Abstract
Vulvovaginal candidiasis during pregnancy is common, but serious complications, including chorioamnionitis, are infrequent. A 41-year-old woman presented at 37 weeks of gestation with reduced fetal movements, and fetal death in utero was subsequently confirmed on ultrasound. Histopathology of the cord and placenta revealed Candida infection and microabscesses on the umbilical cord. Overall, these features are suggestive of ascending infection, consistent with Candida as the causative organism. To the best of our knowledge, this is the first reported case of late stillbirth due to Candida chorioamnionitis. More research is needed to determine the mechanism whereby Candida becomes pathogenic in pregnancy. There is also no clear consensus on how to manage such patients in a subsequent pregnancy.
Keywords: Candida, Chorioamnionitis, Infection, Stillbirth, Case report
Highlights
-
•
Vulvovaginal candidiasis during pregnancy is common, but serious complications, including chorioamnionitis, are infrequent.
-
•
This case describes stillbirth caused by Candida chorioamnionitis.
-
•
Risk factors identified in this case include gestational diabetes mellitus, recent antibiotic use and vaginal douching.
-
•
More research is needed to determine the mechanism whereby Candida becomes pathogenic in pregnancy.
1. Introduction
Vulvovaginal candidiasis during pregnancy is common, but serious complications, including chorioamnionitis, are infrequent. Candida chorioamnionitis is a rare obstetric complication, which has previously been reported to manifest as pre-term premature rupture of membranes (PPROM) or pre-term labour [1].
Known risk factors include foreign bodies such as intra-uterine contraceptive devices [2,3] as well as cervical cerclage [4]. There have also been reported associations with in-vitro fertilisation (IVF) [5] and PPROM [1], causing ascending infection of Candida species. Due to the low frequency of intra-uterine pathogenic Candida infections, the clinical features, progression and treatment of Candida chorioamnionitis are not well described.
2. Case report
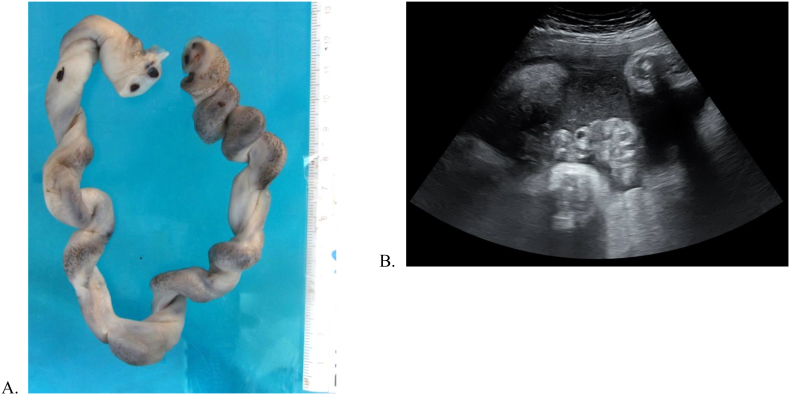
A 41-year-old woman, gravida 4, para 2, presented to a tertiary centre at 37 weeks of gestation with reduced fetal movements for more than 12 h. Her pregnancy was complicated by diet-controlled gestational diabetes mellitus (GDM) as well as subclinical hypothyroidism managed with thyroxine. Several weeks prior to presentation, she had an upper respiratory tract infection treated with oral antibiotics. An obstetric ultrasound at 34 weeks for routine GDM care demonstrated normal fetal growth (with estimated fetal weight on the 48th percentile), no fetal abnormalities, normal umbilical artery Doppler scan and normal biophysical profile. At the time of presentation at 37 weeks, the fetal heart rate could not be auscultated, and fetal death in utero was subsequently confirmed on ultrasound. Ultrasound appearances suggested recent fetal demise. The cord on ultrasound was coiled and thickened with hyperechoic areas (see Fig. 2). There were no clinical signs of chorioamnionitis. The patient proceeded to vaginal delivery of a stillborn female infant after prostaglandin induction of labour on the same day.
Fig. 2.
Punctate cord abscesses (a) macroscopically and (b) on ultrasound.
Genital culture taken prior to delivery was positive for Candida albicans, and microbiological culture of the placenta was also positive for Candida albicans. Histopathology demonstrated fungal spores and hyphae in the umbilical cord, chorionic plate and fetal membranes, with grade II, stage II fetal and maternal inflammatory responses (Fig. 1). Punctate abscesses were seen on the cord macroscopically (Fig. 2). Overall, these features are suggestive of ascending infection, consistent with Candida as the causative organism. The placental disc weight, cord length and cord coil index were within normal limits for the gestational age.
Fig. 1.
(a) Fetal inflammatory response; H&E x40 (b) maternal inflammatory response; H&E x100.
Limited post-mortem examination was conducted, including blood tests and imaging. A full post-mortem examination of the infant was declined by the parents. Cord cytogenetics demonstrated a 21q22.3 duplication, which was found to be a paternally inherited variant of unknown significance. Other post-mortem investigations, including maternal blood tests, fetal magnetic resonance imaging (MRI) and skeletal survey (x-ray) did not demonstrate any abnormalities.
