Abstract
Certain HLA class II genes increase the risk for type 1 diabetes (T1D) development while others provide protection from disease development. HLA class II alleles encode MHC proteins on antigen-presenting cells, which function to present peptides and activate CD4 T cells. The DRB1*15:01 (DR15)-DQA1*01:02-DQB1*06:02 (DQ6) haplotype provides dominant protection across all stages of T1D and is a common haplotype found in Caucasians. However, it is present in <1% of people with T1D. Knowing which metabolic, immunologic, and genetic features are unique to individuals who fail genetic protection and develop T1D is important for defining the underlying mechanisms of DQB1*06:02-mediated protection. We describe a T1D cohort with DQB1*06:02 (n = 50) and compare them to individuals with T1D and without DQB1*06:02 (n = 2,759) who were identified over the last 26 years at the Barbara Davis Center for Diabetes. The age at diagnosis was similar between the cohorts and normally distributed throughout childhood and early adulthood. The average hemoglobin A1c was 10.8 ± 2.8% (95 ± 7 mmol/mol) at diagnosis in those DQB1*06:02 positive. The majority of T1D DQB1*06:02+ individuals were positive for one or more islet autoantibodies; however, there was a greater proportion who were islet autoantibody negative compared with those T1D DQB1*06:02− individuals. Interestingly, DQB1*03:02, which confers significant T1D risk, was present in only those DQB1*06:02+ individuals with islet autoantibodies. This is one of the largest studies examining patients presenting with clinical T1D in the presence of DQB1*06:02, which provides a population to study the mechanisms of failed genetic protection against T1D.
Introduction
Genome-wide association studies have identified over 50 genetic polymorphisms that increase the odds of developing type 1 diabetes (T1D), the immune-mediated form of diabetes (1–4). Over half of the genetic risk for T1D is conferred by HLA genes, especially the class II DR and DQ genes that are in linkage disequilibrium on chromosome 6 (5,6). HLA class II genes encode MHC proteins that are present on antigen-presenting cells, such as B cells, dendritic cells, and macrophages, which function to present processed proteins and activate CD4 T cells (7). Specific HLA alleles are known to increase risk for T1D development, including DRB1*04 (DR4)-DQA1*03:01-DQB1*03:02 (DQ8) and DRB1*03 (DR3)-DQA1*05:01-DQB1*02:01 (DQ2) haplotypes (8,9). Other HLA genes are strongly associated with protection from T1D development, namely, DRB1*15:01 (DR15)-DQA1*01:02-DQB1*06:02 (DQ6), which is a common haplotype found in European Americans with a frequency of about 14% but present in <1% of people with T1D (10–15). Further, genetic studies have mapped the protection to the DQ6 allele, as opposed to the DR15 allele that is in linkage disequilibrium with DQ6 (16,17).
The natural history of T1D occurs in stages that begin with the presence of islet autoantibodies, including those directed against insulin and β-cell proteins (GAD, IA-2, and zinc transporter 8 [ZnT8]), then impaired glucose tolerance, and finally clinical T1D onset marked by hyperglycemia and the need for insulin treatment (18). The Pathway to Prevention Cohort of Type 1 Diabetes TrialNet, an international consortium of investigators studying the natural history of T1D and conducting T1D prevention trials (19), recently identified a cohort of relatives with islet autoantibodies and the DQB1*06:02 allele. From 3,358 autoantibody-positive relatives of people with T1D, 155 (5%) were positive for the DRB1*15:01 (DR15)-DQA1*01:02-DQB1*06:02 (DQ6) haplotype (20). These relatives were less likely to develop additional autoantibodies, e.g., progress from single to multiple autoantibodies, and had little to no metabolic worsening during 2 years of follow up compared with those relatives without DQB1*06:02 (20). These results confirm those of an earlier study in which DQB1*06:02 in the presence of islet autoantibodies protects from clinical T1D development (13). Only 4/155 relatives in the Pathway to Prevention Cohort developed a diagnosis of clinical T1D. This indicates that DQB1*06:02 may not completely protect against the development of islet autoantibodies but does protect against diabetes onset. Given that the onset of diabetes is a rare event with DQB1*06:02, there are limited numbers of DQB1*06:02+ patients with clinical T1D described to date (20–22).
