Abstract
Chickens adapt to P and Ca restriction during the very first days of life by improving P utilisation efficiency. The present study was built to identify the mechanisms underlying this adaptive capacity, and to identify the optimal window of application of the restriction (depletion). A total of 1600 Cobb 500TM male broilers were used. During each phase (from age 0 to 4 d, 5 to 8 d, 9 to 18 d and 19 to 33 d), the animals received either a control diet (H) or a restricted diet (L) with reduced levels of non-phytate P (nPP) and Ca (between −14 and −25 % for both) with four dietary sequences: HHHH, HLHL, LHHL and LLHL. None of the feeding strategies affected growth. Tibia ash content at day 4 and 8 was impaired when the L diet was fed from 0 to 4 and 5 to 8 d, respectively (P = 0⋅038 and P = 0⋅005). Whatever the early restriction period or length between 0 and 8 d of age, the mineralisation delay was compensated by day 18. This was accompanied by an increased mRNA expression of the Ca transporter, CALB1, and an increased apparent ileal digestibility of Ca at day 8 (P < 0⋅001). This adaptation was limited to the starter phase in restricted birds. No effect was seen on P transporters mRNA or protein expression. In conclusion, birds adapted to mineral restriction by increasing Ca and nPP utilisation efficiencies. Depletion−repletion strategies are promising in improving the sustainability of broiler production but need to be validated in phytase-supplemented diets.
Key words: Broiler chickens, Feeding strategies, Calcium, Phosphorus, Adaptation
Abbreviations: AID, apparent ileal digestibility; BW, body weight; H, high non-phytate P and Ca; HU, Hounsfield units; L, low non-phytate P and Ca; nPP, non-phytate P; PPdisp, disappearance of phytate P
Nutritional strategies for improving the efficiency of P use by birds could be implemented to reduce P excretion and avoid P overfeeding. The reduction of dietary P level is possible if the Ca level is simultaneously decreased(1) due to lower propensity for Ca–phosphate and Ca–phytate complexes in the digestive tract. This strategy is particularly interesting in the finisher phase where feed intake and consequently P excretion are high. In addition, broilers fed diets deficient in Ca and non-phytate P (nPP) during an early phase of growth (depletion) exhibit better P and Ca utilisation and are able to compensate for bone mineralisation(1,2). A more complete understanding of the mechanisms involved in this adaptation is required to efficiently use this kind of strategy.
Intestinal enterocytes are constantly exposed to fluctuations in Ca and/or P availability. These fluctuations have been proven to affect gene and protein expression and the activity of transporters through a mechanism driven by parathormone and vitamin D3(3). At low nPP and Ca intake, birds have been shown to adapt by increasing mRNA expression of Ca transporters: CALB1(4), ATP2B1(5,6) and SLC8A1(6) and P transporters: SLC34A2(7,8) and SLC20A1(9) in the small intestine. So far, the majority of these studies have focused on the mRNA expression of no more than three genes at a time, focusing mainly on either Ca or P transporters. Moreover, to our knowledge, only a few studies have measured the impact of these diets on the protein expression of P(9,10) and Ca(4,6) transporters in broilers.
Ashwell & Angel(11) observed that birds fed a deficient diet during the first 90 h of life improved P and Ca apparent absorption compared with control birds. This adaptation was apparent from 90 h and remained even if the birds were fed an adequate diet from 90 h to 38 d of age. It was linked to an increase in SLC34A2 mRNA expression. Thus, those authors made the hypothesis that an imprinting of the birds was possible, involving a long-term adaptation in gene expression. A recent study (MM Van Krimpen, E Willems and HJ Van Harn, unpublished results) failed to observe such a long-term adaptation in birds fed a deficient Ca and nPP diet from 0 to 4 d of age, which showed decreased P and Ca pre-caecal digestibility at day 37, without any variation in the mRNA expression of CALB1, ATP2B1 and SLC34A2. These conflicting results suggest a lack of knowledge about the degree of dietary nPP and Ca reduction, the timing and/or the duration of the depletion required to induce a long-term adaptation in the birds.
The first objective of this experiment was to identify the optimal depletion−repletion strategies. Several conditions (duration and timing) were tested during the depletion phase with the hypothesis that the birds could undergo a long-lasting adaptation with an improved efficiency in mineral utilisation while maintaining growth and bone mineral status. The second objective was to identify and understand the mechanisms related to the adaptation with the hypothesis that it could involve modifications in Ca and P duodenal transporter mRNA and/or protein expression.
Materials and methods
The experiment was conducted under the guidelines of the French Ministry of Agriculture for Animals (Paris, France, authorisation 1153-2015071514404178) at PEAT INRA Poultry Experimental Facility (2018, https://doi.org/10.15454/1.5572326250887292E12).
Experimental diets
The experimental period was divided into four phases: from 0 to 4, 5 to 8, 9 to 18, and 19 to 33 d of age. All experimental diets were based on maize, soyabean meal and wheat. During each phase, the birds received either a high (H) or a low (L) diet (Table 1). The Ca and nPP levels in the H diets were in accordance with the breeder recommendations(12) between 0 and 4 d of age and were at the levels used in commercial conditions for the other phases. The L diets were designed to contain between 14 and 25 % lower Ca and nPP levels compared with the H diet, depending on the phase. For the first three phases, the Ca content was, respectively, 1⋅00, 0⋅92 and 0⋅77 % in the H diets and 0⋅77, 0⋅70 and 0⋅66 % in the L diets. Likewise, nPP contents were, respectively, 0⋅45, 0⋅45 and 0⋅35 % in the H diets and 0⋅35, 0⋅32 and 0⋅30 % in the L diets. The last phase was divided into two dietary periods: 19 to 28 d of age and 29 to 33 d of age. The Ca and nPP contents in the H diet were, respectively, 0⋅64 and 0⋅29 % from 19 to 28 d of age and then 0⋅55 and 0⋅25 % from 29 to 33 d of age. The Ca and nPP contents in the L diet were, respectively, 0⋅53 and 0⋅24 % from 19 to 28 d of age and then 0⋅44 and 0⋅20 % from 29 to 33 d of age. Diets in each phase were combined to form four dietary sequences: (1) HHHH; (2) HLHL; (3) LHHL; and (4) LLHL (see Table 2). The first treatment was the control. Treatments 2, 3 and 4 were designed to study the timing and duration of the first depletion (from 0 to 8 or 0 to 4 or 5 to 8 d of age). During the finisher phase, feed intake and P excretion are at their highest levels. Therefore, the P and Ca levels were reduced during this period based on a previous study indicating the possibility of a complete recovery of the birds at the bone level(1). Calcium carbonate (CaCO3) and dicalcium phosphate (CaHPO4) were added to the diets to adjust the Ca and nPP content. Titanium dioxide (TiO2; 0⋅3 %) was added to the diets from 5 to 18 d of age and from 28 to 33 d of age as an indigestible marker to enable determination of P and Ca apparent ileal digestibility (AID). Diets and water were distributed ad libitum. Diets were distributed as medium crumbs from 0 to 4 d of age and as medium pellets after day 5.
