Abstract
Purpose
To report a case of bilateral pan-uveitis resembling fungal and viral endophthalmitis in a patient who was ultimately diagnosed with sarcoidosis.
Observation
A 64-year-old female presented with a four-day history of painless vision loss in the right eye. She presented with multiple concurrent systemic complaints, including a history of oral and genital sores, patches of hypopigmented skin on her forearms, and occasional shortness of breath. Upon further examination, she was noted to have bilateral pan-uveitis, which was more severe in the right than left eye. Posterior pole examination of the right eye revealed dense vitritis with multiple large whitish round balls that seemed suggestive of fungal or viral endophthalmitis. Initial therapies included intravitreal (IVT) foscarnet and intravenous (IV) acyclovir, followed by IV amphotericin B and oral voriconazole, which did not improve ocular signs and symptoms. Further evaluations ruled out infectious etiologies and lymphoma. Chest computerized tomography (CT) scan revealed findings suggestive of sarcoidosis, which was confirmed with lung biopsy. Anti-viral and -fungal treatments were discontinued, and the patient was started on IV methylprednisolone followed by oral prednisone and mycophenolate mofetil. Ocular symptoms improved, and the patient remained stable after treatment.
Conclusion and Importance
The index report illustrates a case of ocular sarcoidosis that imitated the presentation of infectious endophthalmitis. Though ocular sarcoidosis is known to masquerade as a range of disorders and constitutes part of the differential diagnosis for infectious endophthalmitis, sarcoidosis has not been reported in recent literature to imitate the presentation of fungal endophthalmitis. The index case suggests that ocular sarcoidosis should be considered in the differential diagnoses of fungal endophthalmitis.
Keywords: Pan-uveitis, Sarcoidosis, Infectious endophthalmitis, Fungal endophthalmitis, Vitreous balls, Mimicking
1. Introduction
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology with variable clinical manifestations in multiple organs, including the lungs, skin, lymph nodes, spleen, and eyes. After the lungs, the eyes are the second most commonly involved organ in sarcoidosis, and ocular involvement has been reported in about 20–50% of patients with biopsy-proven disease.1, 2, 3 The visual outcome of sarcoidosis-associated uveitis is devastating, with about 10% of patients ultimately becoming blind in at least one eye.4 The primary cause of visual loss is cystoid macular edema.5,6 Poor visual prognosis has been associated with glaucoma, chronic systemic diseases, and posterior segment involvement, such as the presence of peripheral punched-out lesions.6 Patients who are of African descent or are female are at higher risk for vision loss.6 Ocular sarcoidosis most commonly manifests as a granulomatous bilateral anterior uveitis, which accounts for nearly two-thirds of all cases.7 About 14–28% of patients with ocular sarcoidosis show posterior segment lesions, such as vitritis, periphlebitis, chorioretinitis, and optic nerve granuloma. These findings typically lead to extensive differential diagnoses that include infectious causes, immunologic conditions, and malignancies such as lymphomas.8,9 Although the majority of the cases are bilateral, ocular sarcoidosis may present unilaterally in about 10% of all cases.10, 11, 12 Sarcoidosis-associated uveitis accounts for nearly 5% of all adult cases of uveitis and about 1% of pediatric cases.13 However, these estimates are highly variable and differ substantially in endemic countries.10,12,14, 15, 16, 17, 18, 19, 20, 21 Herein, we report an unusual case of sarcoid-associated acute pan-uveitis that imitated the presentation of fungal endophthalmitis in a patient with no known history of sarcoidosis.
2. Case report
A 64-year-old Caucasian woman working as a real estate agent from Maryland presented to our tertiary Uveitis Clinic by referral due to a four-day history of painless vision loss in the right eye (OD). There was no significant past ocular history. Her past medical history was significant for major depressive disorder, hypercholesterolemia, and bladder surgery for urinary incontinence. She also suffered from oral herpangina roughly once a year, with no history of genital ulcers. The patient also endorsed history of white skin patches on her forearms, easy bruising, sinus issues, and occasional shortness of breath. Medications taken by the patient at the time of presentation included paroxetine, atorvastatin, and vitamin supplements (including calcium). As part of her work as a real estate agent, she had recently been exposed to old homes with damp and moldy environments. She smoked one pack of cigarettes per day and previously lived in Iran, Turkey, and Switzerland. She owned two dogs and owned a cat several years prior.
