Abstract
OBJECTIVE
Corneal nerve fiber length (CNFL) represents a biomarker for diabetic distal symmetric polyneuropathy (DSP). We aimed to determine the reference distribution of annual CNFL change, the prevalence of abnormal change in diabetes, and its associated clinical variables.
RESEARCH DESIGN AND METHODS
We examined 590 participants with diabetes (399 with type 1 diabetes [T1D] and 191 with type 2 diabetes [T2D]) and 204 control patients without diabetes with at least 1 year of follow-up and classified them according to rapid corneal nerve fiber loss (RCNFL) if CNFL change was below the 5th percentile of the control patients without diabetes.
RESULTS
Control patients without diabetes were 37.9 ± 19.8 years old, had median follow-up of three visits over 3.0 years, and mean annual change in CNFL was −0.1% (90% CI −5.9% to 5.0%). RCNFL was defined by values exceeding the 5th percentile of 6% loss. Participants with T1D were 39.9 ± 18.7 years old, had median follow-up of three visits over 4.4 years, and mean annual change in CNFL was −0.8% (90% CI −14.0% to 9.9%). Participants with T2D were 60.4 ± 8.2 years old, had median follow-up of three visits over 5.3 years, and mean annual change in CNFL was −0.2% (90% CI −14.1% to 14.3%). RCNFL prevalence was 17% overall and was similar by diabetes type (64 T1D [16.0%], 37 T2D [19.4%], P = 0.31). RNCFL was more common in those with baseline DSP (47% vs. 30% in those without baseline DSP, P = 0.001), which was associated with lower peroneal conduction velocity but not with baseline HbA1c or its change over follow-up.
CONCLUSIONS
An abnormally rapid loss of CNFL of 6% per year or more occurs in 17% of diabetes patients. RCNFL may identify patients at highest risk for the development and progression of DSP.
Introduction
Diabetic distal symmetric polyneuropathy (DSP) is one of the most prevalent and pervasive diabetes complications that is associated with morbidity and the frequent use of health care resources (1). The natural history of DSP begins with diffuse injury to small unmyelinated sensory nerves (Aδ and C fibers) that include autonomic nerves and those that innervate the skin for conveying pain and temperature sensation. It is generally believed that nerve injury first occurs asymptomatically to small nerve fibers before progressing to involve large nerve fibers (2). While nerve conductions studies (3) are the standard clinical test for DSP, the development of in vivo corneal confocal microscopy (IVCCM) measurement of corneal nerve fiber length (CNFL) represents a measure of small nerve fiber morphology (4–7) that might permit identification of early stages of diabetic DSP and allows for repeated noninvasive monitoring.
While CNFL is a biomarker for DSP risk and disease status, it has been shown to perform well alongside standard clinical tests of small and large fiber function (5,8) and provides the opportunity to identify early changes in DSP (7). Moreover, CNFL has the highest validity and reproducibility compared with other corneal nerve parameters measured by IVCCM (5,9,10). Current research of CNFL has used cross-sectional studies to investigate predictive validity (5,11), which shows the importance of identifying early nerve damage in DSP (12). However, these findings do not provide insight into longitudinal change in CNFL. Understanding the normal distribution of annual change in CNFL might help identify diabetes patients who have initiated a pathological process of small fiber damage, despite their CNFL and small nerve fiber tests showing results within the clinically normal range.
While a single measure of CNFL can serve as a biomarker for DSP, it is imperative to improve on the performance of this measure for identifying patients at risk for rapid DSP progression. Previous work has identified normative values for CNFL by age in control patients without diabetes (13), and smaller studies in type 1 diabetes have suggested a CNFL cut point for identifying patients at risk for developing future DSP (11,14); however, the reference distribution for annual change in CNFL in control patients without diabetes and individuals with diabetes is presently unknown. The objective of this analysis was to determine the reference distribution for the annual change in CNFL in well-characterized control patients without diabetes (N = 204 followed for 3 years), from this distribution determine a threshold for abnormal loss [termed rapid CNFL loss (RCNFL)], and apply this reference threshold to individuals with diabetes to determine the prevalence of abnormal loss in 590 participants with diabetes. The secondary objectives were to identify clinical variables associated with change in CNFL and RCNFL.
Research Design and Methods
Study Design
The study is a post hoc, secondary analysis of a prospective, longitudinal observational study investigating the change in CNFL in individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D) (clinical trial reg. no. NCT02423434, clinicaltrials.gov). The objective of this secondary analysis is to determine the reference range for annual change in CNFL in control patients without diabetes and to identify the prevalence of such abnormal loss in those with diabetes. The protocol and consent procedures at all sites were approved by local research ethics boards, and written informed consent was provided by all study participants or their legal guardians.
