Abstract
Resistant maltodextrin (RMD) from various sources of starch has been extensively studied. However, studies which reported the effects of tapioca RMD (TRM) on glucose and insulin response are lacking. This study investigated the effect of TRM on postprandial plasma glucose and serum insulin in healthy subjects. Additionally, satiety and gastrointestinal tolerability were also evaluated. Sixteen healthy participants received five different treatments on five separate days. Participants received 50 g of either: glucose (GL), tapioca maltodextrin (TM), TRM, MIX15% (7⋅5 g TRM + 42⋅5 g TM) or MIX50% (25 g TRM + 25 g TM). Plasma glucose, serum insulin and subjective appetite responses were measured postprandially over 180 min. Gastrointestinal symptoms were evaluated by questionnaire before and after each test day. Results showed that at 30 min after treatment drinks, plasma glucose after TRM was significantly lowest (104⋅60 (sem 2⋅63 mg/dl) than after GL (135⋅87 (sem 4⋅88) mg/dl; P <0⋅001), TM (127⋅93 (sem 4⋅05) mg/dl; P = 0⋅001), MIX15% (124⋅67 (sem 5⋅73) mg/dl; P = 0⋅039) and MIX50% (129⋅33 (sem 5⋅23) mg/dl; P = 0⋅003) (1 mg/dl = 0⋅0555 mmol/l). In addition, TRM also significantly reduced serum insulin (13⋅01 (sem 2⋅12) μIU/ml) compared with GL (47⋅90 (sem 11⋅93) μIU/ml; P = 0⋅013), TM (52⋅96 (sem 17⋅68) μIU/ml; P = 0⋅002) and MIX50% (33⋅16 (sem 4⋅99) μIU/ml; P = 0⋅008). However, there were no significant differences in subjective appetite between treatments (P > 0⋅05). A single high dose of TRM (50 g) caused flatulence (P < 0⋅05). Tapioca resistant maltodextrin has low digestibility in the small intestine and, therefore, reduced incremental plasma glucose and serum insulin, without affecting satiety in healthy subjects over 180 min. Gastrointestinal tolerability of TRM should be considered when consumed in high doses.
Key words: Tapioca resistant maltodextrin, Plasma glucose, Serum insulin, Satiety, Gastrointestinal tolerability
Abbreviations: GI, gastrointestinal; GL, glucose; GLP-1, glucagon-like peptide-1; iAUC, incremental AUC; MIX15%, 7⋅5 g tapioca resistant maltodextrin + 42⋅5 g tapioca maltodextrin; MIX50%, 25 g tapioca resistant maltodextrin + 25 g tapioca maltodextrin; PYY, peptide YY; RMD, resistant maltodextrin; TM, tapioca maltodextrin; TRM, tapioca resistant maltodextrin
Carbohydrate accounts for more than 50 % of dietary energy intake in Asian countries(1). Previous studies have shown that the quality and quantity of carbohydrate have been linked to non-communicable diseases, such as diabetes and obesity(2,3). The type of carbohydrate has been considered as more important than the amount of carbohydrate as a total percentage of dietary intake(2). Addition of dietary fibre into the daily diet would be beneficial in controlling blood glucose and body weight, and therefore may reduce the risk of type 2 diabetes, as well as obesity(4,5). Among dietary fibre, resistant starch has been a growing interest, which can resist digestion and absorption in the small intestine(6).
Resistant maltodextrin (RMD) is a novel non-viscous soluble dietary fibre which is classified as resistant starch type 5(6–8). It can be derived from various types of starch, such as maize, potato, wheat and also tapioca(7). RMD is produced by several steps, called pyroconversion, including hydrolysis, transglucosidation and repolymerisation. Those reactions produce random 1–2, 1–3, 1–4 and 1–6 α and β glucosidic linkages. However, human digestive enzymes digest only α 1–4 and 1–6 glucosidic linkages. Therefore, RMD is partially resistant to human digestive enzymes and leads to a lower postprandial glycaemic response(9).
Additionally, RMD is promising as a therapeutic agent against obesity and for maintaining body weight. Several studies have reported that RMD stimulates the release of gut hormones, such as peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), which promote satiety(10–12). Fermentation of RMD by gut microbiota in the colon produces SCFA, including acetate, propionate and butyrate. These SCFA act through the activation of GPR41/43 and enhance the release of PYY and GLP-1 from intestinal L-cells(13–15). However, fermentation of RMD may cause adverse effects, such as bloating, abdominal pain and flatulence due to excessive gas production(16). In a meta-analysis study of RMD on glycaemic and insulin responses, the non-viscous soluble polysaccharide was found to attenuate the glycaemic response to carbohydrate foods. In addition, the percentage of attenuation was dose-dependent, and independent of the amount of available carbohydrate co-ingested. Furthermore, the glucose-lowering effect was greater when RMD was incorporated into drinks rather than into solid foods(17).