Table 1.
Composition and chemical analysis of the experimental diets (as-fed basis; g/kg unless otherwise stated)
| Dietary treatments… | 0 to 4 d of age | 5 to 8 d of age | 9 to 18 d of age | 19 to 28 d of age | 29 to 33 d of age | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | L | H | L | H | L | H | L | H | L | |
| Ingredients | ||||||||||
| Maize | 428⋅0 | 442⋅0 | 387⋅9 | 402⋅3 | 436⋅3 | 443⋅5 | 470⋅0 | 477⋅1 | 494⋅9 | 502⋅0 |
| Soyabean meal | 368⋅0 | 366⋅0 | 400⋅1 | 398⋅0 | 358⋅2 | 357⋅1 | 330⋅5 | 329⋅5 | 303⋅4 | 302⋅3 |
| Wheat | 150⋅0 | 150⋅0 | 150⋅0 | 150⋅0 | 150⋅0 | 150⋅0 | 150⋅0 | 150⋅0 | 150⋅0 | 150⋅0 |
| Soya oil | 16⋅00 | 11⋅50 | 24⋅60 | 19⋅90 | 22⋅60 | 20⋅30 | 23⋅80 | 21⋅33 | 25⋅60 | 23⋅30 |
| Dicalcium phosphate | 17⋅80 | 12⋅20 | 15⋅50 | 9⋅94 | 12⋅10 | 9⋅28 | 9⋅00 | 6⋅24 | 7⋅10 | 4⋅32 |
| Calcium carbonate | 3⋅70 | 1⋅70 | 6⋅73 | 4⋅68 | 5⋅40 | 4⋅44 | 4⋅25 | 3⋅35 | 3⋅50 | 2⋅54 |
| Cellulose | 4⋅00 | 4⋅00 | 5⋅00 | 5⋅00 | 5⋅00 | 5⋅00 | 5⋅00 | 5⋅00 | 5⋅00 | 5⋅00 |
| Vitamin and mineral premix* | 3⋅10 | 3⋅10 | 2⋅77 | 2⋅75 | 2⋅67 | 2⋅66 | 2⋅62 | 2⋅61 | 2⋅56 | 2⋅56 |
| Sodium chloride | 2⋅60 | 2⋅60 | 2⋅42 | 2⋅40 | 2⋅23 | 2⋅21 | 2⋅06 | 2⋅06 | 1⋅91 | 1⋅90 |
| dl-Met | 0⋅70 | 0⋅80 | 0⋅75 | 0⋅78 | 1⋅05 | 1⋅06 | 1⋅19 | 1⋅21 | 1⋅33 | 1⋅36 |
| Lys HCl | 0⋅50 | 0⋅50 | 0⋅20 | 0⋅19 | 0⋅23 | 0⋅23 | 0⋅28 | 0⋅28 | 0⋅31 | 0⋅32 |
| l-Threonine | 0⋅60 | 0⋅60 | 0⋅83 | 0⋅86 | 1⋅02 | 1⋅02 | 1⋅10 | 1⋅12 | 1⋅19 | 1⋅20 |
| Anticoccidial | 5⋅00 | 5⋅00 | 0⋅20 | 0⋅20 | 0⋅20 | 0⋅20 | 0⋅20 | 0⋅20 | 0⋅20 | 0⋅20 |
| Titanium dioxide (0⋅05%) | 0⋅00 | 0⋅00 | 3⋅00 | 3⋅00 | 3⋅00 | 3⋅00 | 0⋅00 | 0⋅00 | 3⋅00 | 3⋅00 |
| Calculated (analysed) content† | ||||||||||
| Metabolisable energy (MJ/kg) | 12⋅1 | 12⋅2 | 12⋅1 | 12⋅1 | 12⋅4 | 12⋅4 | 12⋅6 | 12⋅6 | 12⋅8 | 12⋅8 |
| Crude protein (%) | 21⋅5 (22⋅1) | 21⋅5 (22⋅0) | 21⋅5 (21⋅2) | 21⋅5 (22⋅5) | 20⋅0 (20⋅8) | 20⋅0 (21⋅2) | 19⋅0 (19⋅8) | 19⋅0 (19⋅9) | 18⋅0 (18⋅6) | 18⋅0 (18⋅7) |
| Ca | 10⋅0 (9⋅1) | 7⋅7 (7⋅9) | 9⋅2 (10⋅1) | 7⋅0 (7⋅4) | 7⋅7 (7⋅2) | 6⋅6 (6⋅3) | 6⋅4 (6⋅3) | 5⋅3 (5⋅3) | 5⋅5 (5⋅2) | 4⋅4 (4⋅1) |
| Total PP | 7⋅0 (6⋅5) | 6⋅0 (5⋅9) | 6⋅7 (6⋅7) | 5⋅7 (5⋅6) | 5⋅9 (5⋅8) | 5⋅4 (5⋅3) | 5⋅3 (5⋅4) | 4⋅8 (4⋅8) | 4⋅8 (4⋅5) | 4⋅3 (3⋅9) |
| nP | 4⋅6 | 3⋅6 | 4⋅2 | 3⋅2 | 3⋅5 | 3⋅0 | 2⋅9 | 2⋅4 | 2⋅5 | 2⋅0 |
| Ca:nPP | 2⋅2 | 2⋅1 | 2⋅2 | 2⋅2 | 2⋅2 | 2⋅2 | 2⋅2 | 2⋅2 | 2⋅2 | 2⋅2 |
H, high P and Ca; L, low P and Ca; nPP, non-phytate P.
Premix, provided per kg of diet: 15 000⋅00 IU vitamin A; 4300⋅00 IU cholecalciferol; 100⋅00 IU dl-α-tocopherol; 5⋅00 mg menadione; 5⋅00 mg thiamine; 8⋅00 mg riboflavin; 100⋅00 mg niacin; 25⋅00 mg calcium pantothenate; 7⋅00 mg pyridoxine; 0⋅30 μg biotin; 3⋅00 mg folic acid; 0⋅03 μg cyanocobalamin; 50⋅00 mg Fe; 20⋅00 mg Cu; 80⋅00 mg Mn; 90⋅00 mg Zn; 2⋅00 mg iodine, 0⋅20 mg Se, 550⋅00 mg choline chloride.
Calculated from the National Research Council(34). Analysed values in parentheses.
Table 2.
Experimental design
| Treatment name | Diets received per phase | |||
|---|---|---|---|---|
| 0 to 4 d | 5 to 8 d | 9 to 18 d | 19 to 33 d | |
| HHHH | H | H | H | H |
| HLHL | H | L | H | L |
| LHHL | L | H | H | L |
| LLHL | L | L | H | L |
H, high non-phytate P and Ca; L, low non-phytate P and Ca.
