin A1c (mmol/mol) | 38 (36–40) | 76 (56–96) | |
| Normalized troponin | 43.4 (11.3–148.8) | 33.8 (7.9–154.0) | 0.080 |
| Creatinine (mg/dL) | 1.0 (0.9–1.1) | 1.0 (0.8–1.2) | 0.17 |
| eGFR (mL/min/1.73 m2) | 80.6 (19.3) | 78.4 (26.8) | 0.055 |
| Total cholesterol (mg/dL) | 190.3 (46.8) | 200.3 (85.5) | 0.003 |
| LDL cholesterol (mg/dL) | 119.6 (40.7) | 117.4 (66.4) | 0.42 |
| HDL cholesterol (mg/dL) | 37.4 (10.4) | 34.6 (9.4) | <0.001 |
| Triglycerides (mg/dL) | 143.0 (100.0–206.0) | 182.0 (123.0–318.0) | <0.001 |
| In-hospital cardiac procedures and length of stay | |||
| Coronary angiography performed | 1,588 (94.5) | 383 (92.1) | 0.065 |
| Revascularization performed | 1,404 (83.5) | 331 (79.6) | 0.056 |
| PCI | 1,291 (76.8) | 277 (66.6) | <0.001 |
| CABG | 118 (7.0) | 59 (14.2) | <0.001 |
| Length of stay (days) | 3.0 (2.0–5.0) | 4.0 (3.0–8.0) | <0.001 |
Data are n (%), median (IQR), or mean (SD). ASCVD, atherosclerotic cardiovascular disease; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Table 2.
Baseline characteristics: patients with diabetes not receiving insulin and patients with diabetes receiving insulin
| Characteristic | Patients with diabetes not receiving insulin (n = 244, 59%) | Patients with diabetes receiving insulin (n = 172, 41%) | P value |
|---|---|---|---|
| Demographics, diabetes type, and MI type | |||
| Age at MI | 46.0 (42.0–48.0) | 45 (41.5–48.0) | 0.29 |
| Female | 41 (16.8) | 55 (32.0) | <0.001 |
| White | 160 (65.6) | 112 (65.1) | 0.92 |
| Income (thousands of dollars) | 64.2 (50.9–78.2) | 61.4 (50.7–80.3) | 0.29 |
| Insurance | 191 (88.4) | 137 (92.6) | 0.19 |
| Type 1 diabetes | 0 (0) | 39 (23.2) | <0.001 |
| Type 2 diabetes | 244 (100) | 129 (76.8) | <0.001 |
| ST-elevation MI | 137 (56.1) | 75 (43.6) | 0.012 |
| Risk factors | |||
| Hypertension | 165 (69.3) | 111 (64.5) | 0.31 |
| Hyperlipidemia | 231 (94.7) | 156 (90.7) | 0.12 |
| Obesity | 135 (55.3) | 92 (53.5) | 0.71 |
| BMI (kg/m2) | 32.7 (28.6–36.6) | 32.9 (27.5–36.6) | 0.62 |
| Current smoker | 133 (56.1) | 70 (41.2) | 0.003 |
| Alcohol use | 34 (14.4) | 17 (10.1) | 0.19 |
| Substance use | 20 (8.4) | 13 (7.6) | 0.77 |
| PVD | 5 (2.1) | 10 (5.8) | 0.049 |
| Premature CAD in first-degree relative | 64 (26.2) | 38 (22.1) | 0.33 |
| Angina | 212 (89.1) | 142 (84.0) | 0.14 |
| ASCVD score | 10.6 (6.9–17.3) | 8.2 (4.7–14.3) | <0.001 |
| Charlson comorbidity index | 2 (2–3)** | 2 (2–3) | <0.001 |
| Laboratory values | |||
| Hemoglobin A1c (%) | 8.6 (7.1–10.6) | 9.7 (8.0–11.6) | 0.003 |
| Hemoglobin A1c (mmol/mol) | 70 (54–92) | 83 (64–103) | |
| Normalized troponin | 36.3 (9.0–164.3) | 29.8 (7.0–148.3) | 0.32 |
| Creatinine (mg/dL) | 1.1 (0.4) | 1.2 (0.7) | 0.001 |
| eGFR (mL/min/1.73 m2) | 82.6 (25.1) | 72.4 (28.1) | <0.001 |
| Total cholesterol (mg/dL) | 198.5 (73.1) | 202.7 (100.8) | 0.65 |
| LDL cholesterol (mg/dL) | 114.7 (43.5) | 121.2 (89.7) | 0.36 |
| HDL cholesterol (mg/dL) | 33.8 (8.2) | 35.8 (10.8) | 0.045 |