There have been several studies that have investigated the glycaemic and satiety responses to RMD from various sources of starch, such as potato starch(18), maize starch(19–21) and wheat starch(22). However, there are a limited number of studies evaluating the beneficial effects of tapioca RMD (TRM) on glycaemic, insulinaemic and satiety responses. Previous studies have indicated that different sources of starch may have different glycaemic responses(21). Extrinsic factors (i.e. granule surface, porosity and pit formation) and intrinsic factors (i.e. amylose:amylopectin ratio, degree of polymerisation, and branching of glucan polymer) of the starch may influence starch digestibility(23). Starch digestibility is increased with decreasing amylose content since amylose content is positively correlated with the formation of resistant starch(24). The amylose content of tapioca starch is lower (17 %) when compared with other types of starch, such as maize (25 %), wheat (25 %) and potato (20 %)(25). Thus, the digestibility of tapioca starch is highest compared with maize, wheat and potato starch. Moreover, previous studies have shown that tapioca starch is hydrolysed easily by porcine α-amylase, followed by maize and potato starch(26).
Recently, TRM has been developed and it possesses high functionality. The characteristics and functionality of TRM depend on the process, mainly transglucosidation and repolymerisation of the starch(27). The resistant starch content in TRM was found to be 56 % (w/w) and dietary fibre was 86 % (w/w)(25). In our study, we used non-GMO tapioca starch, which contains 90 % dietary fibre. This value is comparable with those commercial RMD available on the market, such as potato RMD with dietary fibre content of approximately 75 %(28), and maize RMD with dietary fibre content of 72–92 %(29,30), and wheat RMD with dietary fibre content of 85 %(31).
Most of the dietary fibre in RMD is water-soluble fibre, which can be used by adding or replacing the readily digestible starch in meals or beverages. However, Wong et al.(32) noted that favourable effects of resistant starch on the glycaemic response were observed when resistant starch replaced readily digestible starch, but not when resistant starch was added to readily digestible starch. To the best of our knowledge, there is limited research on the effects of TRM on postprandial glucose, insulin, subjective appetite and gastrointestinal (GI) symptoms. Therefore, this study aimed to investigate the effects of TRM on postprandial glucose, insulin, subjective appetite and GI symptoms after replacing the digestible maltodextrin and glucose. We hypothesised that TRM would have a minimal effect on plasma glucose and insulin response and replacement of digestible maltodextrin by TRM would lower glycaemic response as well.
Materials and methods
In vitro α-amylase inhibition
The α-amylase inhibition assay was done following the method of Watcharachaisoponsiri et al.(33). Tapioca RMD (50 μl; 5 mg/ml or 10 mg/ml) was incubated with 5 mm-P-nitrophenyl-α-d-maltopentaoside (PNPG-5) (50 μl), and 100 μl of porcine pancreatic α-amylase (EC 3.2.1.1) into a ninety-six-well plate. A blank was prepared by replacing the enzyme using a KPB buffer (50 mm, pH 7⋅0). The reaction was read at absorbance 405 nm in a microplate reader (BioTek Instruments, Inc.) for 90 min with reading intervals at 3 min at 37°C.
A was the initial velocity of the enzyme reaction with the control (without tapioca resistant maltodextrin), a was the initial velocity of the control reaction without the enzyme, B was the initial velocity of tapioca resistant maltodextrin reaction with the enzyme, and b was the initial velocity of the tapioca resistant maltodextrin reaction without the enzyme.
Intervention study
Participants
The inclusion criteria were: subjects should be healthy, aged 18–55 years, and had fasting plasma glucose below 100 mg/dl (5⋅55 mmol/l). Participants who used any medications/dietary supplements/insulin injections to lower plasma glucose, or had any allergies to TRM, or smoked or drank alcohol were excluded from the study. The study was conducted at the Nutrition and Dietetics Department, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok, Thailand.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Research Ethics Review Committee for Research Involving Human Subjects, Chulalongkorn University (protocol no. 196.2/60). Participants were informed about the details of the study, procedures, and adverse effects of the study product. Written informed consent was obtained from all participants prior to enrolment into the study. The anonymity and confidentiality of the participants were preserved.
