Abstract
Studies have begun to emerge showing the protumor effects of tumor-associated neutrophils (TANs) in tumorigenesis, which may involve dysfunction of NK cells. However, the mechanism through which these rebellious neutrophils modulate NK cell immunity in tumor-bearing state remains unclear. In the present study, we demonstrate that neutrophils can impair the cytotoxicity and infiltration capability of NK cells, and downregulate CCR1 resulting in the weakened infiltration capability of NK cells. Moreover, neutrophils can decrease the responsiveness of NK-activating receptors, NKp46 and NKG2D. Mechanistically, enhanced PD-L1 on neutrophils and PD-1 on NK cells, and subsequent PD-L1/PD-1 interactions were the main mechanisms determining the suppression of neutrophils in NK cell immunity. G-CSF/STAT3 pathway was responsible for PD-L1 upregulation on neutrophils, while IL-18 was essential for PD-1 enhancement on NK cells. The crosstalk between neutrophils and NK cells was cell-cell interaction-dependent. These findings suggest that neutrophils can suppress the antitumor immunity of NK cells in tumor-bearing status through the PD-L1/PD-1 axis, highlighting the importance of PD-L1/PD-1 in the inhibitory effect of neutrophils on NK cells. Targeting G-CSF/STAT3 and IL-18 signaling pathway may be potential strategies to inhibit residual tumor in tumor therapy.
Introduction
Contrary to being inconsequential bystanders in tumorigenesis, neutrophils, an important component of the innate immune system, play key roles in antitumor immunity. It has become increasingly clear that neutrophils are a potent source of immune-modulatory cytokines that directly aid in the elimination of tumor cells [1,2] and indirectly augment adaptive immune responses against tumor [[3], [4], [5]]. However, studies showing critical protumorigenic effects of tumor-associated neutrophils (TANs) in tumorigenesis have also begun to emerge. TANs, the double-edged sword of innate immunity, are thus capable of being pro- or anti-tumorigenic depending on the tumor microenvironment [6,7]. Previous reports from our laboratory and others have shown that the inflammatory factors G-CSF/IL-6 [8] induce tumor-promoting neutrophils, while other mediators such as TNF-α and IFN-γ [9] or TGF-β blockade reverse the tumor-promoting effects of neutrophils [6], resulting in the recruitment and activation of TANs with an antitumor phenotype.
Natural killer (NK) cells are the effector lymphocytes of the innate immune system that control several types of tumors and microbial infections by limiting their spread and subsequent tissue damage [10]. Unlike T lymphocytes, NK cell cytotoxicity for tumor cells is decreased in cancer patients and tumor-bearing animal models [11]. The activation of NK cells is determined by a delicate balance between activating and inhibitory receptors [12]. The activating receptor, NKG2D, which recognizes RAE-1, H60, and MULT1 in mice [13], plays an important role in the immune response against cancer [14]. Its ligands are rarely expressed on the surface of healthy cells and tissues but frequently expressed in tumors and tumor cell lines [15]. Additionally, NK cell activation is also controlled by other factors. Evidence for the potential role of neutrophils in NK cell activation, maturation, and homeostasis has been found in mice [16]. Moreover, neutrophils-derived G-CSF may be the inhibitory factor of NK cells [17]. The potential interaction between neutrophils and other leukocytes, including macrophages, dendritic cells (DCs), and T lymphocytes, have been studied [3,18,19]. NK cells and neutrophils are localized in the same areas of spleen and lymph nodes and could form conjugates [20], and neutrophils facilitate the intermediate steps of invasion and metastasis cascade by suppressing NK cell activity [21], suggesting regulatory roles of neutrophils on NK cells. However, how neutrophils modulate NK cell in the tumor microenvironment remains largely unknown. Interestingly, Terme et al. reported that NK cells could express PD-1 [22], which is expressed most in the T cells and transfers the primary inhibitory signal to T cells through PD-L1/PD-1 interactions [23].
The detailed immunological mechanisms through which neutrophils with protumor phenotype modulate NK cells in tumor-bearing state remain unclear. The purpose of the present study was to investigate whether and how rebellious neutrophils modulate the immunity of NK cells in tumor-bearing state and whether neutrophils could suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis mediated by direct cell-cell interaction. Furthermore, the study sought to explore whether the G-CSF/STAT3 signaling pathway is involved in the upregulation of PD-L1 on neutrophils and whether IL-18 mediates the enhancement of PD-1 on NK cells.
Materials and methods
Reagents and antibodies
CCL3 (MIP-1α) and IL-2 were purchased from Millipore (Billerica, MA, USA). Monoclonal antibodies anti-Stat3 (clone: 79D7), anti-phospho-Stat3 (Tyr705) (clone: D3A7), and anti-GAPDH (clone: D16H11) were purchased from Cell Signaling Technology (Beverly, MA). Ly6G mAb (clone 1A8) was from BioExpress. Mouse IL-18 binding protein (IL-18BP) was from BIOHJ Corporation (USA). Anti-NKG2D (MI-6), anti-NKp46 (29A1.4) and anti-G-CSF antibodies were from R&D Systems. G-CSF, GM-CSF, IL-6, and TNF-α were from PeproTech (Rocky Hill, NJ). STAT3 inhibitor (FLLL32) was from Selleck. Rabbit anti-DX5 (clone EPR5788), anti-PD-L1 (clone EPR20529), and anti-PD-1 (clone J43) antibodies were from Abcam. Rabbit anti-mouse CCR1 (clone PA1–41062) and HRP-conjugated Goat anti-Rabbit IgG were from ThermoFisher Scientific. PE-conjugated anti-mouse IFN-γ (clone XMG1.2), PE-conjugated anti-mouse PD-L1 (clone MIH5), PE-conjugated anti-mouse PD-1 (clone RMP1–30) and PE-conjugated anti-mouse DX5 were from eBioscience (San Diego, CA).
Animals and experimental treatment
BALB/c mice (6 weeks old) were obtained from the Center of Medical Experimental Animals of Hubei Province (Wuhan, China). Animals were housed individually in plastic cages with wood chips as bedding, under pathogen-free conditions at 20–25 °C, and 12 L:12 D. Mice were fed a standard laboratory diet and water ad libitum. All studies involving mice were approved by Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology. All animal protocols were conducted according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. For constructing the tumor-bearing mouse model, 1 × 105/100 μl CT-26 cells were injected intramuscularly into the flanks of BALB/c mice and tumor growth was monitored. The animals were used for different experiments on the days indicated, including the isolation of neutrophils or NK cells on day 14 after inoculation.
