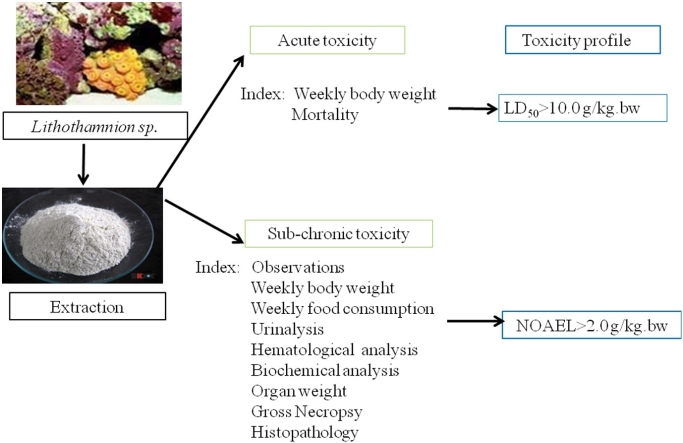
Graphical abstract
Keywords: Lithothamnion sp., Aquamin F, Acute toxicity, Sub-chronic toxicity, Food safety
Highlights
-
•
Lithothamnion sp. is widely available as a marine algae-derived calcium and multi-mineral dietary supplement.
-
•
In an acute toxicity test, Lithothamnion sp. was deemed non-toxic with a LD50 >10 g/kg BW.
-
•
In a standard sub-chronic toxicity study, the no-observed-adverse-effect-level (NOAEL) of Lithothamnion sp. in rats was >2 g/kg BW.
Abstract
Lithothamnion sp., a red algae of the Corallinaceae family, when harvested in its calcareous form, is rich in calcium, magnesium and a variety of trace minerals. It is used as a beneficial dietary mineral supplement across the world (Aquamin F). This study was designed to evaluate the acute and sub-chronic toxicity of Lithothamnion sp. according to the Procedure and Methods of Food Safety Toxicological Assessment GB-15193 (China). In an acute toxicity test, mice (n = 20, 10 male, 10 female) were administered a single dose of 10 g/kg BW of Lithothamnion sp. No mortality, or signs of toxicity were observed. In the sub-chronic toxicity arm of the study, SD rats (n = 80, 40 male, 40 female) were randomly divided into four groups with 10 rats in each group and provided pelleted food containing the algae at either 0.00 %, 0.625 %, 1.25 %, 2.50 % inclusion rates for 90 days. Lithothamnion sp. at all inclusion rates did not cause any mortality, and no treatment-related changes were observed in body weight, organ weight, feed consumption, feed utilization rate, urinalysis, hematological and biochemical blood analysis, gross necropsy or histopathologic examinations. In summary, the median lethal dose (LD50) of Lithothamnion sp. was >10 g/kg BW. The no-observed-adverse-effect-level (NOAEL) for female and male rats of Lithothamnion sp. under these experimental conditions was 2.69 g/kg BW and 2.10 g/kg BW respectively.
1. Introduction
Lithothamnion sp. is a red marine algae of the Corallinaceae family, phylum Rhodophyta. Both the soft living algae, and drifts of its rigid calcareous remains, are found in the cold Atlantic waters off the southwest coast of Ireland and the northwest coast of Iceland. The plant accumulates calcium, magnesium carbonate and a large array of trace elements in its fronds from seawater over its life span. Elements that have the potential to cause harm (e.g. arsenic, lead etc.) are accumulated in such small amounts that they occur well below published safe limits, and as such, are considered harmless (Table 1). The mineralized fronds are harvested from the ocean floor and then utilized as a source of naturally occurring minerals, in food and other industries.
Table 1.
Mineral Composition of Aquamin F (Lithothamnionsp.) c.
| Item | Specification | Analyzed Amount |
|---|---|---|
| Minerala | ||
| Calcium (g/100 g) | 32 % Minimum | 34 |
| Magnesium (g/100 g) | 2.2 % Minimum | 2.9 |
| Arsenic (ppm) | 1.5 Maximum | 0.4 |
| Lead (ppm) | 1.0 Maximum | 0.2 |
| Cadmium(ppm) | 1.0 Maximum | 0.8 |
| Mercury(ppm) | 0.1 Maximum | <0.1 |
| Physical properties | ||
| Moisture(%)b | 5% Maximum | 0.5 |
| pH (1% aqueous solution) | 9.5−10.5 | 9.6 |
| Bulk Density (g/cm3) | 0.7−0.9 | 0.85 |
| Particle Size (μm) | 25 Maximum | 23.3 |
Mineral content analysis was performed using inductively coupled plasma mass spectrometry.
Moisture content analysis was performed in accordance with the guidelines of the Association of Official Analytical Chemists (AOAC).
Aquamin F is a natural product of marine origin that contains numerous trace minerals. The exact composition may vary slightly between batches, and over time.
It has been demonstrated that calcium derived from Lithothamnion sp. has superior bioavailability in humans when compared to a non-plant source of calcium [1]. Besides acting as a natural and bioavailable source of calcium, dietary supplementation with Lithothamnion sp. has also been demonstrated to confer significant anti-inflammatory properties, specifically in the mitigation of discomfort in knee osteoarthritis patients [[2], [3], [4]]. Furthermore, daily supplementation with Lithothamnion sp. with a source of fructo-oligosaccharide in a large cohort of post-menopausal women has been demonstrated to reduce bone turnover markers (compared to placebo), and slow rates of bone loss in women with osteopenia [5]. Lipid markers (LDL and total cholesterol) were also lower in the post-menopausal women treated daily with Lithothamnion sp. than in those in a control group [6].
Globally, micronutrient deficiencies are widespread and have significantly deleterious effects on human health [7]. In China for example, calcium deficiency has been documented in a wide range of age groups, from children and adolescents [8], to elderly people, with 98.2 % of the latter population consuming inadequate calcium [9]. Chronic calcium deficiency is associated with a myriad of adverse health effects, including osteoporosis. Adequate and lifelong consumption of calcium and vitamin D is known to have a key role in prevention of osteoporosis [10].