On examination, her best-corrected visual acuity (BCVA) was counting fingers at 1.5 ft in OD and 20/25 in the left eye (OS). Intraocular pressure (IOP) was within normal limits in both eyes (OU). Slit lamp examination revealed fine keratic precipitates, 2+ conjunctival injection, 3+ cells and aqueous flare, posterior synechiae inferonasally in OD, and 1+ conjunctival injection and no cell and 1+ flare in OS. Initial fundus examination revealed 3+ vitreous haze, “snowballs” and “string of pearls”-like in the vitreous, and a possible chorioretinal lesion in OD, as well as 1+ vitreous haze, mild peripheral retinal hemorrhage, and periphlebitis with no definite retinal infiltrates in OS (Fig. 1). Fluorescein angiography (FA) in OD was inconclusive due to vitreous haze but revealed significant leakage from the disc and inferior vessels in OS (Fig. 2). Initial work-up with complete blood count (CBC), angiotensin-converting enzyme (ACE) levels, lysozyme levels, C-reactive protein, erythrocyte sedimentation rate (ESR), and urinalysis were all within normal limits. B-scan ultrasonography showed hyperechogenicity of the vitreous and possible chorioretinal lesions. Chest x-ray (CXR) showed mild pulmonary abnormalities diffusely, consistent with either a linear or interstitial pattern versus airway wall thickening, suggestive of possible bronchitis, with minimal widening of mediastinum and no evidence of hilar lymphadenopathy (Fig. 3).
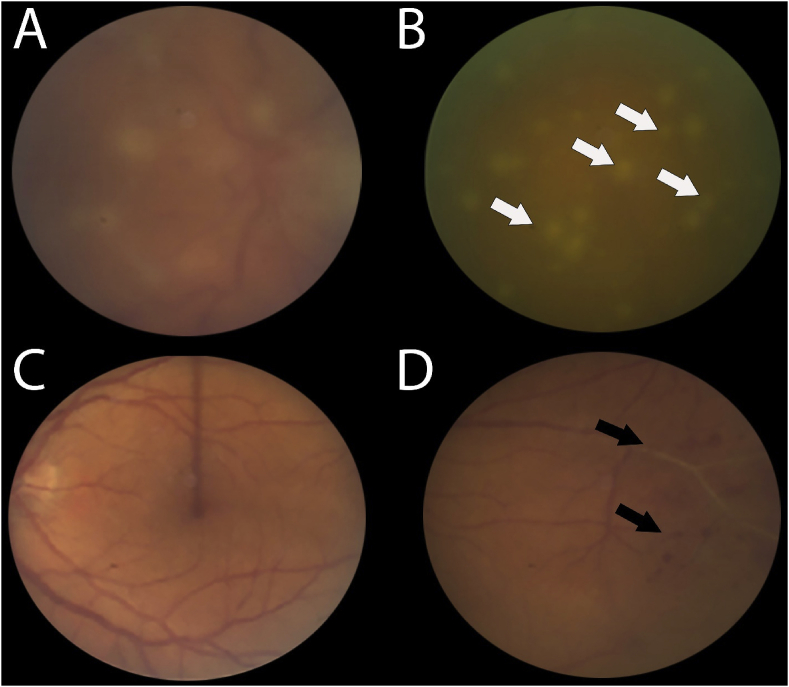
Fig. 1.
Fundus photographs of the right (A, B) and left (C, D) eyes showing dense vitreous haze over the right eye with associated snowball formation. Note the large size of the snowballs with an appearance similar to fungal spores in a “string of pearls” configuration (white arrows). The left eye shows no definitive retinal infiltrates but has evidence of peripheral periphlebitis with associated hemorrhages (black arrows).