Birds and management
A total of 1600 1-d-old male broilers (Cobb 500TM) were raised from 0 to 35 d of age. At their arrival, broilers were weighed and randomly allocated to thirty-two pens (eight pens per treatment). The study consisted of a randomised block design with eight blocks of four pens each (3 m2 per pen). In each block, pens were randomly allocated to a treatment (400 birds per treatment). The room temperature was settled at 33°C upon arrival and decreased progressively to reach 20°C at day 35. Birds were kept under 24 h light on day 1, 23 h from day 2 to day 4 and 18 h thereafter. The broilers were weighed individually at days 0, 8, 18 and 33 (mean weight 2356⋅1 (sd 230⋅2) g). Feed intake per pen was recorded for each phase. Pre-caecal digesta samples from the distal half of the ileum (defined as extending from Meckel's diverticulum to the ileo-caecal junction) were collected from eight birds per pen (eight pens per experimental diet) at days 8, 18 and 33 after pentobarbital injection. Digesta samples were pooled in order to achieve eight pools of eight birds for each treatment. Out of those sixty-four broilers per treatment, twelve broilers per treatment were used to collect intestinal tissue samples immediately after death. The duodenum was isolated and thoroughly washed in ice-cold PBS solution. A 5-cm medial sample was snap-frozen and scraped into liquid N2 before storage at −80°C. The right tibia was collected from twelve birds per treatment at day 4, day 8, day 18 and day 33. At day 33, ten additional birds per treatment were euthanised and stored at −20°C until scanning. The litter from four pens per treatment was weighed at the beginning and the end of the experiment. On the last day of the experiment, a 1⋅5 kg sample was taken after mixing the whole litter to estimate P excretion.
Computed tomography scans
The carcasses at day 33 were scanned using a clinical computed tomography machine (Somatom Definition AS128; Siemens) with scan parameters set at 140 kV tube voltage and 500 mAs current. The image acquisition mode was a matrix size of 32 cm and 512 px and a resolution of 650 μm. The images were converted into DICOM (Digital Imaging and Communications in Medicine) format for analysis using SINGO.VIA (Siemens) software. Bone mineral density was estimated on the right tibia. The bone mineral density was given in Hounsfield units (HU) by the scanner software and later converted to g/cm3 using a phantom (Electron Density Phantom (Model 062 M); Meditest). Total bone was defined in the intervals 200−3000 HU according to Militist et al.(13). Trabecular and cortical bones were defined in the intervals, respectively, of 200−800 HU and 801−3000 HU, according to Sherlock et al.(14).
Chemical and mechanical analysis
Litter and ileal digesta samples were freeze-dried. DM was determined for right tibias (103°C for 12 h) and diets (103°C for 4 h). Samples of the diets, ileal contents, litters and tibias were ashed at 550°C for 12 h. For mineral determination, 0⋅5 g of ashes were solubilised in 6 ml HNO3 and 4 ml of H2SO4 and treated by microwave (room temperature to 210°C for 20 min and at 210°C for 10 min; ETHOSUP, Milestone) and then diluted in 50 ml of water. Ca, P and Ti contents were analysed using an Inductive Coupled Plasma Optical Emission Spectrometer (ICP-OES, iCaP 7000 Series, ThermoScientific; method 990.08; AOAC International, 2006).
To further refine the effects on the pre-caecal digestibility of P when significant, phytate was measured in ileal contents using a commercial assay kit (Megazyme International) and the pre-caecal disappearance of phytate P (PPdisp) determined.
RNA isolation and RT-PCR assay
Total RNA isolation and real-time RT-PCR assay were performed as described previously in Rousseau et al.(1). Concentration and quality of the extracted RNA were assessed by spectrophotometry from 230 to 280 nm, using a Nanodrop 1000 spectrophotometer (Nanodrop Technology). The ratios 260:280 and 260:230 were between 1⋅8 and 2⋅2. The integrity of RNA was assessed by the migration of total RNA on a 1⋅5 % agarose gel. The primers used targeted the following genes (Table 3): P transporters: solute carrier family 20 (phosphate transporter), member 1 (Gallus gallus (chicken)) (SLC20A1), solute carrier family 34 (Na/phosphate), member 2 (Gallus gallus (chicken)) (SLC34A2); Ca transporters: calbindin 1, 28 kDa (Gallus gallus (chicken)) (CALB1), ATPase, Ca2+ transporting, plasma membrane 1 (Gallus gallus (chicken)) (ATP2B1), solute carrier family 8 (Na/Ca exchanger), member 1 (Gallus gallus (chicken)) (SLC8A1). The primers used to study the expression of ATP2B1 and SLC8A1 have been previously described in Jonchere et al.(15). The other primers were specifically designed using Primer BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Amplification products were checked by electrophoresis and further sequenced prior to real-time quantitative PCR analysis.
Table 3.
Primers used for quantitative RT-PCR
| Name | Gene symbol | Genbank accession no. | Forward primer sequence (5′→3′) | Reverse primer sequence (5′→3′) | Product size (bp) |
|---|---|---|---|---|---|
| Na/phosphate co-transporter 2b | SLC34A2 | NM_204474 | GTCCGTTCACTCTGTTGCCT | TGGGTCCTCTTCTTGCCTTG | 242 |
| Na-dependent phosphate transporter 1 | SLC20A1 | XM_003642557 | AGGGCAGAAAGGCGTCAA | CGAGGAAGAAGAGAACAGCAGA | 104 |
| Calbindin 1, 28 kDa | CALB1 | NM_205513 | CAGGGTGTCAAAATGTGTGC | GCCAGTTCTGCTCGGTAAAG | 215 |
| Plasma membrane Ca-transporting ATPase 1 | ATP2B1 | XM_416133 | CTGCACTGAAGAAAGCAGATGTTG | GCTGTCATATACGTTTCGTCCCC | 130 |
| Na/Ca exchanger 1 | SLC8A1 | NM_001079473 | GGATTGTGGAGGTTTGGGAAGG | CTGTTTGCCAGCTCGGTATTTC | 137 |
Western-blot analysis
Duodenal lysates were prepared as described in Coudert et al.(16). Samples were denatured at 75°C for 10 min before loading (80 μg of protein) for migration on 10 % SDS/PAGE gels and then transferred to nitrocellulose membranes. Membranes were blocked in 5 % fat-free milk/PBS, 0⋅1 % Tween for 1 h at room temperature. Membranes were incubated with antibodies against SLC20A1 (ab177147; Abcam®) 1:5000 for 3 h and against CALB1 (ab25085; Abcam®) 1:500 overnight. Membranes were then washed several times and incubated for 1 h with goat anti-rabbit secondary antibodies (A21076, AlexaFluor®; Life Technologies). After stripping, membranes were incubated with anti-vinculin antibody (V9131; Sigma) 1:40 000 for 3 h and then incubated with rabbit anti-mouse secondary antibodies (A21065, AlexaFluor®; Life Technologies). Bands were visualised by IR fluorescence using an Odyssey® Imaging System (LI-COR Inc. Biotechnology) and quantified by Odyssey IR imaging system software (Application software, version 1.2).