| Triglycerides (mg/dL) | 193.0 (128.0–346.0) | 171.0 (121.0–301.5) | 0.53 |
| In-hospital cardiac procedures and length of stay | |||
| Coronary angiography performed | 225 (92.2) | 158 (91.9) | 0.90 |
| Revascularization performed | 199 (81.6) | 132 (76.7) | 0.23 |
| PCI | 169 (69.3) | 108 (62.8) | 0.17 |
| CABG | 32 (13.1) | 27 (15.7) | 0.46 |
| Length of stay (days) | 4.0 (2.0 6.0) | 5.0 (3.0–9.5) | 0.006 |
Data are n (%), median (IQR), and mean (SD). ASCVD, atherosclerotic cardiovascular disease; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Lower of the two.
Baseline Characteristics
The baseline characteristics of patients with diabetes compared with those without diabetes are shown in Table 1. Compared with patients without diabetes, those with diabetes had significantly higher rates of hypertension (67.3% vs. 43.6%; P < 0.001), obesity (54.6% vs. 31.1%; P < 0.001) with higher BMI (32.7 vs. 29.2 kg/m2; P < 0.001), and more prevalent PVD (3.7% vs. 1.6%; P = 0.006). Patients with diabetes had a longer median length of stay (4.0 [interquartile range (IQR) 3.0–8.0] vs. 3.0 [2.0–5.0] days; P < 0.001) and had a lower median income ($62,400 vs. $72,800; P < 0.001).
The baseline characteristics of patients with diabetes receiving insulin therapy compared with patients with diabetes not receiving insulin therapy are shown in Table 2. Compared with patients with diabetes not receiving insulin, those with diabetes receiving insulin were more likely to be female (32.0% vs. 16.8%; P < 0.001), less likely to present with ST-elevation MI (43.6% vs. 56.1%; P = 0.012), had a longer median length of stay (5.0 [IQR 3.0–9.5] vs. 4.0 [2.0–6.0] days; P = 0.006), were less likely to be current smokers (41.2% vs. 56.1%; P = 0.003), and were more likely to have PVD (5.8% vs. 2.1%; P = 0.049).
There was no statistically significant difference in coronary angiography rates between patients with diabetes (92.1%) and those without diabetes (94.5%; P = 0.065) (Table 1). Compared with patients without diabetes, patients with diabetes were significantly less likely to undergo percutaneous coronary intervention (66.6% vs. 76.8%; P < 0.001) and significantly more likely to undergo coronary artery bypass grafting (14.2% vs. 7.0%; P < 0.001).
Long-term Outcomes
Over a median follow-up of 11.2 years (IQR 7.3–14.2 years), diabetes was associated with a higher all-cause mortality (hazard ratio [HR] 2.30 [95% CI 1.77–2.98]; P < 0.001) and cardiovascular mortality (2.68 [1.85–3.89]; P < 0.001). These associations persisted after adjusting for baseline covariates (all-cause mortality: 1.65 [1.14–2.38]; P = 0.008; cardiovascular mortality: 2.10 [1.26–3.49]; P = 0.004) (Supplementary Tables 1 and 2). The adjusted associations also persisted in the sensitivity analysis that excluded patients with newly diagnosed diabetes: Preexisting diabetes was associated with higher adjusted all-cause (1.93 [1.38–2.70]; P < 0.001) and cardiovascular mortality (2.39 [1.40–4.07]; P = 0.001) compared with no diabetes.