Lithothamnion sp. is widely available as a calcium and multi-mineral supplement (Aquamin F), and has a long history of both safety and efficacy. However, published data on the toxicological effects of Lithothamnion sp. calcium are sparse, with the exception of only one study [11] investigating a Brazilian product, which may not be directly relatable to Lithothamnion sp. harvested in North Atlantic waters based on variations in mineral composition and processing techniques. Therefore, this study was designed to examine the acute and sub-chronic toxicity of Lithothamnion sp. (Aquamin F) in order to confirm its safety, at high dose rates (higher than those already published). Techniques used in this study are directly comparable with similar published studies evaluating acute and sub-chronic toxicity of naturally occurring plant-derived substances including Combretum micranthum, black caraway seed essential oil, and Andrographis paniculata leaf extract [[12], [13], [14]].
2. Material and methods
2.1. Test product
The Lithothamnion sp. derived product, Aquamin F, that was evaluated in these toxicology studies was prepared by Marigot Ltd. as follows: calcified fronds of Lithothamnion sp. were harvested from the North Atlantic seabed, and were washed, screened, sterilized, dried and milled. The resulting powder was odorless, neutral, off-white in color, and soluble in weak acids. The composition of the product is shown in Table 1. It is stable under normal conditions for 3 years after manufacture.
2.2. Test product preparation
For the sub-chronic study, Aquamin F (Lithothamnion sp.) test product was incorporated into animal feed at the following addition rates: 0.625 %; 1.25 %; 2.50 %. Pelleted feed containing the test subject was sterilized by 60Co irradiation in vacuum packaging (Beijing Keaoxieli Co., Ltd (No.: SCXK(Jing)2014−0010)). Animal feed without test material was used for the control group.
2.3. Animals
Specific pathogen free (SPF) Kunming (KM) mice (n = 20, 10 male, 10 female) and SPF Sprague Dawley (SD) rats (n = 80, 40 male, 40 female) were provided by the Experimental Animal Center of Shanxi Medical University (No.: SCXK(Jing)2015−0001) and National Institute for Food and Drug Control (No.: SCXK(Jing)2014−0013), respectively. Animal numbers per group were determined based on power studies, and were in accordance with guidelines in the Procedure and Methods of Food Safety Toxicological Assessment GB-15193.3–2014 (China). Animals were housed (5 mice of each sex per cage, one rat per cage) in the SPF animal house of the Toxicology Institute, Shanxi Provincial Center for Disease Control and Prevention (No.: SYXK (Jin) 2015−0002). Rats were singly housed in accordance with the Procedure and Methods of Food Safety Toxicological Assessment GB-15193.3–2014 (China) guidelines. This was deemed necessary as the test substance was provided in-food, and food consumption and utilization were then calculated on an individual animal basis. Animals lived in a temperature-controlled (22 ± 2 °C) environment with relative humidity of 54 ± 8 % and a regular 12-h light-dark cycle. During the experiment, all animals were provided sterilized tap water ad libitum and commercial pelleted feed. Animals were acclimatized for at least 3 days before commencing experiments. As mandated by Chinese legislation on the care and use of laboratory animals, the Animal Experimentation Ethics Committee at Shanxi Provincial Center for Disease Control and Prevention (Taiyuan, China) approved all procedures.
2.4. Acute toxicity evaluation of Lithothamnion sp. In mice
The acute oral toxicity test was carried out in compliance with the Procedure and Methods of Food Safety Toxicological Assessment GB-15193.3–2014 (China). After fasting for 4 h, 20 eligible mice (10 male and 10 female, 18−22 g) were administered Aquamin F (Lithothamnion sp.) at a single dose of 10 g/kg BW by oral gavage in a volume of 20 mL/kg BW. The selected test substance dose, while high, was determined based on preliminary data, and the absence of reported toxicity in the scientific literature. The animals were monitored individually for changes in general behavior, any signs of toxic symptoms and mortality at 30 min, 1 h, 2 h, 4 h, 6 h, 10 h after administration, and at least once daily for the next 13 days. Body weight of each animal was recorded before treatment and on Day 7 and 14. All mice were sacrificed and a gross necropsy was performed at the end of the experiment.
2.5. Sub-chronic toxicity evaluation of Lithothamnion sp. In rats
In accordance with the sub-chronic toxicity method in the Procedure and Methods of Food Safety Toxicological Assessment GB-15193.13–2015 (China), 40 female (60.7–84.5 g) and 40 male (64.0–90.0 g) SD rats up to 6 weeks old were randomly assigned into four groups and provided pelleted feed containing the test subject Aquamin F at one of the following addition rates: 0.625 %; 1.25 %; 2.50 % in each of the 3 treatment groups, or pelleted feed with no added test subject in the control group. Animals were also provided with fresh water ad libitum. The duration of the experiment was 90 days. The selected test substance dosing was designed to exceed average human consumption of Aquamin by 100 fold, to account for species and inter-individual variability. Based on a average feed consumption of 80 g/100 g BW over the course of the experiment, 2.00 g/kg, 1.00 g/kg, 0.50 g/kg BW will be achieved by 2.5 %, 1.25 % and 0.625 % addition rates respectively. Detailed clinical observations of gait, posture, behavior, and mortality were performed for all animals at least once a day. Ophthalmological examination was performed on all rats at the beginning and after the termination of the experiment. The body weight of each animal was recorded before grouping, once a week thereafter and on the day of necropsy. Food consumption of each rat was recorded twice a week to calculate weekly food utilization and average food utilization rate (food consumption / weight gain*100), and the actual dosage of test subject (mg/kg BW/day).
2.5.1. Urinalysis
During the final week of the experiment, fresh urine of animals in each group who had been fasted overnight was collected at the same time for evaluation of gross appearance, protein, specific gravity, pH, glucose and occult blood using test strips and a semi-automatic urine analyzer (URIT-500B, Guilin, China).
2.5.2. Plasma hematological analysis
Before euthanasia, 10 μl blood samples taken from the tail vein diluted in 2 mL of the hematology diluent ISOTONAC 3 MEK-640 were analyzed for hemoglobin concentration (HGB), red blood cell count (RBC), hematocrit (HCT), white blood cell count (WBC) and platelet count (PLT) using a semi-automated hematology analyzer (Photoelectric MK-613, Japan). All animals were euthanized (decapitation) and further blood samples were taken on the 91 st or 92nd day respectively. Serum and plasma samples were centrifuged at 3000 rpm for 10 min, and were used for biochemical and coagulation evaluation (including prothrombin time (PT), activated partial thromboplastin time (APTT)) respectively. The examination for PT and APTT was performed using a semi-automatic coagulation analyzer (URIT-610, Guilin, China).