Calculations and statistical analyses
The P retained in g/kg body weight (BW) produced was calculated per pen as the sum of P ingested in all phases in g/kg BW produced minus the P excreted in the litter in g/kg BW produced. The P retention efficiency was calculated per pen as the P retained divided by P intake in g/kg BW produced per pen. The AID and PPdisp were determined using the following equation: AID or PPdisp (%) = 100 – (100 × (TiDiet × XDigesta)/(TiDigesta × XDiet)) where TiDiet is the Ti concentration of the diet, XDigesta the P, Ca or PP concentration of the ileal digesta, TiDigesta the Ti concentration of the ileal digesta, and XDiet the P, Ca or PP concentration of the diet. Determination of the sample size for the study population was based on a power calculation using the estimated mean and standard deviation (35⋅6 (sd 1⋅3) %) for tibia ash content from a previous broiler study(1). Consequently, it was estimated that in order to detect a change in tibia ash content of 4 % at a significance level of 0⋅05, with a power of 90 %, twelve animals per group should be used. A randomised block design with eight blocks was used to test the effect of dietary treatments on growth performance with the pen as the experimental unit for all criteria, except for bone mineralisation where the bird was the experimental unit. Dietary treatment was included in the model as a fixed effect and the block as a random effect. ANOVA were performed on the studied variables for each of the feeding phases using the MIXED procedure of SAS (version 9.2, 2002; SAS Institute Inc.(17)) after the normality of the variables was checked; multiple mean comparisons were done using Tukey's correction. Litter scores were analysed using χ2 analysis in SAS. Differences were considered significant when P < 0⋅05 and P < 0⋅10 indicated a statistical trend.
Results
Bone mineral status
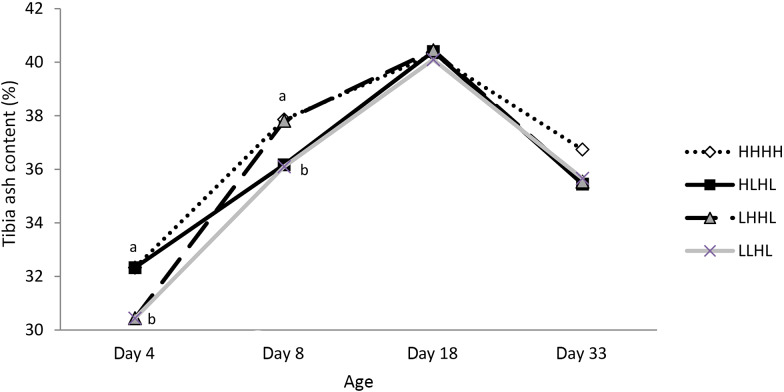
At day 4, tibia ash content decreased with the reduction of P and Ca in the diet (−6 %, P = 0⋅04; Fig. 1). At day 8, birds receiving the L diet between 5 and 8 d of age (HL and LL treatments) had a lower tibia ash content compared with control birds with the HH treatment (respectively, −4⋅46 and −4⋅65 %, P = 0⋅005). Birds receiving LH returned to the level of HH at day 8. At day 18 and day 33, no difference in tibia ash content was observed between the experimental groups.
Fig. 1.
Effect of calcium and non-phytate phosphorus (nPP) diet content on tibia ash content at day 4 (H v. L diets from 0 to 4 d), day 8 (HH and LH v. HL and LL diets from 4 to 8 d), day 18 (HHH, LHH, HLH and LLH diets from 8 to 18 d) and day 33 (HHHH, LHHL, HLHL and LLHL diets from 18 to 33 d). Values are means (n 12). a,b Least-square mean values within a sampling day with unlike letters were significantly different (P < 0⋅05). H, diet high in nPP and calcium; L, diet low in nPP and calcium.
At day 33, the tibia bone mineral density was lower with the LLHL treatment compared with the control treatment (P = 0⋅045; Table 4). Tibia cortical bone mineral density also tended to be reduced with LLHL treatment compared with HLLL treatment (P = 0⋅081). Otherwise, the different depletion−repletion strategies did not affect bone mineral criteria compared with control birds.
Table 4.
Effect of calcium and non-phytate phosphorus (nPP) on tibia mineralisation at day 33
(Least-square mean values and pooled standard errors)
| Dietary treatments*… | HHHH | HLHL | LHHL | LLHL | sem | P |
|---|---|---|---|---|---|---|
| n | 10 | 10 | 10 | 10 | ||
| Total bone BMD (g/cm3) | 1⋅311a | 1⋅298a,b | 1⋅304a,b | 1⋅297b | 0⋅010 | 0⋅045 |
| Trabecular bone BMD (g/cm3) | 1⋅225 | 1⋅223 | 1⋅225 | 1⋅225 | 0⋅003 | 0⋅640 |
| Cortical bone BMD (g/cm3) | 1⋅642 | 1⋅623 | 1⋅644 | 1⋅613 | 0⋅020 | 0⋅081 |
H, high nPP and Ca; L, low nPP and Ca; BMD, bone mineral density.
a,b Least-square mean values within a row with unlike superscript letters were significantly different (P < 0⋅05).
* See Table 2.
Digestibility of phosphorus and calcium and mRNA and protein expression of their transporters
At day 8, the AID of P and Ca as well as PPdisp were higher for birds receiving the L diet between 5 and 8 d of age (HL and LL compared with HH and LH, P ≤ 0⋅001, P ≤ 0⋅001 and P = 0⋅002, respectively; Table 5). In parallel, there was a higher mRNA expression of CALB1 in birds with the LL diet compared with birds receiving HH or LH diets (P ≤ 0⋅001). No effect was observed on the protein expression of P transporters or Ca transporters (P > 0⋅05). At days 18 and 33, diets did not affect P AID or mRNA and protein expression of transporters (Tables 6 and 7). At days 18 and 33, Ca AID was not affected by treatments (P > 0⋅05).
Table 5.