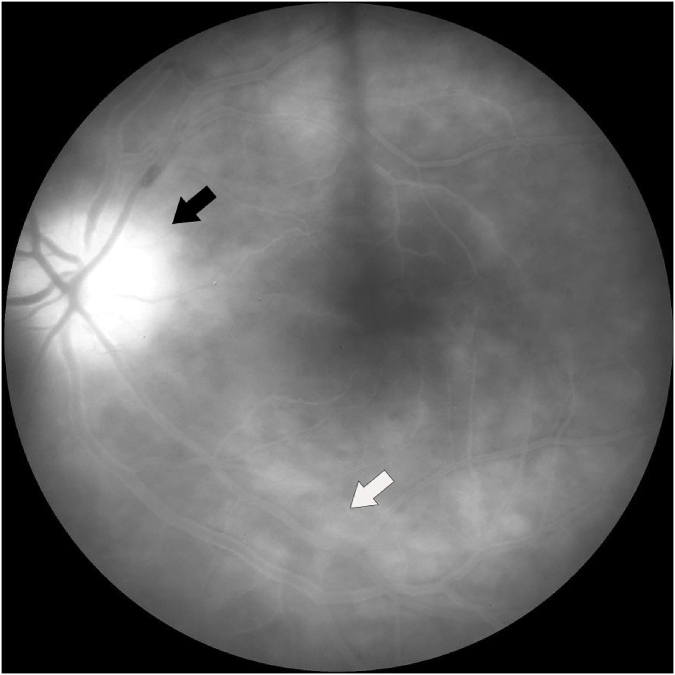
Fig. 2.
Late phase fluorescein angiogram of the left eye demonstrating significant disc leakage (black arrow) with leakage of the inferior retinal vasculature (white arrow).
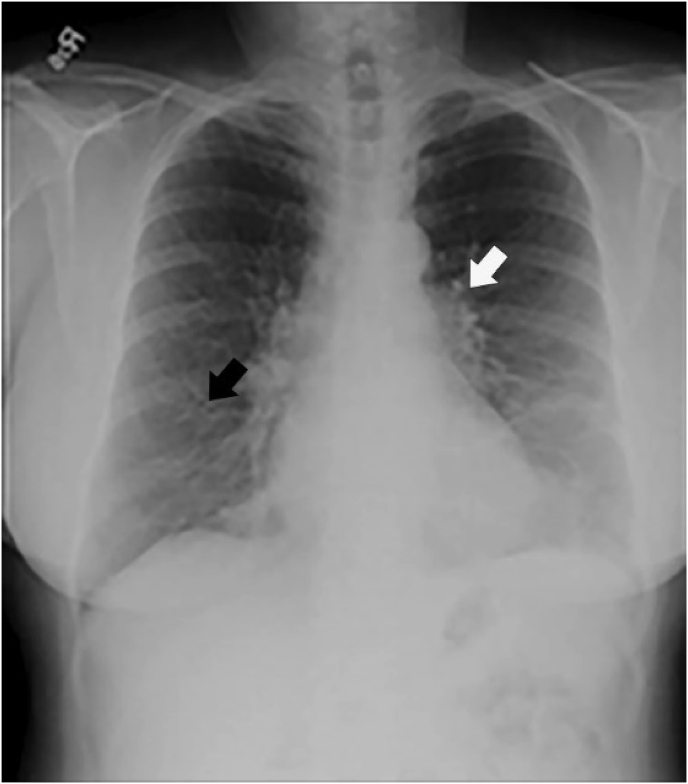
Fig. 3.
Chest radiograph demonstrating fine linear pulmonary infiltrates (black arrow) and interstitial pulmonary infiltrates (white arrow) which are nonspecific for pulmonary disease.
Infectious etiologies such as tuberculosis, treponema pallidum, toxoplasma, Lyme disease, and human immunodeficiency virus (HIV) were ruled out with testing. The patient was admitted to the hospital, and bilateral anterior chamber (AC) tap was performed for gram stain and culture with polymerase chain reaction (PCR). The patient received intravitreal (IVT) foscarnet in OD and intravenous (IV) acyclovir, followed by IV amphotericin B and oral voriconazole, which did not improve ocular signs and symptoms. The patient also noted progressive shortness of breath. Several days later, AC tap for gram stain and culture and PCR for infectious agents including herpes simplex virus (HSV), cytomegalovirus (CMV), varicella zoster virus (VZV), toxoplasmosis, and mycobacterium tuberculosis were all negative. Vitreous biopsy showed vitreous strands with chronic inflammation and epithelioid and multinucleated macrophages suggestive of ocular sarcoidosis, with no signs of fungal infection or lymphoma.