Study design
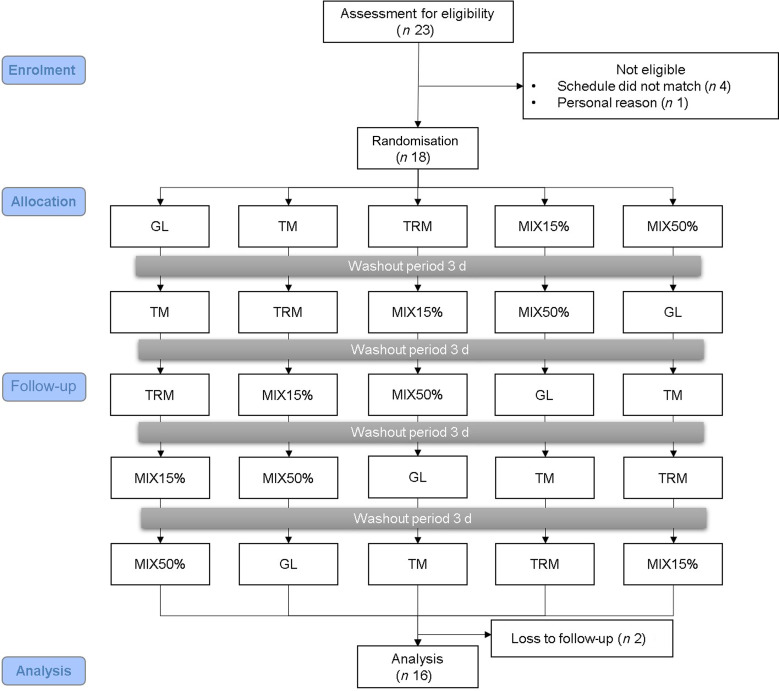
This study was a randomised cross-over controlled trial with a Latin square design. There was a washout period of at least 3 days between treatments (Fig. 1). Eighteen eligible participants were randomly assigned to one of the five treatment drinks including: 50 g of glucose (GL) (The British Dispensary (L.P), Co. Ltd.), 50 g of tapioca maltodextrin (TM) (Banpong Novitat, Co. Ltd.), 50 g of tapioca resistant maltodextrin (TRM) (Banpong Novitat, Co. Ltd.), MIX15% (7⋅5 g tapioca resistant maltodextrin + 42⋅5 g tapioca maltodextrin) or MIX50% (25 g tapioca resistant maltodextrin + 25 g tapioca maltodextrin) (Table 1). All of the intervention starch was dissolved in 100 ml of drinking water.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. GL, 50 g glucose; TM, 50 g tapioca maltodextrin; TRM, 50 g tapioca resistant maltodextrin; MIX15%, 42⋅5 g TM (85 %) + 7⋅5 g TRM (15 %); MIX50%, 25 g TM (50 %) + 25 g TRM (50 %).
Table 1.
Description of treatment drinks
| Group | Glucose (g) | Tapioca maltodextrin (g) | Tapioca resistant maltodextrin (g) |
|---|---|---|---|
| Glucose (GL) | 50 | – | – |
| Tapioca maltodextrin (TM) | – | 50 | – |
| Tapioca resistant maltodextrin (TRM) | – | – | 50 |
| MIX15% | – | 42⋅5 | 7⋅5 |
| MIX50% | – | 25 | 25 |
The experiment began in the morning following an overnight fast. Blood samples were collected from the antecubital vein and transferred into two separated vacutainer tubes: a tube that contains sodium fluoride for plasma glucose analysis and a gel activator tube for serum insulin analysis. Thereafter, participants were advised to consume the test drink within 2 min after the first sip. The first sip was set at 0 min immediately after baseline sampling. Blood samples were taken at 0 (baseline), 30, 60, 120 and 180 min after the first sip for laboratory analysis following the test drink. In addition, participants were asked to rate their subjective appetite at 0 (baseline), 30, 60, 120 and 180 min following test drink consumption.
Plasma glucose and serum insulin analysis
Blood samples were immediately centrifuged (3000 rpm) for 10 min at 4°C and plasma was separated and kept at −80°C for further analysis. Plasma glucose was determined according to the hexokinase method using a clinical chemistry analyser (Beckman Coulter AU480). Serum insulin was analysed by chemiluminescence immunoassay(34).
