Abstract
NKG2D is implicated in autoimmune diabetes. However, the role of this receptor in diabetes pathogenesis is unclear owing to conflicting results with studies involving global inhibition of NKG2D signaling. We found that NKG2D and its ligands are present in human pancreata, with expression of NKG2D and its ligands increased in the islets of patients with type 1 diabetes. To directly assess the role of NKG2D in the pancreas, we generated NOD mice that express an NKG2D ligand in β-islet cells. Diabetes was reduced in these mice. The reduction corresponded with a decrease in the effector to central memory CD8+ T-cell ratio. Further, NKG2D signaling during in vitro activation of both mouse and human CD8+ T cells resulted in an increased number of central memory CD8+ T cells and diabetes protection by central memory CD8+ T cells in vivo. Taken together, these studies demonstrate that there is a protective role for central memory CD8+ T cells in autoimmune diabetes and that this protection is enhanced with NKG2D signaling. These findings stress the importance of anatomical location when determining the role NKG2D signaling plays, as well as when developing therapeutic strategies targeting this pathway, in type 1 diabetes development.
Introduction
Type 1 diabetes is a T cell–mediated autoimmune disorder resulting in the destruction of insulin-producing pancreatic β-cells. β-Cell loss results in an inability of the body to produce insulin. Despite significant progress in type 1 diabetes research, the immune defects that lead to the development of this disease remain poorly understood. The immune receptor NKG2D, expressed by natural killer (NK) cells and subsets of T cells (1–5), is implicated in type 1 diabetes development. However, its role in disease progression remains unclear, with conflicting reports describing pathogenic (6,7), nonpathogenic (8), and protective (9) effects of NKG2D signaling.
NKG2D recognizes multiple NKG2D ligands in both humans and mice. These are endogenous ligands, which are all distantly related to MHC class I in sequence, and are generally believed to be functionally redundant (10,11). NKG2D ligands are considered stress ligands, with their expression induced by cellular stressors, including viral infection or DNA damage (10,11). Engagement of these ligands by NKG2D on NK cells is sufficient to activate NK cell killing of NKG2D ligand-bearing target cells (1). On T cells, NKG2D engagement generally costimulates T-cell receptor–driven activation, enhancing T-cell responses (1,7,9,12–14). In addition, NKG2D signaling has been shown to be important in the development of mouse memory CD8+ T cells (15–17).
Early studies with the NOD mouse model led to the hypothesis that NKG2D signaling, induced by NKG2D ligands expressed on β-islet cells, enhances diabetes development (6,7). However, conflicting results were later reported that brought into question the importance of NKG2D to spontaneous autoimmune diabetes in this preclinical model (8,9). Our previous data implicated differential effects of NKG2D signaling induced by microbiota or islet antigen as a source of this controversy (9). Therefore, new experimental approaches are required to determine the various roles of NKG2D in diabetes.
Here, we show that mRNAs encoding both NKG2D and NKG2D ligands are expressed in human islets, demonstrating that NKG2D signaling in the pancreas is likely relevant to type 1 diabetes pathogenesis. To directly test the effect of NKG2D signaling in the pancreas on autoimmune diabetes, we generated NOD mice with constitutive expression of the mouse NKG2D ligand RAE1ε on β-islet cells (i.e., NOD.RIP-RAE1ε mice). We found that despite earlier infiltration of immune cells into the pancreas, these mice developed significantly less diabetes compared with littermate NOD mice. This diabetes reduction corresponded with a reduced ratio of CD8+ effector T (Teff) and effector memory T (Tem) cells (Teff + em) to CD8+ central memory T cells (Tcm). Correlating with these in vivo data, we found that stimulation of NKG2D on NOD and human CD8+ T cells during in vitro activation resulted in a reduced CD8+ Teff + em:Tcm ratio. Finally, we found that CD8+ Tcm cells actively suppress NOD diabetes development in vivo. Taken together, these results indicate that NKG2D ligand expression in pancreatic islets protects against autoimmune diabetes development by enhancing the generation or survival of a protective CD8+ Tcm population.
Research Design and Methods
Mice
NOD/ShiLtJ (NOD), NOD.CB17-Prkdcscid/J (NOD.Scid), and C57BL/6 (B6) mice were purchased from The Jackson Laboratory (stock numbers 001976, 001303, and 000664, respectively). B6.Cg-Tg(Ins2-cre)25Mgn/J (B6.RIP-cre) mice were purchased from The Jackson Laboratory (stock number 003573). B6.PCCALL-RAE1ε and ubiquitous B6.RAE1ε mice were generated previously (7,18) and provided by Dr. Andrey Shaw (Washington University School of Medicine, St. Louis, MO). The PCCALL-RAE1ε (PCCALL) allele and rat insulin promoter (RIP)-cre transgene were transferred to the NOD genetic background using speed congenic methods (19) by the Washington University School of Medicine Mouse Genetics Core. Both NOD.PCCALL and NOD.RIP-cre strains maintain >98% of the NOD genome, with no alterations in known Idd loci. Experimental NOD.PCCALL-RAE1ε, NOD.RIP-cre, and NOD.RIP-RAE1ε littermates of both sexes were generated by interbreeding NOD.PCCALL and NOD.RIP-cre mice. NOD.B6-Klrk1tm1.1Bpol (NOD.Klrk1−/−) mice were previously generated in our laboratory (9). All mice were housed under specific pathogen–free conditions at either the Washington University School of Medicine or the University of Kansas Medical Center animal facility in accordance with institutional guidelines and with approval from the institutional animal care and use committee.
Human Pancreatic Islet Microarray
Frozen tissue from cadaveric donors was provided by the Network for Pancreatic Organ Donors (nPOD) (20) with approval from the University of Florida Health Center Review Board. The characteristics of the donors used are shown in Supplementary Table 1. Tissue slides were fixed, and laser capture of islets was conducted as previously described (21). All islets in two to five sections of tissue from each donor (a minimum of 30 islets each) were captured and pooled, and RNA was extracted using the Arcturus PicoPure RNA Isolation Kit (Applied Biosystems, Grand Island, NY). An Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) was used to assess quality and quantity of RNA. Samples with sufficient RNA quantity and quality were then subjected to gene expression analysis using Affymetrix expression arrays (GeneChip Human Gene 2.0 ST), and scaled normalized gene expression values were produced as previously described (22).
Insulitis Scoring
Insulitis was scored using standard methods (23). The pancreata were fixed in formalin, and 5 μmol/L sections were generated from the whole tissue and stained with hematoxylin-eosin by the Washington University School of Medicine Histology Core or the Kansas Intellectual and Developmental Disabilities Research Center Histology Core. Thirty islets per pancreas in nonoverlapping sections were scored.
Diabetes Determination
Diabetes was determined by blood glucose measurements taken weekly through tail vein nick. A mouse was defined as diabetic on the date when the first of two consecutive blood glucose measurements ≥250 mg/dL was obtained, as previously described (24).
Flow Cytometry
Spleens were dissociated by pressing through a 40-μm cell strainer in isolation buffer containing PBS, 2% FCS, and 2 mmol/L EDTA. Pancreata were chopped into small sections and digested with 1 mg/mL collagenase IV (Life Technologies) in Iscove’s modified Dulbecco’s medium for 10 min at 37° with shaking, and a single-cell suspension was generated by pressing through a 40-μm cell strainer. The cells were then washed at least five times, followed by antibody staining at 4°C for at least 15 min. After staining, cells were washed and analyzed. Cultured cells were washed once in isolation buffer and stained before similar staining and analysis.