Study Population
The study population was accrued from a consortium of five different cohort studies initiated between 2008 and 2011 (4,5,9,13,15–18). The objectives of the five studies were to determine the role of IVCCM as a biomarker for DSP. The five study centers included Queensland University of Technology, the University of Calgary, the University of Manchester, the University of Michigan, and the University of Toronto. Baseline data have been recently published (7). The current study includes participant-level data from all baseline visits and follow-up data from September 2014 to May 2018.
The presence of diabetes was defined in accordance with the American Diabetes Association guidelines. Neuropathy due to nondiabetic causes, current eye infection or other conditions that precluded IVCCM, or allergy to the ocular anesthetic used during the IVCCM exam were exclusions. Neuropathies due to nondiabetic causes were determined through detailed participant history or through screening of immunoglobulins and B12 levels, depending on each site’s local protocol.
A total of 998 participants with T1D and T2D were recruited for the study, of which 590 (399 T1D and 191 T2D) had at least one follow-up visit and were eligible for this analysis. A total of 204 control patients without diabetes were recruited as a reference population during the same period.
Study Procedures
All procedures noted were performed at each study visit.
IVCCM Procedures
All participants underwent examination of the subbasal nerve plexus of the cornea using the Heidelberg Tomograph Rostock Cornea Module III (Heidelberg Engineering GmbH, Heidelberg, Germany and Heidelberg Engineering, Smithfield, RI) according to published methods (19). In brief, topical anesthetic and a viscous gel medium were applied to the eye, permitting a visual gel bridge between the cornea and the sterile single-use cap on the microscopes’ objective lens. Images were taken through the subbasal layer over a depth of 50 μm using methods that had minor procedural variations between centers (20). The most technically sound images were identified manually by trained site staff, and IVCCM parameters were determined using fully automated software (ACCMetrics Image Analysis Software v2.0, developed by M. Dabbah and X. Chen, University of Manchester, Manchester, U.K.).
Results from up to eight images per eye were averaged. Measured parameters were CNFL, expressed as the total length of nerves in mm/mm2 of image area; corneal nerve branch density (CNBD), expressed as the number of branches/mm2; and corneal nerve fiber density (CNFD), expressed as the number of fibers/mm2. Published data have demonstrated a similar cohort of IVCCM characteristics, reproducibility, and concurrent validity regardless of the study center (5,9,10,15,21,22).
Clinical Evaluation and Electrophysiology
DSP cases were defined by: 1) the presence of one or more neuropathic symptoms and/or 2) the presence of two or more signs of neuropathy, corroborated by 3) the presence of electrophysiological abnormality (11,12). Participants not fulfilling this definition were classified as control subjects. The presence of neuropathic signs and symptoms was determined by a comprehensive neurological examination. Electrophysiological abnormality was determined using clinical nerve conduction studies. Dominant limb peroneal and sural nerves were measured according to the standards of the American Association for Neuromuscular and Electrodiagnostic Medicine. Each center performed examinations and collected data independently and results were sent to the center leading the statistical analysis (Toronto).
Other evaluations included blood pressure, anthropometric measurements, alcohol intake, smoking history, and biochemical tests including glycated hemoglobin A1c (HbA1c), serum lipids, and urinary albumin excretion (generally conducted on the same day as or within 1 week of the neuropathy evaluation). Cooling detection thresholds were determined using Medoc TSA-II NeuroSensory Analyzer (Medoc Advanced Medical Systems, Ramat-Yishai, Israel) along with vibration perception threshold (VPT) (Neurothesiometer; Bail Instruments Limited, Trafford Park, U.K.), using the method of limits (23). The Calgary site used CASE IV (WR Medical Electronics Co., Maplewood, MN) for cooling detection thresholds and VPT testing and these data (n = 73) were excluded from the analysis for lack of comparability.