Characterizing individuals who develop T1D despite genetic protection has important implications for understanding how tolerance is lost to islet antigens. Furthermore, understanding the mechanisms of DQ6-mediated protection from T1D holds the potential to develop therapies that mimic this protection to delay and prevent disease onset. Over the last 26 years, we HLA-typed and measured islet autoantibodies in the vast majority of patients presenting with clinical T1D at our center. From 2,809 T1D patients, we identified 50 patients who had the DQB1*06:02 allele. This is one of the largest studies examining the metabolic and immunologic features of patients presenting with clinical T1D in the presence of DQB1*06:02.
Research Design and Methods
Subjects and Study Design
Subjects were recruited from the Barbara Davis Center for Diabetes Clinics, a major referral center for children and adults with new-onset T1D, between 1993 and 2019. These analyses include 2,809 patients who met the American Diabetes Association diagnostic criteria for diagnosis with diabetes and were diagnosed with T1D. Of the 2,809 patients, we identified 50 patients who required insulin treatment and had at least one DQB1*06:02 allele. Demographic characteristics were collected in a research database, and participants self-reported race and ethnicity. Peripheral blood was obtained for islet autoantibody measurements and HLA typing. All participants provided written informed consent, and the Colorado Multiple Institutional Review Board approved the study.
Laboratory Procedures
Islet autoantibodies to GAD (GADA), IA-2 (IA-2A), ZnT8 (ZnT8A), and insulin (IAA) were measured from serum using radio-binding assays as previously described (23,24). IAA and ZnT8A were measured in patients diagnosed in 1999 or later and 2010 or later, respectively. Harmonized assays for GADA and IA-2A were available starting in 2010, and index values obtained before this time were converted to DK units. The same positive cutoff values were applied to all participants with an index of 0.010 for IAA, 20 DK units for GADA, 5 DK units for IA-2A, and index 0.020 for ZnT8A. HLA typing was performed using sequence-specific oligonucleotide probes for DRB1, DQA1, DQB1, A, and B, as described previously (25).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad Software). Fisher exact tests were used to compare categorical variables between DQB1*06:02+ participants and DQB1*06:02− participants and between DQB1*06:02+ participants who were islet autoantibody positive and negative. The categorical variables analyzed include race/ethnicity, islet autoantibody positivity, number of islet autoantibodies, presence of diabetic ketoacidosis (DKA), and HLA type. As our sample sizes are unequal between the DQB1*06:02+ (n = 50) and DQB1*06:02− (n = 2,759) groups, our power calculations reveal 80% power to detect a medium effect size (based upon a Cohen’s h value of 0.4), which indicates the ability to detect a clinically significant difference in these variables between the groups.
Unpaired t tests were used to compare continuous variables (HbA1c, islet autoantibody levels, and age) between DQB1*06:02+ participants and DQB1*06:02− participants and between DQB1*06:02+ participants who were islet autoantibody positive and negative. Power calculations with our sample sizes indicate that we have 80% power to detect a difference of 0.4 SDs in these continuous variables. P values <0.05 were considered significant.
Data Resource and Availability
All data generated and analyzed during this study are included in the published article and the Supplementary Material.
Results
Characteristics of T1D Participants by HLA-DQB1*06:02 Status
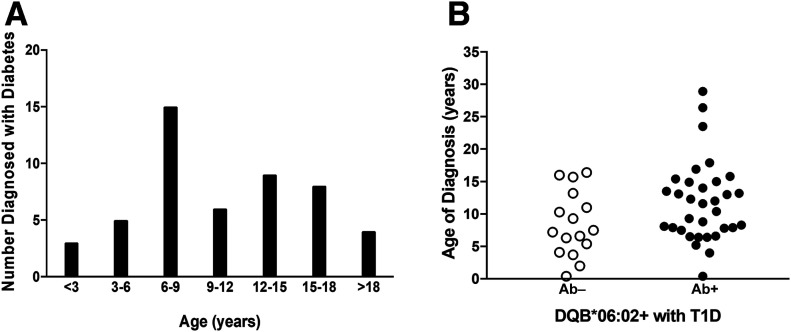
Over the course of 26 years, we identified 50/2,809 (1.8%) individuals with T1D and an HLA-DQB1*06:02 allele (Table 1). The majority of these individuals (62.4%) were seen at initial diagnosis or within 1 year of diagnosis (Supplementary Material). It has been hypothesized that β-cell destruction may occur more slowly and that T1D may develop at an older age in the presence of the strongly protective DQB1*06:02 allele. Interestingly, in our cohort the age of diagnosis with T1D was similar between DQB1*06:02+ (0602+) and DQB1*06:02− (0602−) individuals. The 0602+ patients developed T1D at a mean age of 10.8 ± 6.0 years, and 0602− participants developed T1D at a mean age of 12.5 ± 8.7 years (P = 0.069) (Table 1). The age at diagnosis among 0602+ T1D participants was normally distributed throughout childhood and early adulthood with a peak in T1D diagnosis between ages 6 and 9 years (Fig. 1A). Race and ethnicity were similar between the 0602+ and 0602− groups with the majority of patients being Caucasian; there was no difference in sex between the cohorts (Table 1).
