Abstract
OBJECTIVE
To examine whether low baseline diastolic blood pressure (DBP) modifies the effects of intensive systolic blood pressure (SBP) lowering on cardiovascular outcomes in type 2 diabetes mellitus (T2DM).
RESEARCH DESIGN AND METHODS
The Action to Control Cardiovascular Risk in Diabetes Blood Pressure trial (ACCORD BP), a two-by-two factorial randomized controlled trial, examined effects of SBP (<120 vs. <140 mmHg) and glycemic (HbA1c <6% vs. 7.0–7.9% [<42 vs. 53–63 mmol/mol]) control on cardiovascular events in T2DM (N = 4,731). We examined whether effects of SBP control on cardiovascular composite were modified by baseline DBP and glycemic control.
RESULTS
Intensive SBP lowering decreased the risk of the cardiovascular composite (hazard ratio [HR] 0.76 [95% CI 0.59–0.98]) in the standard glycemic arm but not in the intensive glycemic arm (HR 1.06 [95% CI 0.81–1.40]). Spline regression models relating the effects of the intervention on the cardiovascular composite across the range of baseline DBP did not show evidence of effect modification by low baseline DBP for the cardiovascular composite in the standard or intensive glycemic arms. The relation between the effect of the intensive SBP intervention and baseline DBP was similar between glycemic arms for the cardiovascular composite three-way interaction (P = 0.83).
CONCLUSIONS
In persons with T2DM, intensive SBP lowering decreased the risk of cardiovascular composite end point irrespective of baseline DBP in the setting of standard glycemic control. Hence, low baseline DBP should not be an impediment to intensive SBP lowering in patients with T2DM treated with guideline-recommended standard glycemic control.
Introduction
The 2017 American College of Cardiology/American Heart Association blood pressure (BP) guideline recommends systolic BP (SBP) reduction to <130 mmHg in adults with hypertension regardless of presence of type 2 diabetes mellitus (T2DM) (1). The Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD BP) trial compared intensive (SBP target <120 mmHg) to standard (SBP target <140 mmHg) treatment of hypertension in adults with T2DM. The primary analysis of that trial showed a nonsignificant 12% decrease in the primary composite cardiovascular disease (CVD) end point, along with a nonsignificant 7% higher incidence of all-cause mortality for the intensive treatment group (2). However, a recent analysis of the ACCORD BP data showed evidence of interaction between the intensive SBP and intensive glycemic control interventions (3). There was an increased risk of CVD events and all-cause deaths with intensive SBP control in the setting of intensive control of glycemia (which attenuated after discontinuation of the intensive glycemia intervention) but a lower risk of CVD events and all-cause deaths with intensive SBP control in the setting of standard glycemia. As intensive glycemia treatment is no longer recommended because of its all-cause mortality risk (4), benefits from intensive SBP lowering in the standard glycemia arm of ACCORD BP are clinically relevant for the management of hypertension in patients with T2DM.
Nonetheless, there are concerns about the effects of SBP-lowering interventions on diastolic BP (DBP) in persons with T2DM. The concept of a J-shaped curve between on-treatment levels of DBP and CVD was first reported by Stewart (5) in 1979 and subsequently championed by others (6–11). A principal concern is that intensive SBP reduction in persons with a wide pulse pressure (high SBP and low DBP) could result in adverse consequences due to a further decrease in DBP.
This concern could be amplified in persons with T2DM because diabetes is not only an independent risk factor for micro- and macrovascular complications (12,13) but is also associated with increased arterial stiffness (14) and, consequently, a wide pulse pressure and low DBP. For example, in 257 patients with T2DM in a clinical practice setting, titration of SBP to a goal of <130 mmHg achieved the target SBP in a third of the cohort, but in 57% of the patients, the attained DBP was ≤70 mmHg (15). Low DBP has been reported to be associated with worse CVD outcomes in persons with T2DM (16). Thus, the safety of lowering SBP to <130 mmHg in T2DM has been questioned (15).
