Abstract
OBJECTIVE
Limited information is available about glycemic outcomes with a closed-loop control (CLC) system compared with a predictive low-glucose suspend (PLGS) system.
RESEARCH DESIGN AND METHODS
After 6 months of use of a CLC system in a randomized trial, 109 participants with type 1 diabetes (age range, 14–72 years; mean HbA1c, 7.1% [54 mmol/mol]) were randomly assigned to CLC (N = 54, Control-IQ) or PLGS (N = 55, Basal-IQ) groups for 3 months. The primary outcome was continuous glucose monitor (CGM)-measured time in range (TIR) for 70–180 mg/dL. Baseline CGM metrics were computed from the last 3 months of the preceding study.
RESULTS
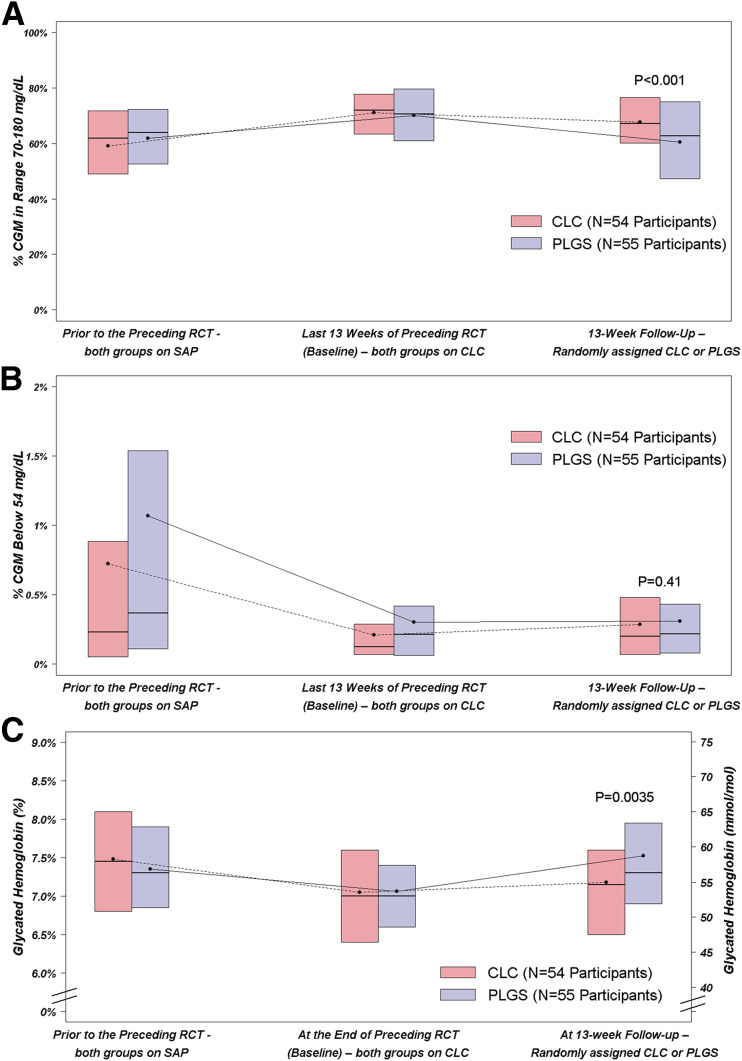
All 109 participants completed the study. Mean ± SD TIR was 71.1 ± 11.2% at baseline and 67.6 ± 12.6% using intention-to-treat analysis (69.1 ± 12.2% using per-protocol analysis excluding periods of study-wide suspension of device use) over 13 weeks on CLC vs. 70.0 ± 13.6% and 60.4 ± 17.1% on PLGS (difference = 5.9%; 95% CI 3.6%, 8.3%; P < 0.001). Time >180 mg/dL was lower in the CLC group than PLGS group (difference = −6.0%; 95% CI −8.4%, −3.7%; P < 0.001) while time <54 mg/dL was similar (0.04%; 95% CI −0.05%, 0.13%; P = 0.41). HbA1c after 13 weeks was lower on CLC than PLGS (7.2% [55 mmol/mol] vs. 7.5% [56 mmol/mol], difference −0.34% [−3.7 mmol/mol]; 95% CI −0.57% [−6.2 mmol/mol], −0.11% [1.2 mmol/mol]; P = 0.0035).
CONCLUSIONS
Following 6 months of CLC, switching to PLGS reduced TIR and increased HbA1c toward their pre-CLC values, while hypoglycemia remained similarly reduced with both CLC and PLGS.
Introduction
Achieving glycemic goals is a challenge not often met in people with type 1 diabetes and requires a balance between lowering glucose into target range, i.e., time in range (TIR), while simultaneously avoiding hypoglycemia (1,2). Insulin delivery systems with low-glucose suspend features are in clinical use to avoid hypoglycemia but, due to their one-sided action, these systems would not be expected to improve TIR or HbA1c (3,4).