Table 1.
Characteristics of T1D participants by HLA-DQB1*06:02 status
| DQB1*06:02+ (n = 50) | DQB1*06:02− (n = 2,759) | P value | |
|---|---|---|---|
| Age at diagnosis, years (mean ± SD) | 10.8 ± 6.0 | 12.5 ± 8.7 | 0.069 |
| Sex (% female) | 52 (n = 26) | 46 (n = 1,266) | 0.395 |
| Race/ethnicity (%) | 0.540 | ||
| White | 86 (n = 43) | 80 (n = 2,213) | |
| Hispanic | 6 (n = 3) | 5 (n = 142) | |
| Other | 6 (n = 3) | 8 (n = 218) | |
| Unknown | 2 (n = 1) | 7 (n = 186) | |
| Islet autoantibody status (%) | |||
| GADA | 55 (27/49) | 52 (1,399/2,676) | 0.773 |
| IA-2A | 39 (19/49) | 60 (1,587/2,663) | 0.005 |
| IAA* | 35 (13/37) | 34 (479/1,398) | 1.00 |
| ZnT8A | 51 (18/35) | 62 (553/898) | 0.289 |
| Number of islet autoantibodies (%)** | 0.009 | ||
| 0 Ab | 30 (14/47) | 14 (247/1,827) | 0.004 |
| 1 Ab | 19 (9/47) | 26 (474/1,827) | 0.398 |
| 2 Ab | 21 (10/47) | 33 (607/1,827) | 0.115 |
| ≥3 Ab | 30 (14/47) | 27 (499/1,827) | 0.741 |
Ab, autoantibodies.
IAA positive values were excluded from analysis if measured >21 days after T1D diagnosis.
Includes samples for which at least three islet autoantibodies were measured; overall P value is reported, followed by those for each category.
Figure 1.
A: Distribution of age at diagnosis with T1D in DQB1*06:02+ participants (n = 50). B: Age at diagnosis of T1D DQB1*06:02+ participants that had islet autoantibodies measured (n = 49). Mean age of autoantibody-negative (Ab−) participants was 8.5 ± 5.0 years (n = 16), and mean age of autoantibody-positive (Ab+) participants was 11.8 ± 6.2 years (n = 33); P = 0.069.
Islet Autoantibody Positivity
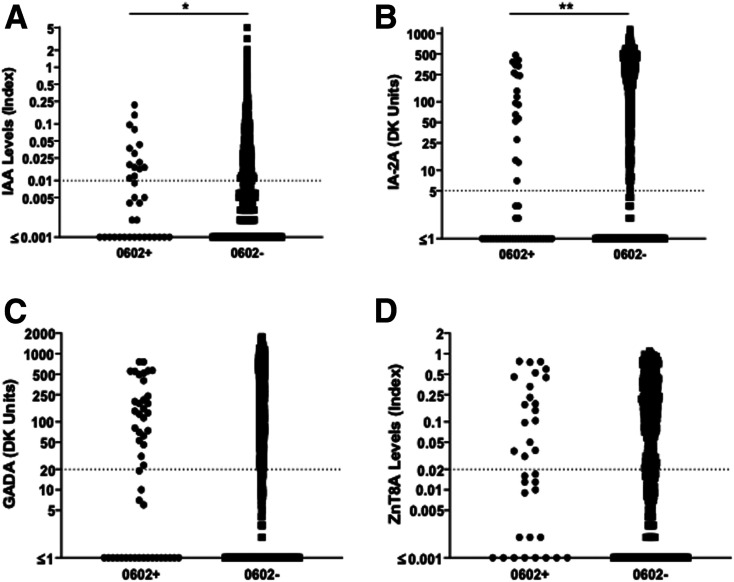
The majority of 0602+ participants (70%) were positive for one or more islet autoantibodies, yet more individuals in the 0602+ group were antibody negative in comparison with the 0602− group (30% vs. 14%, P = 0.004) (Table 1). This resulted in the overall number of islet autoantibodies being lower in the 0602+ group compared with the 0602− group (P = 0.009); however, there was not a difference in the percentage of individuals positive for one or multiple autoantibodies (Table 1). Positivity for GADA, IAA, and ZnT8A was similar between the 0602+ and 0602− participants; IAA was only considered positive if measured within 21 days of T1D diagnosis. However, IA-2A was less frequently positive in 0602+ compared with 0602− individuals (39% vs. 60%, P = 0.005). When examining islet autoantibody levels (Fig. 2A–D), IA-2A levels were lower in the 0602+ group compared with the 0602− with a mean of 70 vs. 161 DK units (P < 0.001). IAA levels were also lower in the 0602+ group compared with 0602− with a mean index of 0.022 vs. 0.050 (P = 0.006).