2.5.3. Serum biochemical analysis
Serum samples were analyzed for alanine transferase (ALT), aspartate transferase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CRE), glucose (GLU), triglyceride (TG), total cholesterol (TCHO), total Protein (TP), albumin (ALB), total bilirubin (T-BIL), chloride (Cl−), potassium (K+), sodium (Na+) and calcium (Ca2+) using an automated biochemistry analyzer (Sysmex BX-3010, Japan).
2.5.4. Necropsy and organ weight
After blood sampling, all animals underwent gross necropsy. The weight of organs including liver, kidneys, adrenals, testes, epididymides, uterus, ovaries, thymus, spleen, cerebrum and heart was recorded as soon as possible, and the coefficient of organ weight to body weight was determined (absolute organ weight/fasting body weight *100 %). Meanwhile, tissue samples from each animal were fixed in 10 % buffered formalin. Fixed tissues included all organs listed above, along with pituitary gland, thyroid, gastrointestinal tract, pancreas, mesenteric lymph nodes, prostate gland and bladder.
2.5.5. Histopathology
Full histopathology was carried out on the preserved organs and tissues (listed above) of rats in both the control and high dose groups. Digital images were obtained by image processing software (Panoramic SCAN, Hungary) and analyzed by a pathologist. These examinations were extended to animals of the remaining treatment groups, if treatment-related differences were observed between the control and high dose groups.
2.6. Statistical analysis
All results are expressed as mean ± standard deviation (SD). Data analysis was performed separately for male and female groups. Homogeneous quantitative data with normal distribution was analyzed by one-way analysis of variance (ANOVA) between control and treated groups, followed by LSD post hoc test using SPSS version 16.0. The non-parametrical Kruskal-Wallis test was used to analyze qualitative data, and quantitative data without normal distribution or homogeneity. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Acute toxicity evaluation of Lithothamnion sp. In mice
No adverse effects, and no mortality was observed in mice administered a single dose of 10 g/kg BW of Aquamin F (Lithothamnion sp.). Body weight increase of the mice over the course of the experiment was within normal range (data not shown). No pathological lesions of liver, kidneys, spleen or intestinal tract were observed. Based on these data, the median lethal dose (LD50) of Lithothamnion sp. was > 10 g/kg BW.
3.2. Sub-chronic toxicity evaluation of Lithothamnion sp. In rats
Aquamin F (Lithothamnion sp.) at all tested doses did not cause adverse effects on skin, fur, eyes, mucous membranes, secretions, excretions and autonomic activity during the 90-day study. No mortality was observed.
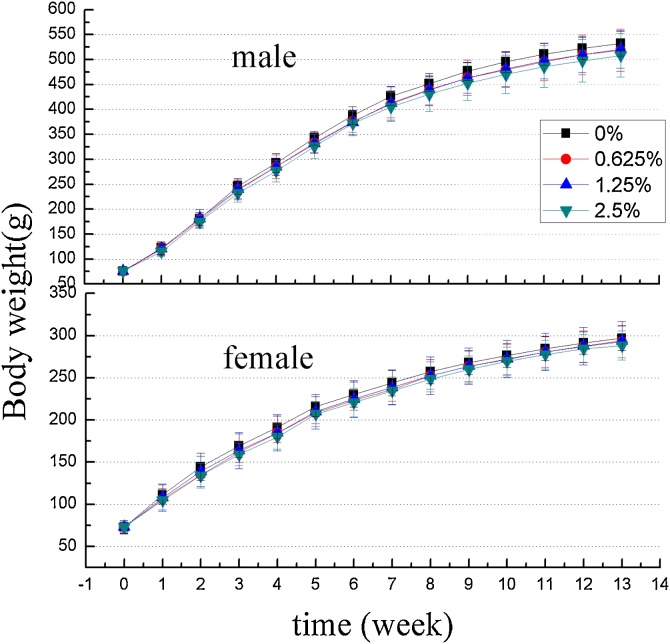
Statistically significant differences in weekly body weight were not observed between treated groups and control group of the same sex (Fig. 1), although there is a trend towards a decrease in body weight gain as the dose increases (Table 2).
Fig. 1.
Effect of Lithothamnion sp. on body weight in rats treated for 13 weeks (n = 10 per group).
Table 2.
Effect of Lithothamnion sp. on total body weight increase, total food consumption, average food utilization rate, actual dosage in rats treated for 13 weeks (mean ± SD) (n = 10 per group).
| Group (%) | Total body weight increase (g) | Total food consumption (g) | Actual dosage (g/kg.BW) | Average food utilization rate (%) |
|---|---|---|---|---|
| Female | ||||
| control | 224.2 ± 15.4 | 2049.6 ± 70.3 | 0 | 10.94 ± 0.74 |
| 0.625 | 220.1 ± 17.4 | 2095.8 ± 60.4 | 0.68 | 10.50 ± 0.76 |
| 1.25 | 220.7 ± 19.5 | 2080.3 ± 43.1 | 1.34 | 10.60 ± 0.77 |
| 2.5 | 215.0 ± 12.3 | 2062.1 ± 61.5 | 2.69 | 10.43 ± 0.45 |
| Male | ||||
| control | 456.5 ± 24.3 | 2600.6 ± 118.2 | 0 | 17.57 ± 0.81 |
| 0.625 | 442.9 ± 38.3 | 2624.4 ± 60.3 | 0.51 | 16.86 ± 1.26 |
| 1.25 | 444.7 ± 31.9 | 2624.4 ± 76.8 | 1.02 | 16.94 ± 1.03 |
| 2.5 | 432.4 ± 37.8 | 2633.3 ± 65.9 | 2.10 | 16.41 ± 1.16 |
Food consumption and food utilization rate of the male and female animals in treatment groups were comparable to those of the control group (Table 2) and did not differ between groups. The actual intake of test substance by diet in the low, medium and high dose treatment groups was 0.68, 1.34 and 2.69 g/kg BW for female rats and 0.51, 1.02 and 2.10 g/kg BW for male rats, respectively.
All urine samples were light yellow in appearance; differences in protein and occult blood index of the treatment groups for male rats were noted in comparison with those of the control group (Table 3).
Table 3.