More recently, closed-loop control (CLC) systems have been developed to improve TIR in addition to decreasing hypoglycemia (5,6). We conducted a 6-month randomized clinical trial (RCT) of a CLC system, Control-IQ, that was demonstrated to safely improve glycemic control compared with sensor-augmented pump therapy (7). This CLC system, which consists of an insulin pump (t:slim ×2 insulin pump with Control-IQ Technology; Tandem Diabetes Care, San Diego, CA) and a continuous glucose monitor without required fingerstick calibration (Dexcom G6; Dexcom, Inc., San Diego, CA), uses an algorithm that is a third-generation descendant of the University of Virginia closed-loop system, later referred to as inControl by TypeZero Technologies (Charlottesville, VA) (7–9). In the trial, mean TIR for glucose of 70–180 mg/dL increased with CLC by 2.6 h/day compared with a sensor-augmented pump (P < 0.001). Significant reductions were also seen in HbA1c and continuous glucose monitor (CGM) metrics for hyperglycemia and hypoglycemia. There were no severe hypoglycemic events in either group, and one episode of diabetic ketoacidosis (DKA) occurred in the CLC group.
At the conclusion of the 6-month RCT, to compare CLC and a state-of-the-art comparator group, participants in the CLC group who elected to continue in the extension study were randomly assigned to either continue CLC or switch to a predictive low-glucose suspend (PLGS) system (Basal-IQ; Tandem Diabetes Care) that used the same insulin pump and CGM. Herein, we report the results from this randomized CLC versus PLGS comparison with further testing of the efficacy of CLC against a comparator group using a device in increasingly common use in type 1 diabetes.
Research Design and Methods
The study was conducted at seven U.S. diabetes centers. The protocol, which was conducted under an investigational device exemption from the U.S. Food and Drug Administration, was approved by a central institutional review board, and written informed consent was obtained as required including a parental consent and assent for participants age 14 to <18 years.
The 6-month RCT preceding this extension study enrolled individuals ≥14 years old with a clinical diagnosis of type 1 diabetes who were treated with insulin for at least 1 year by pump or multiple daily injections. See Brown et al. (7) for a complete listing of eligibility criteria.
Of the 112 participants in the CLC group in the preceding study, 109 consented to continue in the extension phase. Each participant was randomly assigned 1:1, using a permuted block design stratified by clinical site, to continue CLC or to switch to PLGS. The CLC system or PLGS system was provided to each participant. Although fingersticks were not required for calibration of the CGM, participants received a blood glucose meter (Roche Accu-Chek Guide; Roche Diabetes Care, Indianapolis, IN). A blood ketone meter (Abbott Precision Xtra; Abbott Diabetes Care, Alameda, CA) was provided as well.
The primary outcome was CGM-measured TIR during the 13 weeks of the study. Other relevant outcomes included CGM-measured time <70 mg/dL and HbA1c. Phone contacts were made after 1 week and 2 weeks only for the participants switching from CLC to PLGS, with review of downloaded device data and diabetes management changes made as indicated. HbA1c was measured at randomization and after 13 weeks by a central laboratory at the University of Minnesota Advanced Research and Diagnostic Laboratory. Reportable adverse events included serious adverse events, adverse events occurring in association with a study device or procedure, severe hypoglycemia (defined as hypoglycemia requiring assistance due to altered consciousness), DKA as defined by the Diabetes Control and Complications Trial (10), and hyperglycemia with ketonemia for which a health care provider was contacted. After the 13-week visit (which was the only visit in the trial), all participants were given the option to use CLC until the time that it was commercially available (data not reported).
Statistical Methods
As noted in the report on the main RCT results (7), Control-IQ use was temporarily suspended for about 4 weeks during the extension study, which affected 50 of 54 participants in the CLC group. Intention-to-treat analyses were conducted without exclusion of the data from this period, and per-protocol analyses for primary and closed-loop use outcomes were performed with the data from this suspension period excluded.
Baseline CGM metrics were computed using the last 13 weeks of the main RCT. The primary outcome, CGM-measured TIR 70–180 mg/dL, was compared between groups using a linear mixed effects regression model. Similar analyses were conducted for other CGM and HbA1c metrics. Modification of the treatment effect by baseline variables was assessed by including an interaction term in the models described above. All models and reported treatment group differences included adjustment for the baseline level of the dependent variable, age, and clinical center (random effect).
Descriptive statistics include mean with SD and median with interquartile range (IQR) depending on the distribution of data. All P values are two-tailed. There was no formal adjustment for multiplicity. Analyses were performed using SAS 9.4.