Subjective appetite evaluation
Subjective appetite was measured by using a visual analogue scale with pen and paper(35). There were four questions related to hunger, satiety, desire to eat and prospective food consumption, such as ‘how hungry do you feel right now?’; ‘how satiated are you now?’; ‘how strong is your desire to eat?’; and ‘how much food you can eat right now?’. Participants rated their answers on a 100 mm line scale anchored with opposite words at each end of the line (for example: not hungry at all – extremely hungry).
Evaluation of gastrointestinal symptoms
GI symptoms including abdominal pain, nausea, vomiting, bloating and flatulence were evaluated using a questionnaire 1 day before and after the test session(36). The symptoms were observed during 24 h before and after the test session. Participants rated the intensity of symptoms from 0 (none), 1 (mild), 2 (moderate), to 3 (severe). Total score was calculated for the intensity of all symptoms. A score of 0 defined no symptoms at all, while a score of 15 reflected all symptoms rated as severe(36). Stool form was evaluated 24 h before and after the test session using the Bristol Stool Scale with a picture and description for each type of stool form(37).
Statistical analysis
Incremental plasma glucose and serum insulin were calculated as the value at a time point minus the baseline value. Incremental AUC (iAUC) was calculated based on the trapezoidal rule from 0 to 180 min for glucose and insulin, ignoring the area beneath the baseline value(38). Data for subjective appetite and GI symptoms were expressed as mean values, with standard errors of the mean represented as vertical bars. Data were analysed by using the Statistical Package for Social Sciences version 22.0 (IBM Corp.). Normality of data was analysed by using a Shapiro−Wilk test. A repeated-measures ANOVA was performed to analyse the normally distributed data followed by Tukey's honestly significant difference (HSD) test as post hoc analysis. Meanwhile, Friedman's two-way ANOVA was performed to test for differences between treatments in non-normally distributed data. Statistical significance was set at P<0⋅05. Based on a previous study, a minimum sample size of thirteen participants was required to detect a 30 % difference of peak plasma glucose response with 80 % power at a significance level of 0⋅05(39). However, the sample size was increased by 20 % for estimating dropout.
Results
In vitro α-amylase inhibition
In the in vitro study, results showed that the α-amylase inhibitory effect of 5 mg/ml tapioca RMD was 38⋅29 (sem 0⋅54 %, while 10 mg/ml of tapioca RMD inhibited α-amylase activity by 50⋅88 (sem 1⋅91 % (Table 2).
Table 2.
Inhibitory effect of α-amylase by tapioca resistant maltodextrin
(Mean values with their standard errors)
| Tapioca resistant maltodextrin concentration | Inhibition (%) | P | |
|---|---|---|---|
| Mean | sem | ||
| 5 mg/ml | 38⋅29 | 0⋅54 | <0⋅001 |
| 10 mg/ml | 50⋅88 | 1⋅91 | 0⋅001 |
Intervention study
In the randomised cross-over controlled trial, eighteen participants were included in the study. However, two participants lacked follow-up. In total, sixteen participants, including seven males and nine females, completed the study and data were analysed. The mean age of completing participants was 25⋅81 (sem 1⋅19) years and mean BMI was 22⋅88 (sem 0⋅87) kg/m2. The means of fasting plasma glucose and serum insulin were 84⋅60 (sem 1⋅39) mg/dl (4⋅70 (sem 0⋅08) mmol/l) and 4⋅94 (sem 0⋅72) μIU/ml, respectively, at baseline (Table 3).
Table 3.
Baseline characteristics of participants (n 16)
(Numbers of subjects; mean values with their standard errors)
| Mean | sem | |
|---|---|---|
| Sex | ||
| Male (n) | 7 | |
| Female (n) | 9 | |
| Age (years) | 25⋅81 | 1⋅19 |
| Body weight (kg) | 60⋅84 | 3⋅13 |
| BMI (kg/m2) | 22⋅88 | 0⋅87 |
| Fasting plasma glucose (mg/dl)* | 84⋅60 | 1⋅39 |
| Fasting serum insulin (μIU/ml) | 4⋅94 | 0⋅72 |
* To convert glucose in mg/dl to mmol/l, multiply by 0⋅0555.