NOD Diabetes Transfer
Male and female mice were used, with all transfers containing male cells going only into male NOD.Scid recipients. Spleens and lymph nodes from nondiabetic 6- to 12-week-old NOD mice were harvested and dissociated by passing through a 40-μm cell strainer into isolation buffer containing PBS, 2% FCS, and 2 mmol/L EDTA. CD8+ T cells were then enriched by negative selection using magnetic bead separation (cat. no. 558471; BD Biosciences), according to the manufacturer’s instructions. CD8+ T cells were then stained with anti-mCD3-PE-Cy7, anti-mCD8-APC, anti-mCD44-BV650, and anti-mCD62L-BV510 antibodies (BD Biosciences). CD3+CD8+CD44+CD62L− (Teff + em) and CD3+CD8+CD44+CD62L+ (Tcm) populations were separated by FACS by the University of Kansas Medical Center Flow Core using a FACSAria IIIu (BD Biosciences). Separately, splenocytes from nondiabetic 6- to 12-week-old mice were depleted of CD8+ T cells by positive selection using anti-mCD8-PE (BD Biosciences) and anti-PE magnetic bead separation (cat. no. 557899; BD Biosciences); 2.5 × 106 Teff + em, 2.5 × 106 Tcm, or a mixture of 2.5 × 106 Teff + em and 2.5 × 106 Tcm were each combined with 1.5 × 107 CD8+ T cell–depleted NOD splenocytes and adoptively transferred into 2- to 6-month-old NOD.Scid mice by retro-orbital injection.
In Vitro Cell Culture
All cells were grown in Iscove’s modified Dulbecco’s medium supplemented with 0.1 units/mL penicillin, 0.1 units/mL streptomycin, 0.29 μg/mL l-glutamine, 5.5 μmol/L 2-mercaptoethanol, and 10% FCS. Cells were grown at 37°C at 5% CO2.
In Vitro Activation of Mouse CD8+ T Cells
CD8+ T cells were isolated from the spleens and lymph nodes of 6- to 8-week-old NOD mice by negative selection using magnetic bead separation (cat. no. 558471; BD Biosciences), according to the manufacturer’s instructions. Separately, splenocytes from ubiquitous RAE1ε mice or control C57BL/6 mice were dissociated by passing through a 40-μm cell strainer and irradiated with 15 Gy using a 137Cs irradiator. Then, 106 CD8+ T cells were plated with either irradiated C57BL/6 RAE-1ε or C57BL/6 control splenocytes (3 × 106) along with 1 μg/mL anti-mCD3 (clone 2C11) in a 24-well plate.
In Vitro Activation of Human CD8+ T Cells
Total CD8+ T cells were isolated from human peripheral blood mononuclear cells by negative selection using a human CD8 T-cell enrichment set (BD Biosciences), according to manufacturer’s instructions. Twenty-four–well plates were incubated with 3 μg/mL anti-hCD3 (clone: OKT3) and anti-hCD28 (clone: 9.3), along with 20 μg/mL anti-hNKG2D (clone: 149810), or isotype control in PBS overnight at 4°. The plates were washed with PBS, and then the CD8+ T cells were added to the plate (106 cells/well). After 3 days, cells were split 1:2 and maintained in the presence of 20 μg/mL plate-bound anti-hNKG2D. After 5 days of culture, the cells were harvested for analysis.
Statistical Analyses
Data were collected and analyzed with GraphPad Prism (GraphPad Software). The log-rank (Mantel-Cox) test was used for analysis of diabetes incidence or human NKG2D ligand expression incidence. Differences between groups were compared using a two-tailed Mann-Whitney U test unless otherwise indicated. Linear regression analysis was performed to determine relationships in gene expression in human islets. P < 0.05 was considered to be significant and is marked in the figures. Results are shown as mean ± SEM unless otherwise indicated.
Data and Resource Availability
The primary data generated and analyzed during the current study are either included in the published article or are available from the corresponding author upon reasonable request. All resources generated during the current study, including all novel mouse strains, are available from the corresponding author upon reasonable request.
Results
Increased Expression of NKG2D and NKG2D Ligands in Pancreatic Islets of Patients With Type 1 Diabetes
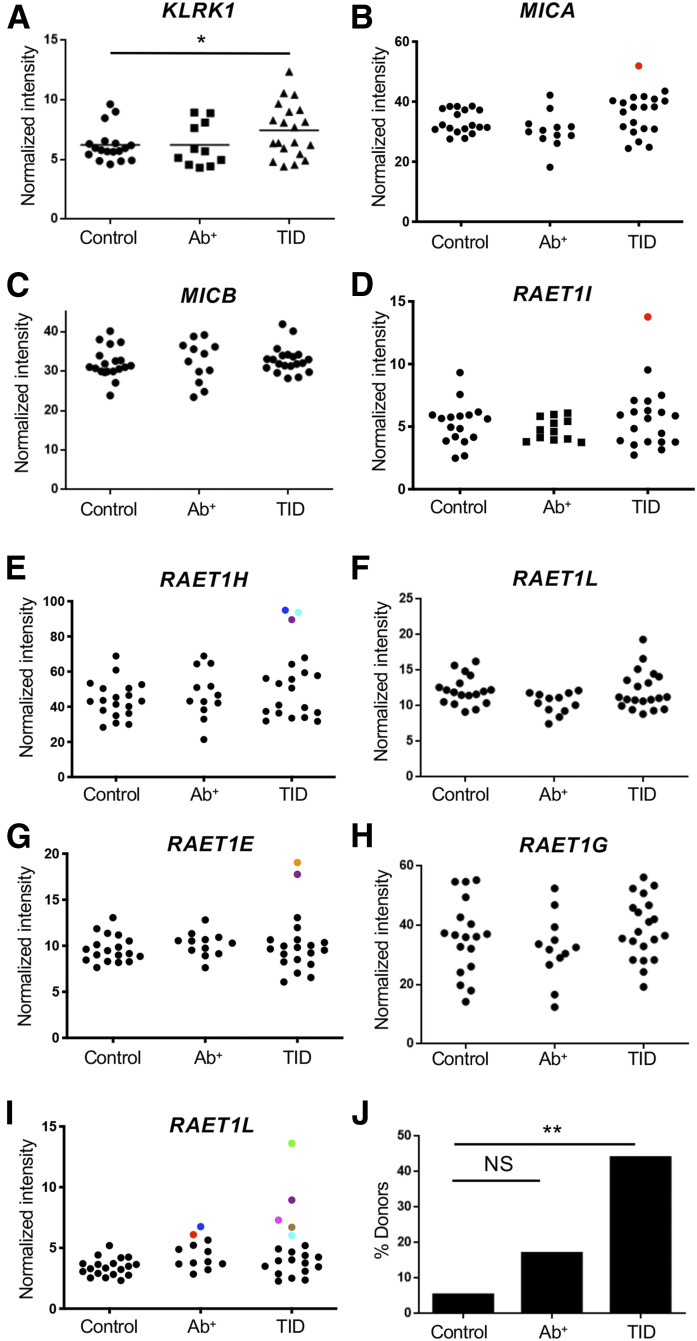
To determine whether studying NKG2D-ligand interaction within the pancreas is relevant to type 1 diabetes, we measured the expression of the mRNAs encoding NKG2D and NKG2D ligands within human pancreatic islets. Pancreatic islets were harvested by laser capture from tissue sections donated to nPOD by people with type 1 diabetes, people with islet antigen-specific autoantibodies but without diabetes (likely prediabetes), or age- and sex-matched control subjects without diabetes. Increased transcripts encoding both NKG2D and NKG2D ligands were detected in pancreatic islets harvested from donors with type 1 diabetes, but not individuals positive for islet-specific antibody, compared with donors without diabetes (Fig. 1). Specifically, the average mean intensity value for the mRNA encoding NKG2D was increased by 20% in the islets from patients with type 1 diabetes compared with those from donors without diabetes (P < 0.05, Mann-Whitney U test) (Fig. 1A). Additionally, we found a positive relationship between NKG2D expression and islet T-cell infiltration (Supplementary Fig. 1A). In the case of NKG2D ligands, 40% of donors with type 1 diabetes expressed levels of mRNA encoding of at least one ligand at a level determined by Grubb’s test to be an outlier from the values of the control subjects compared with 5.3% of control subjects without diabetes (P < 0.009) (Fig. 1B–J). We also found a positive relationship between NKG2D ligand expression and islet T-cell infiltration or NKG2D expression (Supplementary Fig. 1B and C). Together, these data indicate that NKG2D-ligand interactions occur within T cell–infiltrated islets in the pancreas of patients with type 1 diabetes.