Due to the patient's progressive shortness of breath and nonspecific findings on CXR, a chest computerized tomography (CT) scan with contrast (images not available for publication) was performed and revealed multiple lung nodules in the lower lobes bilaterally, with slightly enlarged mediastinal and right hilar lymph nodes and mild interstitial thickening with subtle underlying emphysematous changes. No definitive diagnosis could be made. Hence, lung biopsy was performed at the recommendation of the pulmonology team and revealed reactive lymphocytes and rare benign bronchial epithelium in the right hilar lymph node. The histopathological findings of the lung biopsy supported a diagnosis of sarcoidosis with ocular involvement. The patient began treatment with IV methylprednisolone followed by prednisone, with subsequent improvement. After seven months of therapy, the best-corrected visual acuity was 20/63 in OD and 20/32 in OS. Color fundus photographs showed resolution of vitritis in both eyes (Fig. 4). The patient has since been doing well on mycophenolate mofetil 1000 mg twice a day and prednisone, which has been tapered to 4 mg daily.
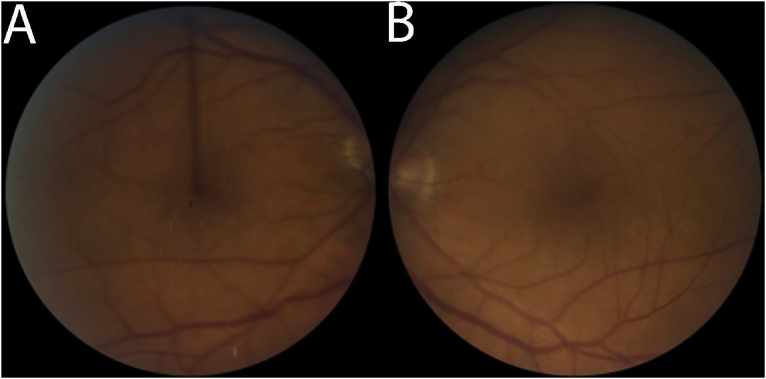
Fig. 4.
Follow up fundus photographs of the right (A) and left (B) eye showing improvement of the previously seen vitreous haze and snowballs in the right eye after treatment with systemic corticosteroids.
3. Discussion
3.1. Generating a diagnosis
We have presented a case of a 64-year-old female who had bilateral pan-uveitis with dense vitritis and multiple large vitreous snowballs (OD > OS). The differential diagnosis for this presentation is broad and can be divided into three general etiologic categories: infectious diseases, autoimmune uveitis, and masquerade syndrome.
Generally, infectious etiologies should be ruled out and treated empirically first while waiting for further investigation. Endogenous fungal endophthalmitis was suspected given the history of various risk factors, including bladder surgery and exposure to moldy environments, as well as the appearance of the dense large vitreous snowballs in OD. However, this patient was not immunocompromised and had no other risk factors for acute fungal endophthalmitis. Other findings, such as posterior synechiae and absence of ocular pain and hypopyon, were also less consistent with fungal infection. Though the vitreous snowballs are atypical for acute retinal necrosis (ARN) syndrome, viral infection cannot be ruled out on presentation if the view of the posterior pole is obscured. HSV, VZV, or an atypical human herpes virus, such as human herpes virus-6 or human T-cell lymphotropic virus, were all possible etiologies and were considered. Endogenous bacterial endophthalmitis, including atypical presentations of Bartonella or Borrelia, were also considered. Parasitic diseases, such as Toxocara and toxoplasmosis, were thought to be less likely in the context of bilateral diseases. Toxoplasmosis is less likely to be bilateral when the infection is acquired as an adult but can be bilateral in congenitally acquired or immunocompromised cases. In this case, the absence of chorioretinitis and retinal scar in OS made toxoplasmosis less likely. While tuberculosis and syphilis should be suspected on any differential diagnosis for uveitis, these were excluded by blood evaluation.