Cell culture
Mouse colon cancer cell line, CT-26, and lymphoma cell line, YAC-1, were obtained from American Type Culture Collection (USA) and cultured according to the guidelines. Cells were cultured at 37 °C in a humidified atmosphere with 5% CO2 in medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin G, and 100 U/ml streptomycin.
NK cytotoxicity assay
The cytotoxicity of NK cells was determined as described previously [24]. YAC-1 cells were labeled with carboxyfluorescein succinimidyl ester (CFSE), co-incubated with NK cells isolated from mice at the indicated ratios, and then incubated at 37 °C for 6 h. The culture medium containing 50 U/ml of IL-2 was used for the incubation of NK cells. The tumor cells incubated without the effector cells were used as control. The cells were then stained with APC-Annexin-V and analyzed by flow cytometry. The cytotoxicity of NK cells was expressed as the percentage of Annexin-V+ cells in CFSE+ cells, which was calculated using the following equation: cytotoxicity % = (Annexin-V+CFSE+ cells/CFSE+ cells in the mixture of tumor cells and effector cells) – (Annexin-V+CFSE+ cells/CFSE+ cells in control tumor cells).
Isolation of neutrophils and NK cells
Neutrophils
The neutrophils were isolated from peritoneal cells as described previously [8]. To acquire un-primed neutrophils, neutrophils were recruited to peritoneal cavity by intra-peritoneal injection of CXCL1-expressing hepatocytes (C-PC) as described previously [8].
NK cells
Spleen cells or PBMCs were isolated from BALB/c mice. NK cells were isolated from spleen cells or PBMCs using anti-DX5 magnetic microbeads and mini MACS columns (Miltenyi Biotec) according to the manufacturers' protocol. The purity of the isolated cells was further identified as described previously [9].
Transfusion of neutrophils and NK cells
Neutrophils were injected intravenously (i.v) into mice at the concentration of 2 × 106 cells/500 μl PBS, once every day for six consecutive days. The mice were then used in subsequent experiments. For the adoptive transfer of NK cells, 3 × 105 CFSE-labeled NK cells from spleen or blood of tumor-bearing mice or normal mice were i.v injected into CT-26 bearing mice at once, and tumor tissues were harvested and prepared for detection by fluorescence microscopy 72 h after the injection.
Immunohistochemistry (IHC)
Tissue sections were prepared and subjected to IHC analysis as described previously [11]. Rabbit anti-mouse DX5 was used as the primary antibody for detecting NK cells. HRP-conjugated Goat anti-Rabbit IgG was used as secondary antibody. Images were obtained using Olympus IX71 microscope at 40 × 10 magnification. The NK cells were counted using Image-Pro Plus 6.0 software. The NK cell density was defined as the number of NK cells per five randomly chosen high-power fields.
Flow cytometry (FCM) analysis
For the detection of CCR-1 expression in NK cells, spleen or PBMC cells were stained with PE-conjugated Rat anti-mouse DX5 and Rabbit anti-mouse CCR1 primary antibodies and Alexa Fluor 488-conjugated Goat anti-Rabbit IgG secondary antibody for FCM analysis. Neutrophils isolated from the blood or peritoneal cavity with or without indicated stimulation factors were stained with PE-conjugated anti-mouse PD-L1 for FCM analysis. NK cells isolated from blood or spleen with or without indicated stimulation factors were stained with PE-conjugated anti-mouse PD-1 for FCM analysis. Parameters were acquired on BD FACS Calibur (BD Biosciences) and analyzed with CellQuest software (BD Biosciences). Percent staining was defined as the percentage of cells in the gate (M1) which was set to exclude ~99% of isotype control cells. Expression index was calculated by using the formula: mean fluorescence × percentage of positive cells.
NKG2D and NKp46 activation assay
Wells of flat-bottomed high protein binding plates (Thermo Fisher Scientific) were coated with 0.5 μg of NKG2D, 0.5 μg of NKp46 antibody or rat IgG2a antibody as control. The cells (106 cells/well) were stimulated with 100 U mouse rIL-2 (Roche) for 5 h, and the intracellular IFN-γ was detected by FCM.
ELISA
G-CSF, GM-CSF, TNF-α, and IL-6 in the serum of mice were detected by ELISA kit according to manufacturer's instructions (Bender MedSystem, Vienna, Austria). Cell-free supernatant of CT-26 was harvested 18 h after culture. IL-18 in the supernatant was quantified using ELISA kit according to manufacturer's instructions (R&D System, Minneapolis, MN).
Western blotting (WB)
IL-18 gene transfection
Plasmid pIL-18, an expression vector carrying the cDNA encoding murine IL-18 was prepared and analyzed as described previously [25]. The plasmid was constructed by the insertion of the cDNA into plasmid pcDNA3.1 (Invitrogen, Carlsbad, CA) in our laboratory. The mice received intramuscular (i.m) injection of pIL-18 (in 100 μl of saline) in the left hind thigh on the indicated day.
RNA interference
CT-26 cells were transfected with IL-18 siRNA (IL-18 Stealth Select RNAi [MSS205424, MSS205426]) or control siRNA (Stealth RNAi Negative Control Med GC; Invitrogen) using lipofectamine RNAiMAX according to manufacturer's instructions. After 6 h of incubation, the transfection mix was removed and replaced with complete medium. After transfection, cells were incubated in incubator at 37 °C with 5% CO2 for 24–72 h. The efficacy of interference was determined by in vitro expression analysis (Supplementary Fig. 1).
IL-18 neutralization in vivo
For the neutralization of IL-18 in vivo, mice were injected intra-peritoneally (i.p) with 20 mg of IL-18 binding protein (IL-18 BP) or saline once every two days from day 0 to day 8.
Neutrophil depletion and PD-L1 neutralization in vivo
Ly6G antibody was used to deplete neutrophils in vivo. Mice were injected i.p. with Ly6G antibody at a dose of 300 μg in 500 μL PBS on days 6, 7, 10, and 14 after tumor inoculation, and once a week in the follow-up observation period. PD-L1 antibody was used to neutralize PD-L1 in vivo. Mice were injected i.p. with 100 μg of PD-L1 antibody in 200 μl PBS on days 5, 9, and 12 after tumor inoculation, and once a week in the subsequent observation period.
