Abstract
OBJECTIVE
Type 2 diabetes has been associated with depression. However, the underlying pathophysiological mechanisms remain unknown. Cerebral small vessel disease, a consequence of diabetes, may lead to depression. Therefore, we evaluated whether cerebral small vessel disease mediates the association between type 2 diabetes and higher depressive symptoms.
RESEARCH DESIGN AND METHODS
We used longitudinal data from the population-based Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study, with examinations from 2002 to 2006 and 5 years later. Type 2 diabetes was defined as self-reported history of type 2 diabetes, use of blood glucose–lowering drugs, or fasting blood glucose level ≥7.0 mmol/L. Cerebral small vessel disease load was quantified in a composite score based on MRI-defined presence of high white matter hyperintensity volume, low total brain parenchyma volume, and subcortical infarcts, cerebral microbleeds, and large perivascular spaces. The 5-year change in the 15-item Geriatric Depression Scale score (GDS-15) was measured between baseline and follow-up.
RESULTS
Included were 2,135 individuals without dementia and baseline depression (baseline age 74.5 [SD 4.6] years, 1,245 women [58.3%], and 197 [9.2%] with diabetes). The GDS-15 score increased 0.4 (SD 1.6) points over time. Baseline diabetes was associated with a greater increase in the GDS-15 score (β = 0.337; 95% CI 0.094; 0.579), adjusted for age, sex, education, and cardiovascular risk factors. Baseline cerebral small vessel disease and change of cerebral small vessel disease statistically significantly mediated a part of this association.
CONCLUSIONS
Type 2 diabetes is associated with a greater increase in depressive symptoms score over 5 years, and cerebral small vessel disease partly explains this association.
Introduction
Depression is an important health concern in type 2 diabetes. It is twice as common in individuals with type 2 diabetes as in the general population and is associated with a higher risk of diabetes-related complications and mortality (1). The factors that mediate the relation between type 2 diabetes and depression are, however, incompletely understood. Cerebral microvascular dysfunction and damage may be such a mediator. Cerebral microvascular dysfunction may disrupt deep and frontal brain structures involved in mood regulation, leading to depressive symptoms (2,3).
Cerebral small vessel disease, a marker of cerebral microvascular dysfunction and damage (4), can be measured on brain MRI as higher white matter hyperintensity volume, lower total brain parenchyma volume, subcortical infarcts, cerebral microbleeds, and large perivascular spaces (4). These MRI features are more prevalent in individuals with type 2 diabetes than in those without (5). In addition, cerebral small vessel disease is associated with a higher incidence of depressive symptoms (6). However, no previous study has investigated whether the association between type 2 diabetes and depressive symptoms is explained or mediated by cerebral small vessel disease.
In view of the above, we investigated, in a large population-based cohort, the association between baseline type 2 diabetes and change in depressive symptoms over time. In addition, we evaluated whether this association was explained by MRI features of cerebral small vessel disease, including higher white matter hyperintensity volume, lower total brain parenchyma volume, subcortical infarcts, cerebral microbleeds, and large perivascular spaces.
Research Design and Methods
Participants
Participants were included from the population-based Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study cohort originating from the Reykjavik Study, described previously (7). Briefly, from 2002 to 2006, 5,764 surviving participants of the Reykjavik Study were examined, and 3,316 (57.5%) were reexamined 5 years later, from 2007 to 2011. Reasons for not attending the follow-up examination included death (n = 1,039), refusal (n = 1,198), and lost to follow-up (n = 211). Individuals who did not participate in the second examination (n = 2,448), compared with individuals who participated in both examinations (n = 3,316), had higher baseline depression symptoms scores (15-item Geriatric Depression Scale [15-GDS] score ≥6: 10.9% vs. 5.1%) and more often used antidepressant medication at baseline (21.2% vs. 12.1%). The AGES-Reykjavik Study was approved by the National Bioethics Committee in Iceland (approval number, VSN-00-063) and by the Institutional Review Board overseeing the National Institute on Aging and the National Institutes of Health. After complete description of the study to the subject, written informed consent was obtained.