Figure 1.
Increased expression of NKG2D and NKG2D ligand mRNA in pancreatic islets of patients with type 1 diabetes. mRNA expression of the genes encoding NKG2D (A) and NKG2D ligands (B–I) in the pancreatic islets from individuals with type 1 diabetes (T1D), individuals without diabetes positive for islet-specific autoantibodies (Ab+), or age- and sex-matched healthy control subjects. *P < 0.05 by Mann-Whitney U test. Data points in panels B–I represent individual samples, with colored circles denoting statistical outliers as determined by Grubb’s test. Each color denotes data from one individual. Percent of donors from each group expressing mRNA for at least one NKG2D ligand at a level determined to be an outlier (J). **P = 0.0084 by a log-rank test.
Generation of a Novel Model to Investigate the Role of NKG2D Signaling Within the Pancreas
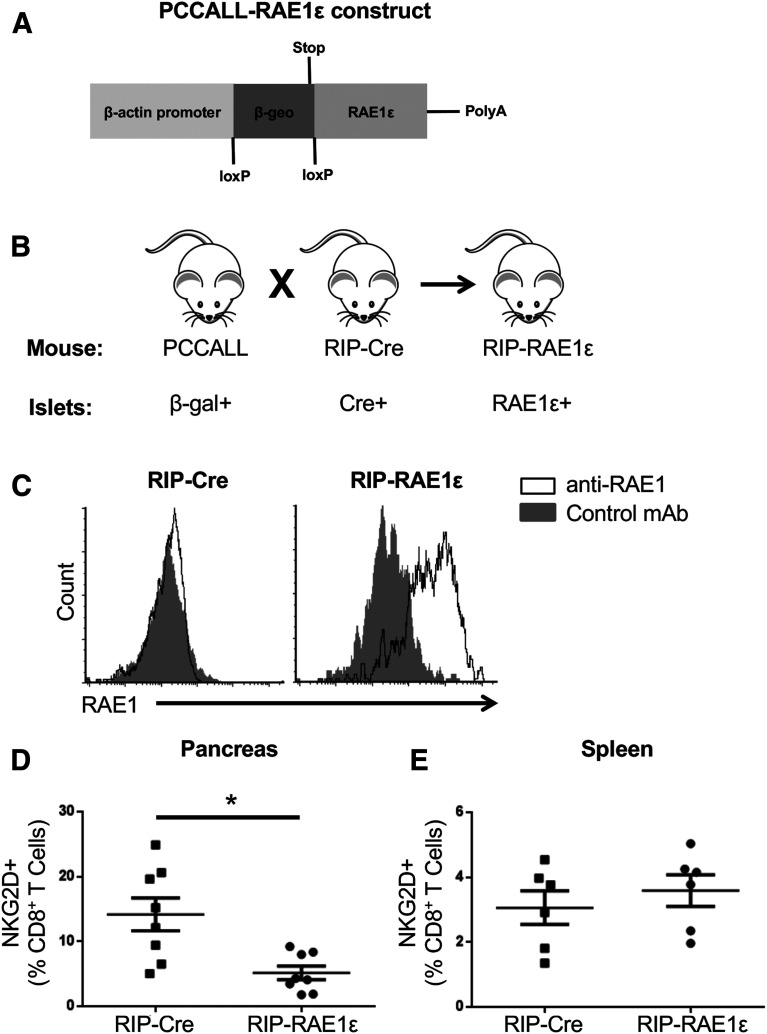
We wished to directly test the effect of NKG2D ligand expression in the pancreas with an animal model. However, because both NKG2D and NKG2D ligands are expressed on immune cells (25–28), we could not eliminate these proteins specifically within the pancreas. We therefore tested this question by increasing NKG2D signaling within the pancreas, which also allowed us to directly test the hypothesis that NKG2D ligand expression on β-islet cells enhances diabetes (6,7). We moved a cre-inducible RAE1ε transgene we generated previously (PCCALL-RAE1ε) (7) and a RIP-cre transgene onto the NOD background. We then interbred these mice to generate NOD mice with constitutive expression of RAE1ε in the pancreatic islets (NOD.RIP-RAE1ε) (Fig. 2A–C).
Figure 2.
Generation of NOD mice with constitutive expression of the NKG2D ligand RAE1ε in the pancreas. A and B: Schematic of part of the vector used to generate the PCCALL-RAE1ε mice (7) (A) and breeding schema for generating NOD mice with constitutive RAE1ε expression in the islets (B). Note that rather than RAE1ε, PCCALL and RIP-cre mice have constitutive expression of β-gal or cre recombinase in islets, respectively. C: Flow cytometric analysis of nonimmune cells in the pancreas of RIP-cre or RIP-RAE1ε mice stained with an antibody specific for RAE1 (open histogram) or a control antibody (solid histogram). These data are representative of three independent experiments. D and E: The percentage of CD8+ T cells with detectable NKG2D expression in the pancreas (D) (n = 8) or spleen (E) (n = 6) of RIP-RAE1ε and RIP-cre mice as determined by flow cytometry. *P < 0.01 by Mann-Whitney U test. mAb, monoclonal antibody.
Engagement of NKG2D by ligand results in not only signaling but also internalization of NKG2D from the cell surface (29). Therefore, to confirm that NKG2D signaling is enhanced in the pancreas of NOD.RIP-RAE1ε mice as it is in B6.RIP-RAE1ε (7), we compared NKG2D expression on CD8+ T cells in the pancreas of NOD.RIP-RAE1ε and single transgenic control NOD mice, NOD.RIP-cre. NKG2D expression was lower on CD8+ T cells in the pancreas of NOD.RIP-RAE1ε mice compared with NOD.RIP-cre (Fig. 2D). By contrast, NKG2D expression was similar within the spleen between the two strains (Fig. 2E). These data demonstrate that NKG2D signaling is enhanced specifically in the pancreas of NOD.RIP-RAE1ε mice.