Co-culture experiments of NK cells and neutrophils
Co-culture with direct contact: neutrophils and NK cells insolated from naïve or tumor-bearing mice were mixed at the indicated ratio in 96 U-bottom plates. Co-culture with lack of contact: neutrophils were added into the lower champer, while indicated ratio of NK cells was added into the up chamber of transwell system. All the cells were cultured in the medium containing 100 U of IL-2 for 3 days.
Statistical analysis
All statistical analyses were done using the SPSS 19.0 software. The results were expressed as mean ± standard deviation (SD) of multiple independent experiments. The means of different groups were compared by employing either Student's t-test or one-way ANOVA followed by post-hoc test. A value of p < 0.05 was considered significant.
Results
Neutrophils impaired the cytotoxicity and infiltration capability of NK cells
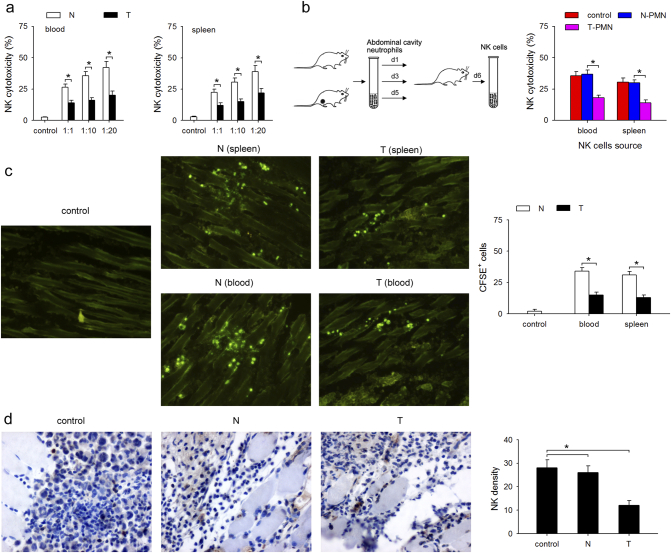
To determine the effect of neutrophils on the cytotoxicity and infiltration capability of NK cells, the CT-26 tumor-bearing mouse model was used. The cytotoxicity of NK cells from CT-26 tumor-bearing mice was weaker than that of NK cells isolated from naïve mice (E:T = 1:1; 1:10; 1:20) in both blood and spleen (Fig. 1a). Next, to investigate whether neutrophils were involved in impairing the cytotoxicity of NK cells, high number of neutrophils (3 × 106 cells, once every day for three days) isolated from normal mice or tumor-bearing mice were injected into normal mice, and the cytotoxicity of NK cells were detected (Fig. 1b left). Interestingly, adoptive transplantation of normal neutrophils had no effects on the cytotoxicity of NK cells, while infusion of neutrophils from the tumor-bearing mice significantly decreased the cytotoxicity of NK cells to YAC-1 cells (Fig. 1b right). To assess the in vivo infiltration ability of NK cells into tumor, CFSE-labeled NK cells from spleen and blood of tumor-bearing mice or normal mice were injected into CT-26 bearing mice and the CFSE-positive cells in the tumor were detected by fluorescence microscopy 72 h after injection. The NK cells from tumor-bearing mice showed less infiltration into tumor than those isolated from normal mice (Fig. 1c). Next, to clarify whether neutrophils could impair the infiltration capability of NK cells, high number of neutrophils isolated from tumor-bearing mice were injected into naïve mice three times before the inoculation of tumor cells, and the infiltration of NK cells was detected. Adoptive transplantation of neutrophils from tumor-bearing mice significantly decreased the infiltration of NK cells on day 5 following CT-26 inoculation (Fig. 1d). Taken together, these results suggest that neutrophils from tumor-bearing mice impair the cytotoxicity and infiltration capability of NK cells.
Fig. 1.
Neutrophils impair the cytotoxicity and infiltration capability of NK cells. (a) Comparison of cytotoxicity of NK cells from tumor-bearing mice and naïve mice. NK cells were isolated from blood or spleen of naïve (N) and tumor-bearing mice (T), and cytotoxicity was measured as described in Materials and Methods. (b) The effect of tumor-associated neutrophils (TANs) on the cytotoxicity of NK cells. Peritoneal neutrophils isolated from naïve mice or CT-26 bearing mice were adoptively transplanted into naïve mice, and cytotoxicity of NK cell was determined as described in Materials and Methods. (c) Comparison of the infiltration of NK cells from tumor-bearing mice and normal mice. NK cells were isolated from spleen and blood of tumor-bearing mice and normal mice, stained with carboxyfluorescein succinimidyl ester (CFSE), and the labeled cells were injected into tumor-bearing mice through the tail veil. The tumor sections were analyzed to assess NK cell infiltration using fluorescence microscope. (d) The effect of TANs on the infiltration of NK cells. Peritoneal neutrophils isolated from naïve mice or tumor-bearing mice were adoptively transplanted into tumor-bearing mice, and NK cell infiltration was determined by immunohistochemistry (IHC), brown: NK cells (DX5 positive), blue: cell nuclei (hematoxylin staining). Results are expressed as mean ± standard deviation (SD) from three independent experiments (n = 8 in each group). *p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Impaired NK cells infiltration was due to downregulation of chemokines receptor CCR1
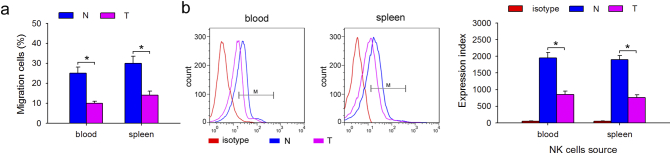
To identify the mechanism underlying the impairment of infiltration of NK cells, in vitro migration assays were conducted using 24-well 6.5 mm transwell plates with 3 mm pore polycarbonate membrane inserts. CCL3, an effective chemokine for NK cells, was previously shown to selectively promote the mobilization of NK cell subsets [26,27]. Therefore, 1 ng/mL CCL3 was used in the migration assays. As shown in Fig. 2a, the same concentration of CCL3 resulted in the recruitment of fewer NK cells isolated from blood and spleen of tumor-bearing mice than from normal mice. We next focused on the reason for decreased NK infiltration by analyzing the receptor expression on NK cells. Although several chemokine receptors were shown to be expressed on NK cells [27], high levels of CCR1 were found on mature NK cells. CCL3, a ligand of CCR1, regulates mature NK cell mobilization through a direct chemotactic action [26]. Therefore, we analyzed the expression of CCR1 on NK cells isolated from blood or spleen. As expected, CCR1 expression was downregulated on NK cells from tumor-bearing mice than from normal mice (Fig. 2b), suggesting that the impairment of infiltration of NK cells may be due to a decrease in CCR1 expression.