Figure 2.
A–D: Islet autoantibody levels in DQB1*06:02+ and DQB1*06:02− T1D individuals for IAA (A), IA-2A (B), GADA (C), and ZnT8A (D). P = 0.006 for IAA, P < 0.001 for IA-2A, P = 0.20 for GADA, and P = 0.76 for ZnT8A. IAA values were included if measured within 21 days of T1D diagnosis. The dotted line is at the cutoff for each autoantibody positivity.
Characteristics of DQB1*06:02+ Patients by Autoantibody Status
The cohort of 0602+ individuals with T1D were diagnosed at ages between 3 months and 28.9 years with no difference between those with and without autoantibodies (P = 0.069) (Fig. 1B). As expected, these individuals were hyperglycemic at the time of T1D diagnosis as measured by hemoglobin A1c levels averaging 10.8 ± 2.8% (95 ± 7 mmol/mol) (Table 2). The rates of DKA were similar between those with and without autoantibodies (Table 2). As there were several individuals with a very early age of diabetes onset (<1 year of age), monogenic forms of diabetes were investigated. Two 0602+ individuals were discovered to have mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 (KCNJ11), which are known to cause neonatal diabetes (26) (Supplementary Table). One participant was autoantibody negative, while the other had an islet autoantibody. A separate autoantibody-negative participant presented with diabetes and later had clinical symptoms suggestive of Wolfram syndrome (27). The participant was found to have a mutation in the WFS1 gene (Supplementary Material).
Table 2.
Characteristics of T1D participants with DQB1*06:02 by autoantibody status
| DQB1*06:02+ (n = 50)* | Ab+ (n = 33) | Ab− (n = 16) | P value | |
|---|---|---|---|---|
| HbA1c at diagnosis, % [mmol/mol] (mean ± SD) | 10.8 ± 2.8 [95 ± 7] (n = 42) | 11.3 ± 2.9 [100 ± 8] (n = 28) | 10.0 ± 2.6 [86 ± 5] (n = 14) | 0.170 |
| DKA at diagnosis (%) | 0.090 | |||
| Yes | 36 (n = 18) | 45 (n = 15) | 19 (n = 3) | |
| No | 44 (n = 22) | 36 (n = 12) | 63 (n = 10) | |
| Unknown | 20 (n = 10) | 18 (n = 6) | 19 (n = 3) | |
| HLA-DQ status (%) | 0.006 | |||
| DQB1*03:02 | 30 (n = 15) | 42 (n = 14) | 0 (n = 0) | 0.002 |
| DQB1*02:01 | 22 (n = 11) | 24 (n = 8) | 19 (n = 3) | 1.00 |
| Other | 48 (n = 24) | 33 (n = 11) | 81 (n = 13) | 0.002 |
Islet autoantibodies were measured in 49/50 0602+ individuals with T1D.