Diabetes Is Delayed in RIP-RAE1ε NOD Mice Through NKG2D Signaling
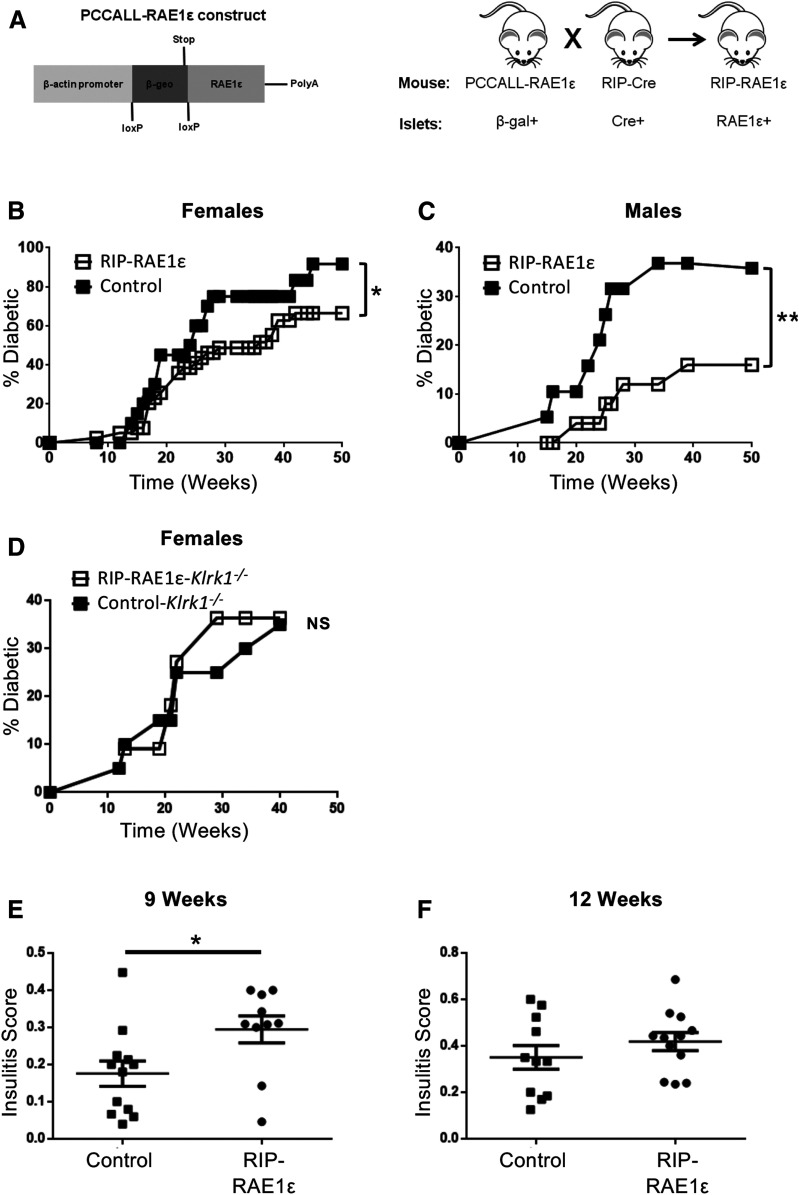
To determine whether overexpression of RAE1ε in β-cells altered NOD islet-specific immunity, we compared diabetes development between NOD.RIP-RAE1ε (RAE1ε+ in islets) and control NOD.PCCALL (RAE1ε− in islets) mice. We observed a significant decrease in diabetes in the NOD.RIP-RAE1ε compared with the NOD.PCCALL littermates (Fig. 3B and C). Because of sexual dimorphism in the NOD model (30–32), a difference in diabetes incidence between male and female mice was expected (Fig. 3B and C). However, in both sexes, RAE1ε expression in the β-islet cells significantly reduced diabetes. NKG2D is the only known receptor to interact with RAE1ε. Therefore, we crossed the RIP-RAE1ε NOD mice to NOD mice genetically deficient in the gene encoding NKG2D (Klrk1) (9,33). Diabetes developed similarly in NOD.RIP-RAE1ε.Klrk1−/− and NOD.PCCALL.Klrk1−/− mice (Fig. 3D). This demonstrates that NKG2D was required for the reduced diabetes in NOD.RIP-RAE1ε mice.
Figure 3.
Diabetes is delayed in RIP-RAE1ε NOD mice through interaction with NKG2D. A: Schematic of part of the vector used to generate the PCCALL-RAE1ε mice and breeding schema for generating NOD mice with constitutive RAE1ε expression in the islets. B and C: Diabetes development in female RIP-RAE1ε (n = 39) and control (PCCALL) (n = 21) and male RIP-RAE1ε (n = 23) and control (PCCALL) (n = 19) NOD mice. D: Diabetes development in female RIP-RAE1ε NKG2D-deficient (Klrk1−/−) (n = 11) and control (PCCALL/NKG2D-deficient [Klrk1−/−]) (n = 20) NOD mice. *P < 0.05, **P < 0.0008 by log-rank test. E and F: Insulitis scores in the pancreata of 9-week-old and 12-week-old RIP-RAE1ε and control (RIP-cre and PCCALL) mice. *P < 0.05 by Mann-Whitney U test.
Earlier Insulitis in RIP-RAE1ε NOD Mice
To determine the mechanism by which diabetes is reduced in NOD.RIP-RAE1ε mice, we first compared insulitis development between NOD.RIP-RAE1ε and control littermates (NOD.PCCALL). Consistent with our earlier studies of the B6.RAE1ε mice (7), we observed increased insulitis in the NOD.RIP-RAE1ε mice compared with NOD.PCCALL mice at 9 weeks of age (Fig. 3E). However, at 12 weeks of age, insulitis was similar in NOD.PCCALL and NOD.RIP-RAE1ε mice (Fig. 3F). Therefore, we concluded that the decrease in diabetes development in the NOD.RIP-RAE1ε mice was not the result of reduced immune infiltration into islets.
Reduced CD8+ Teff + em:Tcm Ratio in RIP-RAE1ε NOD Mice
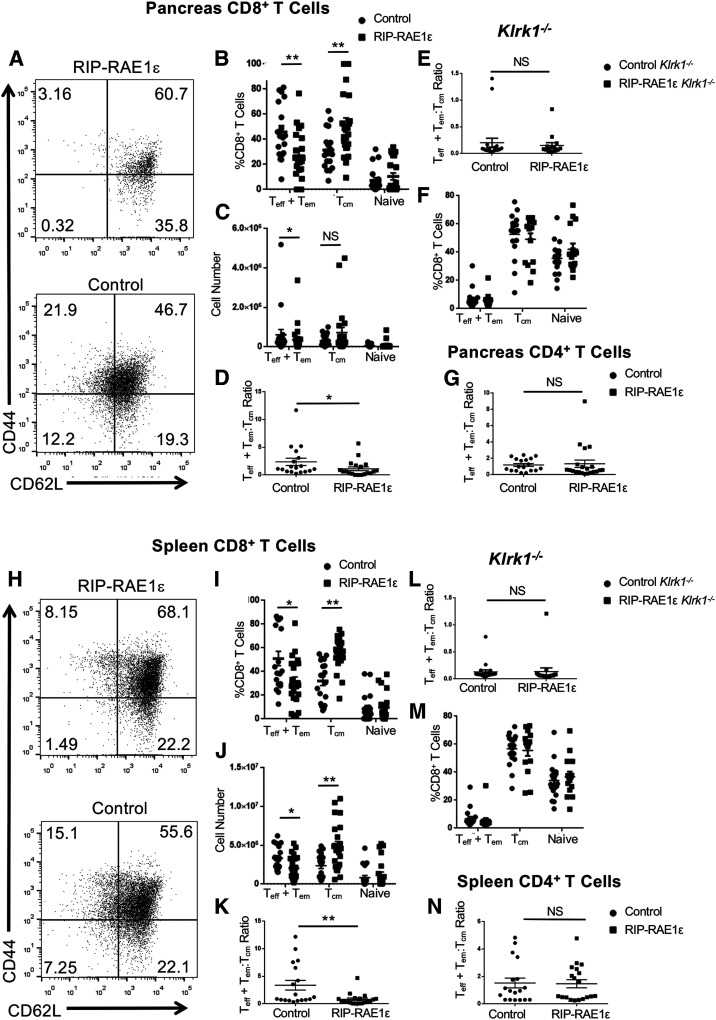
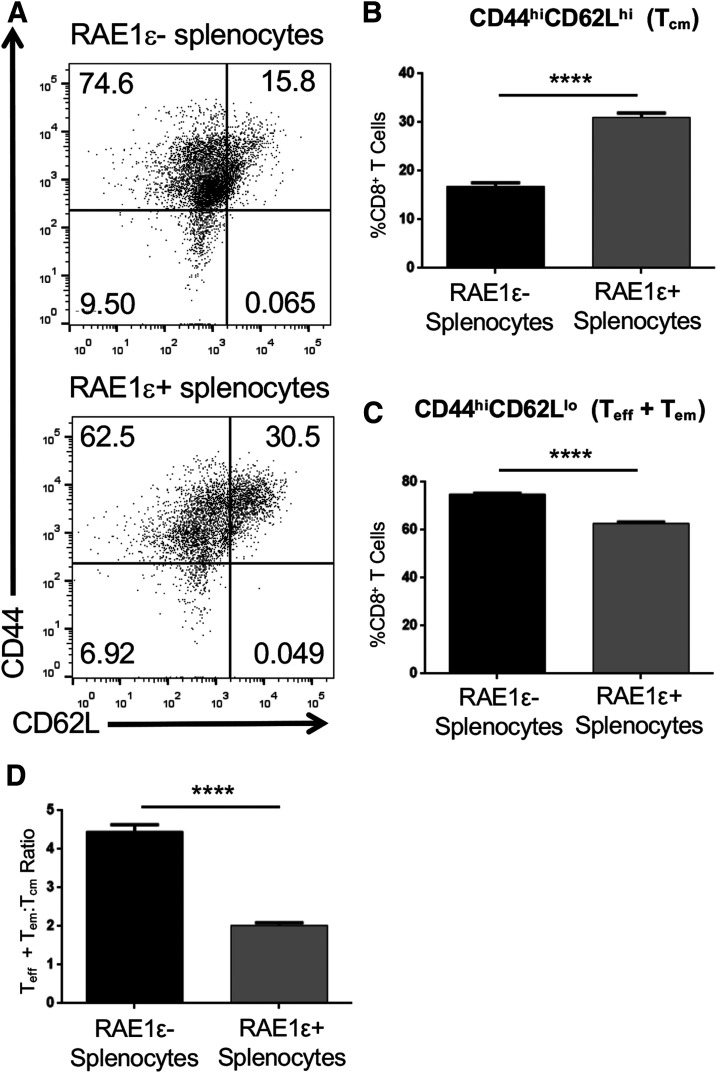
We next compared the immune infiltrate in the pancreas of NOD.RIP-RAE1ε and control littermates (NOD.PCCALL and NOD.RIP-cre) at 12 weeks of age, which is a time at which these strains have similar insulitis and have not yet developed diabetes. We focused on T cells and NK cells because these are the cells on which we detect NKG2D in NOD pancreata and spleens (9). We found similar numbers of T cells, including CD4+ T and CD8+ T, as well as NK cells, in the pancreas (Supplementary Fig. 2A–D) and spleen (Supplementary Fig. 2E–H) of NOD.RIP-RAE1ε and control littermates (NOD.PCCALL and NOD.RIP-cre). However, NOD.RIP-RAE1ε mice had a lower percentage of a CD8+ Teff or Tem phenotype (CD44hiCD62Llo) and a greater percentage of the CD8+ Tcm phenotype (CD44hiCD62hi) in the pancreas (Fig. 4A–C and Supplemental Fig. 5B), resulting in a decreased CD8+ Teff + em:Tcm ratio compared with control littermates (NOD.PCCALL and NOD.RIP-cre) (Fig. 