Effect of calcium and non-phytate phosphorus (nPP) on the apparent ileal digestibility (AID) of calcium and phosphorus, the ileal disappearance of phytate phosphorus (PPdisp) and normalised relative abundance of transporter mRNA at day 8
(Least-square mean values and pooled standard errors)
| Dietary treatments*… | HH | HL | LH | LL | sem | P |
|---|---|---|---|---|---|---|
| n | 8 | 8 | 16 | 16 | ||
| Ca AID (%) | 22⋅72b | 46⋅78a | 22⋅07b | 49⋅64a | 1⋅69 | <0⋅001 |
| P AID (%) | 48⋅34b | 55⋅68a | 47⋅16b | 56⋅57a | 1⋅08 | <0⋅001 |
| PPdisp (%) | 23⋅07b | 31⋅84a | 19⋅35b | 33⋅19a | 2⋅79 | 0⋅002 |
| mRNA expression | ||||||
| n | 12 | 12 | 12 | 12 | ||
| Calb1 | 0⋅76b | 1⋅06a,b | 0⋅92b | 1⋅34a | 0⋅09 | <0⋅001 |
| ATP2B1 | 0⋅96 | 1⋅12 | 0⋅93 | 1⋅11 | 0⋅09 | 0⋅248 |
| SLC8A1 | 0⋅84 | 1⋅26 | 0⋅85 | 1⋅45 | 0⋅28 | 0⋅321 |
| SLC34A2 | 0⋅91 | 0⋅96 | 0⋅96 | 1⋅19 | 0⋅10 | 0⋅213 |
| SLC20A1 | 1⋅26 | 1⋅26 | 1⋅60 | 1⋅37 | 0⋅15 | 0⋅327 |
| Protein expression | ||||||
| n | 6 | 6 | 6 | 6 | ||
| Calb1 | 1⋅31 | 1⋅32 | 0⋅70 | 0⋅79 | 0⋅86 | 0⋅453 |
| SLC20A1 | 2⋅23 | 0⋅82 | 1⋅37 | 0⋅79 | 2⋅08 | 0⋅081 |
H, high nPP and Ca; L, low nPP and Ca.
a,b Least-square mean values within a row with unlike superscript letters were significantly different (P < 0⋅05).
* See Table 2.
Table 6.
Effect of calcium and non-phytate phosphorus (nPP) on the apparent ileal digestibility (AID) of calcium and phosphorus and normalised relative abundance of transporter mRNA at day 18
(Least-square mean values and pooled standard errors)
| Dietary treatments*… | HHH | HLH | LHH | LLH | sem | P |
|---|---|---|---|---|---|---|
| n | 8 | 8 | 8 | 16 | ||
| Ca AID (%) | 35⋅05 | 34⋅77 | 34⋅81 | 37⋅36 | 4⋅94 | 0⋅804 |
| P AID (%) | 51⋅62 | 51⋅75 | 50⋅49 | 53⋅81 | 3⋅61 | 0⋅431 |
| mRNA expression | ||||||
| n | 12 | 12 | 12 | 12 | ||
| Calb1 | 0⋅95 | 0⋅91 | 1⋅00 | 0⋅99 | 0⋅29 | 0⋅440 |
| ATP2B1 | 1⋅17 | 1⋅03 | 1⋅06 | 1⋅17 | 0⋅35 | 0⋅657 |
| SLC8A1 | 0⋅25 | 0⋅23 | 0⋅15 | 0⋅16 | 0⋅15 | 0⋅559 |
| SLC34A2 | 0⋅94 | 0⋅84 | 0⋅97 | 1⋅01 | 0⋅23 | 0⋅392 |
| SLC20A1 | 0⋅29 | 0⋅32 | 0⋅19 | 0⋅24 | 0⋅18 | 0⋅346 |
| Protein expression | ||||||
| n | 6 | 6 | 6 | 6 | ||
| Calb1 | 0⋅52 | 0⋅86 | 0⋅65 | 0⋅73 | 0⋅81 | 0⋅492 |
| SLC20A1 | 1⋅10 | 0⋅75 | 0⋅85 | 1⋅11 | 0⋅54 | 0⋅743 |
H, high nPP and Ca; L, low nPP and Ca.
* See Table 2.
Table 7.
Effect of calcium and non-phytate phosphorus (nPP) on the apparent ileal digestibility (AID) of calcium and phosphorus and normalised relative abundance of transporter mRNA and protein at day 33
(Least-square mean values and pooled standard errors)
| Dietary treatments*… | HHHH | HLHL | LHHL | LLHL | sem | P |
|---|---|---|---|---|---|---|
| n | 8 | 8 | 8 | 8 | ||
| Ca AID (%) | 23⋅84 | 24⋅19 | 17⋅71 | 19⋅37 | 8⋅32 | 0⋅347 |
| P AID (%) | 39⋅27 | 39⋅74 | 37⋅11 | 38⋅54 | 7⋅62 | 0⋅918 |
| mRNA expression | ||||||
| n | 12 | 12 | 12 | 12 | ||
| Calb1 | 1⋅28 | 1⋅42 | 1⋅48 | 1⋅49 | 0⋅40 | 0⋅496 |
| ATP2B1 | 0⋅89 | 1⋅14 | 1⋅63 | 1⋅21 | 0⋅58 | 0⋅553 |
| SLC8A1 | 0⋅43 | 0⋅42 | 0⋅79 | 0⋅54 | 0⋅23 | 0⋅398 |
| SLC34A2 | 0⋅51 | 0⋅43 | 0⋅65 | 0⋅67 | 0⋅38 | 0⋅188 |
| SLC20A1 | 0⋅93 | 0⋅61 | 0⋅91 | 0⋅61 | 0⋅25 | 0⋅072 |
| Protein expression | ||||||
| n | 6 | 6 | 6 | 6 | ||
| Calb1 | 1⋅07 | 0⋅85 | 1⋅03 | 1⋅22 | 0⋅73 | 0⋅451 |
| SLC20A1 | 0⋅70 | 0⋅92 | 1⋅46 | 1⋅35 | 0⋅82 | 0⋅373 |
H, high nPP and Ca; L, low nPP and Ca.
* See Table 2.
Growth performance
Diets did not affect BW, average daily gain, average daily feed intake or mortality (Table 8). Pododermatitis scores (results not shown) were also not different among treatments. Retention efficiency of P was higher with the depletion–repletion strategies compared with control diets (P = 0⋅002), which resulted in lower litter P contents (P = 0⋅008; Table 8).
Table 8.
Effect of calcium and non-phytate phosphorus (nPP) on growth performance of chickens from 0 to 33 d of age and litter quality and phosphorus retention efficiency at day 33
(Least-square mean values and pooled standard errors)
| Dietary treatments*… | HHHH | HLHL | LHHL | LLHL | sem | P |
|---|---|---|---|---|---|---|
| n | 8 | 8 | 8 | 8 | ||
| ADG (g/d) | 64⋅56 | 63⋅99 | 65⋅65 | 65⋅45 | 1⋅91 | 0⋅420 |
| ADFI (g/d) | 92⋅90 | 92⋅99 | 95⋅05 | 94⋅59 | 2⋅69 | 0⋅344 |
| FCR | 1⋅461 | 1⋅471 | 1⋅465 | 1⋅456 | 0⋅015 | 0⋅261 |
| Mortality (%) | 6⋅89 | 7⋅48 | 5⋅27 | 5⋅22 | 3⋅94 | 0⋅587 |
| n | 4 | 4 | 4 | 4 | ||
| Litter DM day 33 (%) | 48⋅09a,b | 42⋅49a | 51⋅41b | 48⋅01a,b | 4⋅14 | 0⋅035 |
| Litter P content (mg/g) | 10⋅19a | 9⋅00b | 8⋅85b | 8⋅19b | 0⋅95 | 0⋅008 |
| Litter Ca content (mg/g) | 13⋅64a | 11⋅51ab | 11⋅86ab | 10⋅75b | 1⋅27 | 0⋅001 |
| P retention efficiency (%) | 50⋅80b | 59⋅46a | 57⋅78a | 59⋅31a | 3⋅03 | 0⋅002 |
H, high nPP and Ca; L, low nPP and Ca; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
a,b Least-square mean values within a row with unlike superscript letters were significantly different (P < 0⋅05).