Fig. 2.
Impaired NK cell infiltration was due to downregulation of the chemokine receptor CCR1. (a) The effect of the chemokine, CCL3, on the migration of NK cells from tumor-bearing mice. Freshly isolated NK cells from blood and spleen of tumor-bearing mice or normal mice were used in standard migration assay. NK cells (3 × 105) were added to the upper well and after 90 min the contents of the lower chemotaxis well were transferred to a polypropylene tube after removal of the transwell insert. Cell migration was calculated as percentage of input cells. (b) The expression of the chemokine receptor, CCR1, on NK cells from tumor-bearing mice. CCR-1 was detected by FCM and expression index of CCR-1 was calculated. Results are expressed as mean ± SD from three independent experiments (n = 6 in each group). *p < 0.05.
Neutrophils suppress NK cell immunity via PD-L1/PD-1
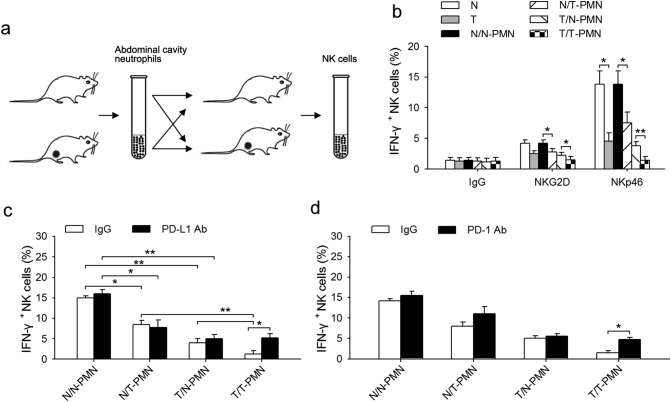
To characterize the mechanism through which neutrophils inhibit NK cell function, a commonly used method of examining the ex vivo responsiveness of NK cell was employed. Neutrophils from normal or tumor-bearing mice were first transfused into naïve or tumor-bearing mice, and NK cells were then collected for ex vivo examining experiments (Fig. 3a). As shown in Fig. 3b, the responsiveness of NK cells from the tumor-bearing mice was decreased after NKG2D or NKp46 stimulation, and neutrophils from tumor-bearing mice further weakened the NK cells responsiveness, especially after NKp46 stimulation. In addition, neutrophils from the tumor-bearing mice decreased the responsiveness of NK cells after transfusion into naïve mice. Unfortunately, normal neutrophils could not recover the responsiveness of NK cells to NKG2D or NKp46 (Fig. 3b). To determine whether PD-L1/PD-1 interaction was involved in the suppression of neutrophils on NK cell immunity, NK cells and neutrophils were sorted from naïve mice or tumor-bearing mice and co-cultured with or without neutralizing antibodies against PD-L1 or PD-1 respectively, followed by analysis of intracellular IFN-γ production. Neutrophils from the tumor-bearing mice decreased the IFN-γ production of both naïve and tumor-bearing NK cells, and PD-L1 neutralizing antibody impeded the inhibitory effect of tumor-bearing neutrophils on tumor-bearing NK cells, but did not attenuate the inhibitory effect of tumor-bearing neutrophils on naïve NK cells (Fig. 3c). Further, PD-1 neutralizing antibody antagonized the inhibitory effect of neutrophils on NK cells (Fig. 3d). These results suggest that neutrophils can suppress NK cell immunity through PD-L1/PD-1 interaction.
Fig. 3.
TANs suppress NK cell immunity by PD-L1/PD-1 interaction. (a) Schematic diagram showing the experimental protocol. Neutrophils from normal or tumor-bearing mice were transfused into naïve or tumor-bearing mice, and NK cells were collected for the activation experiments. (b) The effects of neutrophils on NK cell responsiveness. After experimental treatment as described above in (a), the percent of IFN-γ + NK cells were detected by FCM following in vitro stimulation with NK-activating receptor (NKp46 or NKG2D) antibodies or IgG control. (c) The effect of PD-L1 neutralizing antibody on NK cell responsiveness. Neutrophils and NK cells from naïve or tumor-bearing mice were co-cultured with or without neutralizing antibody against PD-L1 for 12 h, and the percent of IFN-γ + NK cells from the spleen of naïve or tumor-bearing mice was detected by FCM following in vitro stimulation with NK-activating receptor (NKp46 and NKG2D) antibodies or IgG control. (d) The effect of PD-1 neutralizing antibody on NK cell responsiveness. Neutrophils and NK cells from naïve or tumor-bearing mice were co-cultured with or without neutralizing antibody against PD-1 for 12 h, and the percent of IFN-γ + NK cells from the spleen of naïve or tumor-bearing mice was detected by FCM following in vitro stimulation with NK-activating receptor (NKp46 and NKG2D) antibodies or IgG control. Results are expressed as mean ± SD from four independent experiments (n = 6 in each group). *p < 0.05, **p < 0.01.