4D). The CD8+ Teff + em:Tcm ratio was even lower in the spleen of NOD.RIP-RAE1ε mice (Fig. 4H–K and Supplemental Fig. 5B), and this difference was consistently observed in both male and female mice (Supplementary Fig. 3). Demonstrating that NKG2D signaling was required for this effect on CD8+ T-cell populations, no difference was observed between NOD.RIP-RAE1ε-Klrk1−/− versus control littermate NOD.Klrk1−/− (PCCALL/Klrk1−/− and RIP-cre/Klrk1−/−) (Fig. 4E, F, L, and M). In contrast to these CD8+ T-cell population alterations, we did not observe a difference in the CD4+ Teff + em:Tcm ratio when comparing NOD.RIP-RAE1ε and control littermates (NOD. PCCALL and NOD.RIP-cre) (Fig. 4G and N and Supplemental Fig. 4).
Figure 4.
Decreased CD8+ Teff + em:Tcm ratio in RIP-RAE1ε NOD mice. A and H: Representative flow cytometry data gated on CD3+CD8+ cells from the pancreas or spleen of a RIP-RAE1ε (top) or control (bottom) mouse showing CD44 and CD62L expression. The numbers shown are the percentage of cells present in each quadrant of the dot plot. B and I: The percentage of CD8+CD3+ cells that were CD44+CD62L− (Teff + em), CD44+CD62L+ (Tcm), or CD44−CD62L+ (naive) in the pancreas or spleen of RIP-RAE1ε mice (n = 22) and control (RIP-cre and PCCALL) mice (n = 19). C and J: The number of cells that were CD44+CD62L− (Teff + em), CD44+CD62L+ (Tcm), or CD44−CD62L+ (naive) in the pancreas and spleen of RIP-RAE1ε mice (n = 22) and control (RIP-cre and PCCALL) mice (n = 19). D and K: The CD8+ Teff + em:Tcm ratio in the pancreas and spleen of RIP-RAE1ε and control (RIP-cre and PCCALL) mice. E and L: The CD8+ Teff + em:Tcm ratio in the pancreas and spleen of RIP-RAE1ε and control (RIP-cre and PCCALL) Klrk1−/− mice. F and M: The percentage of cells that were CD44+CD62L− (Teff + em), CD44+CD62L+ (Tcm), or CD44−CD62L+ (naive) in the pancreas and spleen of RIP-RAE1ε/Klrk1−/− (n = 15) and control (RIP-cre and PCCALL)/Klrk1−/− (n = 20) mice. G and N: The CD4+ Teff + em:Tcm ratio in the pancreas and spleen of RIP-RAE1ε (n = 22) and control (RIP-cre and PCCALL) (n = 19) mice. *P < 0.05, **P < 0.01 by two-sided Mann-Whitney U test.
CD8+ Tcm Delays NOD Diabetes Development
We hypothesized that the greater number of CD8+ Tcm in the NOD.RIP-RAE1ε was directly responsible for the decreased diabetes development in these mice. To test this, we purified CD8+ Teff + em (CD44+CD62L−) and Tcm (CD44+CD62L+) populations from nondiabetic NOD mice. We then adoptively transferred Teff + em, Tcm, or Teff + em and Tcm, along with CD8+ T cell–depleted NOD splenocytes, into NOD.Scid recipient mice (Fig. 5A). We measured blood glucose weekly, and time to diabetes was determined within each experiment relative to the first mouse to become diabetic. The mice that received the CD8+ Teff + Tem cells developed diabetes significantly earlier compared with the ones that received Tcm or Tcm + Teff + em (Fig. 5B). These results demonstrate that NOD CD8+ Tcm cells not only are less diabetogenic compared CD8+ Teff + Tem but also actively suppress diabetes development.
Figure 5.
CD8+ Tcm cells delay NOD diabetes development. A: Schematic of adoptive transfer experimental design. B: The number of days to diabetes in NOD.Scid recipient mice. The day the first mouse in each experiment developed diabetes was called day 0. The data shown are combined from five independent experiments. Open points denote mice that did not develop diabetes within 30 weeks after adoptive transfer. *P < 0.05, ***P < 0.001 by log-rank test. M, million; WT, wild type.