Frequency of T1D Predisposing HLA Alleles
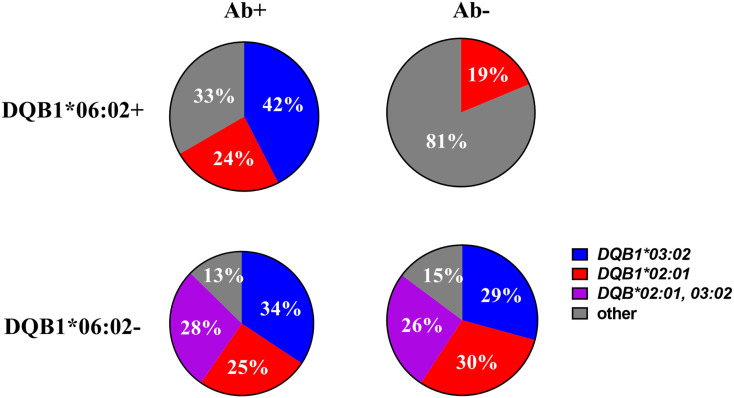
First, the type and frequency of DQB1 alleles were determined in both the 0602− and 0602+ cohorts. As depicted in Fig. 3, the 0602− individuals with T1D have percentages of T1D risk alleles as defined by DQB1*03:02 (DQ8), DQB1*02:01 (DQ2), or both DQ8 and DQ2 that are similar to those reported by other large T1D cohorts (3,5). The percentages of these alleles are independent of autoantibody positivity. However, there are stark differences in the 0602+ cohort based upon the presence of islet autoantibodies. Those 0602 positive with autoantibodies are more likely to have DQB1*03:02 (42%) as the other DQ allele, whereas in the 0602+ group without autoantibodies, the DQB1*03:02 allele was not present (P = 0.002) (Table 2). This is in contrast to DQB1*02:01, which is present in similar frequencies in the 0602+ group independent of autoantibodies (Table 2). When analyzing the 0602+ group with autoantibodies for known T1D class II risk alleles (e.g., DR3, DR4, DQ8, or DQ2), 82% (27/33) had at least one on the other chromosome (Supplementary Material).
Figure 3.
HLA alleles in DQB1*06:02+ and DQB1*06:02− T1D individuals compared by islet autoantibody positive (Ab+) versus autoantibody negative (Ab−) status. n = 33 for 0602+ Ab+, n = 16 for 0602+ Ab−, n = 2,077 for 0602− Ab+, and n = 670 for 0602− Ab−.
Next, to gain insight into further potential genetic risk for T1D with DQB1*06:02, we conducted HLA class I typing for HLA-A and HLA-B alleles. We also conducted class I typing on a subset of 0602− T1D participants (Supplementary Material). Several A and B alleles have been shown to confer T1D risk (28,29), and the frequencies of these alleles in 0602+ and 0602− participants with autoantibodies are depicted in Table 3. Remarkably, 94% (31/33) of 0602+ participants with autoantibodies had at least one A risk allele defined as A*02, A*03, or A*24 compared with 80% (221/276) of those 0602− T1D participants with a trend toward statistical significance (P = 0.058) (Table 3). Known T1D risk B alleles (B*15, 18, 39, 41, 49, and 50) were less frequent in both 0602+ and 0602− groups compared with the A alleles. B risk alleles are more frequent in 0602− T1D individuals compared with 0602+ individuals (51% vs. 24%, P = 0.007) (Table 3).
Table 3.
HLA class I typing in autoantibody-positive participants by DQB1*06:02 status
| DQB1*06:02+ | DQB1*06:02− | P value | |
|---|---|---|---|
| HLA-A allele (%) | |||
| 02 | 36 (24/66) | 33 (184/552) | 0.680 |
| 03 | 20 (13/66) | 11 (61/552) | 0.068 |
| 24 | 8 (5/66) | 10 (56/552) | 0.663 |
| Other | 36 (24/66) | 46 (251/552) | 0.190 |
| Individuals with a risk allele (02, 03, and 24) | 94 (31/33) | 80 (221/276) | 0.058 |
| HLA-B allele (%) | |||
| 15 | 9 (6/66) | 15 (42/276) | 0.240 |
| 18 | 3 (2/66) | 7 (20/276) | 0.273 |
| 39 | 0 (0/66) | 3 (8/276) | 0.362 |
| 41 | 0 (0/66) | 1 (2/276) | 1.00 |
| 49 | 2 (1/66) | 1 (3/276) | 0.578 |
| 50 | 0 (0/66) | 1 (2/276) | 1.00 |
| Other | 86 (57/66) | 72 (199/276) | 0.018 |
| Individuals with a risk allele (15, 18, 39, 41, 49, and 50) | 24 (8/33) | 51 (70/138) | 0.007 |
Taken together, HLA typing indicates that those T1D individuals with DQB1*06:02 have high frequencies of both known T1D risk class II and class I alleles.
Discussion
The HLA-DQB1*06:02 allele is known to provide dominant protection across the stages of T1D and from the initial development of clinical diabetes. As such, only a limited number of 0602+ T1D cases have been described in the literature. Here, we present one of the largest studies to characterize DQB1*06:02+ individuals with clinical T1D to provide insights into the potential mechanisms of failed genetic protection in the presence of a strongly protective HLA allele. These 50 patients were identified over 26 years of performing HLA-DQ typing and measurement of islet autoantibodies in patients presenting to a large tertiary referral center. Less than 2% of our cohort with clinical T1D had the DQB1*06:02 allele, which is evidence of the profound protection afforded by the DQ6 allele against T1D development. Characterizing the rare 0602+ individuals who have developed clinical T1D provides a framework for understanding the underlying mechanisms behind failed genetic protection, which can offer important insights into T1D pathogenesis.