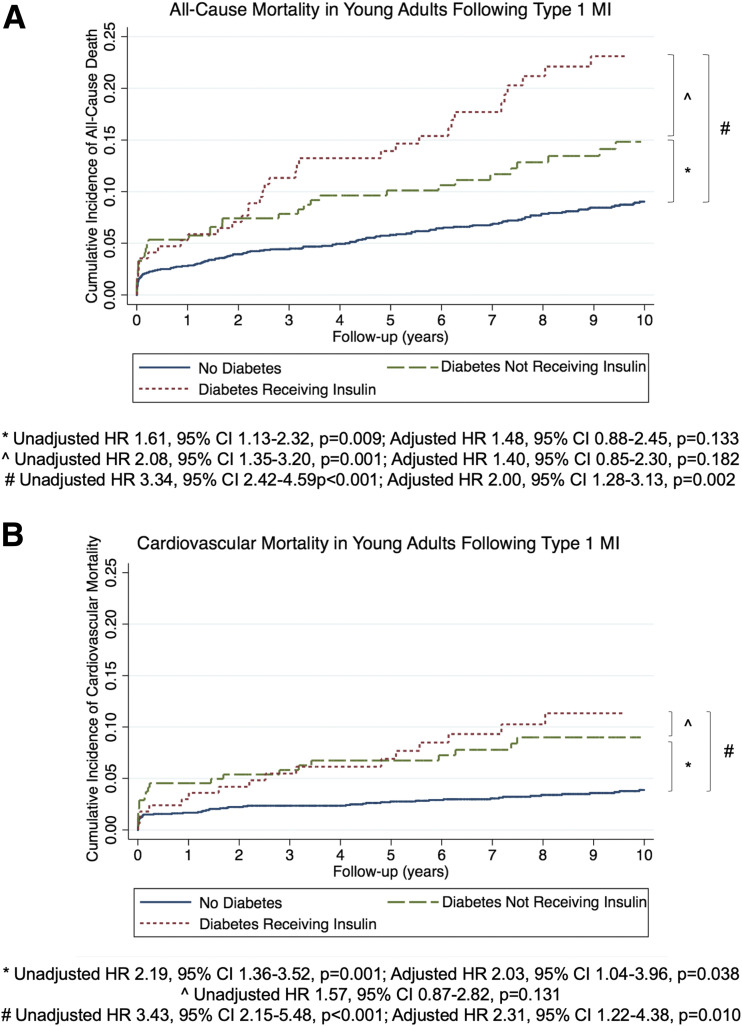
On an unadjusted basis, both diabetes not requiring insulin (HR 1.61 [95% CI 1.13–2.32]; P = 0.009) and diabetes requiring insulin (3.34 [2.42–4.59]; P < 0.001) were associated with significantly higher all-cause mortality compared with no diabetes (Fig. 1A). After adjustment, the association for diabetes requiring insulin (2.00 [1.28–3.13]; P = 0.002) remained significant, but the association for diabetes not requiring insulin (1.48 [0.88–2.45]; P = 0.133) did not.
Figure 1.
Cumulative hazard of all-cause and cardiovascular mortality according to diagnosis of diabetes. A: Kaplan-Meier curves of all-cause mortality in patients without diabetes, with diabetes not receiving insulin, and with diabetes receiving insulin. B: Kaplan-Meier curves of cardiovascular mortality in patients without diabetes, with diabetes not receiving insulin, and with diabetes receiving insulin.
When examining the association of cardiovascular mortality with insulin treatment, the unadjusted HRs for both diabetes not requiring insulin (2.19 [95% CI 1.36–3.52]; P = 0.001) and diabetes requiring insulin (3.43 [2.15–5.48]; P < 0.001) were significantly higher. The association for diabetes requiring insulin (2.31 [1.22–4.38]; P = 0.010) and the association for diabetes not requiring insulin (2.03 [1.04–3.96]; P = 0.038) remained significant after adjustment (Fig. 1B).