Statistical Analysis
To determine the reference distribution of annual CNFL change, the slope of CNFL over time was calculated for each participant. In control patients without diabetes, the slope was calculated using linear regression with log-transformed CNFL values as the dependent variable and time in years as the independent variable (with resulting parameter estimates exponentiated and subtracted from 1 to provide units in percent change per year). To determine a population-level estimate of slope in the control patients without diabetes, and to account for variation in length of follow-up, frequency of follow-up examinations, and site-specific variation in corneal nerve parameter levels, a mixed effects model was used. Time was specified as the random effect (both intercept and slope), and the study site was specified as an additional fixed effect. The threshold for abnormal change in CNFL was set a priori as the 5th percentile of slope values in the control patients without diabetes (i.e., RCNFL). This threshold was applied to the arithmetic slopes of the diabetes participants. While we acknowledge the 2.5th percentile is more commonly used to determine abnormality in clinical variables, given the sample size of our control patients without diabetes (n = 204), this would have resulted in n = 5 with RCNFL. As such, the 5th percentile was used to determine the reference distribution to improve our power such that there were n = 11 participants with RCNFL. However, in the sensitivity analysis, we reported the 2.5th (and 7.5th) percentile values.
The prevalence of RCNFL was compared using the χ2 test. Basic statistical comparisons of other characteristics were made using ANOVA, the Kruskal-Wallis test, or the χ2 test, depending on variable distribution. Linear regression was used to test associations between the change in CNFL (as a continuous dependent variable) and the change in neurological, electrophysiological, and clinical variables (independent variables); univariable regression was used and the resulting regression parameters indicate the change in the given independent variables (measured in unit-change per year) associated with a 1-unit loss of CNFL (measured in percent change per year). Additionally, comparisons of changes in neurological, electrophysiological, and clinical characteristics between participants with diabetes with and without RCNFL were made using a linear mixed model with patient as the random effect. As a descriptive analysis, we tested for the presence of nonlinear relationships between time and CNFL; this was done using a linear random effects model that included quadratic and cubic terms for time. This analysis was performed separately stratified by diabetes and by RCNFL case-control subgroups, and penalized spline curves were used to illustrate the associations. An analytical plan section in the Supplementary Materials further describes the planned analyses performed to generate the Supplementary Tables. An α level of 0.05 was used for tests of statistical significance and statistical analyses were performed using SAS version 9.4 for Windows (SAS Institute, Cary, NC).
Results
The baseline characteristics of the study population are shown in Table 1. Control patients without diabetes were 37.9 ± 19.8 years of age, and 50% were women. Baseline CNFL was 16.5 ± 4.0 mm/mm2 with a change in CNFL of −0.1 ± 3.3% per year measured from a median of three follow-up visits over a median of 3 (2.0, 3.8) years. Those with T1D were 40.1 ± 18.9 years of age, and 49% were women. Baseline CNFL was 13.8 ± 4.2 mm/mm2 with a change in CNFL of −0.7 ± 11.4% per year measured from a median of three follow-up visits over a median of 4.4 (4.0, 7.0) years. Those with T2D were 60.4 ± 8.2 years of age and 54% were women. Baseline CNFL was 13.8 ± 4.3 mm/mm2 with a change in CNFL of −0.1 ± 8.7% per year measured from a median of three follow-up visits over and 5.3 (3.0, 6.0) years.
Table 1.
Baseline characteristics of the 204 control patients without diabetes and 590 patients with diabetes
| Characteristic | Control patients without diabetes | Patients with T1D | Patients with T2D | P value |
|---|---|---|---|---|
| (n = 204) | (n = 399) | (n = 191) | ||
| Female sex | 102 (50) | 206 (48) | 64 (32) | <0.001 |
| Baseline age (year) | 37.61 ± 19.79 | 39.90 ± 18.73 | 60.39 ± 8.16 | <0.001 |
| BMI (kg/m2) | 23.78 ± 5.22 | 25.66 ± 4.91 | 31.38 ± 5.99 | <0.001 |
| HbA1c (%) | 5.48 ± 0.35 | 8.25 ± 1.56 | 7.48 ± 1.34 | <0.001 |
| CNFL (mm/mm2) | 16.48 ± 4.03 | 13.48 ± 4.29 | 13.80 ± 4.41 | <0.001 |
| CNBD (branches/mm2) | 35.36 ± 19.63 | 22.66 ± 16.74 | 26.95 ± 19.98 | <0.001 |
| CNFD (fibers/mm2) | 27.29 ± 9.03 | 20.55 ± 8.98 | 20.54 ± 9.48 | <0.001 |
| CNFL slope (percent/year) | 0.64 ± 9.30 | −0.82 ± 5.05 | −0.25 ± 4.47 | 0.138 |
| CNFL slope 90% CI | −5.9 to 5.0 | −14.0 to 9.9 | −14.1 to 14.3 | — |
| RCNFL (+) | 11 (5.4) | 64 (16.0) | 37 (19.4)* | 0.001 |
| Median follow-up time (years) | 3.0 (2, 3.8) | 4.4 (4.0, 7.0) | 5.3 (3.0, 6.0) | <0.001 |
Data are presented as mean ± SD, n (%), or median (interquartile range). P value for trend comparison between control patients without diabetes and the T1D and T2D subcohorts.