Results
Between 17 January 2019 and 9 April 2019, 109 participants were randomly assigned to continue CLC (N = 54) or to switch to PLGS (N = 55). Mean age was 33 ± 16 years (range 14–72 years), and mean HbA1c was 7.1% ± 0.8% (54 ± 8.7 mmol/mol) (range, 5.5–9.9% [37–85 mmol/mol]). The groups appeared balanced on baseline characteristics (Table 1). The 13-week primary outcome was completed by all 109 participants (Supplementary Fig. 1).
Table 1.
Participant characteristics at time of randomization in this study by treatment group
| PLGS (N = 55) | CLC (N = 54) | |
|---|---|---|
| Age (years) | 34 ± 17 | 32 ± 14 |
| Diabetes duration (years), median (IQR) | 16 (7, 31) | 18 (8, 29) |
| BMI (kg/m2), median (IQR) | 25 (23, 29) | 26 (23, 30) |
| Sex, female | 25 (45) | 28 (52) |
| Race, white* | 46 (87) | 46 (87) |
| Hispanic or Latino ethnicity | 7 (13) | 6 (11) |
| Annual household income† | ||
| <$50,000 | 5 (11) | 5 (12) |
| $50,000 to <$100,000 | 13 (30) | 8 (19) |
| ≥$100,000 | 26 (59) | 29 (69) |
| Highest education level‡ | ||
| Less than a bachelor’s degree | 8 (15) | 8 (15) |
| Bachelor’s degree | 23 (42) | 26 (49) |
| Advanced degree | 24 (44) | 19 (36) |
| Insurance, private§ | 51 (94) | 49 (92) |
| HbA1c, % (mmol/mol) | ||
| Baseline of the preceding RCT | 7.3 ± 0.8 | 7.5 ± 1.1 |
| (56 ± 8.7) | (58 ± 12.0) | |
| At randomization for this trial | 7.1 ± 0.8 | 7.0 ± 0.8 |
| (54 ± 8.7) | (53 ± 8.7) | |
| CGM in TIR 70–180 mg/dL in the preceding RCT (%) | ||
| Baseline | 61.8 ± 16.7 | 59.1 ± 18.3 |
| During 26 weeks | 71.3 ± 12.7 | 71.6 ± 10.7 |
Data are mean ± SD or n (%) unless otherwise indicated.
Two patients in the PLGS group and one in the CLC did not provide race information.
There were 11 patients in the PLGS group and 12 in the CLC group who did not provide income information.
The highest level completed by the patient or by the primary caregiver if the patient was <18 years old. One patient in the CLC group did not provide education information.
One patient in the PLGS group and one in the CLC group did not provide insurance information.
Median CGM use was 97% (IQR 95%, 98%) in the CLC group and 97% (94%, 98%) in the PLGS group. Median closed-loop use in the CLC group was 67% (60%, 79%) overall but 88% (83%, 91%) when excluding the 4-week period in which use of CLC was suspended. Aside from the CLC suspension period, the main reason for CLC being inactive while CGM was in use was related to connectivity issues between the CGM and pump. Mean number of fingersticks per day was 0.8 ± 1.2 in the CLC group and 1.0 ± 1.3 in the PLGS group (median, 0 and 1, respectively).
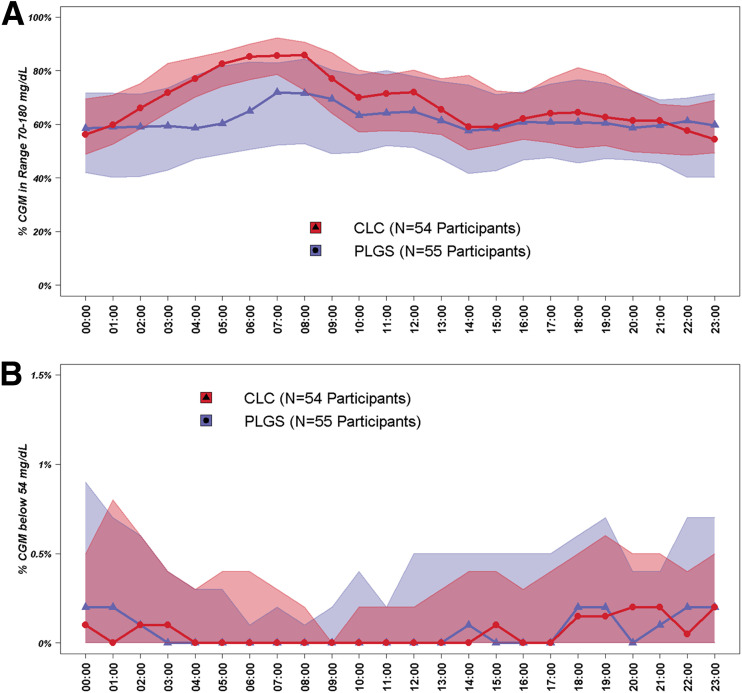
Overall, TIR 70–180 mg/dL was 59.1 ± 18.3% prior to the preceding RCT, 71.1 ± 11.2% during the last 13 weeks of the preceding RCT, and 67.6 ± 12.6% during the 13 weeks of this study in the CLC group; and it was 61.8 ± 16.7%, 70.0 ± 13.6%, and 60.4 ± 17.1%, respectively, in the PLGS group (risk-adjusted difference during 13 weeks of study = 5.9%; 95% CI 3.6%, 8.3%; P < 0.001) (Table 2, Fig. 1A, Fig. 2A, and Supplementary Figs. 2 and 3). In a per-protocol analysis excluding the 4-week period of Control-IQ suspension, the TIR 70–180 mg/dL in the CLC group during this study was 69.1 ± 12.2%, and the risk-adjusted treatment group difference compared with the PLGS group was 7.5% (95% CI 5.3%, 9.8%; P < 0.001). CGM hyperglycemia metrics were lower with CLC than PLGS while hypoglycemia metrics were similar in the two groups (Table 2, Fig. 1B, and Fig. 2B). Similar results were seen for daytime and nighttime (Supplementary Tables 1A and 1B). TIR 70–180 mg/dL outcomes in subgroups are provided in Supplementary Table 2.