Among patients with diabetes, insulin use was associated with higher all-cause mortality (HR 2.08 [95% CI 1.35–3.20]; P = 0.001). After adjusting for baseline covariates, this association attenuated and was no longer significant (1.40 [0.85–2.30]; P = 0.182) (Supplementary Table 3). The unadjusted HR for cardiovascular mortality stratified by insulin use among patients with diabetes was not significant (1.57 [0.87–2.82]; P = 0.131). Among patients with diabetes, the unadjusted HRs for both all-cause and cardiovascular mortality stratified by type 1 versus type 2 diabetes were not significant (all-cause mortality: 1.55 [0.86–2.81]; P = 0.145; cardiovascular mortality: 1.12 [0.44–2.84]; P = 0.815).
Heart Failure Admission
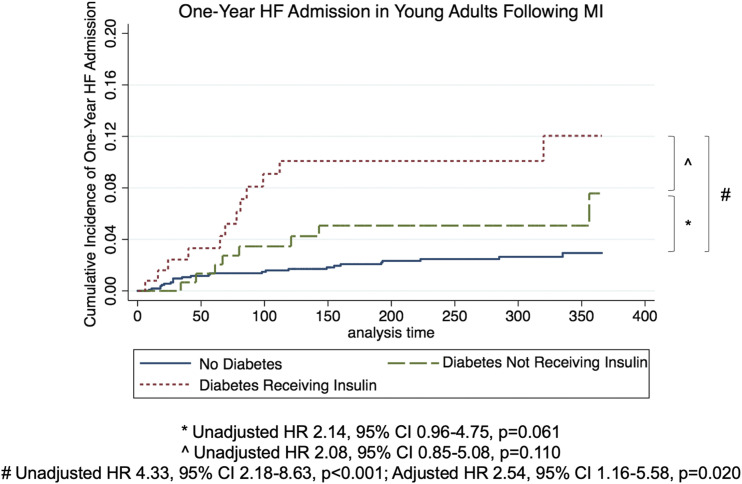
Diabetes was associated with a statistically significant higher rate of admission for heart failure 1 year post-MI on an unadjusted basis (HR 3.07 [95% CI 1.71–5.54]; P < 0.001). After adjustment, the association remained significant (2.12 [1.13–3.99]; P = 0.020). Twenty (4.8%) of the 416 patients with diabetes had an admission for heart failure during the first year after their MI compared with 25 (1.5%) of the 1,681 patients without diabetes (P < 0.001) (Supplementary Table 4). Twelve of the 172 patients (7.0%) with diabetes who used insulin had an admission for heart failure during the first year after their MI compared with 8 (3.3%) of the 244 patients with diabetes not on insulin (P = 0.100). Kaplan-Meier curves of heart failure admission 1 year post-MI in patients without diabetes, diabetes not requiring insulin, and diabetes requiring insulin are shown in Fig. 2.
Figure 2.
Cumulative hazard of heart failure (HF) admission according to diagnosis of diabetes. Kaplan-Meier curves of heart failure admission 1 year post-MI in patients without diabetes, with diabetes not requiring insulin, and with diabetes requiring insulin.
Conclusions
Our study is the largest in our knowledge to examine the prevalence and outcomes of diabetes among patients who experienced an MI at a young age. We found that diabetes was present in 20% of our cohort, with the majority of them having type 2 diabetes. Diabetes was a new diagnosis for 19% of our cohort with diabetes, a finding that reinforces the importance of screening for diabetes among individuals who present with an MI at a young age. Over a median follow-up of 11.2 years, we found that diabetes was associated with a 65% higher all-cause mortality and a doubling in cardiovascular mortality after adjusting for baseline covariates. Supporting recent data about the strong association of diabetes with heart failure, we also found a greater than threefold higher risk of admission for heart failure 1 year post-MI in our patients with diabetes and early-onset MI.
Although prior research has reported outcomes of young patients with diabetes who are diagnosed with CAD (16), our study is the first to examine the effect of diabetes on long-term outcomes of young individuals who have experienced an MI as well as the risk of heart failure admission in this group of patients. Previous studies of cohorts of older patients have reported 30-day and 1-year outcomes of patients with diabetes following acute coronary syndrome (17) or long-term outcomes of patients with diabetes and established or at high risk for atherothrombosis (18). In these studies, patients with diabetes were also at increased risk of both cardiovascular mortality (adjusted HR 1.38) (18) and all-cause mortality (adjusted HR 1.33–1.40) (17,18). However, we found that the adjusted hazard for both cardiovascular mortality (HR 2.10) and all-cause mortality (1.65) as a result of diabetes was higher in younger patients. These findings suggest that diabetes may be an even stronger cardiovascular risk factor in young patients and highlight the importance of even more aggressive risk factor modification in this group.