The combined number of patients with T1D and with T2D who had RCNFL was 101, providing a prevalence of 17.1% (101/590).
The reference distribution for annual percent change in CNFL in the study population of control patients without diabetes had a 5th percentile threshold value of −5.9% indicating that a loss of 6% or more (observed in 11 individuals) identified abnormal change in CNFL, that is, RCNFL. While this study was powered to examine the 5th percentile, sensitivity analysis using instead the 2.5th or 7.5th percentile produced a similar threshold value and similar proportions of diabetes participants who met the criteria for RCNFL (Supplementary Table 1). The control patients without diabetes with RCNFL had lower baseline CNFL (12.97 ± 3.50 vs. 16.66 ± 3.98, P = 0.0029), and the average CNFL slope was lower (−7.68 ± 2.15% vs. 0.28 ± 2.84%, P < 0.001) compared with control patients without diabetes without RCNFL. There were no differences in baseline characteristics other than the baseline level of CNFL between the control patients without diabetes with stable CNFL and those with RCNFL. The reference distribution for annual change in CNFD and CNBD for control patients without diabetes and patients with diabetes are shown in Supplementary Table 3. The threshold for abnormality in these measures was greater in magnitude than the 6% seen for CNFL.
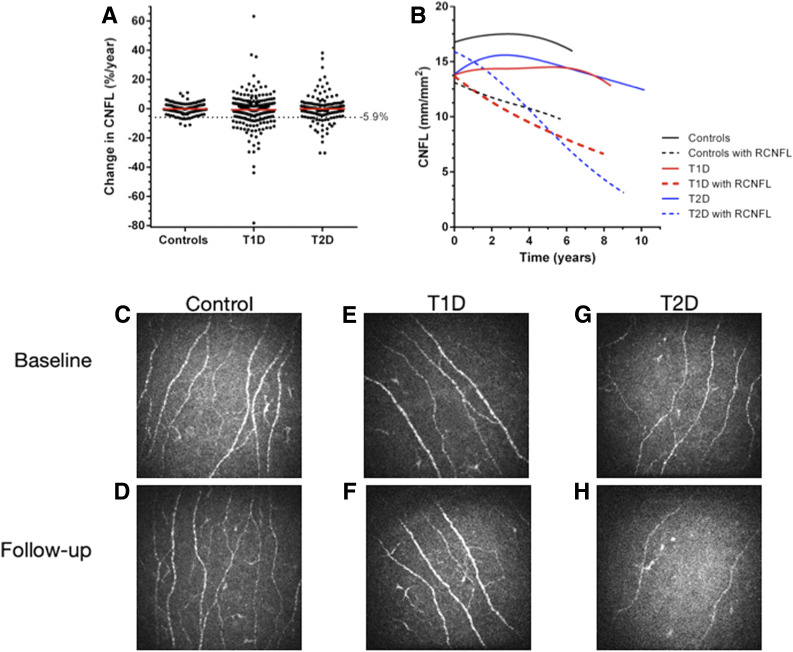
There was a higher proportion of participants with T1D (n = 64, 16.0%) and T2D (n = 37, 19.4%) that met the criteria for RCNFL compared with control patients without diabetes (P < 0.001) (Fig. 1A). The change in CNFL for participants with T1D with RCNFL was −14.67 ± 11.46% per year compared with 2.58 ± 9.93% per year in T1D without RCNFL (P < 0.001). The change in CNFL for participants with T2D, −11.49 ± 6.35% per year, compared with 2.47 ± 7.33% per year in the T2D without RCNFL (P < 0.001). The T1D group had one outlier with a change in CNFL >100% per year that is not shown on the cloud plot in Fig. 1A. Examination of the change in CNFL over time (Fig. 1B) demonstrated a linearrelationship for those with RCNFL. In contrast, T1D and the T2D participants without RCNFL showed a nonlinear progression. In these groups that are initially stable, there is an inflection demonstrating a delayed decrease in CNFL occurring later in time. This change over time may represent stable participants in whom the process of RCNFL is later initiated.
Figure 1.