Effect of Lithothamnion sp. on urinalysis in rats treated for 13 weeks (mean ± SD) (n = 10 per group).
| Group (%) | GLU (mmol/L) | Protein (g/L)a | Specific gravity | pH | Occult blood (cell/ μL)b |
|---|---|---|---|---|---|
| — ± + | — ± + | ||||
| Female | |||||
| control | — | 10 0 0 | 1.015 ± 0.007 | 6.60 ± 0.57 | 820 |
| 0.625 | — | 10 0 0 | 1.018 ± 0.004 | 6.95 ± 0.44 | 910 |
| 1.25 | — | 10 0 0 | 1.016 ± 0.007 | 6.80 ± 0.67 | 9 1 0 |
| 2.5 | — | 10 0 0 | 1.016 ± 0.005 | 7.10 ± 0.21 | 8 2 0 |
| Male | |||||
| control | — | 8 1 1 | 1.014 ± 0.003 | 7.25 ± 0.35 | 9 1 0 |
| 0.625 | — | 10 0 0* | 1.016 ± 0.005 | 7.30 ± 0.35 | 100 0* |
| 1.25 | — | 10 0 0* | 1.014 ± 0.005 | 7.10 ± 0.32 | 10 0 0* |
| 2.5 | — | 10 0 0* | 1.018 ± 0.005 | 7.35 ± 0.53 | 100 0* |
Values in treatment groups of male rats were significantly different from the control group, P < 0.01.
Protein(g/L): —: 0, ±: 0.15, +: 0.3.
Occult blood (cell/μL): —: 0, ±: 10, +: 25.
Treatment with Lithothamnion sp. for 90 days did not induce any significant changes in hematological and biochemical parameters compared to control group (Table 4 and Table 5).
Table 4.
Effect of Lithothamnion sp. on hematological parameters in rats treated for 13 weeks (mean ± SD) (n = 10 per group).
| Group (%) | HGB (g/L) | RBC (×1012/L) | HCT (%) | PLT (×109/L) | PT (s) | APTT (s) | WBC (×109/L) | Classification of WBC |
||
|---|---|---|---|---|---|---|---|---|---|---|
| LYM (%) | NEUT(%) | Others/WBC (%)a | ||||||||
| Female | ||||||||||
| control | 155.6 ± 11.9 | 7.74 ± 0.68 | 41.44 ± 3.80 | 279 ± 82.2 | 11.76 ± 3.78 | 28.15 ± 5.55 | 16.02 ± 2.66 | 73.7 ± 10.5 | 19.4 ± 9.3 | 6.9 ± 2.8 |
| 0.625 | 141.8 ± 14.9 | 7.34 ± 0.48 | 38.57 ± 3.56 | 261 ± 70.6 | 10.48 ± 0.85 | 26.67 ± 3.38 | 14.95 ± 1.96 | 73.2 ± 10.5 | 20.1 ± 10.6 | 6.7 ± 1.8 |
| 1.25 | 148.3 ± 11.6 | 7.77 ± 0.52 | 39.83 ± 3.02 | 259 ± 72.7 | 10.92 ± 1.07 | 23.05 ± 3.51 | 15.84 ± 2.33 | 66.3 ± 8.4 | 27.7 ± 7.9 | 6.0 ± 2.4 |
| 2.5 | 142.2 ± 13.7 | 7.16 ± 0.65 | 36.78 ± 4.63 | 283 ± 73.2 | 12.68 ± 4.43 | 25.03 ± 6.09 | 15.76 ± 2.93 | 68.2 ± 11.5 | 25.7 ± 9.5 | 6.1 ± 3.4 |
| Male | ||||||||||
| control | 149.3 ± 17.3 | 8.03 ± 0.64 | 41.91 ± 3.95 | 236 ± 77.0 | 11.72 ± 0.69 | 26.71 ± 4.79 | 17.27 ± 2.25 | 71.4 ± 5.4 | 22.7 ± 4.8 | 5.9 ± 1.3 |
| 0.625 | 139.7 ± 12.9 | 7.65 ± 0.67 | 39.06 ± 3.77 | 235 ± 69.3 | 11.70 ± 0.37 | 23.04 ± 9.23 | 16.19 ± 3.29 | 66.6 ± 8.8 | 25.3 ± 7.7 | 8.1 ± 2.8 |
| 1.25 | 137.0 ± 18.0 | 7.29 ± 0.83 | 38.22 ± 4.05 | 239 ± 73.3 | 12.05 ± 0.91 | 20.53 ± 4.54 | 17.86 ± 4.95 | 65.1 ± 7.6 | 27.1 ± 7.1 | 7.8 ± 2.7 |
| 2.5 | 135.5 ± 11.0 | 7.18 ± 0.82 | 37.13 ± 4.11 | 257 ± 80.6 | 11.74 ± 0.83 | 20.34 ± 3.67 | 20.59 ± 5.69 | 64.8 ± 5.5 | 28.6 ± 5.8 | 6.6 ± 2.5 |
a Others in WBC include Eosinophil, Basophils and Monocytes.
Abbreviations: HGB: hemoglobin concentration; RBC: red blood cell count; HCT: hematocrit; PLT: platelet count; PT: prothrombin time.
APTT: activated partial thromboplastin time; WBC: white blood cell count; LYM%: percentage of lymphocyte.
NEUT%: percentage of neutrophil granulocyte.
Table 5.
Effect of Lithothamnion sp. on biochemical parameters in rats treated for 13 weeks (mean ± SD) (n = 10 per group).