Several glucose-lowering agents from two drug classes (sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists) improve cardiovascular outcomes in patients with diabetes and at high cardiovascular risk (19–24). On the basis of the existing body of data, drugs from these classes have indications to reduce cardiovascular events, including death (25). There has been an important emphasis for cardiologists to advocate for their patients with diabetes by initiating glucose-lowering therapies that will reduce cardiovascular risk (26,27). A recent American College of Cardiology Expert Consensus Decision Pathway, endorsed by the American Diabetes Association, provides a guide to cardiologists for the initiation and monitoring of these medications with the goal of reducing cardiovascular risk (25). Of note, the mean age in recent trials of these agents was at least 63 years. Given the prevalence of diabetes in younger patients and its association with higher cardiovascular morbidity, it will be important for future trials to include younger patients.
Our study is limited by its retrospective design; however, this allowed us to examine a large number of individuals who experienced an MI at a young age. We estimate that enrolling a similar number of patients in a prospective study would take >10 years to conduct. Because our cohort was limited to individuals who experienced an MI, we were not able to determine the prevalence of diabetes in the at-risk population and, therefore, were unable to provide data on the relative risk of diabetes for causing MI. We do not have information on complications from diabetes, such as neuropathy, nephropathy, and retinopathy. We also do not have data regarding the duration of diabetes for each patient or the use of all diabetes medications. Of note, empagliflozin (December 2016) and liraglutide (August 2017) did not receive their cardiovascular disease indications during this study’s time period. Heart failure admission data were only obtained up to 1 year post-MI, and rates were low. However, we anticipate that over a longer follow-up, the difference that we detected would be even greater.
In conclusion, diabetes was present in 20% of patients with an MI at age ≤50 years and was associated with worse long-term all-cause and cardiovascular mortality over a median follow-up of 11.2 years. Among patients with diabetes, the use of insulin was associated with an even higher all-cause mortality. Diabetes was also associated with an increased risk of heart failure admission 1 year post-MI. These findings highlight the need for implementing more-aggressive therapies aimed at preventing future adverse cardiovascular events in young patients post-MI.
Supplementary Material
Article Information
Funding. S.D. and A.Q. are supported by T32 postdoctoral training grants from the National Heart, Lung, and Blood Institute (T32-HL-094301 andT32-HL-007604, respectively). J.L.J. is supported in part by the Hutter Family Professorship.
Duality of Interest C.P.C. reports research grants from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, and Merck and consulting fees from Alnylam, Amarin, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Janssen, Kowa, Merck, Pfizer, Regeneron, and Sanofi. J.P. reports serving as a consultant for Aegerion, Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi, and Vivus. J.L.J. has received grant support from Roche Diagnostics, Abbott Diagnostics, Singulex, Prevencio, and Cleveland Heart Laboratories; has received consulting income from Roche Diagnostics, MyoKardia, Abbott, and Critical Diagnostics; and has participated in clinical end point committees/data safety monitoring boards for Boehringer Ingelheim, Amgen, AbbVie, Janssen, Abbott, and Siemens Diagnostics. M.F.D.C. has received consulting fees from Sanofi and General Electric. D.L.B. discloses the following relationships: advisory boards for Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; board of directors for Boston VA Research Institute, Society of Cardiovascular Patient Care, and TOBESOFT; chair of the American Heart Association Quality Oversight Committee; data monitoring committees for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the Portopulmonary Hypertension Treatment with Macitentan [PORTICO trial], funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis Requiring Aortic Valve Replacement [ExCEED trial], funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the Edoxaban Compared to Standard Care After Heart Valve Replacement [ENVISAGE] trial, funded by Daiichi Sankyo), and Population Health Research Institute; honoraria from the American College of Cardiology (ACC) (senior associate editor, Clinical Trials and News and ACC.org, and vice-chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention [RE-DUAL PCI] clinical trial steering committee, funded by Boehringer