Annual change in CNFL and representative images. A: The threshold for RCNFL (−5.9%) was determined by the 5th percentile of control patients without diabetes. There was a higher incidence of RCNFL in the diabetes groups compared with control patients without diabetes (P < 0.001). Two outliers beyond 80% change in the control and T1D group are not shown. The red line indicates the mean change in each group. B: Change in CNFL from baseline to follow-up is shown for participants with and without RCNFL, displayed using spline curves. Change in CNFL from baseline to follow-up was as follows: control participants without diabetes 0.17 ± 2.69 mm/mm2; control participants without diabetes with RCNFL −2.00 ± 3.56 mm/mm2; T1D 0.60 ± 2.49 mm/mm2; T1D with RCNFL −4.41 ± 2.50 mm/mm2; T2D 0.81 ± 2.94 mm/mm2; T2D with RCNFL −4.97 ± 2.11 mm/mm2. C and D: Images of control participants without diabetes at baseline (CNFL 17.8 mm/mm2) and with stable CNFL at 2 years follow-up (CNFL 17.9 mm/mm2, slope 0.3% per year). E and F: A participant with T1D with no DSP at baseline (CNFL 13.4 mm/mm2) or 6 years follow-up (CNFL 12.1 mm/mm2, slope −1.6% per year). G and H: A participant with T2D (CNFL 14.3 mm/mm2) who had RCNFL and developed DSP over 5 years of follow-up (CNFL 5.6 mm/mm2, slope −12.6% per year).
We sought to determine the baseline clinical measures that were associated with the presence of RCNFL. Those with RCNFL were not different with respect to female sex (n = 39 [39%] vs. n = 216 [44%], P = 0.30), baseline age (47.33 ± 18.01 years vs. 46.90 ± 18.76 years, P = 0.83), or baseline HbA1c (8.18 ± 1.68% vs. 7.96 ± 1.51%, P = 0.20) compared with the control participants with diabetes but without RCNFL (Table 2).
Table 2.
Comparison of the baseline clinical characteristics and small and large nerve fiber parameters of 101 patients with diabetes and RCNFL and the 489 without
| Characteristic | Diabetes with RCNFL | Diabetes with stable CNFL | P value |
|---|---|---|---|
| (n = 101) | (n = 489) | ||
| Female sex | 39 (39) | 216 (44) | 0.30 |
| Age (year) | 47.33 ± 18.01 | 46.90 ± 18.76 | 0.83 |
| BMI (kg/m2) | 28.56 ± 5.98 | 27.25 ± 5.90 | 0.052 |
| Triglycerides | 1.47 ± 1.08 | 1.29 ± 0.91 | 0.14 |
| T1D/T2D, n | 64/37 | 335/154 | 0.30 |
| Diabetes duration (year) | 19.76 ± 14.85 | 18.70 ± 14.04 | 0.50 |
| HbA1c (%) | 8.18 ± 1.68 | 7.96 ± 1.51 | 0.20 |
| Baseline DSP | 47 (47) | 144 (30) | 0.001 |
| DSP signs | 3.81 ± 3.67 | 2.64 ± 3.09 | 0.004 |
| DSP symptoms | 2.28 ± 3.59 | 1.75 ± 3.35 | 0.13 |
| Small nerve fiber measures | |||
| Baseline CNFL (mm/mm2) | 14.37 ± 4.22 | 13.52 ± 4.32 | 0.073 |
| Cooling detection (°C) | 25.90 (20.00, 28.10) | 27.20 (23.40, 29.20) | <0.001 |
| Large nerve fiber measure | |||
| Vibration perception (V) | 15.72 ± 11.60 | 15.18 ± 16.68 | 0.70 |
| Sural AMP (μV) | 8.07 ± 8.28 | 9.68 ± 8.49 | 0.085 |
| Sural CV (m/s) | 39.62 ± 7.73 | 40.76 ± 7.89 | 0.10 |
| Peroneal AMP (mV) | 3.66 ± 2.33 | 4.13 ± 2.57 | 0.092 |
| Peroneal CV (m/s) | 40.86 ± 7.02 | 42.83 ± 7.20 | 0.013 |
| Peroneal F-wave (ms) | 56.00 (51.40, 71.30) | 54.20 (49.20, 63.95) | 0.030 |
Data are presented as mean ± SD, median (interquartile range), or n (%). P value for comparison between diabetes control patients and the subcohort of patients with diabetes and RCNFL. CV, conduction velocity.
Those with RCNFL were more likely to have baseline DSP (P = 0.001), lower baseline cooling detection threshold (P < 0.001), and slower peroneal conduction velocity (P = 0.013).