Table 2.
Primary and secondary CGM-measured efficacy outcomes
| Prior to the preceding RCT | Last 13 weeks of preceding RCT (baseline) | 13 weeks of follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| CLC (N = 54) | PLGS (N = 55) | CLC (N = 54) | PLGS (N = 55) | CLC (N = 54) | PLGS (N = 55) | Risk-adjusted difference (95% CI)† | P value† | |
| Hours of sensor data | 306 (288, 326) | 307 (283, 327) | 2,127 (2,083, 2,141) | 2,109 (2,074, 2,132) | 2,049 (1,966, 2,162) | 2,030 (1,945, 2,109) | NA | NA |
| Primary outcome | ||||||||
| TIR 70–180 mg/dL (%), mean ± SD | 59.1 ± 18.3 | 61.8 ± 16.7 | 71.1 ± 11.2 | 70.0 ± 13.6 | 67.6 ± 12.6 | 60.4 ± 17.1 | 5.9 (3.6, 8.3) | <0.001 |
| Overall control, mean ± SD | ||||||||
| TIR 70–140 mg/dL (%) | 37.4 ± 15.8 | 39.6 ± 15.5 | 44.9 ± 11.8 | 44.8 ± 12.7 | 42.0 ± 12.5 | 37.1 ± 14.2 | 4.7 (2.5, 7.0) | <0.001 |
| Mean glucose (mg/dL) | 169 ± 34 | 163 ± 31 | 156 ± 17 | 158 ± 23 | 160 ± 20 | 170 ± 30 | −7 (−11, −4) | <0.001 |
| SD (mg/dL) | 61 ± 14 | 60 ± 14 | 52 ± 11 | 54 ± 13 | 55 ± 12 | 60 ± 15 | −3 (−5, −1) | 0.0035 |
| Coefficient of variation (%) | 36 ± 7 | 37 ± 6 | 33 ± 4 | 34 ± 5 | 34 ± 4 | 35 ± 5 | −1 (−2, 1) | 0.32 |
| Hypoglycemia | ||||||||
| % Below 54 mg/dL | 0.23 (0.05, 0.88) | 0.37 (0.10, 1.58) | 0.12 (0.07, 0.29) | 0.21 (0.06, 0.47) | 0.20 (0.07, 0.48) | 0.22 (0.06, 0.46) | ||
| mean ± SD | 0.72 ± 1.28 | 1.07 ± 1.44 | 0.21 ± 0.20 | 0.30 ± 0.37 | 0.29 ± 0.30 | 0.31 ± 0.31 | 0.04 (−0.05, 0.13) | 0.41 |
| % Below 60 mg/dL | 0.60 (0.16, 1.76) | 1.02 (0.35, 2.89) | 0.30 (0.19, 0.65) | 0.44 (0.14, 0.94) | 0.43 (0.19, 0.97) | 0.46 (0.17, 0.96) | 0.09 (−0.07, 0.24) | 0.28 |
| mean ± SD | 1.31 ± 1.91 | 1.77 ± 2.00 | 0.45 ± 0.37 | 0.60 ± 0.64 | 0.61 ± 0.58 | 0.64 ± 0.57 | ||
| % Below 70 mg/dL | 2.39 (0.78, 4.55) | 3.41 (1.06, 5.53) | 1.09 (0.68, 1.79) | 1.30 (0.68, 2.37) | 1.35 (0.73, 2.57) | 1.48 (0.76, 2.67) | 0.13 (−0.18, 0.45) | 0.41 |
| mean ± SD | 3.28 ± 3.43 | 3.88 ± 3.38 | 1.35 ± 0.92 | 1.57 ± 1.26 | 1.75 ± 1.34 | 1.84 ± 1.40 | ||
| Low blood glucose index | 0.69 (0.31, 1.08) | 0.98 (0.38, 1.57) | 0.37 (0.25, 0.57) | 0.43 (0.29, 0.66) | 0.44 (0.25, 0.77) | 0.52 (0.35, 0.81) | −0.01 (−0.08, 0.07) | 0.84 |
| mean ± SD | 0.87 ± 0.79 | 1.07 ± 0.81 | 0.45 ± 0.26 | 0.50 ± 0.30 | 0.53 ± 0.34 | 0.59 ± 0.37 | ||