We used limited-access ACCORD BP data to investigate: 1) whether low baseline DBP in ACCORD BP participants was associated with increased risk of CVD events and all-cause deaths, 2) whether the effects of intensive SBP lowering on CVD events and all-cause deaths were modified by baseline DBP and the glycemia intervention arm, and, finally, 3) whether intensive SBP control was harmful in persons with low baseline DBP in standard and intensive glycemia arms.
Research Design and Methods
Details of the ACCORD BP protocol have been published (2). Briefly, it was a substudy of the ACCORD trial, in which all 10,251 participants were randomly assigned to receive comprehensive intensive therapy targeting an HbA1c level of <6.0% or to receive standard therapy targeting a level of 7.0–7.9% (17,18). With the use of a double two-by-two factorial design, 4,733 ACCORD participants were randomly assigned to lower their blood pressure by receiving either intensive therapy (SBP target <120 mmHg) or standard therapy (SBP target <140 mmHg) in the ACCORD BP trial (2). The remaining 5,518 patients were randomly assigned to fenofibrate or placebo in the ACCORD Lipid trial (18). We obtained limited-access ACCORD BP data from the Biologic Specimen and Data Repository Information Coordinating Center of the National Heart, Lung, and Blood Institute (19).
ACCORD BP Study Population
Participants could be recruited if their HbA1c was ≥7.5% (58 mmol/mol), their SBP was between 130 and 180 mmHg while taking up to three BP medications, and they had <1 g proteinuria/day. Those with known CVD had to be at least 40 years old, whereas those with no evidence of CVD had to be at least 55 years old with at least two CVD risk factors (hypertension, hyperlipidemia, BMI of >32 kg/m2, or smoking) or left ventricular hypertrophy, significant atherosclerosis, or albuminuria. Adults with a serum creatinine >1.5 mg/dL or another serious illness were ineligible.
ACCORD SBP Intervention
Participants were randomly assigned to antihypertensive treatment with an SBP target of <120 mmHg (intensive therapy) or <140 mmHg (standard therapy). In both BP treatment groups, the antihypertensive regimens consisted of Food and Drug Administration–approved medications that were known to improve CVD outcomes in adults with T2DM. Participants in the standard BP treatment group were seen after 1 and 4 months and thereafter every 4 months. Participants in the intensive BP treatment group were followed monthly for the first 4 months or more frequently if needed to achieve their SBP goal and thereafter every 2 months.
ACCORD Glycemia Intervention
Participants were randomly assigned to intensive versus standard glycemic goals of HbA1c of 6% versus 7–7.9% (42 vs. 53–63 mmol/mol). The intensive glycemic intervention was stopped early after a mean follow-up of 3.5 years because of increased mortality risk (17). The SBP intervention was continued until the planned trial end date (2). We used the entire ACCORD BP follow-up for the primary analysis. In sensitivity analyses, we report results with follow-up censored at the end of the intensive glycemic intervention.
Outcomes
We used the primary ACCORD BP CVD composite outcome defined as time to occurrence of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. In additional sensitivity analysis, we defined an expanded CVD composite outcome as time to the ACCORD BP CVD outcome, hospitalization for congestive heart failure, or unstable angina because it has been proposed that lowering SBP in adults with a low DBP may decrease coronary perfusion and cause myocardial damage (20). We also performed additional sensitivity analyses using a nonstroke CVD composite based on the ACCORD primary CVD composite with the exception that stroke was not included.
Statistical Methods
The 33rd and 66th percentiles of baseline DBP were 71 mmHg and 80 mmHg, respectively. We categorized participants based on baseline DBP ≤70, 71–79, or ≥80 mmHg for descriptive purposes and graphical representation. Baseline characteristics were compared among these three DBP groups. We computed mean follow-up DBP for each patient by averaging their BP measurements from month 4 to the last measurement during the follow-up period. We used box plots to display mean follow-up DBP by categories of baseline DBP within the intensive and standard BP treatment groups.