Tumor-derived G-CSF induces PD-L1 expression on neutrophils through the STAT3 pathway
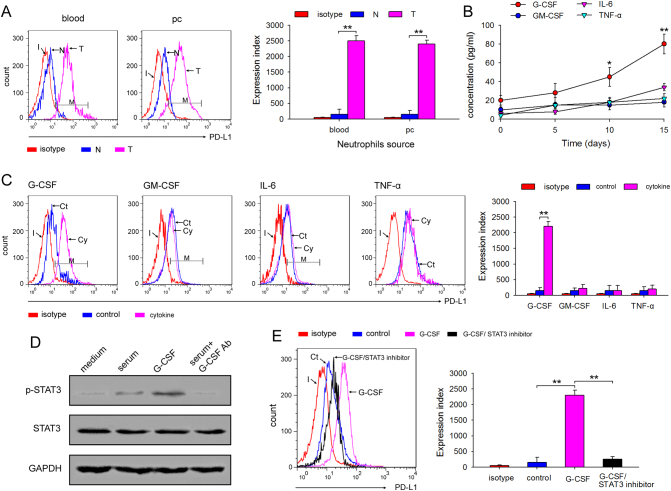
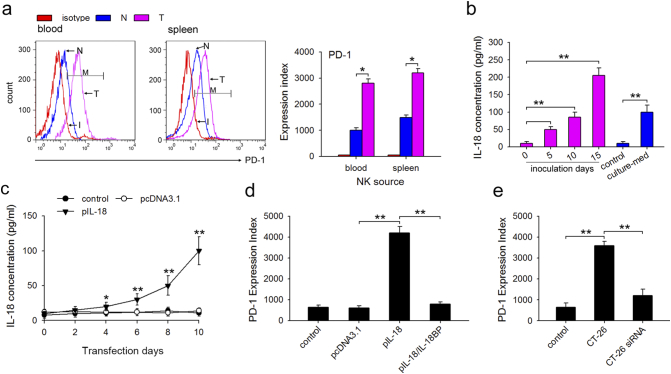
We first demonstrated higher expression of PD-L1 on neutrophils isolated from blood and abdominal cavity of tumor-bearing mice using FCM (Fig. 4a). Since tumor cells secrete various inflammatory cytokines, such as G-CSF, GM-CSF, IL-6, and TNF-α, we screened for these inflammatory cytokines in the serum of tumor-bearing mice to identify the factors required for PD-L1 upregulation on neutrophils. The results showed that during tumor growth only G-CSF and IL-6 increased significantly in the serum (Fig. 4b). Next, to confirm whether G-CSF or IL-6 regulate PD-L1 expression, freshly isolated neutrophils from the abdominal cavity of naïve mice were stimulated with these four cytokines for 12 h and PD-L1 expression was analyzed using FCM. The results showed that only G-CSF significantly upregulated the expression of PD-L1 on neutrophils (Fig. 4c), suggesting that tumor-derived G-CSF plays a key role in PD-L1 induction of neutrophils. Since our previous report revealed that G-CSF induced protumor function of neutrophils through activating STAT3 [8], we analyzed the activation of STAT3. Both G-CSF and serum isolated from tumor-bearing mice stimulated STAT3 activation, while G-CSF neutralizing antibody impeded the STAT3 activating effect of serum from tumor-bearing mice (Fig. 4d). To further study whether PD-L1 expression was modulated by pSTAT3, neutrophils isolated from naïve mice were cultured in medium containing G-CSF with or without the STAT3 phosphorylation inhibitor, FLLL32. As shown in Fig. 4e, FLLL32 almost completely inhibited G-CSF-mediated PD-L1 upregulation on neutrophils. Taken together, these findings suggested that tumor-derived G-CSF induces PD-L1 on neutrophils through the STAT3 pathway.
Fig. 4.
Tumor-derived G-CSF induces PD-L1 expression on neutrophils through the STAT3 pathway. (a) The expression of PD-L1 on neutrophils isolated from tumor-bearing mice. The expression of PD-L1 on neutrophils isolated from blood and abdominal cavity of naïve or tumor-bearing mice was analyzed by FCM, and expression index was calculated. (b) The level of G-CSF, GM-CSF, IL-6, and TNF-α in serum tumor-bearing mice. Mice were inoculated with CT-26 cells, and the serum concentration of G-CSF, GM-CSF, IL-6, and TNF-α was detected by ELISA at the indicated times. (c) The effect of G-CSF, GM-CSF, IL-6, and TNF-α on PD-L1 expression on neutrophils. The neutrophils from C-PC of the indicated mice were cultured for 12 h in the presence of G-CSF, GM-CSF, IL-6, and TNF-α, and the expression of PD-L1 on neutrophils was detected by FCM, and expression index was calculated. (d) The effects of G-CSF on STAT3 activation. The neutrophils from C-PC were stimulated with tumor-bearing mice serum, G-CSF or tumor-bearing mice serum plus G-CSF antibody for 12 h, and phospho-STAT3 was detected by WB. (e) The effect of STAT3 inhibitor on PD-L1 expression on neutrophils. The neutrophils from C-PC were cultured for 12 h in the presence of G-CSF or G-CSF plus STAT3 inhibitor FLLL32, and the expression of PD-L1 on neutrophils was detected by FCM, and expression index was also calculated. Results are expressed as mean ± SD from three independent experiments (n = 6 in each group). *p < 0.05, **p < 0.01.
Tumor-derived IL-18 enhances PD-1 expression on NK cells
We first confirmed higher PD-1 expression on NK cells isolated from blood and spleen of tumor-bearing mice using FCM (Fig. 5a). Cell-autonomous protumorigenic effects of endogenous IL-18 have been reported in B16F10 melanoma [28]. Further, human breast cell line MDA-MB-231 promoted the expression of PD-1 by secreting IL-18 [29]. Therefore, we analyzed IL-18 level in the serum from CT-26 tumor-bearing mice and CT-26 culture medium by ELISA. As shown in Fig. 5b, the concentration of IL-18 increased in the days after the inoculation, and more IL-18 was also detected in the CT-26 culture medium. To clarify the effect of IL-18 on PD-1 expression in vivo, the eukaryotic expression plasmid, pIL-18, was constructed and injected into naïve mice i.m. once every 2 days, and the IL-18 concentration was measured as shown in Fig. 5c. After four injections, FCM revealed that pIL-18 transfection upregulated PD-1 expression on NK cells (Fig. 5d). We found that treatment with IL-18 neutralizing protein (IL-18 BP) downregulated the PD-1 expression compared to the pIL-18 transfection group (Fig. 5d). Next, IL-18 siRNA was constructed and transfected into CT-26 (CT-26 siRNA). The IL-18 siRNA significantly suppressed IL-18 production (Supplementary Fig. 1). IL-18 knockdown in CT-26 significantly downregulated PD-1 expression on spleen NK cells 10 days after CT-26 inoculation (Fig. 5e). Taken together, these results suggest that tumor-derived IL-18 enhances PD-1 expression on NK cells.
Fig. 5.