We also sought to determine if changes from baseline over follow-up in clinical variables were associated with the quantitative change in CNFL (Table 3). The model shows that a 1% per year loss of CNFL was associated with an increase in DSP signs (P = 0.012) and symptoms (P = 0.022), loss of other corneal nerve morphology CNFD (P < 0.001) and CNBD (P < 0.001), as well as measures of large fiber function. Specifically, such decline was associated with a decrease in peroneal amplitude (P = 0.034), slowing in peroneal conduction velocity (P = 0.013) and higher F-wave latency (P = 0.027), but not with the change in HbA1c (P = 0.30). We performed similar analysis for the change from baseline over follow-up in clinical variables associated with the quantitative change in CNFD and CNBD (Supplementary Tables 4 and 5), respectively. These analyses showed similar results except that there were some differences in the specific nerve conduction variables associated with CNFD and CNBD changes.
Table 3.
The estimated change in clinical, neurological, and electrophysiological variables associated with a 1% per year loss of CNFL in the 590 participants with diabetes
| Characteristic | Parameter estimate | Lower CL | Upper CL | P value |
|---|---|---|---|---|
| HbA1c | −1.01 | −2.94 | 0.92 | 0.30 |
| BMI (kg/m2) | 0.13 | −1.12 | 1.38 | 0.83 |
| DSP signs | 1.51 | 0.34 | 2.69 | 0.012 |
| DSP symptoms | 1.66 | 0.24 | 3.07 | 0.022 |
| Small nerve fiber measures | ||||
| CNFD (fibers/mm2) | −2.00 | −2.17 | −1.82 | <0.0001 |
| CNBD (branches/mm2) | −0.96 | −1.06 | −0.87 | <0.0001 |
| Cooling detection (°C) | 0.30 | −0.43 | 0.84 | 0.53 |
| Large nerve fiber measures | ||||
| Vibration perception (V) | 0.027 | −0.13 | 0.18 | 0.73 |
| Sural CV (m/s) | 0.41 | −0.001 | 0.82 | 0.051 |
| Sural AMP (mV) | 0.39 | 0.030 | 0.81 | 0.069 |
| Peroneal AMP (mV) | −1.87 | −3.60 | −0.15 | 0.034 |
| Peroneal CV (m/s) | −0.93 | −1.66 | −0.20 | 0.013 |
| Peroneal F-wave (ms) | 0.34 | 0.04 | 0.63 | 0.027 |
The regression parameter estimate shows the percent change in CNFL associated with a one-unit loss in the given variable. CL, confidence limit; AMP, amplitude.
We also examined the change in clinical variables over follow-up between patients with and without RCNFL. At follow-up, patients with RCNFL showed a greater change in peroneal conduction velocity (−1.1 m/s [−3.6, 0.9] vs. −0.6 m/s [−2.8, 1.5], P = 0.001) and peroneal amplitude (−0.3 mV [−1.1, 0.4] vs. 0.1 mV [−0.8, 1.0], P = 0.0011). There was no change in cooling detection compared with diabetes control patients (0.3 [−1.8, 2.4] vs. 0.0 [−1.6, 2.1], P = 0.18), with no difference in HbA1c (0.0% [−0.6, 0.5] vs. 0.0% [−0.5, 0.6], P = 0.37).
Finally, we explored the presence of RCNFL in participants with diabetes who had DSP at baseline (n = 198), new-onset DSP (n = 84), and no DSP at baseline and remained stable throughout (n = 300). RCNFL was more prevalent in those with DSP at baseline compared with those with new-onset DSP or no DSP (no DSP 36 [12.2%], new-onset DSP 16 [19.0%], and DSP 47 [24.6%], P for trend = 0.0018). The average CNFL slope was not different between these groups (0.3 ± 9.2 vs. −1.6 ± 8.8 vs. −1.0 ± 13.9, P for trend = 0.25).
Conclusions
The current study provides the first reference distribution for annual change in CNFL for control patients without diabetes and those with T1D and T2D. We have called an abnormal change in CNFL RCNFL and have defined it as a change beyond the 5th percentile of the population of control patients without diabetes, or a loss of 6% per year or more. Our analysis shows that RCNFL occurs in 17% of participants with diabetes and is associated with the new onset of DSP among those without neuropathy at baseline, and the progression of DSP among those with neuropathy at baseline. Furthermore, it appears to be independent of the level of baseline glycemia or the change in glycemic exposure over time.
RCNFL was observed in a higher proportion of participants diagnosed with DSP and objective measures of small fiber damage compared with those with new-onset DSP. Of note, RCNFL occurred more often after the gradual progression of subclinical nerve damage as opposed to a change that precedes the diagnosis of DSP. This was evident when we examined the prevalence of RCNFL between participants with diabetes and no DSP, new-onset DSP and DSP at baseline. When we examined the change in clinical characteristics between baseline and follow-up, those with RCNFL showed evidence of progression of large nerve fiber impairment despite no change in HbA1c compared with control subjects. This implies that our reference distribution for annual change can identify patients at risk for rapid disease progression. Moreover, patients at risk for rapidly losing small nerve fiber before impairments in large fiber function may be detected earlier by way of the evaluation of their annual change in CNFL, which is consistent with current understanding of the natural history of DSP (2).