* See Table 2.
Discussion
Optimal timing and duration of the first depletion
One of the objectives of the experiment was to study the best timing (from 0 to 4 d of age v. from 5 to 8 d of age v. from 0 to 8 d of age) and duration (4 d v. 8 d) of the initial depletion to induce a permanent adaptation in the birds. The treatments built to answer this question were LHHL, HLHL and LLHL. As expected, a decrease in nPP and Ca content in the diet between 0 and 4 d of age, 5 and 8 d of age or 0 and 8 d of age decreased bone mineral status during the corresponding phase, regardless of the diets received previously. At day 4 and day 8, our results showed a lower tibia ash content in birds with low P and Ca content in the diet, as in Ashwell & Angel(11) for toe ash content. These results agreed with those of Faridi et al.(18), who observed by meta-analysis that 4⋅0 g/kg nPP are needed to obtain optimal bone mineralisation until 21 d of age, with 7⋅0 g/kg Ca and 2000 μg/kg of vitamin D3.
Birds receiving a diet low in P and Ca increased their P and Ca AID at day 8, but showed lower bone mineralisation than the controls, as observed by Rousseau et al.(1). At day 8, this adaptation appeared to be limited to the diet received during the starter phase (day 5−8) regardless of the feed received during the pre-starter phase (days 0−4) with HL and LL leading to similar results. For Ca, this adaptation was linked to an increase of CALB1 gene expression. Increased AID of Ca linked to increased CALB1 gene expression(1) was also reported in chicks given a low-Ca diet for a 14-d period. Some authors also observed that a decrease of dietary Ca and nPP levels stimulated the mRNA expression of other Ca transporters: SLC8A1 and ATP2B1, and P transporters: SLC20A1 and SLC34A2(3,6,19). Several facts could explain the discrepancy with our results. For the Ca transporters, CALB1 has been proven to be the rate-limiting step in Ca absorption through the epithelium, thus CALB1 expression is highly correlated with intestinal Ca absorption efficiency(20). The lack of effect on protein expression could reflect previous observations that mRNA and protein expression for the same transporter exhibit different temporal patterns(5). Moreover, most experiments find poor correlations between mRNA and protein abundances in the cell due to numerous post-transcriptional regulation mechanisms(21).
As for the lack of effect on P transporter expression, Ca metabolism had been proven to be more closely regulated than P metabolism(22). We hypothesise that Ca was the major mineral deficient in our experiment and drove regulation, thus explaining the lack of effect on P AID at day 18 and day 33 as well as on P transporter protein and mRNA expression. Moreover, P intake through SLC34A2 was defined as the limiting step of P absorption in the intestine(9). Thus, the regulation of P homeostasis would occur mainly by the stimulation of SLC34A2 expression. Yan et al.(7) and Olukosi et al.(8) showed that P content in the diet had to be at 0⋅25 %, lower than used in our L starter diets, in order to enhance the mRNA expression of SLC34A2. The regulation of P absorption could also be at the post-translational levels as suggested by Hattenhauer et al.(23) in mice, with modifications such as glycosylation, ubiquination or palmitoylation. Alternatively, we could hypothesise that the highest P AID was not linked to a mechanism regulating P transporters, but rather to a mechanism regulating phytic P release through endogenous phytase activity. Phytic P hydrolysis being a major component of the P AID, the pre-caecal disappearance of phytic P was determined in order to better understand this effect. Phytic P disappearance at day 8 was higher for birds fed with the L diet between 5 and 8 d compared with birds fed with the H diet, suggesting an increase in endogenous phytase activity. Onyango et al.(24) observed that the activity of mucosal endogenous phytase increased with a reduction of the P content (1⋅2 v. 4⋅6 g/kg of nPP) and the supplementation of cholecalciferol (0 v. 3320 μg/kg) in the diet.
Response to a second depletion
It is possible to lower nPP and Ca content in the diet through all phases without affecting growth performance or mortality. Akter et al.(25) also observed that Ca and nPP content could be reduced (respectively, 6⋅0 and 3⋅0 g/kg from 0 to 35 d of age) without affecting feed conversion ratio. Delezie et al.(26) observed similar results with a reduction of 20−25 % of Ca and nPP dietary contents compared with commercial diets throughout the growth cycle. This result could potentially be explained by the fact that the recommendations are generally set to maximise bone ash(27) which are higher than for growth as reported in a meta-analysis by Faridi et al.(18).
We hypothesised that birds who had been previously depleted could react better to a second depletion occurring in the finisher phase. A second depletion did not have an impact on bone mineral status. At the end of the finisher phase, no differences in mineral AID were observed between the diets, regardless of the initial period and duration of depletion (see treatments LHHL, HLHL and LLHL). P and Ca depletion in an early phase did not induce a permanent adaptation of P and/or Ca transporter genes, protein expression or growth performance contrary to expectations. It is worth noting that the AID of Ca at day 33 was particularly low. Such low values were previously observed in finisher broilers(1). There is no consensus methodology to assess Ca digestibility and there are frequently inconsistencies through measurements(28). In particular, Ca digestibility is reduced with age in broilers(29). Future studies are needed to understand the reasons for the low digestibility values observed with the direct method used here, among them, the effects of dietary P concentrations and Ca:P ratios on Ca digestibility, the indigestible marker, reverse peristalsis, will be relevant to study. Likewise, Van Krimpen et al. (unpublished results) found that birds depleted from 0 to 4 d of age and receiving a control diet from 5 to 37 d of age had the same SLC34A2, CALB1 and ATP2B1 gene expression as control birds at day 21 and day 37. Conversely, other authors have demonstrated that the implementation of a permanent adaptation was possible. Rousseau et al.(1) observed that birds fed with an L diet (0⋅60 % Ca and 0⋅24 % available P) between 10 and 21 d of age and replenished with an H diet (0⋅90 % Ca and 0⋅28 % available P) between 22 and 35 d of age had higher Ca and P AID at day 35 compared with birds fed with diets at recommendations from 10 to 35 d of age. Ashwell & Angel(11) observed that birds depleted during the first 90 h of life had a higher P AID when depleted a second time between day 22 and day 38. These authors used a Ca:nPP ratio of 1⋅64 in the L diet between 0 and 4 d of age, in contrast to 2⋅14 in the present experiment. The Ca and available P contents in the diet between 0 and 4 d of age used by Ashwell & Angel(11) were, respectively, 0⋅59 and 0⋅25 % compared with 0⋅77 and 0⋅36 % in the present experiment. Birds were thus probably more deficient in P than in our experiment. Consequently, it could be hypothesised that P deficiency probably is the inducer of the permanent adaptation.