| Group (%) | ALT (U/L) | AST (U/L) | BUN (mmol/L) | CRE (μmol/L) | GLUC (mmol/L) | ALP (U/L) | TRIG (mmol/L) | CHOL (mmol/L) | TP (g/L) | ALB (g/L) | A/G | K+ (mmol/L) | CL−(mmol/L) | Na+ (mmol/L) | Ca2+ (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | |||||||||||||||
| control | 50.91 ± 15.87 | 118.43 ± 26.42 | 7.34 ± 1.31 | 37.74 ± 4.59 | 6.56 ± 0.82 | 70.47 ± 19.32 | 0.46 ± 0.09 | 2.51 ± 0.28 | 73.70 ± 3.80 | 50.51 ± 2.82 | 2.18 ± 0.08 | 5.94 ± 0.66 | 100.93 ± 2.34 | 151.2 ± 8.59 | 2.69 ± 0.13 |
| 0.625 | 47.33 ± 11.10 | 115.17 ± 29.73 | 7.79 ± 1.29 | 37.71 ± 4.24 | 6.28 ± 0.60 | 68.23 ± 17.09 | 0.39 ± 0.09 | 2.77 ± 0.36 | 73.59 ± 2.89 | 50.65 ± 3.05 | 2.23 ± 0.26 | 6.10 ± 0.92 | 98.91 ± 2.42 | 152.1 ± 8.64 | 2.64 ± 0.09 |
| 1.25 | 48.18 ± 11.33 | 110.96 ± 21.26 | 8.09 ± 1.42 | 39.80 ± 6.04 | 6.29 ± 0.79 | 73.44 ± 22.10 | 0.41 ± 0.12 | 2.61 ± 0.34 | 71.80 ± 4.29 | 49.46 ± 3.26 | 2.23 ± 0.20 | 6.24 ± 0.86 | 98.38 ± 2.13 | 152.3 ± 13.02 | 2.58 ± 0.11 |
| 2.5 | 40.71 ± 8.62 | 107.99 ± 39.68 | 8.49 ± 1.57 | 38.66 ± 6.64 | 6.08 ± 0.47 | 79.71 ± 19.28 | 0.38 ± 0.05 | 2.62 ± 0.26 | 71.56 ± 2.49 | 48.67 ± 2.51 | 2.14 ± 0.24 | 6.46 ± 0.70 | 100.24 ± 2.13 | 155.1 ± 9.41 | 2.61 ± 0.17 |
| Male | |||||||||||||||
| control | 47.56 ± 6.30 | 124.23 ± 23.98 | 6.19 ± 1.19 | 30.46 ± 4.63 | 6.72 ± 0.54 | 102.44 ± 23.67 | 0.65 ± 0.21 | 2.07 ± 0.30 | 67.77 ± 3.07 | 45.05 ± 1.99 | 1.99 ± 0.16 | 6.85 ± 0.87 | 99.70 ± 2.18 | 165.8 ± 7.50 | 2.51 ± 0.08 |
| 0.625 | 46.62 ± 4.48 | 125.94 ± 24.11 | 6.00 ± 0.85 | 29.48 ± 3.38 | 6.56 ± 0.61 | 107.44 ± 21.70 | 0.53 ± 0.17 | 2.08 ± 0.38 | 66.73 ± 2.09 | 44.11 ± 1.27 | 1.96 ± 0.17 | 7.02 ± 0.94 | 98.66 ± 1.24 | 160.1 ± 8.36 | 2.43 ± 0.06 |
| 1.25 | 46.01 ± 5.70 | 114.93 ± 21.86 | 6.07 ± 0.71 | 30.70 ± 4.69 | 6.55 ± 0.44 | 114.83 ± 22.15 | 0.43 ± 0.14 | 1.70 ± 0.32 | 65.14 ± 2.92 | 43.07 ± 1.88 | 1.96 ± 0.19 | 7.11 ± 0.96 | 98.02 ± 1.21 | 162.9 ± 7.85 | 2.44 ± 0.12 |
| 2.5 | 43.61 ± 5.57 | 105.36 ± 25.36 | 6.21 ± 3.85 | 31.88 ± 6.99 | 6.26 ± 0.61 | 126.98 ± 24.07 | 0.52 ± 0.18 | 2.07 ± 0.61 | 64.73 ± 3.33 | 43.27 ± 1.84 | 2.03 ± 0.18 | 7.29 ± 0.84 | 99.14 ± 1.05 | 158.0 ± 8.84 | 2.43 ± 0.09 |
Abbreviations: ALT: alanine transferase; AST: aspartate transferase ; ALP: alkaline phosphatase; BUN: blood urea nitrogen; CRE: creatinine.
GLU: glucose; TG: triglyceride; TCHO: total cholesterol; TP: total protein; ALB: albumin; T-BIL: total bilirubin; Cl−: chloride.
K+: potassium; Na+: sodium;Ca2+: calcium.
Organ weight and coefficient of organ weight of liver, kidneys, adrenals, testes, epididymides, uterus, ovaries, thymus, spleen, brain and heart in treatment groups were similar to those of the control group. No differences were observed between the male and female rats (Table 6a, Table 6b).
Table 6a.
Effect of Lithothamnion sp. on organ weight and coefficient of organ to body weight of rats treated for 13 weeks (mean ± SD) (n = 10 per group).
| Group (%) | Fasting body weight (g) |
Heart |
Spleen |
Kidney |
Liver |
Thymus |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute weight(g) | Relative weight (%) | Absolute weight(g) | Relative weight(%) | Absolute weight(g) | Relative weight (%) | Absolute weight(g) | Relative weight(%) | Absolute weight(g) | Relative weight(%) | ||
| Female | |||||||||||
| control | 273.0 ± 15.3 | 0.97 ± 0.07 | 0.36 ± 0.02 | 0.53 ± 0.09 | 0.193 ± 0.033 | 2.06±0.31 | 0.75 ± 0.08 | 7.51 ± 0.68 | 2.75 ± 0.18 | 0.46 ± 0.13 | 0.169 ± 0.048 |
| 0.625 | 270.5 ± 19.1 | 0.93 ± 0.09 | 0.35 ± 0.03 | 0.49 ± 0.06 | 0.181 ± 0.018 | 2.08 ± 0.30 | 0.77±0.08 | 7.56 ± 0.69 | 2.80 ± 0.18 | 0.51 ± 0.11 | 0.190 ± 0.044 |
| 1.25 | 271.9 ± 22.9 | 0.97 ± 0.07 | 0.36 ± 0.01 | 0.51 ± 0.08 | 0.189 ± 0.024 | 2.12 ± 0.43 | 0.78±0.09 | 7.72 ± 0.94 | 2.83 ± 0.14 | 0.46 ± 0.06 | 0.170 ± 0.022 |
| 2.5 | 261.2 ± 16.1 | 0.93 ± 0.09 | 0.36 ± 0.03 | 0.47 ± 0.06 | 0.179 ± 0.025 | 1.96 ± 0.18 | 0.75±0.04 | 7.35 ± 0.66 | 2.82 ± 0.15 | 0.50 ± 0.10 | 0.191 ± 0.040 |
| Male | |||||||||||
| control | 490.9 ± 24.3 | 1.54 ± 0.13 | 0.31 ± 0.02 | 0.81 ± 0.11 | 0.164 ± 0.016 | 3.41 ± 0.33 | 0.70±0.06 | 13.88 ± 1.51 | 2.82 ± 0.22 | 0.55 ± 0.11 | 0.112 ± 0.021 |
| 0.625 | 484.0 ± 42.8 | 1.55 ± 0.14 | 0.32 ± 0.03 | 0.81 ± 0.09 | 0.168 ± 0.012 | 3.49 ± 0.20 | 0.73±0.07 | 13.25 ± 1.47 | 2.74 ± 0.26 | 0.60 ± 0.09 | 0.124 ± 0.020 |
| 1.25 | 485.7 ± 36.0 | 1.56 ± 0.14 | 0.32 ± 0.03 | 0.79 ± 0.10 | 0.162 ± 0.015 | 3.49 ± 0.35 | 0.72±0.06 | 13.61 ± 1.38 | 2.80 ± 0.18 | 0.57 ± 0.08 | 0.118 ± 0.013 |
| 2.5 | 471.0 ± 40.1 | 1.51 ± 0.16 | 0.32 ± 0.03 | 0.71 ± 0.10 | 0.150 ± 0.017 | 3.46 ± 0.38 | 0.74±0.07 | 12.59 ± 1.28 | 2.68 ± 0.23 | 0.62 ± 0.14 | 0.134 ± 0.035 |
Relative weight to body weight (%) = absolute organ weight/fasting body weight *100 %.