While the measurement of intraepidermal nerve fiber density can be considered the gold standard for assessing small nerve fiber morphology in research and for the diagnosis of small fiber neuropathy (24–26), CNFL offers an alternative. It appears to be a noninvasive and objective biomarker that allows clinicians and researchers to also monitor small nerve fiber morphology. Preliminary research of IVCCM has used cross-sectional studies to demonstrate that a single measures of CNFL <15 mm/mm2 can predict the future incidence of DSP and those with CNFL <11 mm/mm2 are likely to have prevalent DSP (11,12). As such, a single measure of CNFL is important for clinical assessments and establishing baseline DSP monitoring, while our reference distribution will now permit clinicians to interpret normal versus abnormal longitudinal change.
The reference distribution presented herein was developed from the first large-scale longitudinal study of IVCCM. This is an important step to advance the diagnostic validity of CNFL (7) and may permit clinicians to identify patients at risk for DSP. The current analysis expands on this concept in that it implies that patients at risk for new-onset diabetic DSP (with CNFL <15 mm/mm2) could be recruited into intervention trials for DSP as a strategy to improve statistical power and limit recruitment sample size and that the longitudinal slope of CNFL or proportion of patients with RCNFL could be used as a surrogate measure of DSP progression as primary or secondary outcome measures.
The design of the current study minimized bias from recruitment and spectrum bias, though we acknowledge a sample size limitation for the determination of the reference value for change in CNFL. Our population of 204 control patients without diabetes may limit the accuracy of the estimate of the true population and may be susceptible to selection bias. A mixed effects model was used to address this possible limitation, and we note that the mixed effect model may have overestimated the prevalence of RCNFL in the participants with diabetes. The next steps for IVCCM CNFL will be to evaluate the implementation in research trials and clinical practice, such as the development of a harmonized tool within the image analysis software that will automatically calculate change over time.
The reference distribution of annual change in CNFL has been established from the largest multicenter cohort study to date. These findings support the use of IVCCM CNFL as a simple, objective, and noninvasive test for assessing and monitoring DSP. The reference distribution presented herein can be used to help guide the design and inclusion criteria for clinical trials of DSP modifying agents. Such trials could use the CNFL slope or proportion of patients with RCNFL as surrogate outcomes for DSP status. Further research must focus on how CNFL determines treatment decisions, the change in CNFL in response to different therapies and to further refine the proposed reference distribution of annual change in T1D and T2D.
Supplementary Material
Article Information
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) (grant 1DP3-DK-104386-01). Initially, four of the study centers were funded by JDRF (Brisbane, Calgary, Manchester, and Toronto), and one was funded by the Michigan Diabetes Research and Training Center (grant number P30DK020572). Some aspects of the Toronto project were funded by a Diabetes Canada (formerly the Canadian Diabetes Association) operating grant. The generous support of Randy and Jenny Frisch and The Harvey and Annice Frisch Family Fund and the contributions of the Menkes Family Fund supported aspects of this research at the University of Toronto. Other aspects of the Manchester projects have received funding from the NIH (5RO1-NS46259-03, R01DK077903-01A1 NINDS). E.J.H.L. received grants from the Canadian Institutes of Health Research (CIHR). D.P. received grants from the NIH (RFA-DK-13-027) and JDRF during the conduct of the study. N.E. received grants from JDRF and the National Health and Medical Research Council during the conduct of the study. B.A.P. received grants from CIHR, NIH, and JDRF.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding sources played no role in study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Duality of Interest. E.J.H.L. is part owner of Nutarniq Corp., which researches and develops targeted nutritional therapies for chronic diseases and disease complications. B.A.P. receives speaker honoraria from Medtronic, Johnson & Johnson, Insulet, Abbott, Novo Nordisk, and Sanofi; receives research grant support from Medtronic and Boehringer Ingelheim; and serves as a consultant for Boehringer Ingelheim, Insulet, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.J.H.L., N.E., R.A.M., and B.A.P. designed the study. E.J.H.L. and L.E.L. contributed to the data interpretation and wrote the first draft of the manuscript. L.E.L. was responsible for statistical analysis, with contributions from E.J.H.L. All authors participated in data acquisition and interpretation, reviewed the manuscript for scholarly content and accuracy, and gave approval for the final draft. B.A.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018; 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019; the Diabetes Canada and Canadian Society of Endocrinology and Metabolism 21st Professional Conference and Annual Meetings, Halifax, Nova Scotia, Canada, 10–13 October 2018; and 27th Annual Meeting of the Diabetic Neuropathy Study Group of the EASD (NEURODIAB), Rome, Italy, 4–7 September 2018.