Autoimmune causes of uveitis include sarcoidosis, Adamantiades-Behçet disease, and collagen-vascular diseases, such as systemic lupus erythematosus or granulomatosis with polyangiitis. Although the patchy vitiligo on the forearms may suggest Vogt-Koyanagi-Harada disease, this is less likely given the dense vitritis and white race; moreover, ocular findings typically precede skin changes in this disease. Sarcoidosis has a wide range of systemic and ocular manifestations and is often labeled as a great mimicker of many other conditions. Two aspects in this case are particularly unique and deserve special consideration. First, the serologic evaluation for sarcoidosis was non-revealing, with both ACE and lysozyme levels within normal limits. It has previously been reported that an ACE level above 50 units/L yields a sensitivity of 73–84%, specificity of 83–95%, and positive predictive value of 47% for sarcoidosis.23,24 ACE levels above 40 units/L and lysozyme levels above 10 mg/L yield a positive predictive value of 83%. At ACE levels less than 50 units/L, as seen in this index case, the probability of sarcoidosis is less than 1%.23 Second, the patient's clinical presentation was atypical for sarcoidosis. Although vitreous snowballs are common posterior segment manifestations in sarcoidosis and are seen in up to 46% of cases,25 sarcoidosis presenting with dense vitritis, accompanied by snowballs of such a large size, as to mimic the appearance of fungal spores, has, to our knowledge, not been reported. This presentation was more consistent with infectious endophthalmitis secondary to fungal infection, particularly in context of the patient's clinical and exposure history. Numerous reports have described the varied ocular presentations associated with sarcoidosis. Our case presented with bilateral pan-uveitis and vasculitis with abnormalities on CXR but no clear mediastinal or hilar lymphadenopathy. Despite this atypical presentation, sarcoidosis remained on our differential diagnosis. Bilateral ocular inflammation/uveitis as the initial manifestation of sarcoidosis has been reported in 22–38% of cases.11 Many large epidemiologic studies have investigated the incidence of sarcoidosis diagnoses that initially presented as pan-uveitis, with each showing variable results based on sarcoidosis endemicity, from as high as 38% in Turkey to as low as 0.6% in Saudi Arabia. Other international studies have found that the percentage of pan-uveitis cases proven to be secondary to sarcoidosis range from 0.6% to 38% in different countries, with the highest frequencies seen in Turkey, Germany, Italy, and Sri Lanka.10,12,14, 15, 16, 17, 18, 19, 20, 21 In Japan, suspected sarcoidosis accounts for 22.3% of all uveitis cases. Sarcoidosis is also the most common final diagnosis (40.7%) in cases of pan-uveitis of unknown initial etiology.22
Although the patient is fairly young for ocular and central nervous system lymphomas, masquerade syndrome remained on the differential diagnosis. As lymphoma is not an urgent diagnosis, the vitrectomy was pursued primarily to exclude fungal infection. Metastatic disease was less likely. The patient was not taking any medications associated with idiopathic uveitis.
3.2. Treatment
Options for initial empiric therapy for bilateral pan-uveitis and vasculitis of suspected infectious etiology include antibiotics (e.g. vancomycin, ceftazidime), antivirals (e.g. foscarnet for HSV/VZV and ganciclovir for CMV), anti-parasitics (e.g. clindamycin) and antifungals (e.g. amphotericin B]. Given the severe vision loss in OD, immediate empiric treatment was warranted to maximize the chance of restoring visual acuity in OD and preserving visual acuity in OS. Given the high suspicion of fungal endophthalmitis and inability to rule out ARN, the patient was initially treated with an IVT injection of foscarnet (2.4 mg/0.1 ml) and admitted to the hospital for IVT amphotericin B (5 μg/0.1 ml). Paracentesis was performed before IVT injection in OU to allow for gram stain, culture, and PCR testing, and to minimize the risk of elevation of IOP following two consecutive IVT injections. Her IOP was normal after the procedures.
Patients who received both antivirals IVT and systemic therapy compared to systemic therapy alone showed slightly improved visual outcomes and decreased risk of retinal detachment.26 However, systemic antivirals are the mainstay of treatment to arrest progression of retinal necrosis in the affected eye and to prevent involvement of the fellow eye.27,28 While early treatment of ARN is critical to prevent irreversible damage, there is currently no definitive therapeutic protocol. The frequency and duration of injections are subjective, and other data suggests the adjunctive use of IVT agents does not improve clinical outcomes.29
In most cases of fungal endophthalmitis, chorioretinitis and very early endophthalmitis can be treated with systemic antifungal agents without vitrectomy. In more advanced disease, however, therapeutic vitrectomy should be considered. In this case, diagnostic and therapeutic vitrectomy was urgently performed to reduce agent load, clear vitreous media, and obtain enough samples for culture, gram stain, potassium hydroxide test, PCR for fungal agents, as well as cytology, immunohistochemistry, flow cytometry, and gene testing for lymphoma, though less likely. Generally, an AC tap alone will not provide enough samples for all testing; although a higher percentage of true positive diagnoses are thought to be obtained from aqueous than vitreous fluid in PCR testing, this difference has not been shown to be statistically significant.30
If the diagnostic vitrectomy did not yield a diagnosis, chorioretinal biopsy of OD could have been considered, depending on the location of lesions and responsiveness to therapy. Other sites for biopsy should be considered if laboratory testing showed other abnormalities (e.g. liver or kidney dysfunction). In this patient, chest CT revealed hilar lymphadenopathy. A diagnosis of ocular sarcoidosis was eventually reached after a positive lung biopsy by the pulmonology team. Treating intermediate and posterior segment involvement in sarcoidosis generally requires systemic corticosteroids with prednisone (1 mg/kg/day) in adults, followed by oral corticosteroids subsequently tapered to low dose therapy. Steroid-sparing immunomodulatory therapy such as methotrexate, mycophenolate mofetil, and azathioprine may be required to maintain disease quiescence. The patient was treated with IV methylprednisolone, and subsequently prednisone and mycophenolate mofetil, which led to improvement and maintained disease quiescence.