NKG2D Signaling During NOD CD8+ T-Cell Activation Results in a Reduced Teff + em:Tcm Ratio
NKG2D has been shown to be important in CD8+ T-cell memory formation in other mouse strains, especially CD8+ Tcm (15–17). We therefore hypothesized that NKG2D signaling directly in NOD CD8+ T cells was responsible for the decreased CD8+ Teff + em:Tcm ratio in the NOD.RIP-RAE1ε mice. To test this, we purified total CD8+ T cells from wild-type NOD mice and activated them in vitro with splenocytes from B6.RAE1ε, which have ubiquitous transgenic expression of RAE1ε (18), or control B6 splenocytes. Similar to our observation with CD8+ T-cell populations in vivo in the NOD.RIP-RAE1ε mice (Fig. 4), NKG2D stimulation during in vitro activation with the B6.RAE1ε splenocytes resulted in a significantly reduced ratio of Teff + em:Tcm NOD CD8+ T cells (Fig. 6).
Figure 6.
NKG2D signaling in NOD CD8+ T cells increases CD8+ Tcm. A: Representative flow cytometry data gated on in vitro activated NOD CD3+CD8+ cells showing CD44 and CD62L expression. The numbers shown are the percentage of cells present in each quadrant of the dot plot. B and C: The percentage (mean ± SD) of NOD CD8+CD3+ cells that were CD44+CD62L+ (Tcm) (B) or CD44+CD62L− (Teff + em) (C) after activation in vitro in the presence of RAE1ε+ or RAE1ε− splenocytes. D: The ratio (mean ± SD) of NOD CD8+ Teff + em:Tcm generated after activation in vitro in the presence of RAE1ε+ or RAE1ε− splenocytes. These data are representative of three independent experiments. ****P < 0.0001 by two-sided t test.
NKG2D Signaling During Human CD8+ T-Cell Activation Results in a Reduced Teff + em:Tcm Ratio
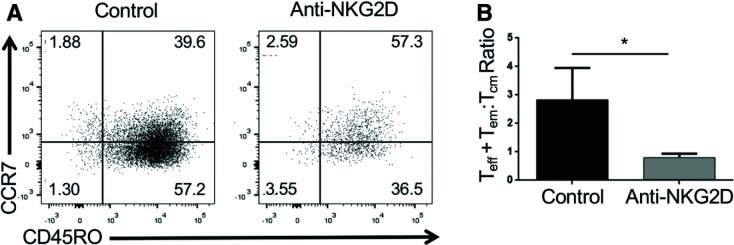
We wanted to determine whether NKG2D stimulation of human CD8+ T cells similarly decreases the ratio of Teff + em:Tcm CD8+ T cells. To do this, we purified total CD8+ T cells from human peripheral blood and activated these cells in vitro for 5 days in the presence of activating anti-NKG2D or control antibody. Similar to the mouse CD8+ T cells, we found that NKG2D stimulation during in vitro activation significantly reduced the ratio of human CD8+ Teff + em:Tcm (Fig. 7).
Figure 7.
NKG2D signaling in human CD8+ T cells increases CD8+ Tcm. A: Representative flow cytometry data gated on in vitro activated human total CD3+CD8+ cells showing CCR7 and CD45RO expression. The numbers shown are the percentage of cells present in each quadrant of the dot plot. B: The ratio of human CD8+ Teff + em (CD45RO+CCR7−):Tcm (CD45RO+CCR7+) generated after activation of human total CD3+CD8+ cells in vitro in the presence of an activating anti-NKG2D or isotype control antibody. These data are the combined results of five independent experiments. *P < 0.05 by one-tailed Mann-Whitney U test.
Discussion
NKG2D and NKG2D ligand expression has been reported in the pancreas of NOD mice (6,9). However, the expression of NKG2D and NKG2D ligands in the human pancreas had not been previously assessed. We demonstrate here that mRNAs encoding NKG2D and NKG2D ligands are detectable in human pancreatic islets, with increased expression in patients with type 1 diabetes compared with age- and sex-matched control subjects without diabetes, and positively correlate with T-cell infiltration into islets. These findings establish that NKG2D signaling likely occurs in the pancreas in inflamed islets during type 1 diabetes, supporting continued study of NKG2D signaling in the pancreas in diabetes pathogenesis.
NKG2D has been implicated in the development of type 1 diabetes. How it affects disease development and whether these effects are positive or negative have been a point of uncertainty. We previously showed a protective effect of NKG2D signaling on CD8+ T cells in NOD diabetes but found evidence that NKG2D signaling in the gut may have detrimental effects on disease development (9). These findings suggest that NKG2D signaling plays different roles in autoimmune diabetes progression in different anatomical locations. In this study, we endeavored to more closely assess the role played by NKG2D signaling within the pancreas in autoimmune diabetes.
Because both NKG2D and NKG2D ligands are expressed on immune cells (25), we could not eliminate these proteins specifically within the pancreas. Therefore, to test the effect of NKG2D signaling in the pancreas, we instead increased NKG2D signaling specifically within the β-islet cells of the pancreas. In our NOD.RIP-RAE1ε model, we selectively increased NKG2D signaling in the islets by transgenically expressing RAE1ε using the RIP. This expression resulted in earlier insulitis in the NOD.RIP-RAE1ε mice, which is consistent with our previous findings with B6.RIP-RAE1ε (7). However, despite this earlier increase, insulitis was similar in NOD.RIP-RAE1ε and control littermates by 12 weeks, and NOD.RIP-RAE1ε mice had reduced diabetes development. This diabetes reduction correlated with a decreased ratio of CD8+ Teff + em cells to CD8+ Tcm cells. Given the central role that CD8+ Teff cells play in autoimmune diabetes pathology (34,35), it is not surprising that reduction in these cells correlated with a reduction in diabetes development. In contrast, the increase in CD8+ Tcm in NOD.RIP-RAE1ε mice was somewhat surprising. This increase is unlikely simply a reflection of the lower percentage of NOD.RIP-RAE1ε mice on their way to developing diabetes. If this was the case, we would have observed a similar increase in CD8+ Tcm in male NOD mice, which have a lower diabetes incidence, compared with female NOD mice, but we did not. Therefore, our data support the hypothesis that this increase in CD8+ Tcm does not just correspond to the reduced diabetes in both female and male NOD.RIP-RAE1ε but also is the cause for the RAE1ε-mediated reduction. This hypothesis is further supported by the results of the NOD.Scid adoptive cell transfer studies that demonstrated suppression of diabetes development by CD8+ Tcm.
The cell adoptive transfer studies revealed that CD8+ Tcm not only blocked diabetes onset but also delayed disease transfer by CD8+ Teff + em. CD8+ Teff + em cells correlate with islet pathology (36), and CD8+ Teff cells play a central role in autoimmune diabetes pathology (34,35). In contrast, CD8+ Tcm cells have reduced cytotoxicity and effector functions (37), correlating with reduced diabetes transfer by these cells. However, the delay in diabetes we observed with cotransfer of CD8+ Tcm cells with CD8+ Teff + em cells demonstrates a regulatory effect of the CD8+ Tcm population. Such a regulatory role for NOD CD8+ Tcm was suggested by previous studies performed by Santamaria and colleagues (38–40) using transgenic T-cell systems; however, this had not been investigated in parental NOD mice. Tcm cells can also be precursors to Teff cells (41). Therefore, the protective cells are likely a subset of the CD8+ Tcm population. CD8+ Tcm cells are a heterogeneous population, which has been shown in other mouse strains to contain a subpopulation of immune regulatory CD8+ T cells (42). Future studies will need to be performed to determine the identity of the protective NOD CD8+ Tcm population, but our results demonstrate that this population is expanded by NKG2D signaling.