In general, T1D can be diagnosed at any age but typically peaks between the ages of 10 and 14 years, which coincides with the start of puberty (30). The 0602+ T1D patients are no exception with an average age at diagnosis of 11 years, which was similar to the 0602− patients in our study. An older age was observed in autoantibody-positive relatives with DQB1*06:02 in the TrialNet cohort; however, only a very small percentage of those individuals progressed to clinical T1D (20). Our data do not suggest that age of diagnosis is delayed by the presence of the DQ6 allele, but our presented results and those from the TrialNet cohort indicate that DQB1*06:02 affords protection in the presence of islet autoimmunity. However, as we report here, rare instances where the DQB1*06:02 allele fails to provide genetic protection from T1D do occur. Therefore, protection via the presence of the DQ6 allele is not absolute.
In fact, approximately two-thirds of our 0602+ T1D patients had islet autoantibodies. It is also appreciated that autoantibody-negative T1D can encompass different types of diabetes including monogenic forms of diabetes (31), and we did identify monogenic forms of diabetes with mutations in the KCNJ11 and WFS1 genes in three 0602+ individuals. Maturity-onset diabetes of the young (MODY) is another monogenic form of diabetes, which is more common in autoantibody-negative patients (32). Comprehensive testing for monogenic forms of diabetes including MODY needs to be completed in the 0602+ patients without antibodies, as this subset of individuals does not have genetic risk for T1D, to discern whether these individuals truly have autoantibody-negative T1D. Finally, type 2 diabetes can be difficult to distinguish from autoantibody-negative T1D. Although we cannot completely exclude this possibility, the majority of our autoantibody-negative T1D participants were prepubertal, and all participants required insulin treatment at diagnosis, thus making a diagnosis of type 2 diabetes less likely.
The autoantibody profiles of those with 0602+ and 0602− T1D showed equivalent percentages of patients positive for autoantibodies directed against GAD65 and ZnT8 with similar levels between the groups. However, IAA and IA-2A levels were lower in the T1D 0602+ group compared with the 0602− group. Interestingly, a much lower percentage of autoantibodies directed against IA-2 was observed in the 0602+ T1D patients. This finding could indicate less autoimmunity directed against IA-2, or the potential exists that there are autoantibodies directed against the extracellular domain of IA-2 (33), as the measured antibodies in our assay bind to intracellular IA-2 epitopes. A recent report describes antibodies to a novel variant of IA-2 with three amino acid substitutions, which has been shown to increase the prediction of T1D in autoantibody-positive relatives (34). It will be important to measure these alternate IA-2 autoantibodies in our 0602+ T1D patients.
The potential mechanisms of failed genetic protection in the presence of DQB1*06:02 are important to define. Our HLA typing data indicate that both known class I and II T1D risk alleles are frequently present in the 0602+ T1D group. This implicates islet-reactive CD8 and CD4 T cells in disease development as class I and II molecules function to present peptides to these respective T-cell subsets. Remarkably, 94% of the autoantibody-positive 0602+ group had an A risk allele with much lower frequencies of B risk alleles, implicating the role of HLA-A molecules in 0602+ T1D patients. CD8 T cells reactive to islet peptides presented by HLA-A2 are known to exist in the peripheral blood of patients with T1D (35,36) and within the residual islets of T1D organ donors (37,38). Thus, the presence, frequency, and function of islet-reactive CD8 T cells will be important to measure in 0602+ T1D patients.
When examining the other DQ allele in addition to 0602, we observed a high proportion of T1D risk-conferring alleles, mainly DQ8 (DQB1*03:02) and DQ2 (DQB1*02:01). These class II risk alleles did segregate by autoantibody status in that those with islet autoantibodies were more likely to have DQ8 on the other chromosome. In fact, DQ8 was only observed in those T1D 0602+ individuals with autoantibodies. Preproinsulin peptides have been eluted from antigen-presenting cells expressing DQ6 and DQ8, indicating that both heterodimers are capable of binding and presenting insulin peptides (39,40). Interestingly, an epitope within the signal peptide was presented by both DQ8 and DQ6, albeit in different binding registers (40). It will be important to study the function and activation status of CD4 T cells that respond to similar insulin peptides presented by DQ6 and DQ8 in individuals with and without T1D.