Tumor-derived IL-18 enhances PD-1 expression on NK cells. (a) The expression of PD-1 on NK cells from tumor-bearing mice. The expression of PD-1 on NK cells isolated from blood and spleen of normal and tumor-bearing mice was detected by FCM, and expression index was also calculated. (b) The levels of IL-18 in serum of CT-26 tumor-bearing mice or CT-26 culture supernatant. IL-18 was detected by ELISA. (c) IL-18 expression in serum of pIL-18-transfected mice. Mice received intra-muscular (i.m) injection of eukaryotic expression plasmid pIL-18 as described in Materials and Methods, and the concentration of IL-18 was measured using ELISA kits at indicated times after pIL-18 transfection. (d) The effect of pIL-18 transfection and its neutralizing protein IL-18BP on PD-1 expression on NK cells. PD-1 expression on NK cells isolated from spleen was detected by FCM, and expression index was calculated. (e) The effect of IL-18 knockdown in CT-26 on PD-1 expression on NK cells isolated from spleen. The expression of PD-1 on NK cells isolated from CT-26 or IL-18 siRNA-transfected CT-26 -bearing mice was detected by FCM, and expression index was calculated. Results are expressed as mean ± SD from three independent experiments (n = 6 in each group). *p < 0.05, **p < 0.01.
The inhibition of neutrophils on NK cells was dependent on direct cell-cell contact
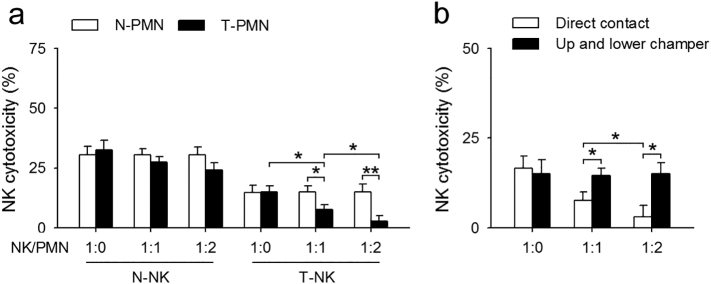
To further understand the mechanism of action of neutrophils on NK cells, neutrophils and NK cells insolated from naïve or tumor-bearing mice were co-cultured in the medium containing 100 U of IL-2 for 3 days, and CFSE-labeled YAC-1 cells (in logarithmic phase) were used as target cells to detect the cytotoxicity of NK cells. Neutrophils from tumor-bearing mice did not change the cytotoxicity of normal NK cells significantly. Decrease in NK cytotoxicity has been observed in tumor-bearing state (as shown in Fig. 1a), and neutrophils from tumor-bearing mice could further downregulate the cytotoxicity of tumor-bearing NK cells with the rise of neutrophils/NK ratio (Fig. 6a). It is noteworthy that neutrophils alone showed very limited cytotoxicity to YAC-1 cells (Supplementary Fig. 2). Interestingly, neutrophils-mediated inhibition of NK cell cytotoxicity was lost when NK-neutrophils were co-cultured in different chambers of the transwell system (Fig. 6b). These results indicate that neutrophil-mediated inhibition of antitumor immunity of NK cells was dependent on cell-cell contact.
Fig. 6.
The inhibition of neutrophils on NK cells was dependent on direct cell-cell contact. (a) The effect of neutrophils on the cytotoxicity of NK cells. Neutrophils and NK cells were isolated from naïve or tumor-bearing mice, 1 × 105 NK cells were co-cultured with neutrophils at the indicated ratios for 6 h, and then incubated with YAC-1 cells (1 × 105). NK cells cytotoxicity was detected as described in Materials and Methods. (b) The effect of neutrophil-NK cell contact on NK cell cytotoxicity. Neutrophils and NK cells were co-cultured in a direct contact in a well or in different chambers of a transwell system, and NK cell cytotoxicity was detected as described in Materials and Methods. Results are expressed as mean ± SD from four independent experiments (n = 6 in each group). *p < 0.05, **p < 0.01.
Neutrophil depletion cooperated with anti-PD-L1 improve the survival of tumor-bearing mice
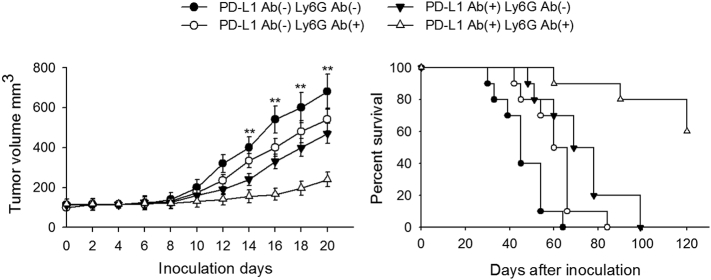
Previous studies reported that increase in peripheral neutrophil count was associated with poor clinical outcomes and short overall survival, and that depletion of neutrophils could improve survival [30,31]. Thus, to further confirm whether neutrophil depletion along with PD-L1 neutralization could improve the survival of tumor-bearing mice, we observed the therapeutic effect of combining PD-L1 blockade and neutrophil depletion. When naïve mice were inoculated with tumor cells, neutrophil depletion combined with PD-L1 blockade was more efficient in suppressing tumor growth as evaluated by tumor volume and survival in mice (Fig. 7).
Fig. 7.
Neutrophils depletion combined with anti-PD-L1 improves the survival of tumor-bearing mice. Naïve mice were inoculated with CT-26 tumor cells. The effect of neutrophil depletion along with PD-L1 blockade on tumor growth was evaluated by analyzing tumor volume and mice survival as shown. Tumor volume was calculated on the indicated days (left), and mice survival rate was recorded in parallel experiments (right). Results are expressed as mean ± SD from four independent experiments (n = 8 in each group; left), or expressed as mean ± SD from two independent experiments (n = 12 in each group; right). **p < 0.01.
Discussion
Studies showing critical roles for neutrophils in tumorigenesis have begun to emerge. Neutrophils have a significant impact on tumor by modulating the microenvironment as well as by influencing immune cell recruitment and activation. To our knowledge, the present study is the first to demonstrate that neutrophils suppress the antitumor immunity of NK cells in a cell-cell interaction-dependent manner through the PD-L1/PD-1 axis. Our data showed that neutrophils from tumor-bearing mice impair the cytotoxicity and weaken the infiltration capability of NK cells that was explained by CCR1 downregulation, and meanwhile, could inhibit the responsiveness of NK-activating receptor NKp46 and NKG2D. Mechanistically, enhanced expression of PD-L1 on neutrophils and PD-1 on NK cells as well as PD-L1/PD-1 interaction was the underlying cause of the suppression of neutrophils on NK cell immunity. G-CSF/STAT3 pathway and IL-18 were responsible for the upregulation of PD-L1 and PD-1 on neutrophils and NK cells, respectively.