In summary, the index case report emphasizes the importance of maintaining a targeted but broad differential diagnosis in managing uveitis. Many cases that initially present most consistent with one etiology may ultimately receive very different or unexpected diagnoses. The index case reinforces the reputation of ocular sarcoidosis as a great masquerader with particularly protean clinical manifestations. To our knowledge, this report is the first in recent literature to describe a case of ocular sarcoidosis that initially imitated the presentation of fungal endophthalmitis with dense vitritis and fungal ball-like vitreous opacities.
Patient consent
Verbal consent was obtained from the patient to publish this case.
Written consent to publish was not obtained. The index report does not contain any personal identification information.
Funding
Research to Prevent Blindness Departmental Challenge Award, National Eye Institute of the National Institutes of Health P30 Grant (EY026877), and an unrestricted educational grant from the Ocular Imaging Research and Reading Center (OIRRC) have been awarded to the Byers Eye Institute at Stanford University
Authorship
All authors attest that they meet the current ICMJE criteria.
Declaration competing of interest
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Jamilloux Y., Kodjikian L., Broussolle C., Seve P. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13(8):840–849. doi: 10.1016/j.autrev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Crick R.P., Hoyle C., Smellie H. The eyes IN sarcoidosis. Br J Ophthalmol. 1961;45(7):461–481. doi: 10.1136/bjo.45.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sungur G., Hazirolan D., Bilgin G. Pattern of ocular findings in patients with biopsy-proven sarcoidosis in Turkey. Ocul Immunol Inflamm. 2013;21(6):455–461. doi: 10.3109/09273948.2013.775311. [DOI] [PubMed] [Google Scholar]
- 4.Rothova A., Suttorp-van Schulten M.S., Frits Treffers W., Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stavrou P., Linton S., Young D.W., Murray P.I. Clinical diagnosis of ocular sarcoidosis. Eye. 1997;11(Pt 3):365–370. doi: 10.1038/eye.1997.77. [DOI] [PubMed] [Google Scholar]
- 6.Ohno S.A.K., Usui M., Uchio E. Uveitis Today. Elsevier; Amsterdam: 1998. A posterior segment involvement in sarcoidosis; pp. 207–210. [Google Scholar]
- 7.Rothova A. Ocular involvement in sarcoidosis. Br J Ophthalmol. 2000;84(1):110–116. doi: 10.1136/bjo.84.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum J.T. Uveitis. An internist's view. Arch Intern Med. 1989;149(5):1173–1176. doi: 10.1001/archinte.149.5.1173. [DOI] [PubMed] [Google Scholar]
- 9.Milman N., Hoffmann A.L. Childhood sarcoidosis: long-term follow-up. Eur Respir J. 2008;31(3):592–598. doi: 10.1183/09031936.00011507. [DOI] [PubMed] [Google Scholar]
- 10.Grajewski R.S., Caramoy A., Frank K.F. Spectrum of uveitis in a German tertiary center: review of 474 consecutive patients. Ocul Immunol Inflamm. 2015;23(4):346–352. doi: 10.3109/09273948.2014.1002567. [DOI] [PubMed] [Google Scholar]
- 11.Pasadhika S., Rosenbaum J.T. Ocular sarcoidosis. Clin Chest Med. 2015;36(4):669–683. doi: 10.1016/j.ccm.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uyama M. Uveitis in sarcoidosis. Int Ophthalmol Clin. 2002;42(1):143–150. doi: 10.1097/00004397-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Sepah Y.J., Agarwal A., Jabs D.A., Nguyen Q.D. In: Sarcoidosis. Schachat A.P., editor. 2014. [Google Scholar]
- 14.Wong A., McKelvie J., Slight C., Sims J. Land of the long white cloud: the spectrum of uveitis at a tertiary referral center in New Zealand. Ocul Immunol Inflamm. 2017;25(sup1):S115–S121. doi: 10.1080/09273948.2016.1203957. [DOI] [PubMed] [Google Scholar]