Postprandial plasma glucose response
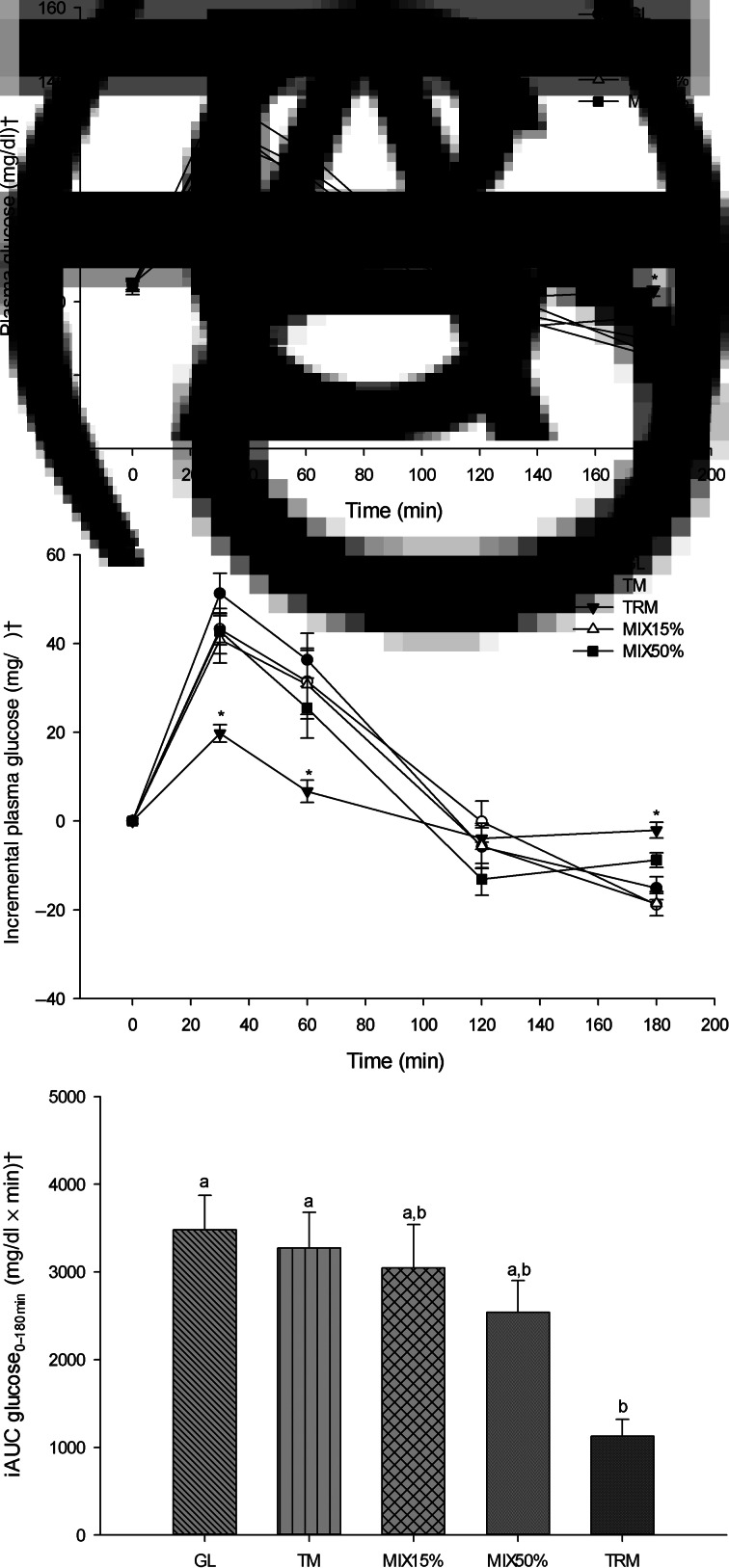
The peak of plasma glucose after every treatment drink was reached at 30 min (Fig. 2(A)). At 30 min after treatment drinks, plasma glucose concentration of TRM was lowest (104⋅60 (sem 2⋅63) mg/dl) compared with those after GL (135⋅87 (sem 4⋅88) mg/dl; P = <0⋅001), TM (127⋅93 (sem 4⋅05) mg/dl; P = 0⋅001), MIX15% (124⋅67 (sem 5⋅73) mg/dl; P = 0⋅039) and MIX50% (129⋅33 (sem 5⋅23) mg/dl; P = 0⋅003). In addition, mean plasma glucose concentrations after the TRM (91⋅53 (sem 2⋅70) mg/dl) treatment at 60 min time points were significantly lower when compared with the GL treatment (120⋅93 (sem 6⋅11) mg/dl; P = 0⋅005) (Table 4). (To convert glucose in mg/dl to mmol/l, multiply by 0⋅0555.)
Fig. 2.