Type 2 Diabetes
Baseline type 2 diabetes was defined as self-reported history of type 2 diabetes, use of blood glucose-lowering drugs, or a fasting blood glucose level ≥7.0 mmol/L (8).
Depressive Symptoms
Depressive symptoms were assessed with the GDS-15 (score range 0–15) at baseline and at follow-up 5 years later (9,10). We calculated the 5-year change in the GDS-15 score over time by subtracting the GDS-15 score at baseline from the GDS-15 score at follow-up. In addition, we computed separately an apathy subscale from the three apathy items of the GDS-15 (GDS-3A, range 0–3) and a subscale including the remaining depression items (GDS-12D, range 0–12), as described previously (11). Additionally, use of antidepressant medication (tricyclics, selective serotonin reuptake inhibitors, other nontricyclics, and monoamine oxidase inhibitors) was assessed from medication bottles brought to the clinic at baseline and at follow-up.
Brain MRI Measures
Image Acquisition
All eligible participants were offered high-resolution 1.5T brain MRI (Signa TwinSpeed; General Electric Medical Systems). A standardized imaging protocol, described previously (12,13), was used at baseline and follow-up and included the following sequences: 3-dimensional spoiled-gradient recalled T1-weighted, proton density/T2-weighted fast spin-echo, fluid-attenuated inversion recovery (FLAIR) and T2*-weighted gradient-echo type echoplanar image (GRE-EPI). All images were acquired to give full brain coverage with slices angled parallel to the anterior commissure–posterior commissure line to give reproducible image views in the oblique-axial plane.
Image Analysis
Five features of cerebral small vessel disease were evaluated: white matter hyperintensity volume, total brain parenchyma volume, subcortical infarcts, cerebral microbleeds, and large perivascular spaces. White matter hyperintensity volume and total brain parenchyma volume were computed automatically with a previously described image analysis pipeline (14) and were expressed as the percentage of total intracranial volume. Subcortical infarcts were defined as subcortical brain parenchyma defects, as described previously (13), with a diameter of ≥4 mm and a signal intensity similar to cerebrospinal fluid on all pulse sequences (T2-weighted, proton density-weighted, and FLAIR), surrounded by an area of high signal intensity on FLAIR images and without evidence of hemosiderin on T2*-weighted GRE-EPI sequence. No upper limit was used for the size of subcortical infarcts. Cerebral microbleeds were defined as focal areas of signal void visible on the T2*-weighted GRE-EPI sequence (12). Large perivascular spaces were defined as defects in the subcortical area without a rim or area of high signal intensity on FLAIR and without evidence of hemosiderin on the T2*-weighted GRE-EPI sequence (15).
Confounding Variables
Selection of confounding and covarying measures was based on previous studies (16,17) showing their association with type 2 diabetes or depressive symptoms. All covariates were determined at baseline, unless otherwise specified. The following variables were assessed by questionnaire: education level (primary, secondary, and college/university education), smoking history (never, former, current), and alcohol use. Alcohol use was assessed with the following questions: “How often do you drink (alcohol) in a month?” and “How many drinks do you consume on a single occasion?” We multiplied the number of occasions by the amount consumed on each occasion to calculate the approximate number of drinks consumed per month. BMI was calculated as measured weight in kilograms divided by height in centimeters squared. Hypertension was defined as systolic pressure ≥140 mmHg, diastolic pressure ≥90 mmHg, or use of antihypertensive medication. Total cholesterol–to–HDL cholesterol ratio and coronary artery disease were determined as described previously (7). Strokes (i.e., symptomatic brain infarct or hemorrhage) prevalent at baseline were obtained from medical records. Incident strokes that occurred between the baseline and follow-up examination were adjudicated by a dementia neurologist, a stroke neurologist, and a neuroradiologist, as described previously (7). Dementia was diagnosed according to international guidelines (18) by a panel that included a geriatrician, a neurologist, a neuropsychologist, and a neuroradiologist, as described previously (7).
Analytic Sample
Of the 3,316 participants who attended the follow-up examination, 207 were excluded because of a diagnosis of dementia at baseline (n = 47) or at follow-up (n = 160). In addition, participants were excluded if they had missing data on brain MRI (n = 701) or the GDS-15 score at baseline or at follow-up (n = 149), or confounders (n = 18). Finally, we excluded participants with baseline clinically relevant depressive symptoms, defined as a GDS-15 score ≥6 (n = 106). In the main analysis, we did not exclude participants using antidepressant medication, because these medications are also prescribed for indications other than depression (e.g., diabetic polyneuropathy). The final study sample for the main analysis consisted of 2,135 participants.
Statistical Analysis
We calculated the mean (SD) or median (interquartile range) of the characteristics for the total study population, according to presence of type 2 diabetes, and for those included and excluded for the present analysis.
We summarized the baseline cerebral small vessel disease features and the 5-year change in cerebral small vessel disease features over time into a composite score of baseline cerebral small vessel disease and a composite score of cerebral small vessel disease change, because we hypothesize that each cerebral small vessel disease feature may mediate the association between type 2 diabetes and depressive symptoms according to similar mechanisms. A composite score reduces the influence of the biological variability of its components (19) and requires fewer statistical tests. We calculated a composite score of baseline cerebral small vessel disease, as described previously (3). One point per cerebral small vessel disease feature at baseline was assigned based on the following cutoffs: for high white matter, hyperintensity volume quartile 4 versus quartiles 1–3; for low total brain parenchyma volume, quartile 1 versus quartiles 2–4; and for subcortical infarcts, cerebral microbleeds, and large perivascular spaces, presence versus absence. The points for each feature were summed to compute the composite score of baseline cerebral small vessel disease (range 0–5). Subsequently, we calculated a composite score of cerebral small vessel disease change between the baseline and follow-up examination by assigning 1 point for each cerebral small vessel disease feature based on the following cutoffs: for high increase in white matter hyperintensity volume, quartile 4 versus quartiles 1–3; for high decrease in total brain parenchyma, quartile 4 versus quartiles 1–3; and for new subcortical infarcts, cerebral microbleeds, and large perivascular spaces, one or more new lesion(s) versus no new lesions.
The statistical analysis proceeded in two stages. First, multivariable linear regression analyses were used to estimate the associations between type 2 diabetes at baseline, the composite score of baseline cerebral small vessel disease, the composite score of cerebral small vessel disease change, and the 5-year change in the GDS-15 score. Analyses were adjusted for baseline age, sex, education level (model 1), and additionally for baseline alcohol use, smoking history, BMI, hypertension, and the total cholesterol–to–HDL cholesterol ratio (model 2).
Second, we performed a mediation analysis to test the hypothesis that cerebral small vessel disease features explain the association between type 2 diabetes at baseline and change in the GDS-15 score. The mediation model quantifies the degree to which a variable statistically explains the association between a determinant and an outcome variable. We tested separately the explained associations of the baseline composite score of cerebral small vessel disease and the composite score of cerebral small vessel disease change. We used bootstrapping (10,000 samples) to calculate bias-corrected 95% CIs of the explained associations using the PROCESS 3.4 statistical package for PASW Statistics software (20). PROCESS is a path analysis modeling tool that uses ordinary least squares and logistic regression for estimating the regression coefficients and corresponding 95% CIs of total, direct, and indirect effects in a mediation model.
We did several secondary analyses to examine the robustness of our associations. First, we repeated the analysis with adjustment for each individual cerebral small vessel disease feature in the model separately, and we calculated which percentage of the explained effect by the composite scores was accounted for by the individual cerebral small vessel disease features.
Second, we evaluated the association between type 2 diabetes at baseline and the GDS-15 score at follow-up as the outcome instead of the 5-year change in the GDS-15 score and with additional adjustment for the GDS-15 score at baseline.
Third, we evaluated the association between type 2 diabetes at baseline and change in the GDS-15 score after excluding participants using antidepressant medication at baseline.
Fourth, it has been suggested that cerebral small vessel disease features may be most strongly associated with apathy-related symptoms of depression (11). Therefore, we tested the association between type 2 diabetes at baseline with change in the GDS-3A and GDS-12D scores, respectively, and evaluated whether the composites scores of cerebral small vessel disease mediated this association.
Fifth, we evaluated the association between baseline type 2 diabetes and change in the GDS-15 score, adjusting for baseline coronary artery disease. Data on baseline coronary artery disease were missing in 220 participants.
Sixth, we repeated the analysis additionally adjusting for baseline stroke or incident stroke during follow-up, which may further mediate or confound our associations with cerebral small vessel disease (21,22).
Seventh, we repeated the analysis after excluding individuals with a baseline or incident subcortical infarct with a size ≥15 mm to limit the potential confounding effect by large vessel disease.
All analyses were done with PASW Statistics (version 23). A P value of <0.05 was considered statistically significant.
Results
The mean age of the participants at baseline was 74.5 (SD 4.6) years, 58.7% were women, and 9.2% had type 2 diabetes (Table 1). The mean time between baseline and follow-up was 5.2 (SD 0.2) years. On average, the GDS-15 score increased 0.4 (SD 1.6) points over 5 years. The GDS-15 score increased 0.7 (SD 1.9) points in participants with type 2 diabetes and 0.3 (SD 1.6) points in participants without type 2 diabetes. Characteristics of the individuals excluded from the analyses are provided in Supplementary Table 1.
Table 1.
Characteristics of the total study population and according to the presence of type 2 diabetes at baseline
| Total study population | Participants without type 2 diabetes | Participants with type 2 diabetes | |
|---|---|---|---|
| Participants, n (%) | 2,135 (100) | 1,938 (90.8) | 197 (9.2) |
| Age at baseline (years) | 74.5 (4.6) | 74.5 (4.6) | 74.6 (4.3) |
| Women, n (%) | 1,245 (58.3) | 1,151 (59.4) | 94 (47.7) |
| Education level, n (%) | |||
| Primary | 410 (19.2) | 377 (19.5) | 33 (16.8) |
| Secondary | 1,106 (51.8) | 1,003 (51.8) | 103 (52.3) |
| College/university | 619 (29.0) | 558 (28.8) | 61 (31.0) |
| Former smoker, n (%) | 985 (46.1) | 884 (45.6) | 101 (51.3) |
| Current smoker, n (%) | 220 (10.3) | 202 (10.4) | 18 (9.1) |
| High alcohol use, n (%)* | 881 (41.3) | 803 (41.4) | 78 (39.6) |
| BMI (kg/m2) | 27.2 (4.0) | 27.1 (4.0) | 28.8 (4.0) |
| HbA1c (mmol/mol) | 38 (5) | 37 (3) | 47 (10) |
| HbA1c (%) | 5.6 (0.5) | 5.6 (0.3) | 6.5 (0.9) |
| Total cholesterol–to–HDL cholesterol ratio | 3.7 (1.1) | 3.7 (1.1) | 3.9 (1.2) |
| Baseline coronary artery disease, n (%)† | 488 (26.2) | 426 (22.0) | 62 (31.5) |
| Stroke, n (%) | |||
| Baseline | 94 (4.4) | 85 (4.4) | 9 (4.6) |
| Follow-up | 24 (1.1) | 20 (1.0) | 4 (2.0) |
| Hypertension, n (%) | 1,651 (77.3) | 1,472 (76.0) | 179 (90.9) |
| Incident depressive symptoms (GDS-15 score ≥6), n (%) | 92 (4.3) | 79 (4.1) | 13 (6.6) |
| GDS-15 score | |||
| Baseline, median (IQR) | 1 (0–3) | 1 (1–3) | 1 (1–2) |
| 5-year change | +0.4 (1.6) | +0.3 (1.6) | +0.7 (1.9) |
| Use of antidepressant medication, n (%) | |||
| Baseline | 215 (10.1) | 196 (10.1) | 19 (9.6) |
| Follow-up | 287 (13.4) | 264 (13.6) | 23 (11.7) |
| Composite scores of cerebral small vessel disease features | |||
| Baseline | 0.8 (0.9) | 0.7 (0.9) | 0.9 (1.0) |
| 5-year change | +0.6 (0.8) | +0.6 (0.7) | +0.8 (0.9) |
| Total brain parenchyma volume, mL | |||
| Baseline | 1,097 (102) | 1,097 (101) | 1,088 (114) |
| 5-year change | −33 (17) | −31 (17) | −35 (18) |
| White matter hyperintensity volume, mL | |||
| Baseline, median (IQR) | 11 (6–21) | 11 (6–20) | 12 (7–22) |
| 5-year change | +6 (7) | +5 (7) | +6 (8) |
| Subcortical infarcts, n (%) | |||
| Baseline | 154 (7.2) | 123 (6.3) | 31 (15.7) |
| Incident | 87 (4.1) | 74 (3.8) | 13 (6.6) |
| Cerebral microbleeds, n (%) | |||
| Baseline | 355 (16.6) | 317 (16.4) | 38 (19.3) |
| Incident | 377 (17.7) | 332 (17.1) | 45 (22.8) |
| Large perivascular spaces, n (%) | |||
| Baseline | 341 (16.0) | 302 (15.6) | 39 (19.8) |
| Incident | 60 (2.8) | 50 (2.6) | 10 (5.1) |
Data are presented as mean (SD) unless otherwise stated. IQR, interquartile range.
High alcohol use was defined as alcohol use above median, stratified by sex. †Data missing in n = 220.
Participants with type 2 diabetes compared with those without had a greater increase in the GDS-15 score between the baseline and follow-up examination after adjustment for age, sex, education, alcohol use, smoking history, BMI, hypertension, and the total cholesterol–to–HDL cholesterol ratio (Table 2). In addition, participants with type 2 diabetes had a higher composite score of baseline cerebral small vessel disease and a higher composite score of cerebral small vessel disease change (Table 2). A higher composite score of baseline cerebral small vessel disease and a higher composite score of cerebral small vessel disease change were associated with greater increase in the GDS-15 score over time (Table 2).
Table 2.
Associations between type 2 diabetes at baseline, baseline and change in composite score of cerebral small vessel disease and change in the GDS-15 score
| Change in the GDS-15 score over time | Composite score of baseline | Composite score of change over time | ||
|---|---|---|---|---|
| Model | β (95% CI) | β (95% CI) | β (95% CI) | |
| Type 2 diabetes vs. no diabetes at baseline | 1 | 0.339 (0.099; 0.579) | 0.219 (0.093; 0.345) | 0.205 (0.094; 0.316) |
| 2 | 0.337 (0.094; 0.579) | 0.217 (0.089; 0.344) | 0.222 (0.110; 0.335) | |
| Composite score of baseline | 1 | 0.094 (0.013; 0.175) | — | — |
| 2 | 0.095 (0.014; 0.176) | — | — | |
| Composite score of change | 1 | 0.198 (0.107; 0.290) | — | — |
| 2 | 0.194 (0.102; 0.285) | — | — |
Model 1, adjusted for age, sex, and education level; model 2, model 1 plus alcohol use, smoking history, BMI, hypertension, and total cholesterol–to–HDL cholesterol ratio. Analyses with cerebral small vessel disease as determinant were also adjusted for type 2 diabetes.
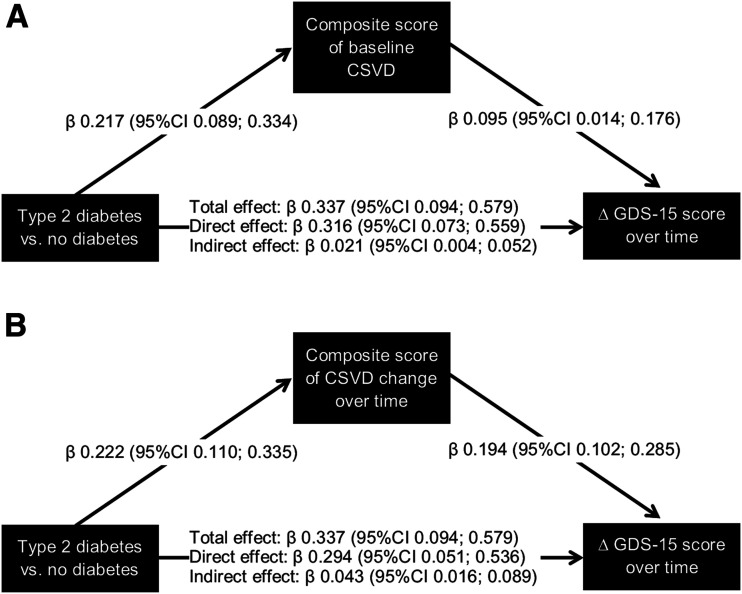
Mediation analysis showed that the composite scores of baseline cerebral small vessel disease and cerebral small vessel disease change each statistically significantly explained part of the total association between type 2 diabetes and the greater increase in the GDS-15 score over time (Fig. 1A and B).
Figure 1.
Association between type 2 diabetes at baseline and change in the GDS-15 score, and the mediating effect by the composite score of baseline cerebral small vessel disease (CVSD) (A) and the composite score of cerebral small vessel disease change (B). Total effect is the association between type 2 diabetes at baseline and change in the GDS-15 score without adjustment for the composite score, direct effect is the association between type 2 diabetes and change in the GDS-15 score after adjustment for the composite score, and indirect effect is the explained or mediated effect by the composite score. Associations are given as regression coefficients (β) and corresponding 95% CIs. All associations are adjusted for age, sex, education level, alcohol use, smoking history, BMI, hypertension, and total cholesterol–to–HDL cholesterol ratio.
Secondary Analyses
Most individual cerebral small vessel disease features attenuated the association between baseline type 2 diabetes and the greater increase in the GDS-15 score over time (Supplementary Figs. 1 and 2). Results were qualitatively similar when we used the GDS-15 score at follow-up as the outcome instead of the 5-year change in the GDS-15 score and with additional adjustment for the GDS-15 score at baseline (Supplementary Table 2). The association between type 2 diabetes and change in the GDS-15 score was qualitatively similar when we excluded participants who used antidepressant medication at baseline (n = 215) (Supplementary Table 3). Type 2 diabetes was associated with a greater increase in both the GDS-3A and GDS-12D scores (Supplementary Table 4). In addition, the composite scores of cerebral small vessel disease explained part of the total association of type 2 diabetes and a greater increase in the GDS-3A score and the GDS-12D score (Supplementary Figs. 3 and 4). The association between type 2 diabetes and change in the GDS-15 score was comparable when we additionally adjusted for baseline coronary artery disease or for baseline stroke and incident stroke during follow-up (Supplementary Table 5). In addition, results were similar when we excluded individuals with a baseline or incident subcortical infarct with a size ≥15 mm (n = 41) (Supplementary Fig. 5).
Conclusions
In this study, type 2 diabetes was independently associated with a greater increase in depressive symptoms over time, and cerebral small vessel disease features explained or mediated part of this association.
The study findings are in accordance with the vascular depression hypothesis (2) and suggest that cerebral microvascular damage may contribute to the development of depressive symptoms in individuals with type 2 diabetes. A previous meta-analysis found that cerebral small vessel disease features are associated with a higher risk of incident depressive symptoms (6). In addition, a previous systematic review found that type 2 diabetes is associated with the presence of cerebral small vessel disease features (5). This study extends these previous studies by showing that the association between type 2 diabetes and a greater increase in depressive symptoms over time is partially explained by cerebral small vessel disease features.
Type 2 diabetes may lead to cerebral small vessel disease via various mechanisms, including impaired insulin-dependent arteriolar dilation, advanced glycation, excessive oxidative stress, and epigenetic changes (23,24). The cerebral microvasculature, in turn, is involved in the regulation of many cerebral processes, including cerebral perfusion, neurovascular coupling, blood-brain barrier permeability, and neurogenesis (24). Impairment of these processes may lead to neuronal dysfunction, ischemia, and cell death, which may ultimately contribute to depressive symptoms via damage in deep and frontal brain structures involved in mood regulation (24).
Other mechanisms may, however, explain the observed associations. First, cerebral small vessel disease may indirectly lead to depression through incident stroke. However, adjusting for stroke at baseline or incident stroke during follow-up did not change our results. Second, cerebral small vessel disease may indirectly lead to depression through cognitive impairment. For the current study, however, we excluded individuals with dementia at baseline or at follow-up. Third, other confounding factors may explain the association between type 2 diabetes, cerebral small vessel disease features, and change in depressive symptoms over time, such as lower socioeconomic status and cardiovascular factors. However, the associations between type 2 diabetes, cerebral small vessel disease, and depressive symptoms were independent of education level and cardiovascular risk factors (25,26). Nevertheless, we cannot exclude the possibility of residual confounding.
Some part of the association between type 2 diabetes and higher depressive symptoms over time remained unexplained after taking into account the effect of cerebral small vessel disease features. This remaining association may be due to microvascular dysfunction that is not directly captured in the MRI scans in the current study (e.g., microinfarctions, increased blood-brain permeability, and lower cerebral vasoreactivity). In addition, it is possible that only a subset of individuals with type 2 diabetes develop depressive symptoms that are related to cerebral small vessel disease. Depressive symptoms in type 2 diabetes may be related to other mediators such as psychosocial factors (1), diabetes-related comorbidities (1), and glucose neurotoxicity (27).
Strengths of the current study are the large population-based sample of older participants, the comprehensive brain MRI assessment of various cerebral small vessel disease features at baseline and follow-up 5 years later, and the extensive adjustment for a series of potential confounders.
Our study has certain limitations. First, the construction of a composite score of cerebral small vessel disease assumes that all of its components are of equal importance in the association between type 2 diabetes and higher depressive symptoms over time. However, we found that most individual cerebral small vessel disease features contributed to the effect of cerebral small vessel disease on the association between type 2 diabetes and higher depressive symptoms over time.
Second, death or attrition between the baseline and follow-up examination may have resulted in a disproportional loss of people who were at high risk for depression. The associations between diabetes, cerebral small vessel disease, and depressive symptoms found in the current study sample may be biased if associations substantively differed in people who were lost to follow-up. We consider this biased attrition unlikely, in which case the loss of the individuals reduces our power to detect existing associations.
Third, we lacked power to investigate the association of baseline type 2 diabetes to incident depressive symptoms (GDS-15 score ≥6).
Fourth, because we studied associations of change in depressive symptoms with change in cerebral small vessel disease features, we cannot investigate the temporality of the association. However, it is likely that only many repeated measures made at short intervals will resolve temporal relations.
In view of the increased risk of depression in type 2 diabetes, efforts to favorably influence cerebral microvascular function through lifestyle and pharmacological therapy might help to prevent or treat microvascular dysfunction-related depression (24,28). Evidence suggests that weight loss and exercise may improve microvascular function and symptoms of depression (24). In addition, drugs, such as renin-angiotensin-aldosterone system inhibitors and antihyperglycemic agents (i.e., metformin and glucagon-like peptide 1 receptor agonists), may improve microvascular function, possibly beyond their blood pressure- or glucose-lowering effects (24).
In conclusion, the current study found that type 2 diabetes is independently associated with a greater increase in depressive symptoms over time and that this association is partly explained by MRI features of cerebral small vessel disease.