| Hypoglycemic event rate per week* | 3.6 (1.8, 6.6) | 5.3 (2.6, 8.6) | 2.5 (1.5, 3.9) | 2.8 (1.4, 4.6) | 3.0 (1.5, 4.9) | 3.1 (1.6, 5.3) | 0.1 (−0.3, 0.6) | 0.58 |
| mean ± SD | 4.6 ± 3.5 | 5.9 ± 4.3 | 2.9 ± 1.8 | 3.2 ± 2.4 | 3.3 ± 2.1 | 3.5 ± 2.4 | ||
| Hyperglycemia | ||||||||
| % Above 180 mg/dL | 35 (26, 48) | 32 (22, 45) | 26 (21, 35) | 27 (19, 38) | 32 (22, 39) | 36 (22, 51) | −6.04 (−8.40, −3.68) | <0.001 |
| mean ± SD | 38 ± 20 | 34 ± 18 | 28 ± 11 | 28 ± 14 | 31 ± 13 | 38 ± 18 | ||
| % Above 250 mg/dL | 8.6 (4.6, 18.9) | 8.3 (3.6, 15.8) | 4.7 (2.4, 9.8) | 6.0 (2.3, 10.4) | 7.1 (3.2, 11) | 9.3 (3.6, 18) | −2.46 (−3.92, −1.01) | 0.001 |
| mean ± SD | 13.1 ± 13.4 | 11.6 ± 12.5 | 6.7 ± 5.5 | 8.2 ± 8.8 | 8.4 ± 7.1 | 12.8 ± 12.2 | ||
| % Above 300 mg/dL | 2.2 (1.0, 7.4) | 2.3 (0.6, 6.2) | 1.2 (0.4, 3.0) | 1.3 (0.4, 3.7) | 1.8 (0.5, 3.7) | 2.5 (0.8, 6.1) | −0.89 (−1.76, −0.01) | 0.05 |
| mean ± SD | 5.4 ± 7.6 | 4.6 ± 7.1 | 2.1 ± 2.3 | 3.1 ± 4.9 | 2.8 ± 3.6 | 5.1 ± 7.0 | ||
| High blood glucose index | 7.4 (5.6, 11.6) | 7.0 (4.6, 10.4) | 5.8 (4.5, 8.3) | 5.7 (4.3, 8.6) | 6.9 (4.6, 8.6) | 7.6 (5.2, 11.6) | −1.3 (−2, −0.7) | <0.001 |
| mean ± SD | 9.0 ± 5.8 | 8.2 ± 5.1 | 6.2 ± 2.7 | 6.8 ± 3.9 | 7.0 ± 3.3 | 9.0 ± 5.2 | ||
Data are median (IQR) unless otherwise indicated. NA, not available.
†Adjusted for baseline value of the dependent variable plus age, and clinical center (random effects).
At least 15 consecutive minutes <70 mg/dL.
Figure 1.
CGM % TIR 70–180 mg/dL (A) and % time <54 mg/dL (B) prior to preceding RCT, during last 13 weeks of preceding RCT, and during 13 weeks of current RCT. Corresponding HbA1c values (C).
Figure 2.
Envelope plots during current RCT. CGM % TIR 70–180 mg/dL (A) and % time <54 mg/dL (B).
Mean HbA1c was 7.48 ± 1.10% (58 ± 12 mmol/mol) prior to the start of the preceding RCT, 7.05 ± 0.78% (54 ± 8.5 mmol/mol) at the start of this study, and 7.18 ± 0.80% (55 ± 8.7 mmol/mol) at 13 weeks in the CLC group and 7.35 ± 0.83% (57 ± 9.1 mmol/mol), 7.06 ± 0.77% (54 ± 8.4 mmol/mol) and 7.53 ± 1.14% (59 ± 12.5 mmol/mol), respectively, in the PLGS group (adjusted treatment group difference −0.34% (95% CI −0.57%, −0.11%; P = 0.0035) (Fig. 1C and Supplementary Table 3). There was no difference between groups in daily insulin amount (P = 0.25) or weight change (P = 0.39) (Supplementary Table 4).