Footnotes
This article contains supplementary material online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0951/-/DC1.
E.J.H.L. and L.E.L. are co-first authors.
References
- 1.Pop-Busui R, Boulton AJ, Feldman EL, et al. . Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breiner A, Lovblom LE, Perkins BA, Bril V. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014;37:1418–1424 [DOI] [PubMed] [Google Scholar]
- 3.England JD, Gronseth GS, Franklin G, et al.; American Academy of Neurology; American Association of Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207 [DOI] [PubMed] [Google Scholar]
- 4.Tavakoli M, Quattrini C, Abbott C, et al. . Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010;33:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Bril V, Orszag A, et al. . Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care 2012;35:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petropoulos IN, Alam U, Fadavi H, et al. . Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care 2013;36:3646–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins BA, Lovblom LE, Bril V, et al. . Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia 2018;61:1856–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivaskandarajah GA, Halpern EM, Lovblom LE, et al. . Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care 2013;36:2748–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacaud D, Romanchuk KG, Tavakoli M, et al. . The reliability and reproducibility of corneal confocal microscopy in children. Invest Ophthalmol Vis Sci 2015;56:5636–5640 [DOI] [PubMed] [Google Scholar]
- 10.Ostrovski I, Lovblom LE, Farooqi MA, et al. . Reproducibility of in vivo corneal confocal microscopy using an automated analysis program for detection of diabetic sensorimotor polyneuropathy. PLoS One 2015;10:e0142309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovblom LE, Halpern EM, Wu T, et al. . In vivo corneal confocal microscopy and prediction of future-incident neuropathy in type 1 diabetes: a preliminary longitudinal analysis. Can J Diabetes 2015;39:390–397 [DOI] [PubMed] [Google Scholar]
- 12.Jiang MS, Yuan Y, Gu ZX, Zhuang SL. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. Br J Ophthalmol 2016;100:9–14 [DOI] [PubMed] [Google Scholar]
- 13.Tavakoli M, Ferdousi M, Petropoulos IN, et al. . Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care 2015;38:838–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TM, Bril V. Using in vivo corneal confocal microscopy to identify diabetic sensorimotor polyneuropathy risk profiles in patients with type 1 diabetes. BMJ Open Diabetes Res Care 2017;5:e000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stem MS, Hussain M, Lentz SI, et al. . Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J Diabetes Complications 2014;28:658–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom 2012;95:348–354 [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Ahmed A, Bril V, et al. . Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med 2012;29:e297–e303 [DOI] [PubMed] [Google Scholar]
- 18.Dehghani C, Pritchard N, Edwards K, et al. . Morphometric stability of the corneal subbasal nerve plexus in healthy individuals: a 3-year longitudinal study using corneal confocal microscopy. Invest Ophthalmol Vis Sci 2014;55:3195–3199 [DOI] [PubMed] [Google Scholar]
- 19.Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp 2011;47:2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hume DA, Lovblom LE, Ahmed A, et al. . Higher magnification lenses versus conventional lenses for evaluation of diabetic neuropathy by corneal in vivo confocal microscopy. Diabetes Res Clin Pract 2012;97:e37–e40 [DOI] [PubMed] [Google Scholar]
- 21.Halpern EM, Lovblom LE, Orlov S, Ahmed A, Bril V, Perkins BA. The impact of common variation in the definition of diabetic sensorimotor polyneuropathy on the validity of corneal in vivo confocal microscopy in patients with type 1 diabetes: a brief report. J Diabetes Complications 2013;27:240–242 [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Graham J, Dabbah MA, et al. . Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015;38:1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy D, Abraham R, Reid G. A comparison of two methods for measuring thermal thresholds in diabetic neuropathy. J Neurol Neurosurg Psychiatry 1989;52:1072–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panoutsopoulou IG, Wendelschafer-Crabb G, Hodges JS, Kennedy WR. Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology 2009;72:1205–1210 [DOI] [PubMed] [Google Scholar]
- 25.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol 1998;55:1513–1520 [DOI] [PubMed] [Google Scholar]
- 26.Smith AG, Howard JR, Kroll R, et al. . The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci 2005;228:65–69 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.