Glycaemic responses following 50 g of starch ingestion in healthy participants (n 16): 50 g glucose (GL); 50 g tapioca maltodextrin (TM); 50 g tapioca resistant maltodextrin (TRM); 42⋅5 g TM (85 %) + 7⋅5 g TRM (15 %) (MIX15%); and 25 g TM (50 %) + 25 g TRM (50 %) (MIX50%). (A) Plasma glucose over 180 min (mg/dl), (B) incremental plasma glucose over 180 min (mg/dl), and (C) incremental AUC (iAUC) of glucose from 0 to 180 min (iAUC glucose0–180min). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that for GL (P < 0⋅05). a,b Mean values with unlike letters were significantly different (P < 0⋅05). † To convert glucose in mg/dl to mmol/l, multiply by 0⋅0555.
Table 4.
Postprandial glucose following different treatments in healthy participants
(Mean values with their standard errors)
| 0 min | 30 min | 60 min | 120 min | 180 min | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sem | P* | Mean | sem | P* | Mean | sem | P* | Mean | sem | P* | Mean | sem | P* | |
| Postprandial plasma glucose (mg/dl)† | |||||||||||||||
| GL | 84⋅60 | 1⋅39 | NS | 135⋅87 | 4⋅88 | <0⋅001 | 120⋅93 | 6⋅11 | 0⋅005 | 78⋅80 | 4⋅86 | NS | 69⋅47 | 2⋅71 | 0⋅007 |
| TM | 84⋅67 | 1⋅30 | NS | 127⋅93 | 4⋅05 | 0⋅001 | 116⋅13 | 7⋅79 | NS | 84⋅53 | 4⋅53 | NS | 65⋅80 | 2⋅54 | <0⋅001 |
| TRM | 84⋅87 | 1⋅50 | – | 104⋅60 | 2⋅63 | – | 91⋅53 | 2⋅70 | – | 80⋅93 | 1⋅62 | – | 82⋅80 | 1⋅34 | – |
| MIX15% | 83⋅73 | 1⋅78 | NS | 124⋅67 | 5⋅73 | 0⋅039 | 114⋅47 | 8⋅00 | NS | 78⋅13 | 4⋅98 | NS | 65⋅13 | 3⋅10 | <0⋅001 |
| MIX50% | 84⋅33 | 1⋅61 | NS | 129⋅33 | 5⋅23 | 0⋅003 | 112⋅00 | 6⋅97 | NS | 72⋅40 | 3⋅48 | NS | 75⋅80 | 1⋅60 | NS |
| Incremental plasma glucose (mg/dl)† | |||||||||||||||
| GL | 0⋅00 | 0⋅00 | NS | 51⋅27 | 4⋅56 | <0⋅001 | 36⋅33 | 5⋅97 | 0⋅002 | −5⋅80 | 4⋅78 | NS | −15⋅13 | 2⋅57 | 0⋅018 |
| TM | 0⋅00 | 0⋅00 | NS | 43⋅27 | 3⋅58 | 0⋅002 | 31⋅47 | 7⋅43 | 0⋅027 | −0⋅13 | 4⋅61 | NS | −18⋅87 | 2⋅50 | <0⋅001 |
| TRM | 0⋅00 | 0⋅00 | – | 19⋅73 | 1⋅98 | – | 6⋅67 | 2⋅52 | – | −3⋅93 | 2⋅44 | – | −2⋅07 | 1⋅80 | – |
| MIX15% | 0⋅00 | 0⋅00 | NS | 40⋅93 | 5⋅34 | 0⋅032 | 30⋅73 | 7⋅70 | 0⋅027 | −5⋅60 | 5⋅11 | NS | −18⋅60 | 2⋅73 | <0⋅001 |
| MIX50% | 0⋅00 | 0⋅00 | NS | 45⋅00 | 5⋅06 | 0⋅003 | 27⋅67 | 6⋅75 | NS | −11⋅93 | 3⋅61 | NS | −8⋅53 | 1⋅67 | NS |
GL, glucose; TM, tapioca maltodextrin; TRM, tapioca resistant maltodextrin; MIX15%, 7⋅5 g tapioca resistant maltodextrin + 42⋅5 g tapioca maltodextrin; MIX50%, 25 g tapioca resistant maltodextrin + 25 g tapioca maltodextrin.
* P value when compared with TRM (NS = not significant; P>0⋅05).
† To convert glucose in mg/dl to mmol/l, multiply by 0⋅0555.