Evaluation of the Effect of the Intensive SBP Intervention in Relation to the Glycemia Intervention and Baseline DBP
We performed separate Cox regression analyses within the standard and intensive glycemic control groups to estimate overall hazard ratios (HRs), which compared the CVD composite between the intensive versus standard SBP interventions within the two glycemic control groups, irrespective of the level of baseline DBP. Subsequently, we expanded the two Cox regression models by adding baseline DBP and the linear interaction between baseline DBP as a continuous variable and the randomized SBP group to test if the effects of the intensive SBP intervention varied by the level of baseline DBP within the two glycemic control groups. Similar models with cubic spline terms for baseline DBP and its interaction with the randomized SBP group were used for graphical displays of the HR for the intensive versus standard SBP intervention in relation to categories of baseline DBP. In the next step, we evaluated the three-way interaction among intensive SBP intervention, glycemic control, and a linear term for baseline DBP to test if the dependence of the effect of intensive SBP intervention on baseline DBP differed between the two glycemic control groups. The three-way interaction model was evaluated in the full cohort with baseline hazard stratified by glycemic control. A similar series of Cox regressions was performed with baseline DBP dichotomized according to whether DBP was ≤70 mmHg versus >70 mmHg.
We repeated the Cox regression analyses described above for all-cause death, the expanded CVD composite, nonstroke CVD composite, and congestive heart failure. Baseline hazard functions of all Cox proportional hazards regression models were also stratified by ACCORD’s clinical center networks. Analyses of scaled Schoenfeld residuals (21) indicated no evidence of nonproportional hazards. The glycemia intervention was stopped before the end of the main trial; therefore, as a sensitivity analysis, we repeated the above analyses with additional censoring of each patient’s follow-up at the time corresponding to the termination of the glycemia intervention.
We analyzed all data using Stata version MP 14.0 or SAS version 9.4. We conducted hypothesis testing using a two-sided α of 0.05 without adjustment for multiple comparisons. We compared numeric baseline characteristics between baseline DBP tertiles using one-way ANOVA and categorical variables using χ2 tests.
Results
The current analysis included 4,731 ACCORD BP trial participants (Supplementary Fig. 1). In Table 1, participant characteristics are summarized by tertiles of baseline DBP. In general, participants with DBP ≤70 mmHg were older and more likely to be white and have a history of current or past smoking, heart failure, chronic kidney disease, stroke, or myocardial infarction, and a longer duration of diabetes.
Table 1.
Baseline characteristics by DBP tertiles
| Tertile 1 | Tertile 2 | Tertile 3 | P value | |
|---|---|---|---|---|
| DBP ≤70 mmHg (N = 1,402) | DBP 71–79 mmHg (N = 1,638) | DBP ≥80 mmHg (N = 1,691) | ||
| DBP, mmHg | 64.0 (5.1) | 75.0 (2.6) | 86.3 (4.8) | |
| Age, years | 65.1 (6.8) | 62.5 (6.6) | 61.0 (6.0) | <0.001 |