A decrease in dietary Ca and nPP has been shown to induce regulation in the kidney and bones(3). The expression of Ca and P transporters in the kidney is increased to enhance the Ca and P renal reabsorption and bone resorption is stimulated to release P and Ca(6). A long-term adaptation to the early depletion might have occurred in our experiment in the kidney and bones. Indeed, P retention efficiency was higher for birds that received depletion−repletion treatments while no effect was seen on P AID at day 18 and day 33. The higher P retention efficiency could be linked to higher renal reabsorption of P or increased bone turnover rather than a change in digestibility/absorption.
In the present experiment, we did not observe a long-term adaptation of the animals to an early depletion, while P retention efficiency was around 51 % in the control diet. Van Krimpen et al. (unpublished results) also did not observe a long-term adaptation with similar P retention efficiency values. In contrast, Ashwell & Angel(11) observed that birds depleted during the first 90 h of life exhibited higher P absorption at 38 d compared with control birds when both groups were fed with a low finisher diet. The P retention efficiency in this study was 35⋅3 % in the control diet, compared with 47⋅1 % in the low pre-starter diet accompanied by a higher total P intake for the control birds (11⋅9 g/kg BW). As suggested by Van Krimpen et al. (unpublished results) the apparent long-term adaptation observed by Ashwell & Angel(11) could result from a reduced P retention efficiency in the control diet rather than an enhancement resulting from the depletion during the pre-starter phase.
Necessity of a repletion
One of our preliminary hypotheses was that a repletion phase was necessary in order to catch up in bone mineralisation. Birds depleted from 0 to 4 d of age had the same bone mineral status at day 8 compared with control birds when fed with the H diet between 5 and 8 d of age. Likewise, birds depleted from 0 to 8 d of age or 5 to 8 d of age showed similar bone criteria at day 18 compared with control diets, when replenished between 9 and 18 d of age. The birds seemed to be able to catch up in bone mineralisation after 4 or 8 d on a control diet. Whatever the type of initial depletion, repletion with the H diet led to similar P and Ca AID at 18 d of age. As a result, higher Ca and nPP content in the diet was the explanation of a catch up in bone mineralisation rather than a permanent adaptation of birds to increase P and Ca digestive utilisation.
In the finisher phase, the birds were able to maintain a mineral status at the control levels when fed the L diet. It had been demonstrated that P and Ca requirements for bone growth and metabolism decrease with age(30) and the dietary Ca and nPP content were reduced accordingly in our experiment. Moreover, the decrease in nPP and Ca dietary content was less severe for the birds during the finisher phases 1 and 2 (respectively, −21 and −25 % compared with −30 % during the pre-starter and starter phase). Interestingly, the most depleted programme, LLHL, led to lower tibia bone mineral density without effects on tibia ash content. Bone mineral content discriminated between the dietary treatments with more sensitivity than classical bone measures as seen in Angel et al.(31) and Bradbury et al.(22). Interestingly, cortical bone mineral density followed the same trend to discriminate between the treatments compared with total bone mineral density. However, trabecular bone mineral density was not affected by dietary treatment. Likewise, Kim et al.(32) and Jendral et al.(33) found that total and cortical bone mineral density discriminated between the treatments with no effect on trabecular bone mineral density in, respectively, broiler chicks at day 21 and hens at 65 weeks of age. As stated by these authors, these results could be due to excessive bone resorption from the endosteal surface located at the inner edge of the cortical shell. The analysed feed composition showed that the Ca:nPP ratio in the L diet during the finisher phase was 2⋅65 compared with 2⋅20 as formulated. As a result, the Ca intake compared with the nPP intake was much higher than formulated during this phase. The higher ratio could reduce P availability and then increase bone resorption.
Conclusion
Reducing P and Ca content in the diet did not have an impact on growth performance. Despite reduced bone mineralisation during the initial depletion phase, the birds were able to catch up in bone mineralisation at day 8 or at day 18, after being fed a diet at commercial Ca and nPP levels during, respectively, the starter or grower phase. It is then possible to lower nPP and Ca in the diet during the finisher period, without affecting growth performance or bone mineralisation. Our results demonstrated that it was not necessary to replenish the diets during the finishing period in order to ensure acceptable bone mineralisation, thus limiting the environmental impact of the production. The birds adapted to a diet low in P and Ca content by increasing their P and Ca AID accompanied by stimulation of the mRNA expression of the Ca transporter, CALB1. This adaptation appeared to be limited to the starter phase for birds receiving the depleted diet. None of our initial depletion strategies led to a long-lasting adaptation. All of the depletion–repletion strategies used improved litter quality. However, the LLHL programme showed the most promising results, with optimal growth performance and the largest decrease in environmental and economic impacts.
Acknowledgements
The authors gratefully acknowledge PEAT INRA Poultry Experimental Facility (2018, https://doi.org/10.15454/1.5572326250887292E12) for conducting the animal experiment. We are grateful to the CIRE platform for their technical support in performing the laboratory analysis.
The present study was supported by a CRD research grant from NSERC, with Nutreco Canada, Prorec, Aliments Breton, and MixScience as industry partners.
The contributions of each author to the study were as follows: A. S. V., A. N., S. K., G. P. and M. P. L.-M. participated in the design and planning of the study; A. S. V., L. L., F. L., S. M.-C. and N. M. participated in the laboratory analysis of the data; A. S. V., A. N. and M. P. L.-M. participated in the analysis and interpretation of the data; A. S. V., A. N., M. J. D., F. L., S. K., G. P. and M. P. L.-M. participated in the writing and editing of the article.
There are no conflicts of interest.