As shown in Fig. 2(B), incremental plasma glucose had a similar trend to plasma glucose which shows attenuation at 30 min following ingestion of TRM (19⋅73 (sem 1⋅98) mg/dl) when compared with GL (51⋅27 (sem 4⋅56) mg/dl; P <0⋅001), TM (43⋅27 (sem 3⋅58) mg/dl; P = 0⋅002), MIX15% (40⋅93 (sem 5⋅34) mg/dl; P = 0⋅032) and MIX50% (45⋅00 (sem 5⋅06) mg/dl; P = 0⋅003). At 60 min, incremental plasma glucose after 50 g TRM ingestion (6⋅67 (sem 2⋅52) mg/dl) was significantly lower compared with GL (36⋅33 (sem 5⋅97) mg/dl; P = 0⋅002), TM (31⋅47 (sem 7⋅43) mg/dl; P = 0⋅027) and MIX15% (30⋅73 (sem 7⋅70) mg/dl; P = 0⋅027).
Tapioca resistant maltodextrin significantly reduced iAUC glucose0–180min when compared with GL and TM (iAUC glucose0–180min of TRM, GL and TM was 1126⋅00 (sem 191⋅51) mg/dl × min; 3481⋅00 (sem 391⋅48) mg/dl × min, P = 0⋅001; and 3273⋅00 (sem 406⋅42) mg/dl × min, P = 0⋅004, respectively). Replacement of TM by TRM tended to decrease, but not significantly, iAUC glucose0–180min of MIX15% (3045⋅00 (sem 497⋅47) mg/dl × min; P = 0⋅943) and MIX50% (2541⋅43 (sem 362⋅67) mg/dl × min; P = 0⋅198) when compared with GL (Fig. 2(C)).
Postprandial serum insulin response
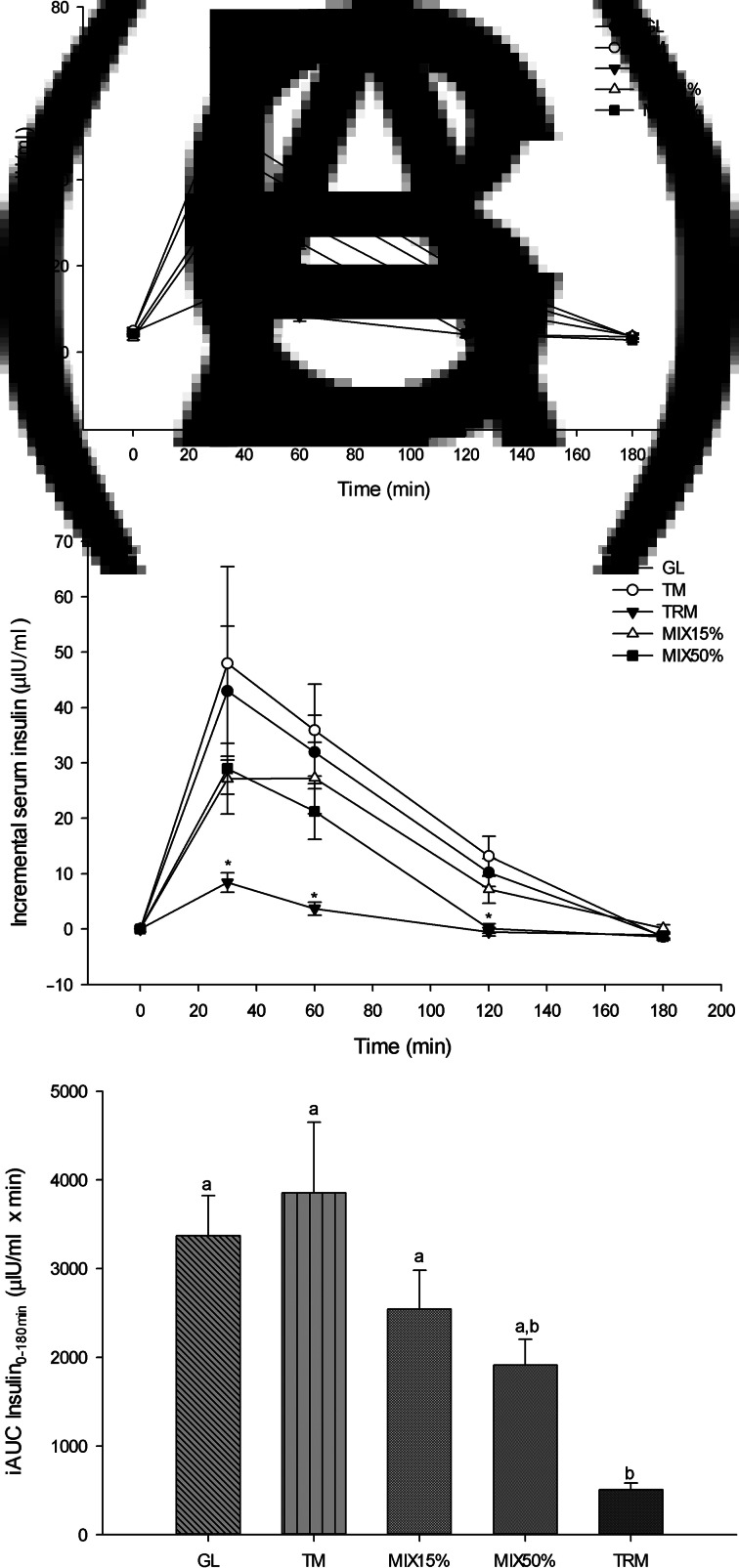
Similar to the glycaemic response, at 30 min TRM significantly decreased serum insulin (13⋅01 (sem 2⋅12) μIU/ml) when compared with GL (47⋅90 (sem 11⋅93) μIU/ml; P = 0⋅013), TM (52⋅96 (sem 17⋅68) μIU/ml; P = 0⋅002) and MIX50% (33⋅16 (sem 4⋅99) μIU/ml; P = 0⋅008). At 60 min, TRM (8⋅29 (sem 1⋅16) μIU/ml) significantly attenuated serum insulin when compared with GL (36⋅91 (sem 7⋅12) μIU/ml; P = 0⋅001), TM (40⋅91 (sem 8⋅62); P = 0⋅008) and MIX15% (30⋅65 (sem 6⋅79); P = 0⋅028), however, there were no significant differences when compared with MIX50% (25⋅41 (sem 5⋅08); P = 0⋅943) (Fig. 3(A)).
Fig. 3.