NK cell impairment or deficiency has been associated with increased incidence of various cancers in patients and animal models [32], indicating that targeting NK cell regulation would be key immune strategy against tumor. Immune cells modulate the antitumor immunity of NK cells through direct cell-cell interaction in tumor microenvironment. Neutrophils and NK cells are the first immune cells to infiltrate the tumor, and localize in the same areas of spleen, lymph nodes and tumor, suggesting an increased possibility of interaction between them [20,33]. Although there are few previous reports demonstrating different and in some cases contradictory conclusions with regard to the modulation of neutrophils on NK cells in tumor [16,34], we confirmed that neutrophils suppress the antitumor immunity of NK cells in a cell-cell interaction-dependent manner, including impairing the cytotoxicity of NK cells, weakening the infiltration capability of NK cells, as well as inhibiting the responsiveness of NK-activating receptors. Spiegel et al. showed that neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells by inhibiting NK cell function [21], but the detailed molecular mechanisms were not revealed. PD-L1/PD-1 signaling pathway transfers negative signal for antitumor immunity, and mediates immunosuppression in tumor microenvironment. The contribution of PD-L1/PD-1 axis to T cells exhaustion has been investigated extensively [35]. Antibodies against PD-L1 and PD-1 have proved to be clinically useful, and the rational for using them mostly comes from the evidence of tumor specific CD8+ T cells enhancement. Interestingly, a previous work reported that peri-tumoral neutrophils negatively regulate adaptive immunity through the PD-L1/PD-1 signaling pathway in hepatocellular carcinoma [36]. Additionally, the PD-1/PD-L1 axis modulates NK cells versus multiple myeloma effect [37]. However, whether neutrophils could inhibit the antitumor immunity of NK cells through the PD-L1/PD-1 pathway needed to be confirmed. In the present study, we demonstrated that neutrophils suppress the antitumor immunity of NK cells through PD-L1/PD-1 axis in a manner dependent on cell-cell interaction. The therapeutic effects of PD antibodies or inhibitors have been shown to be associated with PD expression. Here, we showed that enhanced expression of PD-L1 on neutrophils and PD-1 on NK cells as well as PD-L1/PD-1 interaction were the main mechanisms determining the suppression of neutrophils on NK cells immunity. It is also noteworthy that there may exist crosstalks between other immune cells and NK cells in addition to the interaction between neutrophils and NK cells that are responsible for NK cell impairment, and may act in concert to stimulate tumor development. For example, in TS/A cells-bearing mice, MDSCs could suppress NK cells cytotoxicity through inhibiting perforin production in a cell-cell contact dependent manner [24]. Additionally, MDSCs could activate NK cells through RAE-1, a NK cell activating ligand, suggesting an unexpected novel activating role of MDSCs on NK cells [38]. Thus, whether neutrophils can modulate antitumor immunity of other immune cells or whether other immune cells can affect the antitumor immunity of neutrophils to exert specific protumor effects require further investigation. Further studies are also needed to clarify the detailed mechanisms of interaction between these immune cells.
G-CSF is a pluripotent inflammatory cytokine that regulates hemopoiesis as well as immune responses. G-CSF-secreting tumors, including colorectal, lung, bladder, and cervical carcinomas, are among the most rapidly growing cancers due to immunosuppression [39]. Interestingly, we previously reported that G-CSF induced the protumor function of neutrophils [8]. Here, we identified G-CSF as an immunosuppressive factor secreted by tumor cells, confirming that tumor-derived G-CSF could effectively induce PD-L1 expression on neutrophils through the activation of STAT3 pathway, which led to the inhibition of neutrophils on the antitumor immunity of PD-1-expressing T cells [40] and NK cells [41]. In this manner, neutrophils appear to contribute to tumor progression through the G-CSF-PD-L1 pathway, providing a new mechanism for neutrophils-induced protumor effects. In the future, therapeutic strategies aimed at interfering with TANs or the G-CSF-PD-L1 immunosuppressive pathway may be developed to provide a novel direction for cancer immunotherapy. The inflammatory cytokine, IL-18, which plays a major role in activating NK cells [42], has been shown to be accumulated in cancer patients, but its pathophysiological role in tumor immunity remains largely unclear. Recently, Park et al. reported that IL-18 could induce PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer, and tumor-derived IL-18 was associated with bad prognosis of triple-negative breast cancer patients [29]. In this study, we showed that IL-18, either exogenous or tumor-derived, promoted the expression of immuno-inhibitory receptor PD-1 on NK cells, which may lead to the suppression of NK-mediated tumor immunosurveillance. Although the exact mechanism of IL-18-induced PD-1 expression on NK cells was not identified in this study, abnormal PD-1 expression on NK cells provides evidence for the suppression of IL-18 on NK cells. Further studies may be needed to clarify the molecular mechanisms determining the effects of IL-18 on PD-1 expression and NK immunity.
In conclusion, based on the results obtained using a tumor-bearing mice model and an in vitro immune cell model, we demonstrate that neutrophils exert protumor effects through suppressing the antitumor immunity of NK cells in a PD-L1/PD-1 dependent manner, thereby providing new insights into the pathogenesis of colon cancer development. Additionally, our data demonstrate a new mechanism of impairment of NK immunity in tumor-bearing status, and, further, describes in detail the mechanism responsible for neutrophils-mediated inhibition of NK antitumor immunity, which may provide clues for better understanding of the pathogenesis of cancer and contribute to development of potential therapeutic strategies by targeting and remodeling neutrophils and NK cells in preventing tumor. Targeting G-CSF/STAT3 and IL-18 signaling pathway may also be potential strategies to inhibit residual tumor in antitumor therapy.
Declaration of competing interest
None.
Acknowledgments
Acknowledgments
We thank Dr. Yongchao Li (Department of Biochemistry and Molecular biology, Tongji Medical College, Huazhong University of Science and Technology) for his help with the experiments.
Funding sources
This work was supported by the National Natural Science Foundation of China [Grant numbers 81701469 and 81502461]; the Huazhong University of Science and Technology “Double Top” Construction Project of International Cooperation [Grant number 540-5001540013]; the Natural Science Foundation of Hubei Province [Grant numbers 2016CFB340 and 2015CFB179]; the Key Project of Wuhan Health and Family Planning Commission [Grant number WX15A08]; the Young and Mid-Aged Key Medical Personnel Training Project of Wuhan City; and the Young Key Talent Project of Wuhan Fourth Hospital.