References
- 1.Rousseau X, Valable AS, Létourneau-Montminy MP, et al. (2016) Adaptive response of broilers to dietary phosphorus and calcium restrictions. Poult Sci 95, 2849–2860. [DOI] [PubMed] [Google Scholar]
- 2.Yan F, Angel R, Ashwell C, et al. (2005) Evaluation of the broiler's ability to adapt to an early moderate deficiency of phosphorus and calcium. Poult Sci 84, 1232–1241. [DOI] [PubMed] [Google Scholar]
- 3.Proszkowiec-Weglarz M & Angel R (2013) Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility. J Appl Poult Res 22, 609–627. [Google Scholar]
- 4.Nie W, Yang Y, Yuan J, et al. (2012) Effect of dietary non-phytate phosphorus on laying performance and small intestinal epithelial phosphate transporter expression in dwarf pink-shell laying hens. J Anim Sci Biotechnol 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Q, Chandler JS, Wasserman RH, et al. (1993) Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc Nat Acad Sci U S A 90, 1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centeno VA, Diaz de Barboza GA, Marchionattia AN, et al. (2004) Dietary calcium deficiency increases Ca2+ uptake and Ca2+ extrusion mechanisms in chick enterocytes. Comp Biochem Phys A 139, 133–141. [DOI] [PubMed] [Google Scholar]
- 7.Yan F, Angel R & Ashwell CM (2007) Characterization of the chicken small intestine type IIb sodium phosphate cotransporter. Poult Sci 86, 67–76. [DOI] [PubMed] [Google Scholar]
- 8.Olukosi O, Adedokun SA, Ajuwon KM, et al. (2011) Early responses of sodium-dependent phosphate transporter type IIb in broiler chicks to dietary phosphorus intervention. Br Poult Abstr 7, 39. [Google Scholar]
- 9.Huber K, Zeller E & Rodehutscord M (2015) Modulation of small intestinal phosphate transporter by dietary supplements of mineral phosphorus and phytase in broilers. Poult Sci 94, 1009–1017. [DOI] [PubMed] [Google Scholar]
- 10.Fang R, Xiang Z, Cao M, et al. (2012) Different phosphate transport in the duodenum and jejunum of chicken response to dietary phosphate adaptation. Asian-Aust J Anim Sci 25, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwell CM & Angel R (2010) Nutritional genomics: a practical approach by early life conditioning with dietary phosphorus. R Bras Zootec 39, 268–278. [Google Scholar]
- 12.Cobb-Vantress (2015) Performances et recommandations nutritionnelles Cobb500TM (Nutritional performance and recommendations Cobb500TM). https://www.cobb-vantress.com/fr_FR/products/cobb500/ (accessed June 2020).
- 13.Militist G, Donkó T, Dalle Zotte A, et al. (2013) Application of computed tomography to assess the effect of egg yolk ratio on body composition in chickens of different genotypes and gender at hatch and during the rearing period. Br Poult Sci 54, 611–619. [DOI] [PubMed] [Google Scholar]
- 14.Sherlock ML, Demmers TGM, Goodship AE, et al. (2010) The relationship between physical activity and leg health in the broiler chickens. Br Poult Sci 51, 22–30. [DOI] [PubMed] [Google Scholar]
- 15.Jonchere V, Brionne A, Gautron J, et al. (2012) Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiol 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coudert E, Pascal G, Dupont J, et al. (2015) Phylogenesis and biological characterization of a new glucose transporter in the chicken (Gallus gallus), GLUT12. PLOS ONE 10, e0139517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SAS Institute Inc. (2003) Statistical Analysis System, version 9.0. Cary, NC: SAS Institute Inc. [Google Scholar]
- 18.Faridi A, Gitoee A & France J (2015) A meta-analysis of the effects of non-phytate phosphorus on broiler performance and tibia ash concentration. Poult Sci 94, 2753–2762. [DOI] [PubMed] [Google Scholar]
- 19.Bar A (2009) Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Comp Biochem Phys A 152, 447–469. [DOI] [PubMed] [Google Scholar]
- 20.Joost G, Hoenderop J, Nilius B, et al. (2005) Calcium absorption across epithelia. Physiol Rev 85, 373–322. [DOI] [PubMed] [Google Scholar]
- 21.Maier T, Güell M & Serrano L (2009) Correlation of mRNA and protein in complex biological samples. FEBS Lett 583, 3966–3973. [DOI] [PubMed] [Google Scholar]
- 22.Bradbury EJ, Wilkinson SJ, Cronin GM, et al. (2014) Nutritional geometry of calcium and phosphorus in broiler chicks. Growth performance, skeletal health and intake arrays. Animal 8, 1071–1079. [DOI] [PubMed] [Google Scholar]
- 23.Hattenhauer O, Traebert M, Murer H, et al. (1999) Regulation of small intestinal Na-Pi type IIb cotransporter by dietary phosphate intake. Am J Physol Gastrointest Liver Physiol 277, 756–762. [DOI] [PubMed] [Google Scholar]
- 24.Onyango EM, Asem EK & Adeola O (2007) Dietary cholecalciferol and phosphorus influence intestinal mucosa phytase activity in broiler chicks. Br Poult Sci 47, 632–639. [DOI] [PubMed] [Google Scholar]
- 25.Akter M, Graham H & Iji PA (2016) Response of broiler chickens to different levels of calcium, non-phytate phosphorus and phytase. Br Poult Sci 57, 799–809. [DOI] [PubMed] [Google Scholar]
- 26.Delezie E, Bierman K, Nollet L, et al. (2015) Impacts of calcium and phosphorus concentration, their ratio, and phytase supplementation level on growth performance, foot pad lesions, and hock burn of broiler chickens. J Appl Poult Res 24, 115–126. [Google Scholar]
- 27.Driver JP, Pesti GM, Bakalli RI, et al. (2006) The effect of feeding calcium- and phosphorus-deficient diets to broiler chickens during the starting and growing–finishing phases on carcass quality. Poult Sci 85, 1939–1946. [DOI] [PubMed] [Google Scholar]
- 28.Anwar N & Ravindran V (2016) Measurement of calcium digestibility in feed ingredients for poultry – methodology and challenges In Phytate Destruction: Consequences for Precision Animal Nutrition, pp. 191–206 [Walk CL, Kühn I, Stein HH, Kidd MT and Rodehutscord M, editors]. Wageningen: Wageningen Academic Publishers. [Google Scholar]
- 29.Angel R, Proszkowiec-Weglarz M, Jimenez-Moreno E, et al. (2013) Impact of time and dietary calcium and phosphorus deficiencies on their digestibilities in single ingredients Proceedings of the European Symposium of Poultry Nutrition, Potsdam, Germany.
- 30.Li J, Yuan J, Miao Z, et al. (2017) Effects of age on intestinal phosphate transport and biomechanical values of broiler chickens. Asian-Aust J Anim Sci 30, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angel R, Saylor WW, Mitchell AD, et al. (2006) Effect of dietary phosphorus, phytase and 25-hydroxycholecalciferol on broiler chicken bone mineralization, litter phosphorus and processing yields. Poult Sci 85, 1200–1211. [DOI] [PubMed] [Google Scholar]
- 32.Kim WK, Bloomfield SA & Ricke SD (2011) Effects of age, vitamin D3, and fructooligosaccharies on bone growth and skeletal integrity of broiler chicks. Poult Sci 90, 2425–2432. [DOI] [PubMed] [Google Scholar]
- 33.Jendral MJ, Korver DR, Church JS, et al. (2008) Bone mineral density and breaking strength of white leghorns housed in conventional, modified, and commercially available colony battery cages. Poult Sci 87, 828–837. [DOI] [PubMed] [Google Scholar]
- 34.National Research Council (1994) Nutrient Requirements of Poultry, 9th revised ed Washington, DC: The National Academies Press. [Google Scholar]