Our results indicate that the reduced Teff + em:Tcm ratio in NOD.RIP-RAE1ε mice is due to enhanced NKG2D signaling directly in CD8+ T cells. We found that NKG2D stimulation during in vitro activation of wild-type NOD CD8+ T cells drove a similar decrease in the Teff + em:Tcm ratio as observed in NOD.RIP-RAE1ε mice. This finding that NKG2D stimulation drives a shift toward CD8+ Tcm in the NOD mouse is consistent with a role for NKG2D in promoting memory cell development or survival, particularly Tcm, demonstrated by others with C57BL/6 mice (15–17,43). Importantly, demonstrating the translatability of the findings of these mouse studies to the human, we showed that NKG2D signaling during the activation of human CD8+ T cells also favors Tcm generation over Tem/Teff. Our findings presented here were from studies performed with total NOD and human CD8+ T cells. In similar studies we performed beginning with naive CD8+ T cells, we did not observe this increase in Tcm. This is consistent with the findings of Wensveen et al. (15), who found that NKG2D signaling in C57BL/6 CD8+ T cells increased CD8+ Tcm production by promoting the survival of precursor memory cells rather than by affecting cell proliferation or differentiation. However, additional studies need to be done to confirm that the change in CD8+ Teff + em:Tcm ratio in vivo is entirely dependent on NKG2D signaling directly in NOD CD8+ T cells, as well as when during the process of memory cell differentiation NKG2D signaling is important. Further, although our data demonstrate that constitutive high expression of an NKG2D ligand in pancreatic islet cells reduces NOD diabetes and the CD8+ Teff + em:Tcm ratio, it still needs to be determined whether endogenous NKG2D ligand expressed in the pancreas (9) has similar effects.
Initial reports demonstrated that RAE1 family members were expressed by β-islet cells in NOD mice (6), that RAE1ε expression by β-islet cells enhanced the recruitment of CD8+ T cells to pancreatic islets (7), and that NKG2D inhibition reduced diabetes in both the NOD and the RIP-lymphocytic choriomeningitis virus mouse models of autoimmune diabetes alone or together with regulatory CD4+ T cells, respectively (6,44). This led to the hypothesis that inappropriate NKG2D ligand expression by β-islet cells contributes to autoimmune diabetes development or progression. However, we and others have been unable to detect RAE1 expression in the pancreas of NOD mice and do not detect NKG2D ligand expression by β-islet cells in our NOD colony (9,45,46). The reason for these differing reports of RAE1 expression in islets of NOD mice from different mouse colonies is unclear but may be the result of variation that is due to genetic drift or differences in microbiota composition. Regardless, the lack of RAE1 expression in the islets of mice in our NOD colony afforded us a model with which we could directly test the hypothesis that NKG2D ligand expression on β-islet cells promotes NOD diabetes. In contrast to the original hypothesis, our data demonstrate that RAE1ε expression by islet cells is protective against NOD diabetes and acts through interaction with NKG2D.
Although constitutive exposure to ligand can lead to NKG2D downregulation and dysfunction (47,48), our data are consistent with enhanced, not reduced, NKG2D signaling in pancreatic islets, resulting in reduced diabetes in the NOD.RIP-RAE1ε mice. While we observed reduced NKG2D expression on CD8+ T cells of NOD.RIP-RAE1ε mice in the pancreas, NKG2D expression by CD8+ T cells in the spleen was unaffected. This is indicative of increased local NKG2D signaling because NKG2D is internalized upon ligand engagement and signaling. Additionally, the reduced diabetes in NOD.RIP-RAE1ε mice correlates with the increased rate of diabetes in NKG2D-deficient NOD mice that we observed with treatment with gut microbiota-depleting antibiotics in previous studies (9). Finally, our in vitro data demonstrate that NKG2D signaling in CD8+ T cells enhances Tcm generation or survival, which correlates with the increase in CD8+ Tcm that is observed in NOD.RIP-RAE1ε mice. NKG2D is not expressed on mouse CD8+ T cells until 3–4 days following initial activation (9,14). This is a time when these cells start to migrate to the site of antigen (49). Taken together, these data support the conclusion that NKG2D signaling in islet-specific CD8+ T cells present in the islets during activation enhances Tcm generation or survival.
In conclusion, we found an increased presence of NKG2D and NKG2D ligand mRNA within the pancreatic islets of patients with type 1 diabetes and developed a novel mouse model, NOD.RIP-RAE1ε, to test the effects of NKG2D signaling specifically within the pancreas. We showed that increased NKG2D signaling within the pancreas of NOD mice had a protective effect, delaying diabetes in NOD.RIP-RAE1ε mice. This correlated with a decrease in the CD8+ Teff + em:Tcm ratio in NOD.RIP-RAE1ε mice. We demonstrated that NKG2D signaling on CD8+ T cells drives a similar shift in both human and NOD CD8+ T-cell populations in vitro. Finally, we found that CD8+ Tcm cells transferred significantly less NOD diabetes than CD8+ Teff + em cells and had a protective effect, delaying diabetes when cotransferred with CD8+ Teff + em cells. We therefore propose a protective role for NKG2D signaling within the pancreas in autoimmune diabetes by increasing a protective CD8+ Tcm population relative to the more pathogenic CD8+ Teff + em population. These findings reiterate the importance of NKG2D in autoimmune diabetes and further stress the importance of anatomical location when determining the role that NKG2D signaling plays in disease development. This is particularly critical when designing therapeutic strategies that target NKG2D or NKG2D-expressing cells, such as T cells.
Article Information
Acknowledgments. Organ procurement organizations partnering with nPOD to provide research resources are listed at https://www.jdrfnpod.org//for-partners/npod-partners.
Funding. This research was performed with the support of nPOD (RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R), and The Leona M. & Harry B. Helmsley Charitable Trust (grant number 2018PG-T1D053). This work made use of the University of Kansas Medical Center Flow Cytometry Core Laboratory (supported in part by National Institute of General Medical Sciences [NIGMS] Centers of Biomedical Research Excellence grant P30-GM-103326 and National Cancer Institute Cancer Center Support grant P30-CA-168524) and the Kansas Intellectual Developmental Disabilities Research Center Histology Core (supported by National Institute of Child Health and Human Development grant U54-HD-090216). Funding for this work was provided by The University of Kansas Biomedical Research Training Program (to A.P.T. and K.L.K.), JDRF (JDRF 17-2012-595 to I.C.G. and C.E.M.), American Association of Immunologists (to N.S. and M.A.M.), the American Diabetes Association (1-12-JF-41 to M.A.M.), the Washington University School of Medicine Diabetes Research Center (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] grant P30-DK-020579 to M.A.M.), the Molecular Regulation of Cell Development and Differentiation Center of Biomedical Research Excellence (NIGMS grant P20 GM104936/P30-GM-122731 to M.A.M.), and NIDDK grants UC4-DK-104155 (to I.C.G. and C.E.M.) and UC4-DK-104167 (to C.E.M.).
The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD.
Duality of Interest. M.A.M. is a consultant for Johnson & Johnson Global Services. No other conflicts of interest relevant to this article were reported.
Author Contributions. A.P.T., K.L.K., and M.A.M. designed and planned the experiments. A.P.T., K.L.K., N.S., I.C.G., and M.A.M. performed the experiments. A.P.T., K.L.K., N.S., C.E.M., and M.A.M. analyzed and discussed the data. A.P.T. and M.A.M. wrote the manuscript. All authors reviewed the manuscript. M.A.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this article were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12311675.
A.P.T. and K.L.K. contributed to this work equally.