Author contributions
D.Z and J.F. conceived the project. R.S., Y.X., H.L., C.G., and J.W. performed the experiments. R.S., Y.X., H.L., L.S., X.Y. D.Z., and J.F. analyzed the results. R.S., L.S., X.Y., D.Z. and J.F. wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2020.100825.
Contributor Information
Dongxin Zhang, Email: dxzhang@hust.edu.cn.
Jueping Feng, Email: fengjuepingpuai@163.com.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Gregory A.D., Houghton A.M. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 2.Scapini P., Lapinet-Vera J.A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 3.Beauvillain C., Delneste Y., Scotet M., Peres A., Gascan H., Guermonprez P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 4.Harwood N.E., Barral P., Batista F.D. Neutrophils—the unexpected helpers of B-cell activation. EMBO Rep. 2012;13:93–94. doi: 10.1038/embor.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C.Y., Tai J.A., Li S., Nishikawa T., Kaneda Y. Virus-stimulated neutrophils in the tumor microenvironment enhance T cell-mediated anti-tumor immunity. Oncotarget. 2016;7:42195–42207. doi: 10.18632/oncotarget.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridlender Z.G., Albelda S.M. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 8.Yan B., Wei J.J., Yuan Y., Sun R., Li D., Luo J. IL-6 cooperates with G-CSF to induce protumor function of neutrophils in bone marrow by enhancing STAT3 activation. J. Immunol. 2013;190:5882–5893. doi: 10.4049/jimmunol.1201881. [DOI] [PubMed] [Google Scholar]
- 9.Sun R., Luo J., Li D., Shu Y., Luo C., Wang S.S. Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal NK cells. Oncotarget. 2014;5:12621–12634. doi: 10.18632/oncotarget.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 11.Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 12.Lanier L.L. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raulet D.H. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 14.Smyth M.J., Swann J., Cretney E., Zerafa N., Yokoyama W.M., Hayakawa Y. NKG2D function protects the host from tumor initiation. J. Exp. Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diefenbach A., Jamieson A.M., Liu S.D., Shastri N., Raulet D.H. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 16.Sporri R., Joller N., Hilbi H., Oxenius A. A novel role for neutrophils as critical activators of NK cells. J. Immunol. 2008;181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 17.Su Y.C., Li S.C., Hsu C.K., Yu C.C., Lin T.J., Lee C.Y. G-CSF downregulates natural killer cell-mediated cytotoxicity in donors for hematopoietic SCT. Bone Marrow Transplant. 2012;47:73–81. doi: 10.1038/bmt.2011.22. [DOI] [PubMed] [Google Scholar]
- 18.van Gisbergen K.P., Sanchez-Hernandez M., Geijtenbeek T.B., van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva M.T. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 20.Costantini C., Calzetti F., Perbellini O., Micheletti A., Scarponi C., Lonardi S. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFNγ: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–1686. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel A., Brooks M.W., Houshyar S., Reinhardt F., Ardolino M., Fessler E. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terme M., Ullrich E., Aymeric L., Meinhardt K., Desbois M., Delahaye N. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 23.Greenwald R.J., Latchman Y.E., Sharpe A.H. Negative co-receptors on lymphocytes. Curr. Opin. Immunol. 2002;14:391–396. doi: 10.1016/s0952-7915(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu C., Yu S., Kappes J., Wang J., Grizzle W.E., Zinn K.R. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng H., Zhang G.M., Li D., Zhang H., Yuan Y., Zhu H.G. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J. Immunol. 2006;176:1411–1420. doi: 10.4049/jimmunol.176.3.1411. [DOI] [PubMed] [Google Scholar]
- 26.Bernardini G., Sciume G., Bosisio D., Morrone S., Sozzani S., Santoni A. CCL3 and CXCL12 regulate trafficking of mouse bone marrow NK cell subsets. Blood. 2008;111:3626–3634. doi: 10.1182/blood-2007-08-106203. [DOI] [PubMed] [Google Scholar]
- 27.Gregoire C., Chasson L., Luci C., Tomasello E., Geissmann F., Vivier E. The trafficking of natural killer cells. Immunol. Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taieb J., Chaput N., Menard C., Apetoh L., Ullrich E., Bonmort M. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 29.Park I.H., Yang H.N., Lee K.J., Kim T.S., Lee E.S., Jung S.Y. Tumor-derived IL-18 induces PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer. Oncotarget. 2017;8:32722–32730. doi: 10.18632/oncotarget.16281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houghton A.M. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9:1732–1737. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- 31.Michael M., Goldstein D., Clarke S.J., Milner A.D., Beale P., Friedlander M. Prognostic factors predictive of response and survival to a modified FOLFOX regimen: importance of an increased neutrophil count. Clin. Colorectal Cancer. 2006;6:297–304. doi: 10.3816/CCC.2006.n.048. [DOI] [PubMed] [Google Scholar]
- 32.Orange J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda R., Narumi K., Hashimoto H., Miyakawa R., Okusaka T., Aoki K. Interaction of natural killer cells with neutrophils exerts a significant antitumor immunity in hematopoietic stem cell transplantation recipients. Cancer Med. 2016;5:49–60. doi: 10.1002/cam4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaeger B.N., Donadieu J., Cognet C., Bernat C., Ordonez-Rueda D., Barlogis V. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J. Exp. Med. 2012;209:565–580. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon S., Labarriere N. PD-1 expression on tumor-specific T cells: friend or foe for immunotherapy? Oncoimmunology. 2017;7 doi: 10.1080/2162402X.2017.1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He G., Zhang H., Zhou J., Wang B., Chen Y., Kong Y. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015;34:141. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benson D.M., Jr., Bakan C.E., Mishra A., Hofmeister C.C., Efebera Y., Becknell B. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nausch N., Galani I.E., Schlecker E., Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aliper A.M., Frieden-Korovkina V.P., Buzdin A., Roumiantsev S.A., Zhavoronkov A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014;3:737–746. doi: 10.1002/cam4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callahan M.K., Postow M.A., Wolchok J.D. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44:1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Della C.M., Pesce S., Muccio L., Carlomagno S., Sivori S., Moretta A. Features of memory-like and PD-1(+) human NK cell subsets. Front. Immunol. 2016;7:351. doi: 10.3389/fimmu.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaix J., Tessmer M.S., Hoebe K., Fuseri N., Ryffel B., Dalod M. Cutting edge: priming of NK cells by IL-18. J. Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures