Abstract
Adipose tissue serves as the body’s primary energy storage site; however, findings in recent decades have transformed our understanding of the multifaceted roles of this adaptable organ. The ability of adipose tissue to undergo energy expenditure through heat generation is termed adaptive thermogenesis, a process carried out by thermogenic adipocytes. Adipocytes are the primary parenchymal cell type in adipose tissue, yet these cells are sustained within a rich stromal vascular microenvironment comprised of adipose stem cells and progenitors, immune cells, neuronal cells, fibroblasts, and endothelial cells. Intricate cross talk between these diverse cell types is essential in regulating the activation of thermogenic fat, and the past decade has shed significant light on how this intercellular communication functions. This review will draw upon recent findings and current perspectives on the sophisticated repertoire of cellular and molecular features that comprise the adipose thermogenic milieu.
Introduction
We are amid a global epidemic of obesity and metabolic syndrome. Crucial to these pathologies is adipose tissue. However, not all fat is involved in energy storage. Instead, there are functionally distinct types of fat. White adipose tissue (WAT) is the principal site of triglyceride storage, while thermogenic fat, which consists of classical brown and inducible beige/brite adipocytes, specializes in thermogenic energy expenditure. The past decade has seen great strides made in our understanding of thermogenic brown and beige adipocytes. Major catalysts in this process were the rediscovery of brown adipose tissue (BAT) in adult humans and the identification of distinct inducible beige adipocytes.
In many tissues, heterogeneous populations of cell types interact to achieve the physiological role of the tissue and sustain homeostasis. Adipose tissue is no exception. The heterogeneous cellular nature of adipose tissue, as well as dynamic communications among its various resident cell types, is critical for its diverse activities (1). It has become apparent that the stromal microenvironment, in which thermogenic adipocytes emerge, plays a fundamental role in thermogenic function.
The Adipose Tissue Microenvironment
Advances in high-throughput technologies and single-cell profiling have helped to uncover the heterogeneity in adipocyte progenitor populations, providing a more comprehensive and unbiased approach to studying the adipose stromal compartment. Classical brown adipocytes first originate embryonically from dermomyotomal precursors expressing Myf5 and Pax7, before PRDM16-driven commitment to the brown adipocyte lineage (2,3). Major recent findings from independent groups have better elucidated the developmental hierarchy of adipocyte progenitors and their niche in adipose tissue (4–7). These studies uncovered congruent adipogenic precursor populations that exist both in rodents and humans, represented broadly by multipotent DPP4+ progenitors and ICAM1+ and CD142+ preadipocytes.
The BATLAS resource was developed using bulk RNA sequencing to identify unique gene signatures of mature brown, white, and beige adipocytes in mice and humans (8), giving us further insights into the transcriptomic similarities and differences among these distinct adipose cell types and allowing us to better study how they interact with their microenvironment. The in situ proximity of adipocytes to vasculature and neurite projections suggests that signals derived from the circulation and nervous system are important for determining adipocyte maturation. Interestingly, it has been demonstrated that MyoD+ progenitors can give rise to glycolytic beige adipocytes in the absence of adrenergic signaling from the sympathetic nervous system (SNS) (9). Recent reports using single nuclei adipocyte RNA sequencing defined a metabolically active subtype of murine adipocytes in subcutaneous fat (termed “type nine adipocytes”) that exhibit hallmarks of thermogenic beige adipocytes in response to cold (10). Transcriptomic profiling of clonal populations of adipocytes derived from human subcutaneous mesenchymal progenitors revealed four major adipocyte clusters, each purported to undertake distinct metabolic and physiological functions (11). The second cluster identified corresponded to thermogenic beige cells, exhibiting features such as iron accumulation and resistance to oxidative stress, for facilitating mitochondrial biogenesis and thermogenic activity.
As we continue to make advances with finer-resolution approaches and integration of human data with other models, our understanding of the rich adipogenic niche that gives rise to thermogenic fat cells will continue to develop.
Intercellular Cross Talk in Thermogenic Activation
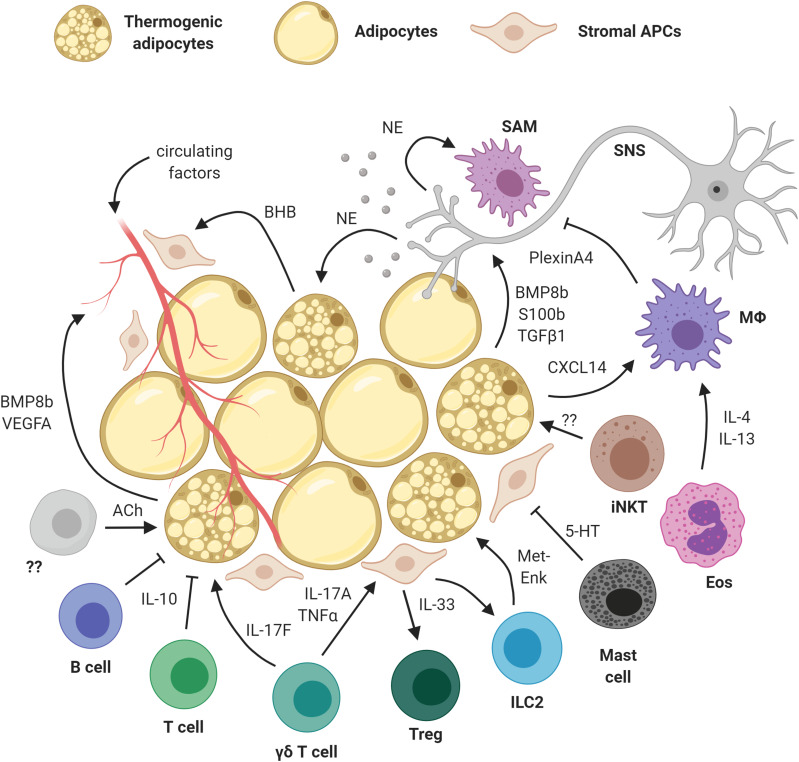
As our understanding of thermogenic adipocytes has evolved, so too has our appreciation of the diverse cellular ensemble that sustains and regulates thermogenic phenomena. These phenomena, in response to cues such as temperature, exercise, and diet, would not manifest without the intricate cross talk that takes place within the adipose milieu as illustrated in Fig. 1.
Figure 1.
Intercellular cross talk in the thermogenic microenvironment. A diverse ensemble of stromal vascular cells interact with adipocytes and their precursors to orchestrate adipose thermogenesis. Created with BioRender.com. 5-HT, serotonin; ACh, acetylcholine; APCs, adipose precursor cells; BHB, β-hydroxybutyrate; BMP, bone morphogenetic proteins; Eos, eosinophil; ILC2, group 2 innate lymphoid cell; iNKT, invariant natural killer T cells; MΦ, macrophage; Met-Enk, methionine enkephalin peptide; NE, norepinephrine; SAM, sympathetic neuron-associated macrophage; TGFβ1, transforming growth factor β1; TNFα, tumor necrosis factor α; VEGFA, vascular endothelial growth factor A.
Neuronal-Adipocyte Cross Talk
Innervation of WAT and BAT by the SNS is well established, providing a direct and immediate link between the central nervous system and its output in fat. Catecholamines, namely norepinephrine and epinephrine, are the primary neurotransmitters responsible for potentiating adrenergic signaling driven by the SNS. More abundant SNS innervation in BAT facilitates its heightened thermogenic capacity over subcutaneous or visceral WAT, with shared overlap existing in the SNS circuitry between these depots (12). Disruption of bone morphogenetic protein (BMP) signaling in BAT leads to increased subcutaneous WAT innervation (13), likely in an effort to retain the lost thermogenic capacity from the BAT depot. BMP8b, produced by activated brown and beige adipocytes, can promote sympathetic innervation, providing a feedback loop from thermogenic adipocytes to regulate local neuronal growth (14). BMP8b has also been shown to increase BAT thermogenesis by sensitizing brown adipocyte responsiveness to adrenergic signaling (15). Sympathetic neurite density appears to cluster closely with thermogenic adipocytes, and this phenomenon is PRDM16 dependent (16). S100b, a brown adipocyte–derived neurotrophic factor, has recently been shown to promote SNS innervation (17). Calsyntenin 3β, a novel endoplasmic reticulum (ER)-localized protein unique to mammals, is responsible for orchestrating the export of S100b. The clustering effect of thermogenic adipocytes can partly be explained as an effort to propagate the output of SNS afferents in adipose tissue. These findings affirm the fundamental importance of SNS-derived signals in mediating thermogenic activation.
Vascular-Adipocyte Cross Talk
The adipose vasculature is crucial for mediating physiological (and pathophysiological) phenomena such as inflammation, nutrient balance, and thermogenesis. Angiogenesis governs the extent and remodeling of adipose vasculature and has thus been identified as a promising therapeutic target for treating metabolic pathologies. Proangiogenic factors appear to be important for optimal thermogenic function, and enhanced beiging is observed in subcutaneous WAT of mice with inducible adipocyte-specific overexpression of the vascular endothelial growth factor VEGFA (18). On the contrary, adenoviral-mediated neutralization of VEGF results in reduced vascularization and decreased thermogenic activity (19). Blocking VEGFR2 diminished cold-induced angiogenesis and thermogenic capacity in brown fat, whereas blockade of VEGFR1 had the opposite effect (20). This specificity has implications for strategies targeting vasculature remodeling in adipose-based therapies. In addition to promoting sympathetic innervation described above, BMP8b can also induce vascularization and thereby regulate remodeling of the adipose niche in response to thermogenic demand (14).
Immune-Adipocyte Cross Talk
A rich hematopoietic niche resides within the stromal vascular fraction of adipose tissue. Our awareness of tissue-resident immune cells and their involvement in adipose homeostasis and energy balance has been an area of intense research interest for well over a decade now. Given their pleiotropic functions and broad spectrum of activity, adipose tissue macrophages (ATMs) have garnered substantial interest. Alternative (M2) activation of ATMs was implicated in driving beige fat thermogenesis via production of catecholamines (21). However, conflicting evidence has since arisen over whether ATMs produce catecholamines, instead pointing toward a role for ATMs in norepinephrine uptake and clearance (22–24). Now, given our deeper appreciation for the heterogeneity of the macrophage activity spectrum, interest in ATMs remains steadfast, and recent findings have shed light on their importance in thermogenic brown fat through controlling sympathetic innervation (25) and their recruitment in response to CXCL14 chemokine secretion by thermogenic brown adipocytes (26). Additionally, given the high metabolic demand of brown adipocytes undergoing thermogenesis, protective mechanisms have evolved to maintain cellular integrity and proteostasis and to limit immune cell–mediated inflammation. Nuclear factor erythroid 2-like 1 (NFE2L1/NRF1) is a transcription factor localized to the ER that was recently identified to orchestrate the adaptive cellular response in brown adipocytes during high metabolic activity, mitigating ER stress and preventing the accumulation of proinflammatory M1 macrophages (27).
Adipose-resident eosinophils comprise another, albeit less well-understood, aspect of the type 2 immune signaling network in fat, with reports implicating these cells in adipose function and thermogenesis (28). Serotonin secretion from mast cells has been shown to inhibit PDGFRα+ progenitor proliferation and differentiation into beige adipocytes, with a recent report suggesting that mast cell inactivation can promote thermogenesis (29). These findings emerged in light of reports of a non-neuronal cholinergic circuitry in beige fat activation, in which immune cell–derived acetylcholine sustained thermogenic tone in beige adipocytes expressing the nicotinic acetylcholine receptor subunit CHRNA2 (30). It will be interesting to uncover the immune cells responsible for driving this cholinergic circuitry and shed further light on non-neuronal cholinergic signaling given the established importance of adrenergic function in thermogenic fat.
Innate lymphoid cells hail from the common lymphoid progenitor but lack T- or B-cell receptors and do not fall neatly under the traditional hematopoietic lineages. Group 2 innate lymphoid cells were first thrust into the metabolic spotlight with the discovery that these cells orchestrate eosinophil recruitment (via production of interleukin-5 [IL-5]) and M2 macrophage polarization (via IL-13) while mediating the beneficial thermogenic action of methionine enkephalin (31). Other lymphocyte subsets have similarly been noted for their importance in adaptive thermogenesis. Invariant natural killer T cells, which share features of natural killer cells and T lymphocytes, can be activated to proliferate and stimulate release of FGF21 from adipocytes; however, the precise invariant natural killer T cell–derived molecule(s) mediating this interaction have yet to be pinpointed (32). Regulatory T cells (Tregs) can be induced from CD4 T-cell precursors in brown and white fat via a STAT6/PTEN axis following thermogenic stimuli ranging from cold exposure to acute high-fat diet and pharmacological β3-adrenergic receptor activation (33). An adipose-enriched γδ T-cell population is responsible for controlling Treg expansion and invoking secretion of the alarmin cytokine IL-33 by stromal cells (34). This pathway relies upon production of the inflammatory cytokines IL-17A and TNF-α by resident γδ T cells, which act upon stromal cells and subsequently influence the Treg niche. New evidence has since emerged demonstrating that γδ T cell–derived IL-17F signals directly to adipocytes expressing the IL-17 receptor C (IL-17RC), eliciting sympathetic innervation of adipose tissue in a TGFβ1-dependent manner (35). Building upon previous work showing that bone marrow–derived IL-10 repressed transcription of thermogenic genes in adipocytes (36), Tontonoz and colleagues have since demonstrated that IL-10 produced by T and B lymphocytes impaired the activation of IL10Rα-expressing beige precursors, modulating the induction of thermogenesis and systemic energy balance (10).
Integrated Regulation of the Thermogenic Response
Thermogenesis is a facultative mechanism that manifests itself in response to various external stresses such as temperature, exercise, and diet, and evolutionary fine-tuning has resulted in an array of adaptive pathways that regulate the thermogenic response (Table 1). Exposure to cold temperature is the best-characterized stimulus of thermogenesis. Modulating ambient temperature remains the most widespread approach for studying brown and beige fat thermogenesis today in an effort to better understand and mimic the physiological response to cold for therapeutic purposes. Food intake has also been proposed to dictate adaptive energy expenditure. Pioneering work by Rothwell and Stock demonstrated that voluntary overeating in rats did not result in the extent of weight gain anticipated (37). The authors observed changes in the activity of the SNS and BAT, leading to the proposal of a phenomenon termed diet-induced thermogenesis.
Table 1.
Regulation of thermogenic fat by environmental cues
| Thermogenic stimuli | Molecules | References |
|---|---|---|
| Cold | Adipocyte derived: 12,13-diHOME, 12-HEPE, BHB, BMPs, cardiolipin, CXCL14, S100b, TGFβ1, VEGFA | 14,15,17,18,20,26,35,50,53,55–57 |
| Adipocyte receiving: acetylcholine, catecholamines, IL-10, IL-17A, IL-17F, lactate, Met-Enk, succinate, TNF-α | 10,16,22–24,30,31,34–36,51,52 | |
| Other (e.g., exercise, diet, cachexia, burns) | Adipocyte derived: 12,13-diHOME, BMPs, EPDR1, FGF21, IL-6 | 13,32,40,49,54 |
| Adipocyte receiving: IL-6, irisin, meteorin-like, serotonin | 28,29,38,39,42,43 |
BHB, β-hydroxybutyrate; EPDR1, ependymin-related protein 1; Met-Enk, methionine enkephalin peptide.
The endocrine and nervous systems play a primary role in integrating environmental cues and then setting in motion an appropriate physiological response in adipose tissue. In response to environmental changes such as exposure to cold temperature, exercise, or caloric intake, hormones and neurons work independently and in tandem to mediate the dynamic changes that occur in thermogenic fat.
Originating from the term cytokine, an explosion of different -kine portmanteaus has emerged in biology to describe molecules and hormones that regulate physiological signaling. Through study of the transcriptional coactivator PGC-1α, muscle-derived hormones such as meteorin-like were discovered to promote thermogenesis (28,38,39). IL-6, also regarded as an exercise-induced myokine, appears necessary for the beneficial metabolic effects of BAT transplantation (40) and is secreted by subcutaneous adipocytes and preadipocytes independently of macrophage-derived IL-6 (41). IL-6 is also noted for its role in mediating browning induced from burn injuries and cancer cachexia (42,43). Several other central and peripheral molecules have been proposed to mediate exercise-induced adipose thermogenesis (44,45). However, while the benefits of exercise in adipose function and beiging have been well-documented in rodents, further studies in humans are still warranted as our understanding of this regulation evolves (46–48).
Proteomic screens have led to the generation of adipose secretomes that provide a global landscape of secreted molecules, greatly advancing the breadth of our understanding of adipokines and related molecules. A recent study shed light on the comparative secretomes of WAT and BAT and revealed ependymin-related protein 1 as an important determinant of brown adipocyte precursor commitment (49). Further, aging-induced impairment of beiging has been linked to a switch toward a more fibrotic adipose precursor phenotype, driven by diminished expression of the beige and brown fat master regulator PRDM16 (50). The cellular metabolites lactate (51) and succinate (52) have also been implicated in thermogenic activation following cold exposure.
Lipid species, termed lipokines, have risen to prominence for their role in intercellular cross talk too. Lipidomic analyses identified BAT as the source of circulating 12,13-diHOME both in mice and in humans following exercise and cold exposure, enhancing fuel uptake and thermogenesis (53,54). Lipidomics also revealed that the lipoxygenase product 12-HEPE is induced during cold exposure to regulate glucose homeostasis in BAT (55). Generation of a “lipid bio-signature” in thermogenic fat revealed cardiolipin, a mitochondrial membrane-localized phospholipid, to be strongly induced during cold stress and critical for thermogenic fat activity (56,57).
Exosomes have emerged as crucial intercellular carriers of molecular cargo. These heterogeneous extracellular vesicles have become a recent area of interest in adipose tissue homeostasis, with several studies highlighting a role for exosomes in thermogenic brown and beige fat. Within the adipose tissue microenvironment, exchange of cellular material is mediated by extracellular vesicles in response to metabolic cues (58). Adipose-derived circulating exosomes can transport various payloads, including miRNAs, which can dictate gene expression programs in distal tissues such as the liver (59). BAT-derived exosomes containing miRNA may prove to be useful therapeutic targets for treating metabolic disease and provide us with insights into BAT activity (60). Continued investigation of the intricate cross talk mediated through exosomes will further illustrate how local and systemic factors influence brown and beige fat activity.
Conclusion and Perspective
Although adipocytes comprise the majority of tissue mass, their thermogenic function is supported by a rich stromal vascular niche comprised of neurons, hematopoietic cells, vasculature, and various other stromal cell types. Together, these findings constitute a rapidly evolving corpus of knowledge on the accessory cell types and their secreted mediators that are fundamental to adipose thermogenesis, especially in response to environmental challenges. Ultimately, this knowledge aids us in understanding the pathogenesis of obesity and other metabolic disorders. It also opens up new therapeutic strategies to fight the epidemic of obesity and metabolic syndrome by fine-tuning the thermogenic microenvironment.
Article Information
Acknowledgments. We apologize to those whose work is not cited owing to space limitations.
Funding. This work was supported by the National Institutes of Health (R01DK107583 to J.W. and R01DK077097 and R01DK102898 to Y.-H.T.), the American Diabetes Association (1-18-IBS-281 to J.W.), and a Michigan Life Science Fellowship to A.J.K.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Lynes MD, Tseng Y-H. Deciphering adipose tissue heterogeneity. Ann N Y Acad Sci 2018;1411:5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lepper C, Fan C-M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 2010;48:424–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seale P, Bjork B, Yang W, et al. . PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burl RB, Ramseyer VD, Rondini EA, Pique-Regi R, Lee YH, Granneman JG. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab 2018;28:300–309.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrick D, Sakers A, Irgebay Z, et al. . Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 2019;364:eaav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwalie PC, Dong H, Zachara M, et al. . A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018;559:103–108 [DOI] [PubMed] [Google Scholar]
- 7.Hepler C, Shan B, Zhang Q, et al. . Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 2018;7:e39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perdikari A, Leparc GG, Balaz M, et al. . BATLAS: deconvoluting brown adipose tissue. Cell Rep 2018;25:784–797.e4 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Ikeda K, Yoneshiro T, et al. . Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 2019;565:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajbhandari P, Arneson D, Hart SK, et al. . Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife 2019;8:e49501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min SY, Desai A, Yang Z, et al. . Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc Natl Acad Sci U S A 2019;116:17970–17979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen NLT, Barr CL, Ryu V, Cao Q, Xue B, Bartness TJ. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol 2017;312:R132–R145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz TJ, Huang P, Huang TL, et al. . Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 2013;495:379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellegrinelli V, Peirce VJ, Howard L, et al. . Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat Commun 2018;9:4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittle AJ, Carobbio S, Martins L, et al. . BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012;149:871–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi J, Wu Z, Choi CHJ, et al. . Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab 2018;27:226–236.e3 [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, Ye M, Resch JM, et al. . Innervation of thermogenic adipose tissue via a calsyntenin 3β-S100b axis. Nature 2019;569:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J, Kim M, Sun K, An YA, Gu X, Scherer PE. VEGF-A–expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4–independent metabolic improvements. Diabetes 2017;66:1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagchi M, Kim LA, Boucher J, Walshe TE, Kahn CR, D’Amore PA. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J 2013;27:3257–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue Y, Petrovic N, Cao R, et al. . Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 2009;9:99–109 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen KD, Qiu Y, Cui X, et al. . Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011;480:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirzgalska RM, Seixas E, Seidman JS, et al. . Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 2017;23:1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camell CD, Sander J, Spadaro O, et al. . Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 2017;550:119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer K, Ruiz HH, Jhun K, et al. . Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med 2017;23:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf Y, Boura-Halfon S, Cortese N, et al. . Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat Immunol 2017;18:665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cereijo R, Gavaldà-Navarro A, Cairó M, et al. . CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab 2018;28:750–763.e6 [DOI] [PubMed] [Google Scholar]
- 27.Bartelt A, Widenmaier SB, Schlein C, et al. . Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat Med 2018;24:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao RR, Long JZ, White JP, et al. . Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014;157:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Wang X, Yin H, et al. . Functional inactivation of mast cells enhances subcutaneous adipose tissue browning in mice. Cell Rep 2019;28:792–803.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jun H, Yu H, Gong J, et al. . An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat Med 2018;24:814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brestoff JR, Kim BS, Saenz SA, et al. . Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015;519:242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch L, Hogan AE, Duquette D, et al. . iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab 2016;24:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kälin S, Becker M, Ott VB, et al. . A Stat6/Pten axis links regulatory T cells with adipose tissue function. Cell Metab 2017;26:475–492.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohlgruber AC, Gal-Oz ST, LaMarche NM, et al. . γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol 2018;19:464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu B, Jin C, Zeng X, et al. . γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 2020;578:610–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajbhandari P, Thomas BJ, Feng AC, et al. . IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell 2018;172:218–233.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979;281:31–35 [DOI] [PubMed] [Google Scholar]
- 38.Boström P, Wu J, Jedrychowski MP, et al. . A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H, Wrann CD, Jedrychowski M, et al. . Irisin mediates effects on bone and fat via αV integrin receptors. Cell 2018;175:1756–1768.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanford KI, Middelbeek RJW, Townsend KL, et al. . Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013;123:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reilly SM, Ahmadian M, Zamarron BF, et al. . A subcutaneous adipose tissue-liver signalling axis controls hepatic gluconeogenesis. Nat Commun 2015;6:6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patsouris D, Qi P, Abdullahi A, et al. . Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep 2015;13:1538–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petruzzelli M, Schweiger M, Schreiber R, et al. . A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 2014;20:433–447 [DOI] [PubMed] [Google Scholar]
- 44.Cao L, Choi EY, Liu X, et al. . White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011;14:324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J 2013;27:1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norheim F, Langleite TM, Hjorth M, et al. . The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 2014;281:739–749 [DOI] [PubMed] [Google Scholar]
- 47.Vosselman MJ, Hoeks J, Brans B, et al. . Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes 2015;39:1696–1702 [DOI] [PubMed] [Google Scholar]
- 48.Stanford KI, Middelbeek RJ, Townsend KL, et al. . A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015;64:2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deshmukh AS, Peijs L, Beaudry JL, et al. . Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab 2019;30:963–975.e7 [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Ishibashi J, Trefely S, et al. . A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab 2019;30:174–189.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrière A, Jeanson Y, Berger-Müller S, et al. . Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 2014;63:3253–3265 [DOI] [PubMed] [Google Scholar]
- 52.Mills EL, Pierce KA, Jedrychowski MP, et al. . Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018;560:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynes MD, Leiria LO, Lundh M, et al. . The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med 2017;23:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanford KI, Lynes MD, Takahashi H, et al. . 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 2018;27:1111–1120.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leiria LO, Wang C-H, Lynes MD, et al. . 12-lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from brown fat. Cell Metab 2019;30:768–783.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sustarsic EG, Ma T, Lynes MD, et al. . Cardiolipin synthesis in brown and beige fat mitochondria is essential for systemic energy homeostasis. Cell Metab 2018;28:159–174.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynes MD, Shamsi F, Sustarsic EG, et al. . Cold-activated lipid dynamics in adipose tissue highlights a role for cardiolipin in thermogenic metabolism. Cell Rep 2018;24:781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crewe C, Joffin N, Rutkowski JM, et al. . An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 2018;175:695–708.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomou T, Mori MA, Dreyfuss JM, et al. . Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017;542:450–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Buyel JJ, Hanssen MJW, et al. . Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun 2016;7:11420. [DOI] [PMC free article] [PubMed] [Google Scholar]