There were no severe hypoglycemia or DKA events in either group. Three reportable episodes of hyperglycemia with ketosis occurred in the PLGS group, with two being due to infusion set failure and one being associated with illness (kidney stone), and none occurred in the CLC group (Supplementary Table 5).
Conclusions
This multicenter randomized trial in people with type 1 diabetes is the first large randomized trial comparing PLGS versus CLC, with both systems using the same sensor and insulin pump. Thus, the only difference between the two systems was the control strategy (predictive PLGS vs. CLC). The trial found that participants who continued using CLC for an additional 3 months, on average, generally maintained the TIR improvement seen during the prior RCT, while participants who transitioned to use of PLGS for 3 months had, on average, approximately a 10% reduction in their TIR to levels similar to their pre-CLC levels. There were no differences in hypoglycemia between the two systems; thus, the TIR improvement on CLC was primarily due to reduction in hyperglycemia via active control of insulin delivery compared with passive PLGS. The improved TIR in the continued CLC group was accompanied by lower HbA1c levels compared with the PLGS group. Therefore, this multicenter study provides further evidence of efficacy and safety of this CLC system versus a state-of-the art comparator group.
Studies using PLGS report similar hypoglycemia reduction, and most (11,12) but not all studies (3) report no substantial increase in hyperglycemia. CLC in contrast has the potential to further improve TIR and reduce hyperglycemia, and these results are consistent with prior studies using the same CLC (7). In addition, the specific CLC algorithm has a design feature of tightening overnight control by lowering the target to 112.5–120 mg/dL in the morning and, as such, has more pronounced improvements in glycemic control overnight compared with PLGS. These results are consistent with a prior study of evening versus daytime use of CLC (13), and they show that there is a significant opportunity for daytime CLC improvement via more aggressive postprandial strategies as long as hypoglycemia reduction is maintained.
Similar to the main RCT, CLC was active close to a median of 90% of the time (when the CLC suspension period due to a software issue was excluded), and median CGM use was more than 95% of the time. The results were achieved with performance of very few blood glucose meter measurements. Given users’ concerns over the complexity of CLC systems, these results are relevant and may reflect the ease of use of the system.
Strengths of the study included a protocol that mirrored the amount of contact that might occur in real-world use of a CLC system, i.e., a single visit after 3 months, no remote monitoring, a high participant retention rate, and a high degree of adherence to the treatment assignment. In addition, no other large studies have compared CLC to PLGS systems that are in clinical use and none to our knowledge that are using the same platforms. As noted in reporting the results of the RCT preceding this study, the interpretation of the results must be viewed in the context of the participant characteristics and the setting of a university-based diabetes centers, as well as the fact that all participants were experienced with the use of a CGM and this specific CLC system, having completed the prior RCT.
In conclusion, the results of this study demonstrate that switching to PLGS following 6-months of CLC reduced TIR and increased HbA1c toward their pre-CLC values, while hypoglycemia remained similarly reduced with both CLC and PLGS.
Article Information
Funding. This project was funded by National Institute of Diabetes and Digestive and Kidney Diseases grant UC4 108483. The University of Virginia Strategic Investment Fund Project number 88 provided institutional and regulatory support. Tandem Diabetes Care provided the experimental closed-loop systems used in the trial, system-related supplies including the Dexcom CGM and Roche glucometer, and technical expertise.
Tandem Diabetes Care was not involved in data analysis and was provided a copy of the manuscript for review prior to publication.
Duality of Interest. S.A.B. reports receiving grant support and supplies, paid to her institution, from Tandem Diabetes Care, Insulet, and Tolerion and supplies, provided to her institution, from Dexcom and Roche Diagnostics. R.W.B. reports receiving consulting fees, paid to his institution, from Insulet, Bigfoot Biomedical, and Eli Lilly; grant support and supplies, provided to his institution, from Tandem and Dexcom; and supplies from Ascenia and Roche. B.A.B. reports receiving grant support and advisory board fees from Medtronic Diabetes and ConvaTec, grant support and presentation fees from Insulet, advisory board fees from Novo Nordisk and Profusa, grant support from Eli Lilly, grant support and equipment from Dexcom, and holding patent 61197230 on a hypoglycemia prediction algorithm. L.M.L. reports receiving consulting fees from Dexcom, Sanofi, Eli Lilly, Novo Nordisk, Roche, Boehringer Ingelheim, Johnson & Johnson, Insulet, Insulogic, ConvaTec, and Merck. R.P.W. reports receiving grant support, consulting fees, and supplies, provided to his institution, from Dexcom; advisory fees from Medtronic; grant support, provided to his institution, from Tandem Diabetes Care and Bigfoot Biomedical; grant support, paid to his institution, advisory board fees, and supplies, provided to his institution, from Eli Lilly; and grant support, paid to his institution, and supplies, provided to his institution, from MannKind and Novo Nordisk. Y.C.K. reports receiving supplies from Dexcom, Roche Diabetes, and Tandem Diabetes Care; grant support from Medtronic Diabetes; consulting fees from Novo Nordisk; and holding patent US9486172B2 on estimation of insulin sensitivity from CGM and subcutaneous insulin delivery in type 1 diabetes. C.J.L. reports receiving advisory board fees from Sanofi and grant support, paid to her institution, from Dexcom, Tandem Diabetes Care, Insulet, Abbott Diabetes, Senseonics, and Lexicon Pharmaceuticals. J.E.P. reports receiving grant support, provided to his institution, consulting