Ingelheim), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor, associate editor), Population Health Research Institute (for the Cardiovascular Outcomes for People Using Anticoagulation Strategies [COMPASS] Operations Committee, Publications Committee, and Steering Committee, and U.S. national co-leader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (continuing medical education steering committees); other relationships with Clinical Cardiology (deputy editor), NCDR-ACTION Registry Steering Committee (chair), and Veterans Administration Clinical Assessment, Reporting and Tracking System for Cath Labs [VA CART] Research and Publications Committee (chair); research funding from Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, and The Medicines Company; royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator for Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; trustee for the ACC; and unfunded research for FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.D., A.S., D.B., B.L.C., D.L.B., and R.B. researched the data and wrote the manuscript. J.Y., E.M.D., M.R., A.Q., J.H., and J.K. researched the data and reviewed/edited the manuscript. C.P.C., D.M.P., J.P., K.N., J.L.J., and M.F.D.C. reviewed/edited the manuscript. S.D. and R.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were published in abstract form at the 68th Annual Scientific Session and Expo of the American College of Cardiology, New Orleans, LA, 16–18 March 2019.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0998/-/DC1.
See accompanying article, p. 1679.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017. Atlanta, GA, U.S. Dept of Health and Human Services, 2017 [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 3.Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol 2018;6:69–80 [DOI] [PubMed] [Google Scholar]
- 4.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med 2012;29:453–463 [DOI] [PubMed] [Google Scholar]
- 6.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care 2003;26:2999–3005 [DOI] [PubMed] [Google Scholar]
- 8.Fournier JA, Sánchez A, Quero J, Fernández-Cortacero JA, González-Barrero A. Myocardial infarction in men aged 40 years or less: a prospective clinical-angiographic study. Clin Cardiol 1996;19:631–636 [DOI] [PubMed] [Google Scholar]
- 9.Singh A, Collins B, Qamar A, et al. Study of young patients with myocardial infarction: design and rationale of the YOUNG-MI Registry. Clin Cardiol 2017;40:955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS, et al.; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Biomarker Subcommittee; ECG Subcommittee; Imaging Subcommittee; Classification Subcommittee; Intervention Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; ESC Committee for Practice Guidelines (CPG); Document Reviewers . Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–159822958960 [Google Scholar]
- 11.Stagg V. CHARLSON: Stata module to calculate Charlson index of comorbidity. EconPapers 2017. Available at https://econpapers.repec.org/software/bocbocode/s456719.htm. Accessed 1 March 2019
- 12.U.S. Census Bureau Median Household Income in the Past 12 Months (in 2015 inflation-adjusted dollars). Suitland-Silver Hill, MD, U.S. Census Bureau, 2015 [Google Scholar]
- 13.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–469 [DOI] [PubMed] [Google Scholar]
- 14.Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2016;375:1868–1877 [DOI] [PubMed] [Google Scholar]
- 15.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole JH, Miller JI III, Sperling LS, Weintraub WS. Long-term follow-up of coronary artery disease presenting in young adults. J Am Coll Cardiol 2003;41:521–528 [DOI] [PubMed] [Google Scholar]
- 17.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007;298:765–775 [DOI] [PubMed] [Google Scholar]
- 18.Cavender MA, Steg PG, Smith SC Jr., et al.; REACH Registry Investigators . Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015;132:923–931 [DOI] [PubMed] [Google Scholar]
- 19.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 20.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 22.Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 23.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 24.Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:2099. [DOI] [PubMed] [Google Scholar]
- 25.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol 2018;72:3200–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassif ME, Kosiborod M. Are we ready to bell the cat? A call for cardiologists to embrace glucose-lowering therapies proven to improve cardiovascular outcomes. Circulation 2018;138:4–6 [DOI] [PubMed] [Google Scholar]
- 27.Opingari E, Partridge ACR, Verma S, Bajaj HS. SGLT2 inhibitors: practical considerations and recommendations for cardiologists. Curr Opin Cardiol 2018;33:676–682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.