| Female sex, % | 46.5 | 49.6 | 46.9 | 0.17 |
| White race, % | 64.4 | 58.1 | 54.6 | <0.001 |
| SBP, mmHg | 130.7 (14.7) | 137.8 (13.0) | 146.9 (13.9) | <0.001 |
| Number of BP medications | 1.8 (1.1) | 1.7 (1.1) | 1.6 (1.1) | <0.001 |
| Participants randomized to intensive BP control, % | 50.6 | 49.1 | 50.1 | 0.72 |
| Participants randomized to intensive glycemia control, % | 50.3 | 50.2 | 49.8 | 0.95 |
| Never smoker, % | 40.3 | 48.0 | 43.9 | <0.001 |
| History of MI, % | 18.0 | 10.9 | 12.9 | <0.001 |
| History of CVD, % | 41.7 | 33.1 | 27.7 | <0.001 |
| History of CHF, % | 5.4 | 3.4 | 4.3 | 0.026 |
| History of stroke, % | 8.0 | 5.7 | 5.9 | 0.025 |
| Duration of diabetes, years | 13.4 (8.5) | 10.7 (7.7) | 9.3 (6.7) | <0.001 |
| Fasting plasma glucose, mg/dL | 167.7 (52.8) | 172.7 (54.4) | 179.8 (55.7) | <0.001 |
| BMI | 31.2 (5.3) | 32.3 (5.5) | 32.8 (5.5) | <0.001 |
| HbA1c, % [mmol/mol] | 8.2 (0.9) [66 (10)] | 8.3 (1.0) [67 (11)] | 8.4 (1.1) [68 (12)] | <0.001 |
| MDRD eGFR, mL/min/1.73 m2 | 86.0 (23.6) | 91.2 (23.0) | 93.6 (22.3) | <0.001 |
| CKD (eGFR <60 mL/min/1.73 m2) | 13.1 | 8.3 | 4.9 | <0.001 |
| Urine ACR, mg/g | 1.5 (0.7–4.6) | 1.3 (0.7–4.1) | 1.6 (0.7–5.1) | 0.003 |
Data are percentages for all categorical factors and means (SD) for all numeric factors with the exception of urine ACR, which is summarized with medians (interquartile range) due to positive skewness and compared by using the Kruskal-Wallis test. Other numeric variables are compared by using one-way ANOVA and categorical variables by using χ2 tests.
ACR, albumin-to-creatinine ratio; CHF, congestive heart failure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction.
Achieved SBP and DBP by Intervention Arm
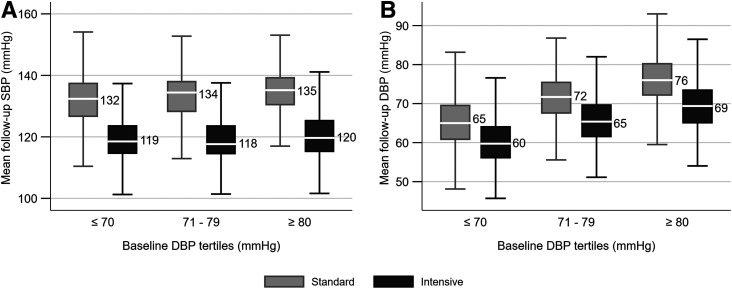
In both the standard and intensive therapy groups, the mean achieved SBP was similar across tertiles of baseline DBP (Fig. 1A), and the SBP separation was achieved with the intensive SBP intervention irrespective of baseline DBP. The mean achieved DBP (Fig. 1B) was higher in the standard compared with intensive therapy groups within each tertile of baseline DBP. For example, the achieved mean DBPs in the intensive and standard therapy groups were 60 ± 6 mmHg and 65 ± 6 mmHg, respectively, for those in the lowest tertile of DBP at baseline. The mean arterial pressures and the mean follow-up pulse pressures were lower in the intensive compared with standard therapy groups across the baseline tertiles of DBP (Supplementary Fig. 2).
Figure 1.
Box plots of mean follow-up SBP (A) and DBP (B) by SBP intervention and baseline DBP tertiles. Shows first quartile − 1.5 interquartile range (IQR), first quartile, median, third quartile, and third quartile + 1.5 IQR. IQR is the third quartile to first quartile. A total of 78 participants (39 in standard and 39 in intensive BP arm) had missing follow-up BP data after 2 months and were not included.
Interactions of SBP Lowering and Baseline DBP as a Continuous Variable on the CVD Composite in the Settings of Standard or Intensive Glycemic Control
In the entire analytic cohort, 694 CVD composite events occurred over 21,466 patient-years of follow-up and 292 all-cause deaths over 23,413 patient-years of follow-up.