fees, and speaker fees from Tandem Diabetes Care; grant support, provided to his institution, and advisory board fees from Medtronic; grant support, provided to his institution, and consulting fees from Eli Lilly; grant support and supplies, provided to his institution, from Insulet; and supplies, provided to his institution, from Dexcom. E.D. reports receiving equipment and supplies from Tandem Diabetes Care, LifeScan, and Dexcom; consulting fees, royalties, equipment, and supplies from Insulet; fees for serving on a speakers’ bureau, equipment, and supplies from Roche; consulting fees from Eli Lilly; grant support and drugs from Xeris; consulting fees and royalties from Mode AGC; holding patents 9,984,773 and 2,957,432 on moving-horizon state-initializer from control applications, for which royalties are received; holding patent 9,907,515 on a health monitoring system, for which royalties are received; holding patent 2,897,189 on a model-based personalization scheme of an artificial pancreas for type 1 diabetes applications, for which royalties are received; holding patents 9,700,708, 6,062,859, and 2,816,388 on maintaining multiple defined physiological zone using model predictive control, for which royalties are received; holding patents 2,897,925 and 2014800152784 on daily periodic target-zone modulation in the model predictive control problem for artificial pancreas for type 1 diabetes applications, for which royalties are received; holding patent 2,789,630 on systems, devices, and methods to deliver biological factors or drugs to a subject, for which royalties are received; holding patent 2,521,483 B1 on a system to deliver insulin to a subject, for which royalties are received; holding patent 8,762,070 on systems, devices, and methods to deliver biological factors or drugs to a subject, for which royalties are received; holding pending patent 14/734,994 on systems and method of variable dose glucagon delivery; holding patents 10,327,681 and 2014349078 on glucose rate increase detector, a meal detection module for the health monitoring system, for which royalties are received; holding pending patent 61/751,942 on daily periodic target-zone modulation in the model predictive control problem for artificial pancreas for type 1 diabetes applications, for which royalties are received; and holding pending patent 61/751,941 on model-based personalization scheme of an artificial pancreas for type 1 diabetes applications, for which royalties are received. F.J.D. reports receiving equity, holding licensed intellectual property, and serving on an advisory board for Mode AGC; holding patents 9,984,773 and 2,957,432 on moving-horizon state-initializer for control applications, for which royalties are received; holding patent 9,907,515 on health monitoring system, for which royalties are received; holding patent 2,897,189 on model-based personalization scheme of an artificial pancreas for type 1 diabetes applications, for which royalties are received; holding patent 2,957,432 on moving-horizon stat-initializer for control applications, for which royalties are received; holding patents 9,700,708, 6,062,859, and 2,816,388 on maintaining multiple defined physiological zones using model predictive control, for which royalties are received; holding patents 2,897,925 and 2014800152784 on daily periodic target-zone modulation in the model predicative control problem for artificial pancreas for type 1 diabetes applications, for which royalties are received; holding patent 2,789,630 on systems, devices, and methods to deliver biological factors or drugs to a subject, for which royalties are received; holding pending patent 14/734,994 on systems and method of variable dose glucagon delivery; holding patents 10,327,68 and 2014349078 on glucose rate increase detector, a meal detection module for the health monitoring system, for which royalties are received; holding pending patent 61/751,942 on daily periodic target-zone modulation in the model predictive control problem for artificial pancreas for type 1 diabetes application, for which royalties are received; and holding pending patent 61/751,941 on model-based personalization scheme of an artificial pancreas for type 1 diabetes applications, for which royalties are received. L.A.-O. has received consulting fees from Dexcom. S.M.A. reports receiving grant support from Medtronic. G.P.F. reports receiving grants support and lecture fees from Medtronic, MiniMed, Insulet, and Tandem; grant support from Abbott; and grant support and consulting fees from Eli Lilly. C.K. has received consulting fees, paid to his institution, from Bigfoot Biomedical and grant support and supplies, provided to his institution, from Tandem and Dexcom. J.W.L. reports receiving consulting fees, paid to his institution, from Animas Corporation, Bigfoot Biomedical, Tandem Diabetes Care, and Eli Lilly. B.P.K. reports receiving lecture fees and equipment, provided to the University of Virginia, from Dexcom; grant support, paid to the University of Virginia, advisory board fees, and consulting fees from Sanofi; consulting fees and equipment, provided to the University of Virginia, from Tandem Diabetes Care; holding patents 8,562,587 and 9,750,438 B2 on CGM-based prevention of hypoglycemia via hypoglycemia risk assessment and smooth reduction of insulin delivery, licensed to Dexcom, for which royalties are received; and holding patent 9,4,30,022 B2 on the method and apparatus for modular power management and protection of critical services in ambulatory medical devices, licensed to Dexcom, for which royalties are received. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A.B. was involved in study design and wrote and edited the manuscript. R.W.B., B.A.B., L.M.L., R.P.W., Y.C.K., C.J.L., J.E.P., E.D., F.J.D., L.A.-O., S.M.A., M.M.C., L.E., G.P.F., C.L., V.S., M.D.B., C.K., and J.W.L. researched data, contributed to discussion, and reviewed/edited the manuscript. D.R. performed statistical analyses and wrote and edited the manuscript. B.P.K. was involved in study design and wrote and edited the manuscript. J.W.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A listing of the iDCL Trial Research Group members appears in the supplementary material.