An alternate mechanism includes epitope stealing in which DQ6 preferentially binds T1D autoantigens such that DQ8 cannot present these antigens thus leading to less effector T-cell responses. Binding and peptide elution data from DQ6 and DQ8 expressing antigen-presenting cells are consistent with this mechanism (40,41). Epitope stealing may be lacking in the ∼50% of our 0602+ T1D cases with DQ8; however, additional mechanisms may still be involved for those with a strongly protective HLA allele to develop T1D.
In conclusion, we describe a very rare cohort of individuals that developed clinical T1D in the presence of HLA-DQB1*06:02, which is ordinarily known to confer strong protection against disease development. Studying the clinical, immunologic, and genetic features of individuals that failed genetic protection holds promise for understanding mechanisms that ultimately lead to β-cell destruction in T1D.
Article Information
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK-095995, DK-108868, DK-110845, DK-032083, DK-094712), JDRF, the Children’s Diabetes Foundation (2-SRA-2016-202-5-B), and the Colorado Clinical and Translational Science Institute (TR001082).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.M.S., L.Y. and A.W.M. designed the studies and wrote the manuscript. A.M.M., A.A.A., K.A.M., E.E.B., and T.A. performed experiments and reviewed the manuscript. L.P. conducted independent statistical analysis on the study data. A.W.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12311162.
References
- 1.Concannon P, Erlich HA, Julier C, et al.; Type 1 Diabetes Genetics Consortium . Type 1 diabetes: evidence for susceptibility loci from four genome-wide linkage scans in 1,435 multiplex families. Diabetes 2005;54:2995–3001 [DOI] [PubMed] [Google Scholar]
- 2.Todd JA, Walker NM, Cooper JD, et al.; Genetics of Type 1 Diabetes in Finland; Wellcome Trust Case Control Consortium . Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 2007;39:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett JC, Clayton DG, Concannon P, et al.; Type 1 Diabetes Genetics Consortium . Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradfield JP, Qu HQ, Wang K, et al. . A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS Genet 2011;7:e1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erlich H, Valdes AM, Noble J, et al.; Type 1 Diabetes Genetics Consortium . HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu X, Deutsch AJ, Lenz TL, et al. . Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet 2015;47:898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama M, Simmons KM, Michels AW. Molecular interactions governing autoantigen presentation in type 1 diabetes. Curr Diab Rep 2015;15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009;360:1646–1654 [DOI] [PubMed] [Google Scholar]
- 9.Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med 2012;2:a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 1987;329:599–604 [DOI] [PubMed] [Google Scholar]
- 11.Morel PA, Dorman JS, Todd JA, McDevitt HO, Trucco M. Aspartic acid at position 57 of the HLA-DQ beta chain protects against type I diabetes: a family study. Proc Natl Acad Sci U S A 1988;85:8111–8115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baisch JM, Weeks T, Giles R, Hoover M, Stastny P, Capra JD. Analysis of HLA-DQ genotypes and susceptibility in insulin-dependent diabetes mellitus. N Engl J Med 1990;322:1836–1841 [DOI] [PubMed] [Google Scholar]
- 13.Pugliese A, Kawasaki E, Zeller M, et al. . Sequence analysis of the diabetes-protective human leukocyte antigen-DQB1*0602 allele in unaffected, islet cell antibody-positive first degree relatives and in rare patients with type 1 diabetes. J Clin Endocrinol Metab 1999;84:1722–1728 [DOI] [PubMed] [Google Scholar]
- 14.Redondo MJ, Kawasaki E, Mulgrew CL, et al. . DR- and DQ-associated protection from type 1A diabetes: comparison of DRB1*1401 and DQA1*0102-DQB1*0602*. J Clin Endocrinol Metab 2000;85:3793–3797 [DOI] [PubMed] [Google Scholar]
- 15.Klitz W, Maiers M, Spellman S, et al. . New HLA haplotype frequency reference standards: high-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens 2003;62:296–307 [DOI] [PubMed] [Google Scholar]
- 16.Erlich HA, Griffith RL, Bugawan TL, Ziegler R, Alper C, Eisenbarth G. Implication of specific DQB1 alleles in genetic susceptibility and resistance by identification of IDDM siblings with novel HLA-DQB1 allele and unusual DR2 and DR1 haplotypes. Diabetes 1991;40:478–481 [DOI] [PubMed] [Google Scholar]