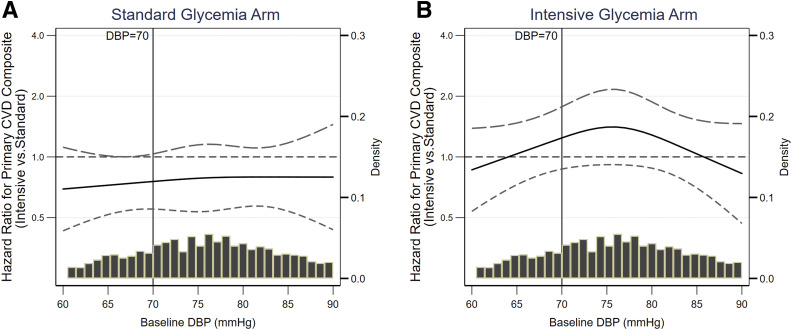
In the standard glycemic arm, intensive SBP lowering significantly decreased the risk of the CVD composite (HR 0.76 [95% CI 0.59–0.98]). Spline regression relating the effects of the intervention on the CVD composite across the range of baseline DBP is displayed in Fig. 2A. The effect of the SBP intervention in the standard glycemic arm did not appear to be related to baseline DBP for the CVD composite (linear interaction term for baseline DBP as a continuous variable and SBP intervention arm, P = 0.67).
Figure 2.
Spline regressions relating baseline DBP with the effects of intensive SBP lowering on CVD composite outcome during the entire follow-up duration. The x-axis depicts 5th to 95th percentile of baseline DBP. A: CVD composite end point in standard glycemia arm; linear interaction P = 0.67. B: CVD composite end point in intensive glycemia arm; linear interaction P = 0.85.
In the intensive glycemic arm, intensive SBP lowering did not reduce the risk of the CVD composite (HR 1.06 [95% CI 0.81–1.40]). In the intensive glycemic arm, the effect of the intensive SBP intervention on the CVD composite did not appear to be related to baseline DBP as a continuous variable as shown in Fig. 2B (linear interaction P = 0.85).
The relation between the effect of the intensive SBP intervention and baseline DBP was similar between the two glycemic control groups for the CVD composite (three-way interaction P = 0.83).
In sensitivity analyses that censored follow-up at the time the glycemia intervention was stopped, results were similar to the main analyses in the standard and intensive glycemia arms (Sup‐plementary Fig. 3A and B) with a three-way interaction P value for the CVD composite of 0.59.
Interactions of SBP Lowering and Baseline DBP as a Continuous Variable on All-Cause Mortality in the Settings of Standard or Intensive Glycemic Control
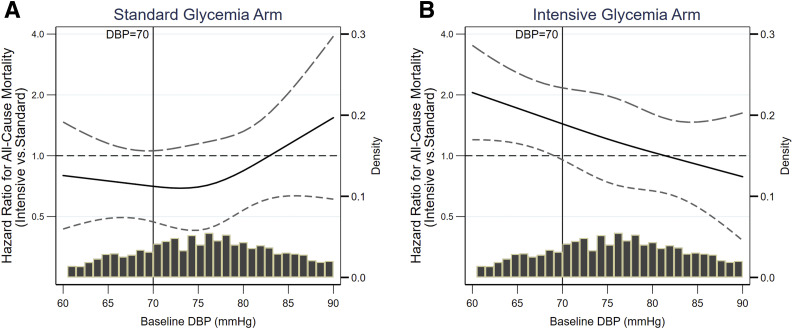
Spline regressions relating the effects of the intervention on all-cause mortality with baseline DBP in standard glycemia arm (Fig. 3A) and intensive glycemia arm (Fig. 3B) are presented in Fig. 3. Intensive SBP lowering did not reduce the risk of all-cause death in the standard glycemia arm (HR 0.84 [95% CI 0.60–1.17]) or the intensive glycemia arm (HR 1.34 [95% CI 0.98–1.85]). While there was no evidence of interaction between baseline DBP and BP intervention on all-cause mortality in the standard glycemia arm (linear interaction, P = 0.39), intensive SBP lowering appeared to increase the risk of all-cause death at lower levels of baseline DBP in the intensive glycemia arm (linear interaction P = 0.010). Results were similar when the analyses were limited to follow-up until the glycemic intervention was terminated (Supplementary Fig. 3C and D).