Clinical trial reg. no. NCT03591354, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.12240968.
Contributor Information
Collaborators: iDCL Trial Research Group, Boris Kovatchev, Stacey Anderson, Emma Emory, Mary Voelmle, Katie Conshafter, Kim Morris, Mary Oliveri, Linda Gondor-Fredrick, Harry Mitchell, Kayla Calvo, Christian Wakeman, Marc Breton, Lori Laffel, Elvira Isganaitis, Louise Ambler-Osborn, Emily Flint, Kenny Kim, Lindsay Roethke, Jordan Pinsker, Mei Mei Church, Camille Andre, Molly Piper, Carol Levy, David Lam, Grenye O’Malley, Camilla Levister, Selassie Ogyaadu, Jessica Lovett, Yogish C. Kudva, Vinaya Simha, Vikash Dadlani, Shelly McCrady-Spitzer, Corey Reid, Kanchan Kumari, R. Paul Wadwa, Greg Forlenza, G. Todd Alonso, Robert Slover, Emily Jost, Laurel Messer, Cari Berget, Lindsey Towers, Alex Rossick-Solis, Bruce Buckingham, Laya Ekhlaspour, Tali Jacobson, Marissa Town, Ideen Tabatabai, Jordan Keller, Evalina Salas, Francis Doyle, III, Eyal Dassau, John Lum, Roy Beck, Samantha Passman, Tiffany Campos, Dan Raghinaru, Craig Kollman, Carlos Murphy, Nandan Patibandla, Sarah Borgman, Guillermo Arreza-Rubin, Thomas Eggerman, Neal Green, Boris Kovatchev, Sue Brown, Stacey Anderson, Marc Breton, Lori Laffel, Jordan Pinsker, Carol Levy, Yogish C. Kudva, R. Paul Wadwa, Bruce Buckingham, Francis Doyle III, Eric Renard, Claudio Cobelli, Yves Reznik, Guillermo Arreza-Rubin, John Lum, Roy Beck, Robert Janicek, Deanna Gabrielson, Steven H. Belle, Jessica Castle, Jennifer Green, Laurent Legault, Steven M. Willi, Carol Wysham, and Thomas Eggerman
References
- 1.American Diabetes Association 6. Glycemic targets: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl. 1):S66–S76 [DOI] [PubMed] [Google Scholar]
- 2.Foster NC, Beck RW, Miller KM, et al. . State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care 2017;40:764–770 [DOI] [PubMed] [Google Scholar]
- 4.Chen E, King F, Kohn MA, Spanakis EK, Breton M, Klonoff DC. A review of predictive low glucose suspend and its effectiveness in preventing nocturnal hypoglycemia. Diabetes Technol Ther 2019;21:602–609 [DOI] [PubMed] [Google Scholar]
- 5.Bergenstal RM, Garg S, Weinzimer SA, et al. . Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 6.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 7.Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown S, Raghinaru D, Emory E, Kovatchev B. First look at control-IQ: a new-generation automated insulin delivery system. Diabetes Care 2018;41:2634–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keith-Hynes P, Guerlain S, Mize B, et al. . DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 2013;7:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 11.Abraham MB, Nicholas JA, Smith GJ, et al.; PLGM Study Group . Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care 2018;41:303–310 [DOI] [PubMed] [Google Scholar]
- 12.Forlenza GP, Li Z, Buckingham BA, et al. . Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care 2018;41:2155–2161 [DOI] [PubMed] [Google Scholar]
- 13.Kovatchev BP, Kollar L, Anderson SM, et al. . Evening and overnight closed-loop control versus 24-7 continuous closed-loop control for type 1 diabetes: a randomised cross-over trial. Lancet Digit Health 2020;2:e64–e73 [DOI] [PMC free article] [PubMed] [Google Scholar]