- 17.Hoover ML, Marta RT. Molecular modelling of HLA-DQ suggests a mechanism of resistance in type 1 diabetes. Scand J Immunol 1997;45:193–202 [DOI] [PubMed] [Google Scholar]
- 18.Insel RA, Dunne JL, Atkinson MA, et al. . Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sims EK, Geyer S, Johnson SB, et al.; Type 1 Diabetes TrialNet Study Group . Who is enrolling? The path to monitoring in type 1 diabetes TrialNet’s Pathway to Prevention. Diabetes Care 2019;42:2228–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugliese A, Boulware D, Yu L, et al.; Type 1 Diabetes TrialNet Study Group . HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 haplotype protects autoantibody-positive relatives from type 1 diabetes throughout the stages of disease progression. Diabetes 2016;65:1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeliszewski D, Tiercy JM, Boitard C, et al. . Extensive study of DRB, DQA, and DQB gene polymorphism in 23 DR2-positive, insulin-dependent diabetes mellitus patients. Hum Immunol 1992;33:140–147 [DOI] [PubMed] [Google Scholar]
- 22.Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet 1996;59:1134–1148 [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L, Rewers M, Gianani R, et al. . Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]
- 24.Wenzlau JM, Juhl K, Yu L, et al. . The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rewers A, Babu S, Wang TB, et al. . Ethnic differences in the associations between the HLA-DRB1*04 subtypes and type 1 diabetes. Ann N Y Acad Sci 2003;1005:301–309 [DOI] [PubMed] [Google Scholar]
- 26.Gloyn AL, Pearson ER, Antcliff JF, et al. . Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004;350:1838–1849 [DOI] [PubMed] [Google Scholar]
- 27.Urano F. Wolfram syndrome: diagnosis, management, and treatment. Curr Diab Rep 2016;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nejentsev S, Howson JM, Walker NM, et al.; Wellcome Trust Case Control Consortium . Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 2007;450:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noble JA, Valdes AM, Varney MD, et al.; Type 1 Diabetes Genetics Consortium . HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes 2010;59:2972–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Chan L. Monogenic diabetes: what it teaches us on the common forms of type 1 and type 2 diabetes. Endocr Rev 2016;37:190–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chambers C, Fouts A, Dong F, et al. . Characteristics of maturity onset diabetes of the young in a large diabetes center. Pediatr Diabetes 2016;17:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acevedo-Calado M, James EA, Morran MP, et al. . Identification of unique antigenic determinants in the amino terminus of IA-2 (ICA512) in childhood and adult autoimmune diabetes: new biomarker development. Diabetes Care 2017;40:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acevedo-Calado MJ, Pietropaolo SL, Morran MP, et al.; Type 1 Diabetes TrialNet Study Group . Autoantibodies directed toward a novel IA-2 variant protein enhance prediction of type 1 diabetes. Diabetes 2019;68:1819–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velthuis JH, Unger WW, Abreu JR, et al. . Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes 2010;59:1721–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James EA, Abreu JRF, McGinty JW, et al.; Immunology of Diabetes Society T Cell Workshop Committee . Combinatorial detection of autoreactive CD8+ T cells with HLA-A2 multimers: a multi-centre study by the Immunology of Diabetes Society T Cell Workshop. Diabetologia 2018;61:658–670 [DOI] [PubMed] [Google Scholar]
- 37.Coppieters KT, Dotta F, Amirian N, et al. . Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Duque S, Azoury ME, Colli ML, et al. . Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naïve CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab 2018;28:946–960.e6 [DOI] [PubMed] [Google Scholar]
- 39.Astill TP, Ellis RJ, Arif S, Tree TI, Peakman M. Promiscuous binding of proinsulin peptides to Type 1 diabetes-permissive and -protective HLA class II molecules. Diabetologia 2003;46:496–503 [DOI] [PubMed] [Google Scholar]
- 40.van Lummel M, Buis DTP, Ringeling C, et al. . Epitope stealing as a mechanism of dominant protection by HLA-DQ6 in type 1 diabetes. Diabetes 2019;68:787–795 [DOI] [PubMed] [Google Scholar]
- 41.Eerligh P, van Lummel M, Zaldumbide A, et al. . Functional consequences of HLA-DQ8 homozygosity versus heterozygosity for islet autoimmunity in type 1 diabetes. Genes Immun 2011;12:415–427 [DOI] [PubMed] [Google Scholar]