References
- 1.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727–729 [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Song Y, Bakker AB, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 1999;285:730–732 [DOI] [PubMed] [Google Scholar]
- 3.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 2002;17:19–29 [DOI] [PubMed] [Google Scholar]
- 4.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A 2003;100:9452–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai Z, Turtle CJ, Booth GC, et al. Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus. J Exp Med 2009;206:793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogasawara K, Hamerman JA, Ehrlich LR, et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 2004;20:757–767 [DOI] [PubMed] [Google Scholar]
- 7.Markiewicz MA, Wise EL, Buchwald ZS, et al. RAE1ε ligand expressed on pancreatic islets recruits NKG2D receptor-expressing cytotoxic T cells independent of T cell receptor recognition. Immunity 2012;36:132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerra N, Pestal K, Juarez T, et al. A selective role of NKG2D in inflammatory and autoimmune diseases. Clin Immunol 2013;149:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trembath AP, Sharma N, Raju S, Polić B, Markiewicz MA. A protective role for NKG2D-H60a interaction via homotypic T cell contact in nonobese diabetic autoimmune diabetes pathogenesis. Immunohorizons 2017;1:198–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev 2010;235:267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013;31:413–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 2004;21:357–366 [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich LI, Ogasawara K, Hamerman JA, et al. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J Immunol 2005;174:1922–1931 [DOI] [PubMed] [Google Scholar]
- 14.Markiewicz MA, Carayannopoulos LN, Naidenko OV, et al. Costimulation through NKG2D enhances murine CD8+ CTL function: similarities and differences between NKG2D and CD28 costimulation. J Immunol 2005;175:2825–2833 [DOI] [PubMed] [Google Scholar]
- 15.Wensveen FM, Lenartic M, Jelencic V, et al. NKG2D induces Mcl-1 expression and mediates survival of CD8 memory T cell precursors via phosphatidylinositol 3-kinase. J Immunol 2013;191:1307–1315 [DOI] [PubMed] [Google Scholar]
- 16.Zloza A, Kohlhapp FJ, Lyons GE, et al. NKG2D signaling on CD8⁺ T cells represses T-bet and rescues CD4-unhelped CD8⁺ T cell memory recall but not effector responses. Nat Med 2012;18:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez C, Prajapati K, Burke B, Plaza-Rojas L, Zeleznik-Le NJ, Guevara-Patino JA. NKG2D signaling certifies effector CD8 T cells for memory formation. J Immunother Cancer 2019;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheney EE, Wise EL, Bui JD, et al. A dual function of NKG2D ligands in NK-cell activation. Eur J Immunol 2012;42:2452–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakeland E, Morel L, Achey K, Yui M, Longmate J. Speed congenics: a classic technique in the fast lane (relatively speaking). Immunol Today 1997;18:472–477 [DOI] [PubMed] [Google Scholar]
- 20.Campbell-Thompson M, Wasserfall C, Kaddis J, et al.; Network for Pancreatic Organ Donors with Diabetes . Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev 2012;28:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson SJ, Rodriguez-Calvo T, Gerling IC, et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 2016;59:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Kakoola DN, Lenchik NI, Desiderio DM, Marshall DR, Gerling IC. Molecular phenotyping of immune cells from young NOD mice reveals abnormal metabolic pathways in the early induction phase of autoimmune diabetes. PLoS One 2012;7:e46941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiter EH. The NOD mouse: a model for insulin-dependent diabetes mellitus. Curr Protoc Immunol 2001;Chapter 15:Unit 15.9. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson MA. Evaluating preclinical efficacy. Sci Transl Med 2011;3:96cm22. [DOI] [PubMed] [Google Scholar]
- 25.Trembath AP, Markiewicz MA. More than decoration: roles for natural killer group 2 member D ligand expression by immune cells. Front Immunol 2018;9:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molinero LL, Fuertes MB, Rabinovich GA, Fainboim L, Zwirner NW. Activation-induced expression of MICA on T lymphocytes involves engagement of CD3 and CD28. J Leukoc Biol 2002;71:791–797 [PubMed] [Google Scholar]
- 27.Zwirner NW, Fernández-Viña MA, Stastny P. MICA, a new polymorphic HLA-related antigen, is expressed mainly by keratinocytes, endothelial cells, and monocytes. Immunogenetics 1998;47:139–148 [DOI] [PubMed] [Google Scholar]
- 28.Rabinovich BA, Li J, Shannon J, et al. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol 2003;170:3572–3576 [DOI] [PubMed] [Google Scholar]
- 29.Molfetta R, Quatrini L, Zitti B, et al. Regulation of NKG2D expression and signaling by endocytosis. Trends Immunol 2016;37:790–802 [DOI] [PubMed] [Google Scholar]
- 30.Makino S, Kunimoto K, Muraoka Y, Katagiri K. Effect of castration on the appearance of diabetes in NOD mouse. Jikken Dobutsu 1981;30:137–140 [DOI] [PubMed] [Google Scholar]
- 31.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 1992;51:285–322 [DOI] [PubMed] [Google Scholar]
- 32.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world--recent facts and figures. Immunol Today 1993;14:193–196 [DOI] [PubMed] [Google Scholar]
- 33.Zafirova B, Mandarić S, Antulov R, et al. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity 2009;31:270–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrack AL, Martinov T, Fife BT, Cell-Mediated Beta Cell Destruction T. T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol (Lausanne) 2017;8:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol 2008;100:79–124 [DOI] [PubMed] [Google Scholar]
- 36.Chee J, Ko HJ, Skowera A, et al. Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol 2014;192:572–580 [DOI] [PubMed] [Google Scholar]
- 37.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol 2014;14:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khadra A, Tsai S, Santamaria P, Edelstein-Keshet L. On how monospecific memory-like autoregulatory CD8+ T cells can blunt diabetogenic autoimmunity: a computational approach. J Immunol 2010;185:5962–5972 [DOI] [PubMed] [Google Scholar]
- 39.Tsai S, Shameli A, Yamanouchi J, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 2010;32:568–580 [DOI] [PubMed] [Google Scholar]
- 40.Shameli A, Clemente-Casares X, Wang J, Santamaria P. Development of memory-like autoregulatory CD8+ T cells is CD4+ T cell dependent. J Immunol 2011;187:2859–2866 [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004;22:745–763 [DOI] [PubMed] [Google Scholar]
- 42.Li S, Xie Q, Zeng Y, et al. A naturally occurring CD8(+)CD122(+) T-cell subset as a memory-like Treg family. Cell Mol Immunol 2014;11:326–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wensveen FM, Jelenčić V, Polić B. NKG2D: a master regulator of immune cell responsiveness. Front Immunol 2018;9:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Belle TL, Ling E, Haase C, Bresson D, Ursø B, von Herrath MG. NKG2D blockade facilitates diabetes prevention by antigen-specific Tregs in a virus-induced model of diabetes. J Autoimmun 2013;40:66–73 [DOI] [PubMed] [Google Scholar]
- 45.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One 2013;8:e59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angstetra E, Graham KL, Zhao Y, et al. An indirect role for NK cells in a CD4(+) T-cell-dependent mouse model of type I diabetes. Immunol Cell Biol 2012;90:243–247 [DOI] [PubMed] [Google Scholar]
- 47.Oppenheim DE, Roberts SJ, Clarke SL, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol 2005;6:928–937 [DOI] [PubMed] [Google Scholar]
- 48.Wiemann K, Mittrücker HW, Feger U, et al. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol 2005;175:720–729 [DOI] [PubMed] [Google Scholar]
- 49.Kinjyo I, Qin J, Tan SY, et al. Real-time tracking of cell cycle progression during CD8+ effector and memory T-cell differentiation. Nat Commun 2015;6:6301. [DOI] [PMC free article] [PubMed] [Google Scholar]