- 15.Rautenbach W., Steffen J., Smit D., Lecuona K., Esterhuizen T. Patterns of uveitis at two university-based referral centres in Cape Town, South Africa. Ocul Immunol Inflamm. 2017:1–7. doi: 10.1080/09273948.2017.1391954. [DOI] [PubMed] [Google Scholar]
- 16.Manandhar A. Patterns of uveitis and Scleritis in Nepal: a tertiary referral center study. Ocul Immunol Inflamm. 2017;25(sup1):S54–S62. doi: 10.3109/09273948.2016.1161804. [DOI] [PubMed] [Google Scholar]
- 17.Al Dhibi H.A., Al Shamsi H.N., Al-Mahmood A.M. Patterns of uveitis in a tertiary care referral Institute in Saudi Arabia. Ocul Immunol Inflamm. 2017;25(3):388–395. doi: 10.3109/09273948.2015.1133836. [DOI] [PubMed] [Google Scholar]
- 18.Perkins E.S., Folk J. Uveitis in London and Iowa. Ophthalmologica. 1984;189(1-2):36–40. doi: 10.1159/000309382. [DOI] [PubMed] [Google Scholar]
- 19.Cimino L., Aldigeri R., Marchi S. Erratum to: changes in patterns of uveitis at a tertiary referral center in Northern Italy: analysis of 990 consecutive cases. Int Ophthalmol. 2018;38(1):143. doi: 10.1007/s10792-017-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.Y., Kim D.Y., Woo S.J. Clinical patterns of uveitis in tertiary ophthalmology centers in Seoul, South Korea. Ocul Immunol Inflamm. 2017;25(sup1):S24–S30. doi: 10.1080/09273948.2016.1206203. [DOI] [PubMed] [Google Scholar]
- 21.Siak J., Kumaradas M., Chee S.P. The pattern of uveitis in Sri Lanka. Ocul Immunol Inflamm. 2017;25(sup1):S63–S68. doi: 10.1080/09273948.2017.1313991. [DOI] [PubMed] [Google Scholar]
- 22.Shirahama S., Kaburaki T., Nakahara H. Epidemiology of uveitis (2013-2015) and changes in the patterns of uveitis (2004-2015) in the central Tokyo area: a retrospective study. BMC Ophthalmol. 2018;18(1):189. doi: 10.1186/s12886-018-0871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baarsma G.S., La Hey E., Glasius E., de Vries J., Kijlstra A. The predictive value of serum angiotensin converting enzyme and lysozyme levels in the diagnosis of ocular sarcoidosis. Am J Ophthalmol. 1987;104(3):211–217. doi: 10.1016/0002-9394(87)90406-5. [DOI] [PubMed] [Google Scholar]
- 24.Power W.J., Neves R.A., Rodriguez A., Pedroza-Seres M., Foster C.S. The value of combined serum angiotensin-converting enzyme and gallium scan in diagnosing ocular sarcoidosis. Ophthalmology. 1995;102(12):2007–2011. doi: 10.1016/s0161-6420(95)30763-4. [DOI] [PubMed] [Google Scholar]
- 25.Khalatbari D., Stinnett S., McCallum R.M., Jaffe G.J. Demographic-related variations in posterior segment ocular sarcoidosis. Ophthalmology. 2004;111(2):357–362. doi: 10.1016/S0161-6420(03)00793-0. [DOI] [PubMed] [Google Scholar]
- 26.Flaxel C.J., Yeh S., Lauer A.K. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2013;111:133–144. [PMC free article] [PubMed] [Google Scholar]
- 27.Palay D.A., Sternberg P., Jr., Davis J. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112(3):250–255. doi: 10.1016/s0002-9394(14)76725-x. [DOI] [PubMed] [Google Scholar]
- 28.Aizman A., Johnson M.W., Elner S.G. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007;114(2):307–312. doi: 10.1016/j.ophtha.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 29.Tibbetts M.D., Shah C.P., Young L.H., Duker J.S., Maguire J.I., Morley M.G. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824. doi: 10.1016/j.ophtha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Harper T.W., Miller D., Schiffman J.C., Davis J.L. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009;147(1) doi: 10.1016/j.ajo.2008.07.043. 140-7.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]