Figure 3.
Spline regressions relating baseline DBP with the effects of intensive SBP lowering on all-cause mortality during the entire follow-up duration. The x-axis depicts 5th to 95th percentile of baseline DBP. A: All-cause mortality in standard glycemia arm; linear interaction P = 0.39. B: All-cause mortality in intensive glycemia arm; linear interaction P = 0.01.
Interactions of SBP Lowering and Baseline DBP (as a Dichotomous Variable; ≤70 vs. > 70 mmHg) on CVD Composite and All-Cause Deaths in the Settings of Standard or Intensive Glycemic Control
Within the standard glycemia arm (Supplementary Fig. 4A), the HR for the primary CVD composite outcome with intensive SBP lowering was similar between those with baseline DBP ≤70 mmHg (HR 0.76 [95% CI 0.50–1.17]) and those with baseline DBP >70 mmHg (HR 0.78 [95% CI 0.56–1.07]); the interaction P value comparing the HRs between the baseline DBP groups was 0.96. Results were similar for all-cause mortality (Supplementary Fig. 4A).
Within the intensive glycemia arm, the HR comparing the primary CVD composite outcome between the intensive and standard SBP interventions was similar between those with a baseline DBP ≤70 mmHg (HR 1.04 [95% CI 0.67–1.61]) and those with baseline DBP >70 mmHg (HR 1.07 [95% CI 0.74–1.53)], with an interaction P value of 0.92. In contrast, for all cause-mortality, there was a nearly twofold increase in hazard (HR 1.93 [95% CI 1.18–3.14]) with intensive SBP lowering in the intensive glycemic arm participants with baseline DBP ≤70 mmHg (Supplementary Fig. 4B), but no statistically significant effect of intensive SBP control in the participants with baseline DBP >70 mmHg (HR 0.99 [95% CI 0.64–1.53]). The interaction P value comparing these two HRs was 0.04.
Expanded CVD Composite Outcome, Nonstroke CVD Outcome, and Heart Failure Outcome
These are summarized in Supplementary Table 1. In general, intensive BP therapy appeared beneficial for these outcomes in the standard but not the intensive glycemic arm. There was no evidence that intensive SBP lowering was harmful in those with a low baseline DBP who were assigned to standard glycemic control.
Conclusions
The current series of secondary analyses indicate that among persons with T2DM, an intensive SBP-lowering intervention further reduced DBP but still decreased the risk of CVD events across the range of baseline DBP in the setting of standard glycemic control. Thus, there was no evidence that low baseline DBP modified the cardiovascular effects of intensive SBP lowering with guideline-recommended glycemic control.
Despite the well-documented value of treatment in adults with high levels of DBP, the effects of DBP lowering on outcomes have been controversial. Many, but not all (22,23), analyses of the relationship between on-treatment levels of DBP and CVD events have reported a J- or U-shaped curve with higher levels of CVD in those with lower levels of DBP (5–11). Biological plausibility for the J-curve phenomenon has been proposed (6,24). As most of the ventricular myocardial perfusion occurs during diastole, particularly in persons with left ventricular hypertrophy (increased oxygen [O2] demand) and coronary artery disease (decreased O2 supply), lower DBP could potentially lead to myocardial hypoperfusion and associated damage. Indeed, in the Atherosclerosis Risk in Communities (ARIC) cohort, lower DBP was associated with increased serum concentrations of cardiac troponin T, a marker of myocardial injury (20).
Based on the above observational studies of cohort or postrandomization-achieved BP data sets, strong causal inferences on the effects of lowering DBP have been drawn, perhaps erroneously (7–10,20,24–26). For instance, depending upon the observed threshold in a given study, various lower bounds of DBP have been proposed below which DBP lowering is considered deleterious (27).