Article Information
Funding. The AGES-Reykjavik Study was supported by the Icelandic Heart Association, Intramural Research Program at the National Institute on Aging (grants N01-AG-12100 and HHSN271201200022C), the Althingi (the Icelandic Parliament), and the Icelandic Centre for Research (RANNIS) (grant 141101-051). T.T.v.S. is supported by a Veni research grant (916.19.074) from the Netherlands Organisation for Scientific Research (NWO) and the Netherlands Organisation for Health Research and Development (ZonMw) and by a Dutch Heart Foundation research grant (2018T025).
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.P.R., T.T.v.S., and L.J.L. researched and analyzed data, wrote the first version of the manuscript, reviewed and revised the manuscript, and wrote the final version. J.D., S.S., C.D.A.S., and V.G. contributed to the discussion and reviewed and edited the manuscript. L.J.L. is the guarantor of this work, and as such, had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12273077.
References
- 1.Bădescu SV, Tătaru C, Kobylinska L, et al. . The association between diabetes mellitus and depression. J Med Life 2016;9:120–125 [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–922 [DOI] [PubMed] [Google Scholar]
- 3.van Sloten TT, Sigurdsson S, van Buchem MA, et al. . Cerebral small vessel disease and association with higher incidence of depressive symptoms in a general elderly population: the AGES-Reykjavik Study. Am J Psychiatry 2015;172:570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Smith EE, Biessels GJ, et al.; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015;3:75–89 [DOI] [PubMed] [Google Scholar]
- 6.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018;90:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris TB, Launer LJ, Eiriksdottir G, et al. . Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes 2017;9:320–324 [DOI] [PubMed] [Google Scholar]
- 9.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999;14:858–865 [DOI] [PubMed] [Google Scholar]
- 10.Yesavage JA, Brink TL, Rose TL, et al. . Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982–1983;17:37–49 [DOI] [PubMed] [Google Scholar]
- 11.Ligthart SA, Richard E, Fransen NL, et al. . Association of vascular factors with apathy in community-dwelling elderly individuals. Arch Gen Psychiatry 2012;69:636–642 [DOI] [PubMed] [Google Scholar]
- 12.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. . Cerebral microbleeds in the population based AGES-Reykjavik Study: prevalence and location. J Neurol Neurosurg Psychiatry 2008;79:1002–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher AI, Gudmundsson LS, Sigurdsson S, et al. . Migraine headache in middle age and late-life brain infarcts. JAMA 2009;301:2563–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurdsson S, Aspelund T, Forsberg L, et al. . Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik Study. Neuroimage 2012;59:3862–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Sigurðsson S, Jónsson PV, et al. . Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol 2017;74:1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oladeji BD, Gureje O. The comorbidity between depression and diabetes. Curr Psychiatry Rep 2013;15:390. [DOI] [PubMed] [Google Scholar]
- 17.Renn BN, Feliciano L, Segal DL. The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev 2011;31:1239–1246 [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, DSM-IV. 4th ed. Washington, DC, American Psychiatric Press Inc., 1994 [Google Scholar]
- 19.O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics 1984;40:1079–1087 [PubMed] [Google Scholar]
- 20.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. A Regression-Based Approach. New York, Guilford Press, 2017 [Google Scholar]
- 21.Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin North Am 2014;43:103–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-Fabela L, Melano-Carranza E, Aguilar-Navarro S, García-Lara JM, Gutiérrez-Robledo LM, Avila-Funes JA. Hypertension as a risk factor for developing depressive symptoms among community-dwelling elders. Rev Invest Clin 2009;61:274–280 [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013;93:137–188 [DOI] [PubMed] [Google Scholar]
- 24.Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes 2018;67:1729–1741 [DOI] [PubMed] [Google Scholar]
- 25.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88:1973–1998 [DOI] [PubMed] [Google Scholar]
- 26.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–1757 [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci 2008;9:36–45 [DOI] [PubMed] [Google Scholar]
- 28.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005;19:113–122 [DOI] [PubMed] [Google Scholar]