Table 6b.
Effect of Lithothamnion sp. on organ weight and coefficient of organ to body weight of rats treated for 13 weeks (mean ± SD) (n = 10 per group).
| Group (%) | Fasting weight (g) |
Ovary(Testis) |
Uterus(Epididymis) |
Cerebrum |
Adrenal gland |
||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute weight(g) | Relative weight (%) | Absolute weight(g) | Relative weight(%) | Absolute weight(g) | Relative weight (%) | Absolute weight(g) | Relative weight (%) | ||
| Female | |||||||||
| control | 273.0 ± 15.3 | 0.1391 ± 0.0340 | 0.0510 ± 0.0120 | 0.59 ± 0.12 | 0.218 ± 0.048 | 1.41 ± 0.07 | 0.52 ± 0.03 | 0.0686 ± 0.0129 | 0.0251 ± 0.0040 |
| 0.625 | 270.5 ± 19.1 | 0.1310 ± 0.0274 | 0.0484 ± 0.0089 | 0.58 ± 0.04 | 0.216 ± 0.015 | 1.41 ± 0.07 | 0.52 ± 0.04 | 0.0730 ± 0.0072 | 0.0270 ± 0.0015 |
| 1.25 | 271.9 ± 22.9 | 0.1462 ± 0.0324 | 0.0535 ± 0.0085 | 0.60 ± 0.08 | 0.221 ± 0.021 | 1.40 ± 0.08 | 0.52 ± 0.04 | 0.0708 ± 0.0125 | 0.0259 ± 0.0029 |
| 2.5 | 261.2 ± 16.1 | 0.1138 ± 0.0343 | 0.0434 ± 0.0120 | 0.55 ± 0.08 | 0.213 ± 0.029 | 1.41 ± 0.08 | 0.54 ± 0.03 | 0.0667 ± 0.0193 | 0.0254 ± 0.0070 |
| Male | |||||||||
| control | 490.9 ± 24.3 | 3.69 ± 0.20 | 0.75 ± 0.06 | 0.61 ± 0.05 | 0.125 ± 0.015 | 1.50 ± 0.10 | 0.31 ± 0.03 | 0.0671 ± 0.0170 | 0.0138 ± 0.0041 |
| 0.625 | 484.0 ± 42.8 | 3.65 ± 0.35 | 0.76 ± 0.05 | 0.63 ± 0.05 | 0.131 ± 0.020 | 1.54 ± 0.07 | 0.32 ± 0.04 | 0.0687 ± 0.0100 | 0.0144 ± 0.0031 |
| 1.25 | 485.7 ± 36.0 | 3.71 ± 0.28 | 0.77 ± 0.07 | 0.61 ± 0.07 | 0.127 ± 0.020 | 1.52 ± 0.10 | 0.32 ± 0.04 | 0.0672 ± 0.0166 | 0.0139 ± 0.0037 |
| 2.5 | 471.0 ± 40.1 | 3.43 ± 0.56 | 0.73 ± 0.11 | 0.64 ± 0.07 | 0.138 ± 0.019 | 1.57 ± 0.06 | 0.33 ± 0.03 | 0.0698 ± 0.0090 | 0.0149 ± 0.0021 |
Relative weight to body weight (%) = absolute organ weight/fasting body weight *100 %.
Macroscopic evaluation did not show any novel pathological lesions between the experimental and control groups. Therefore, histopathological examination was performed only on animals in the high-dose and control groups. A small number of sporadic, spontaneous changes were observed, including one scattered necrosis lesion and one inflammatory cell aggregation lesion in livers of control group rats, one mild focal necrosis lesion in a renal tubule of a control group female rat, and one white cell infiltration lesion in the lamina propria of a uterus of an animal in the high-dose group. Based on these data, and the actual intake of test substance, the no-observed-adverse-effect-level (NOAEL) for female and male rats under these experimental conditions was 2.69 g/kg BW and 2.10 g/kg BW respectively.
4. Discussion
A large number of animal and human studies have described the effect of Lithothamnion sp. on a range of important parameters including, but not limited to bone strength, composition and metabolism [5,[15], [16], [17]], mitigation of the clinical signs of knee osteoarthritis [[2], [3], [4]], and calcium metabolism itself [1], particularly during exercise [5,18,19]. However, published comprehensive toxicological data on Lithothamnion sp. calcium are sparse, excepting one other publication [11], and given the wide-ranging applications of this bioavailable source of calcium and other minerals, completing and publishing these studies was deemed important.
The acute toxicity study demonstrated that the median lethal dose (LD50) for Lithothamnion sp. was more than 10 g/kg BW, as there were no toxic effects, or histopathologic changes observed in mice. Mice are often used in acute toxicity studies9 [14] and were also deemed appropriate for the purposes of this study. Significantly, this is a higher dose level than the LD50 level already published (2 g/kg BW in rats) [11]. Although acute toxicity studies often test doses in the range of 5 g/kg BW [[12], [13], [14]], a higher dose was chosen for this study based on preliminary studies that indicated very low toxicity for this particular product. Furthermore, the product that had been tested was Brazilian in origin, and it is possible that it differs significantly in its composition from the test substance in question (harvested from North Atlantic / Icelandic waters).