In contrast, the underlying processes (such as increased arterial stiffness) that lead to a decline in DBP rather than the level of DBP per se might be the reason for the observed associations of worse outcomes with lower DBP. Statistical modeling and multivariable regressions are typically used in observational analyses, but residual confounding is still a concern. The most direct and valid method of testing the J-curve hypothesis is to assess the effect of an active intervention that lowers DBP, particularly in persons with a starting DBP <70 mmHg. If lowering DBP is deleterious below a certain DBP, one would expect that the effects of lowering SBP on CVD outcomes and death would be modified by baseline level of DBP.
In the current study of persons with T2DM and hypertension, the beneficial effects of intensive SBP lowering on CVD outcome in the standard glycemia arm was not modified by level of baseline DBP. Results were similar when a nonstroke CVD composite or heart failure was examined as the end point of interest. These observations in ACCORD BP participants with standard glycemic control were similar to our earlier report in Systolic Blood Pressure Intervention Trial (SPRINT) participants without diabetes (28).
There is a rational biological basis for these observations. Myocardial O2 balance depends upon both myocardial O2 demand and O2 supply (29). Lowering SBP might preserve or improve myocardial O2 balance based on a decrease in myocardial O2 demand by lowering systolic pressure-time index (29) and a decrease in afterload with consequent regression of left ventricular hypertrophy and left ventricular mass, which compensates for the potential deleterious effects of lower coronary blood flow.
In contrast to the beneficial effects of SBP lowering in persons with T2DM and low DBP on standard glycemic control or previous findings in adults without diabetes (28), the current study suggests that intensive SBP lowering does not appear to confer cardiovascular benefits in the setting of intensive glycemic control. Indeed, there is a suggestion that intensive SBP lowering might increase the risk of all-cause mortality in the setting of intensive glycemic control, particularly when baseline DBP is low. Because of the post hoc nature of the current study and the borderline significant P value for the three-way interaction term for all-cause mortality, these findings should be considered as hypothesis generating rather than definitive proof that intensive SBP lowering is harmful in the setting of intensive glycemia in persons with T2DM, hypertension, and low baseline DBP.
In summary, in persons with T2DM, intensive SBP lowering decreased the risk of CVD composite end point irrespective of baseline DBP in the setting of standard glycemia. Hence, low baseline DBP should not be an impediment to intensive SBP lowering in the setting of standard glycemia. In contrast, caution might be warranted regarding intensive SBP lowering in the setting of intensive glycemic control when baseline DBP is low.
Article Information
Funding. Statistical analyses and preparation of this manuscript were supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (R21-HL-145494), National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-091437), and the University of Utah Study Design and Biostatistics Center (funded in part by U.S. Public Health Service research grants UL1-RR-025764 and C06-RR-11234 from the National Center for Research Resources). ACCORD BP was funded by the NHLBI and National Institutes of Health.
This manuscript was prepared using ACCORD research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of ACCORD or the NHLBI/National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. O.L.I., T.G., G.W., R.E.B., and S.B. were responsible for conception and study design. T.G., G.W., and R.E.B. were responsible for analysis of the data. All authors participated in interpretation of data. O.L.I., T.G., P.K.W., G.W., R.E.B., and S.B. were responsible for the literature search. O.L.I., T.G., G.W., R.E.B., and S.B. drafted the manuscript. All authors performed critical revision of the manuscript for important intellectual content and gave final approval of the manuscript. T.G., G.W., R.E.B., and W.A. provided statistical expertise. T.G. and S.B. obtained funding with grant R21-HL-145494 and T.G., A.K.C., G.M.C., and S.B. with grant R01-DK-091437. W.A. was an ACCORD BP investigator. T.G. and S.B. provided administrative, technical, or logistic support. All authors take responsibility for their contributions to this work. S.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the American Society of Nephrology Kidney Week 2018, San Diego, CA, 23–28 October 2018.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12026865.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
See accompanying article, p. 1684.