3. Discussion
While several case reports of Candida chorioamnionitis have been published [1,3,[6], [7], [8]], none has been associated with late stillbirth. Most other reports have described co-existing risk factors such as IVF [5,9], cervical cerclage [4] or intra-uterine contraceptive devices [2], none of which was present in this case. Candida chorioamnionitis may cause various fetal and neonatal outcomes; a 27% birth rate before 22 weeks and a 29% mortality rate of singletons born after 22 weeks were reported in one literature review [1]. Other cases describe congenital Candida infection, presenting with extensive white and elevated patches [2], or red maculo-papular rash on the fetal skin [10], although neither of these was evident on limited external examination of the stillborn infant in our case. However, there are reports of microabscesses in the umbilical cord, which is not dissimilar to the findings in this case (see Fig. 2) [10].
Because vaginal candidiasis occurs frequently in pregnancy, the specific mechanism which causes the organism to become pathogenic is unknown. In this case, potential contributing factors include gestational diabetes, recent antibiotics given for an upper respiratory tract infection, and possibly even vaginal douching. The patient had been douching vaginally daily with a commercially available douche throughout the pregnancy. Although not previously reported in the literature, this suggests a hypothesis of an associated change in the vaginal microflora and pH, thus increasing susceptibility to Candida with ascending infection. Of note is that this patient had also used frequent vaginal douches in her 2 previous pregnancies with good outcomes, but that this pregnancy was the patient's first while affected by GDM. The 21q22.3 duplication found on cord cytogenetics has no known association with stillbirth.
In conclusion, this case describes a late stillbirth due to Candida albicans chorioamnionitis and funisitis with the presence of multiple cord abscesses seen on histopathology. It is surmised that the actual cause of death would be infection and inflammation causing cord vessel occlusion, which in retrospect is suggested by the appearance of thickened, coiled hyperechogenic areas of the umbilical cord. Further research is required to determine the altered susceptibility in the host and the trigger to the significant pathogenicity (microabscess formation) of Candia albicans. The management in a subsequent pregnancy is a particular challenge.
Acknowledgments
Contributors
H.M. Obermair contributed to conception and design, acquisition of data and drafting of the manuscript.
G. Bhagwanani contributed to conception and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript.
R. Caldas contributed to acquisition of data and critical revision of the manuscript.
H. Doyle contributed to acquisition of data, analysis and interpretation of data, and critical revision of the manuscript.
J. Smoleniec contributed to acquisition of data, analysis and interpretation of data, and critical revision of the manuscript.
A. Adno contributed to conception and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Obtained.
Provenance and peer review
This case report was peer reviewed.
References
- 1.Maki Y., Fujisaki M., Sato Y., Sameshima H. Candida chorioamnionitis leads to preterm birth and adverse Fetal-neonatal outcome. Infect. Dis. Obstet. Gynecol. 2017;2017:1–11. doi: 10.1155/2017/9060138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bittencourt A., dos Santos W., de Oliveira C. Placenta and fetal candidiasis - presentation of a case of an abortus. Miycopathologia. 1984;87:181–187. doi: 10.1007/BF00436906. [DOI] [PubMed] [Google Scholar]
- 3.Roque H., Abdelhak Y., Young B.K. Intra amniotic candidiasis. Case report and meta-analysis of 54 cases. J. Perinat. Med. 1999;27(4):253–262. doi: 10.1515/JPM.1999.036. [DOI] [PubMed] [Google Scholar]
- 4.Poliquin V., Al-Sulmi E., Menticoglou S. Intra-amniotic infection involving Candida albicans subsequent to emergency cerclage: a case series. Can. J. Infect. Dis. Med. Microbiol.= Journal canadien des maladies infectieuses et de la microbiologie medicale. 2015;26(5):245–246. doi: 10.1155/2015/589078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackel D., Lai K. Candida glabrata Sepsis associated with chorioamnionitis in an in vitro fertilization pregnancy: case report and review. Clin. Infect. Dis. 2013;56(4):555–558. doi: 10.1093/cid/cis936. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Flores J., Cruceyra M., Cañamares M., Garicano A., Nieto O., Tamarit I. Candida chorioamnionitis: report of two cases and review of literature. J. Obstet. Gynaecol. 2016;36(7):843–844. doi: 10.1080/01443615.2016.1196479. [DOI] [PubMed] [Google Scholar]
- 7.Rode M., Morgan M., Ruchelli E., Forouzan I. Candida Chorioamnionitis after serial therapeutic amniocenteses: a possible association. J. Perinatol. 2000;20:335–337. doi: 10.1038/sj.jp.7200381. [DOI] [PubMed] [Google Scholar]
- 8.Kusanovic J.P., Romero R., Martinovic C., Silva K., Erez O., Maymon E. Transabdominal collection of amniotic fluid “sludge” and identification of Candida albicans intra-amniotic infection. J. Matern. Fetal Neonatal Med. 2017;31(10):1279–1284. doi: 10.1080/14767058.2017.1315095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang M., Cham E.M., Eppes C.S., Gerber S.E., Reed K.D., Ernst L.M. Placental and fetal findings in intrauterine Candida Lusitaniae infection following in vitro fertilization and embryo transfer. Pediatr. Dev. Pathol. 2014;15(2):127–131. doi: 10.2350/11-04-1019-CR.1. [DOI] [PubMed] [Google Scholar]
- 10.Diana A., Epiney M., Ecoffey M., Pfister R. “White dots on the placenta and red dots on the baby”: congential cutaneous candidiasis--a rare disease of the neonate. Acta Paediatr. 2004;93(7):996–999. doi: 10.1111/j.1651-2227.2004.tb02701.x. [DOI] [PubMed] [Google Scholar]