The value of using animal studies to predict toxic effects in humans has been confirmed to have a high predictive value [20], and depending on regulatory requirements, sub-chronic oral toxicity studies can be carried out over a course of up to 90 days [12,13,21]. As is standard practice, changes in general behavior and body weight were used as preliminary markers for toxicity evaluation, and in this study, no mortality, no physical appearance abnormalities or changes in behavioral patterns were observed in all treatment groups (both male and female). Furthermore, mean body weight, food consumption and food utilization rate of both genders in all treatment groups showed no significant differences, confirming that the test product (Aquamin F) did not negatively effect the growth of rats, although a trend towards a decrease in body weight gain as the dose increased was observed. With high inter-individual variability in weight gain, this trend is difficult to interpret, although it may indicate that nutrient absorption is being affected at the high dose range, as feed consumption and utilization did not vary between groups. In the absence of gastro-intestinal lesions, and hematology and biochemistry abnormalities, the extent of nutrient malabsorption, if it exists, is difficult to qualify.
Changes in hematological parameters are a more specific index for toxicity assessment, and assessing these values in animals has a high predictive value for human toxicity [20]. Changes in these parameters can be caused by sub-chronic administration of naturally occurring substances [13], however, in this sub-chronic study, no significant alterations in in hematologic values were observed, illustrating that the test product (Aquamin F) did not adversely affect animal health using a further metric (hematology).
The liver is the main organ for drug metabolism and biotransformation, and liver function and structure should always be thoroughly evaluated in toxicity studies. The serum biochemical markers used to assess liver tissue damage and functions include levels of certain liver specific enzymes (ALT, AST and ALP) and proteins (PT, ALB, the ratio of ALB/GLB). As the liver is also involved in cholesterol metabolism and glucose synthesis [22], TCHO and GLU in serum may also be used as indirect indices of liver health. The current study demonstrated no differences in the above biochemical parameters of rats treated with test substance at any dose when compared to the control group, demonstrating that Lithothamnion sp. caused no hepatotoxicity in SD rats at the tested doses.
As is the case with the liver, kidneys are often affected by toxic substances, and are evaluated by testing for the presence of proteinuria, hematuria etc., and serum BUN, Cr and electrolyte levels [23]. In the present study, the absence of significant differences in these parameters in treated groups when compared with the control group confirmed that renal function was preserved throughout the study. Moreover, it was determined that the rats in treatment groups were able to adequately regulate calcium digestion, absorption and metabolism, as no increase in serum calcium concentration was observed. Urinalysis performed at the end of the sub-chronic study showed that male rats of treatment groups actually had a decrease in urine protein and occult blood, further confirming that the test product did not harm the urinary system. Interestingly, these results are not in accordance with the results reported in another Lithothamnion sp. toxicity study in which increases in serum creatinine were observed at the higher treatment dose [11]. The reason for this discrepancy is not clear at this time, although the test substances in these studies may differ in their composition, as this particular study was performed on a Brazilian product. Furthermore, the normal urinalysis results from the current study (an evaluation which was not performed in the work by Almeida et al. [11]) confirm our interpretation of normal renal function in the test subjects.
Individual organ weight will correspondingly increase with growth, and relative weight (coefficient) of organ to body weight is therefore predictable. Variations in this ratio may result from organ enlargement (congestion, edema) or reduction in organ size (necrosis, atrophy) associated with toxicity. In the current study, organ weight and coefficient of organ to body weight between treatment groups and control group remained within normal ranges.
Macroscopic evaluation of all rats in treated and control groups was normal. Histopathological results demonstrated a small number of sporadic lesions in liver and kidney of the control group (both sexes) and uterus (n = 1) in the high-dose group. These findings were deemed clinically insignificant and sporadic in nature. More significant histopathologic changes have been reported in sub-chronic toxicity studies of naturally-occurring substances, such as black caraway seed essential oil [13].
Based on these data, it is surmised that dietary administration of Lithothamnion sp. (Aquamin F) at doses up to 2.69 g/kg BW and 2.10 g/kg BW for female and male rats for 90-days was safe and did not result in any significant alterations in clinical observations, body weight, food consumption, food utilization rate, hematology and clinical biochemistry analysis, urinalysis, absolute and relative organ weight, macroscopic evaluation and histopathology analysis. In the absence of significant adverse findings, and no changes in plasma calcium concentrations, it must be considered that the product was not absorbed by the rats, and therefore could not have been expected to cause toxicity in the first place. While this is possible, it is considered unlikely, as previous studies indicate significant (positive) changes in rats specifically and compared to controls, when exposed to much lower doses of the same product. These include effects on bone [17,24] suggesting significant absorption of the product.
Application of a 100-fold safety/uncertainty factor to these data in order to account for species and inter-individual variability is considered standard practice in most cases, and may be regarded as a safe coefficient in this instance [25]. The high dose at 2000 mg/kg BW in this study is more than 140-fold greater than 14 mg/kg BW/day commonly used by the general population. Therefore, this study can be used by regulatory bodies to support the statement that this Lithothamnion sp. supplement, Aquamin F may be preliminarily regarded as a safe product for human consumption. However, this study has not comprehensively evaluated the toxicity of Lithothamnion sp., as it is considered necessary and prudent to evaluate potential mutagenicity and teratogenicity in future studies.
5. Conclusion
This study confirms that Lithothamnion sp. (Aquamin F) was a non-toxic substance with an LD50 greater than 10 g/kg BW. The no-observed-adverse-effect-level (NOAEL) of Lithothamnion sp. (Aquamin F) in SD rats was 2100 mg/kg BW for male rats and 2690 mg/kg BW for female rats under these experimental conditions of a standard sub-chronic toxicity study.
CRediT authorship contribution statement
Ying Zhang: Writing - original draft, Conceptualization, Investigation, Software, Formal analysis. Ruotao Tian: Supervision, Project administration, Conceptualization, Resources. Haili Wu: Writing - review & editing, Visualization. Xuemin Li: Validation, Investigation, Data curation, Conceptualization. Shuqin Li: Investigation, Methodology. Linxiu Bian: Investigation, Methodology.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are highly thankful to Mrs. Laping Meng for providing the composition data of the test subject.