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. . ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:2199–226929146533 [Google Scholar]
- 2.Cushman WC, Evans GW, Byington RP, et al.; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beddhu S, Chertow GM, Greene T, et al. . Effects of intensive systolic blood pressure lowering on cardiovascular events and mortality in patients with type 2 diabetes mellitus on standard glycemic control and in those without diabetes mellitus: reconciling results from ACCORD BP and SPRINT. J Am Heart Assoc 2018;7:e009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association 6. Glycemic targets: Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S48–S56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart IM. Relation of reduction in pressure to first myocardial infarction in patients receiving treatment for severe hypertension. Lancet 1979;1:861–865 [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581–584 [DOI] [PubMed] [Google Scholar]
- 7.Messerli FH, Mancia G, Conti CR, et al. . Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006;144:884–893 [DOI] [PubMed] [Google Scholar]
- 8.Vidal-Petiot E, Ford I, Greenlaw N, et al.; CLARIFY Investigators . Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388:2142–2152 [DOI] [PubMed] [Google Scholar]
- 9.Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J Am Coll Cardiol 2014;64:588–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangalore S, Messerli FH, Wun C-C, et al.; Treating to New Targets Steering Committee and Investigators . J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J 2010;31:2897–2908 [DOI] [PubMed] [Google Scholar]
- 11.Kang Y-Y, Wang J-G. The J-curve phenomenon in hypertension. Pulse (Basel) 2016;4:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77 [Google Scholar]
- 13.Tancredi M, Rosengren A, Svensson A-M, et al. . Excess mortality among persons with type 2 diabetes. N Engl J Med 2015;373:1720–1732 [DOI] [PubMed] [Google Scholar]
- 14.Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis 2015;238:370–379 [DOI] [PubMed] [Google Scholar]
- 15.Osher E, Stern N. Diastolic pressure in type 2 diabetes: can target systolic pressure be reached without “diastolic hypotension”? Diabetes Care 2008;31(Suppl. 2):S249–S254 [DOI] [PubMed] [Google Scholar]
- 16.Rönnback M, Isomaa B, Fagerudd J, et al.; Botnia Study Group . Complex relationship between blood pressure and mortality in type 2 diabetic patients: a follow-up of the Botnia Study. Hypertension 2006;47:168–173 [DOI] [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute Action to Control Cardiovascular Risk in Diabetes (ACCORD) data set. Available from https://biolincc.nhlbi.nih.gov/studies/accord/. Accessed 21 April 2020
- 18.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginsberg HN, Elam MB, Lovato LC, et al.; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus [published correction appears in N Engl J Med 2010;362:1748]. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEvoy JW, Chen Y, Rawlings A, et al. . Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016;68:1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526 [Google Scholar]
- 22.Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527–1535 [DOI] [PubMed] [Google Scholar]
- 23.Adamsson Eryd S, Gudbjörnsdottir S, Manhem K, et al. . Blood pressure and complications in individuals with type 2 diabetes and no previous cardiovascular disease: national population based cohort study. BMJ 2016;354:i4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489–495 [PubMed] [Google Scholar]
- 25.Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 1999;159:2004–2009 [DOI] [PubMed] [Google Scholar]
- 26.Fagard RH, Staessen JA, Thijs L, et al. . On-treatment diastolic blood pressure and prognosis in systolic hypertension. Arch Intern Med 2007;167:1884–1891 [DOI] [PubMed] [Google Scholar]
- 27.Williams B, Mancia G, Spiering W, et al.; ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104 [DOI] [PubMed] [Google Scholar]
- 28.Beddhu S, Chertow GM, Cheung AK, et al.; SPRINT Research Group . Influence of baseline diastolic blood pressure on effects of intensive compared with standard blood pressure control. Circulation 2018;137:134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman JIE, Buckberg GD. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc 2014;3:e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]