References
- 1.Zenk J.L., Frestedt J.L., Kuskowski M.A. Effect of calcium derived from Lithothamnion sp. On markers of calcium metabolism in premenopausal women. J. Med. Food. 2018;21(2):154–158. doi: 10.1089/jmf.2017.0023. [DOI] [PubMed] [Google Scholar]
- 2.Frestedt J.L., Kuskowski M.A., Zenk J.L. A natural seaweed derived mineral supplement (Aquamin F) for knee osteoarthritis: a randomised, placebo controlled pilot study. Nutr. J. 2009;8:7. doi: 10.1186/1475-2891-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frestedt J.L., Walsh M., Kuskowski M.A., Zenk J.L. A natural mineral supplement provides relief from knee osteoarthritis symptoms: a randomized controlled pilot trial. Nutr. J. 2008;7:9. doi: 10.1186/1475-2891-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffernan S.M., McCarthy C., Eustace S., FitzPatrick R.E., Delahunt E., Vito G.D. Mineral rich algae with pine bark improved pain, physical function and analgesic use in mild-knee joint osteoarthritis, compared to Glucosamine: a randomized controlled pilot trial. Complementary Ther. Med. 2020;50 doi: 10.1016/j.ctim.2020.102349. [DOI] [PubMed] [Google Scholar]
- 5.Slevin M.M., Allsopp P.J., Magee P.J., Bonham M.P., Naughton V.R., Strain J.J., Duffy M.E., Wallace J.M., McSorley E.M. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J. Nutr. 2014;144(3):297–304. doi: 10.3945/jn.113.188144. [DOI] [PubMed] [Google Scholar]
- 6.Cronin B.E., Allsopp P.J., Slevin M.M., Magee P.J., Livingstone M.B.E., Strain J.J., McSorley E.M. Effects of supplementation with a calcium-rich marine-derived multi-mineral supplement and short-chain fructo-oligosaccharides on serum lipids in postmenopausal women. Br. J. Nutr. 2016;115(4):658–665. doi: 10.1017/S0007114515004948. [DOI] [PubMed] [Google Scholar]
- 7.Bailey R.L., West K.P., Jr, Black R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015;66(Suppl 2):22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 8.Wang H.J., Wang D.T., Ouyang Y.F., Huang F.F., Ding G.Q., Zhang B. Do Chinese Children Get Enough Micronutrients? Nutrients. 2017;9(4):397. doi: 10.3390/nu9040397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z., Zhao L.Y., Man Q.Q., Wang J.Z., Zhao W.H., Zhang J. Dietary Micronutrients Intake Status among Chinese Elderly People Living at Home: Data from CNNHS 2010-2012. Nutrients. 2019;11(8):1787. doi: 10.3390/nu11081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy, March 7-29, 2000: highlights of the conference. South. Med. J. 2001;94(6):569–573. [PubMed] [Google Scholar]
- 11.Almeida F., Schiavo L.V., Vieira A.D., Araújo G.L., Queiroz-Junior C.M., Teixeira M.M., Cassali G.D., Tagliati C.A. Gastroprotective and toxicological evaluation of the Lithothamnion calcareum algae. Food Chem. Toxicol. 2012;50(5):1399–1404. doi: 10.1016/j.fct.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Kpemissi M., Metowogo K., Melila M., Veerapur V.P., Negru M., Taulescu M., Potârniche A.V., Suhas D.S., Puneeth T.A., Vijayakumar S., Eklu-Gadegbeku K. Acute and subchronic oral toxicity assessments of Combretum micranthum (Combretaceae) in Wistar rats. Toxicol. Rep. 2020;7:162–168. doi: 10.1016/j.toxrep.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabarraei H., Hassan J., Parvizi M.R., Golshahi H. Evaluation of the acute and sub-acute toxicity of the black caraway seed essential oil in Wistar rats. Toxicol. Rep. 2019;6:869–874. doi: 10.1016/j.toxrep.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worasuttayangkurn L., Nakareangrit W., Kwangjai J., Sritangos P., Pholphana N., Watcharasit P., Rangkadilok N., Thiantanawat A., Satayavivad J. Acute oral toxicity evaluation of Andrographis paniculata-standardized first true leaf ethanolic extract. Toxicol. Rep. 2019;6:426–430. doi: 10.1016/j.toxrep.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslam M.N., Bergin I., Jepsen K., Kreider J.M., Graf K.H., Naik M., Goldstein S.A., Varani J. Preservation of bone structure and function by Lithothamnion sp. Derived minerals. Biol. Trace Elem. Res. 2013;156(1–3):210–220. doi: 10.1007/s12011-013-9820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslam M.N., Kreider J.M., Paruchuri T., Bhagavathula N., DaSilva M., Zernicke R.F., Goldstein S.A., Varani J. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcif. Tissue Int. 2010;86(4):313–324. doi: 10.1007/s00223-010-9340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan O., Sweeney J., O’Meara B., Widaa A., Bonnier F., Byrne H.J., O’Gorman D.M., O’Brien F.J. A Natural, Calcium-Rich Marine Multi-mineral Complex Preserves Bone Structure, Composition and Strength in an Ovariectomised Rat Model of Osteoporosis. Calcif. Tissue Int. 2017;101(4):445–455. doi: 10.1007/s00223-017-0299-7. [DOI] [PubMed] [Google Scholar]
- 18.Barry D.W., Hansen K.C., van Pelt R.E., Witten M., Wolfe P., Kohrt W.M. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med. Sci. Sports Exerc. 2011;43(4):617–623. doi: 10.1249/MSS.0b013e3181f79fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shea K.L., Barry D.W., Sherk V.D., Hansen K.C., Wolfe P., Kohrt W.M. Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med. Sci. Sports Exerc. 2014;46(10):2007–2013. doi: 10.1249/MSS.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W., Dorato M., Van Deun K., Smith P., Berger B., Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 21.OECD . Organization for Economic Cooperation and Development; Paris, France: 1998. Test No. 408: Repeated Dose 90-day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals. [Google Scholar]
- 22.Bechmann L.P., Hannivoort R.A., Gerken G., Hotamisligil G.S., Trauner M., Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012;56(4):952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Gowda S., Desai P.B., Kulkarni S.S., Hull V.V., Math A.A., Vernekar S.N. Markers of renal function tests. N. Am. J. Med. Sci. (Boston) 2010;2(4):170–173. [PMC free article] [PubMed] [Google Scholar]
- 24.Bae Y.J., Bu S.Y., Kim J.Y., Yeon J.Y., Sohn E.W., Jang K.H., Lee J.C., Kim M.H. Magnesium supplementation through seaweed calcium extract rather than synthetic magnesium oxide improves femur bone mineral density and strength in ovariectomized rats. Biol. Trace Elem. Re. 2011;144(1-3):992–1002. doi: 10.1007/s12011-011-9073-2. [DOI] [PubMed] [Google Scholar]
- 25.Renwick A.G. The use of safety or uncertainty factors in the setting of acute reference doses. Food Addit. Contam. 2000;17(7):627–635. doi: 10.1080/026520300412555. [DOI] [PubMed] [Google Scholar]