Serum insulin response following 50 g of starch ingestion in healthy participants (n 16): 50 g glucose (GL); 50 g tapioca maltodextrin (TM); 50 g tapioca resistant maltodextrin (TRM); 42⋅5 g TM (85 %) + 7⋅5 g TRM (15 %) (MIX15%); and 25 g TM (50 %) + 25 g TRM (50 %) (MIX50%). (A) Serum insulin over 180 min (μIU/ml), (B) incremental serum insulin over 180 min (μIU/ml), and (C) incremental AUC of serum insulin from 0 to 180 min (iAUC insulin0–180min). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that for GL (P < 0⋅05). a,b Mean values with unlike letters were significantly different (P < 0⋅05).
As shown in Fig. 3(B), incremental serum insulin was significantly lower following TRM (8⋅40 (sem 1⋅77) μIU/ml) consumption when compared with GL (42⋅96 (sem 11⋅74) μIU/ml; P = 0⋅003), TM (47⋅99 (sem 17⋅48) μIU/ml; P = 0⋅013) and MIX50% (28⋅98 (sem 4⋅58) μIU/ml; P = 0⋅013) at 30 min. Reduction of incremental serum insulin of TRM was also observed at 60 and 120 min when compared with GL and TM (P < 0⋅05). Likewise, MIX50% (0⋅09 (sem 0⋅88) μIU/ml) attenuated incremental serum insulin at 120 min compared with GL (10⋅20 (sem 2⋅54) μIU/ml; P = 0⋅023) and TM (13⋅14 (sem 3⋅58) μIU/ml; P = 0⋅010).
The iAUC insulin0–180min of TRM was significantly lower (505⋅29 (sem 75⋅14) μIU/ml × min) when compared with GL (3372⋅03 (sem 452⋅46) μIU/ml × min; P <0⋅001), TM (3854⋅54 (sem 798⋅26) μIU/ml × min; P = 0⋅001) and MIX15%(2543⋅94 (sem 438⋅17) μIU/ml × min; P = 0⋅008), but was not significantly different when compared with MIX50% (1913⋅26 (sem 292⋅13) μIU/ml × min; P = 0⋅314). Replacement of tapioca maltodextrin by tapioca resistant maltodextrin tended to lower iAUC insulin0–180min of MIX15% by 34⋅0 % and MIX50% by 50⋅4 %, when compared with iAUC insulin0–180min of tapioca maltodextrin (P > 0⋅05) (Fig. 3(C)).
Subjective appetite
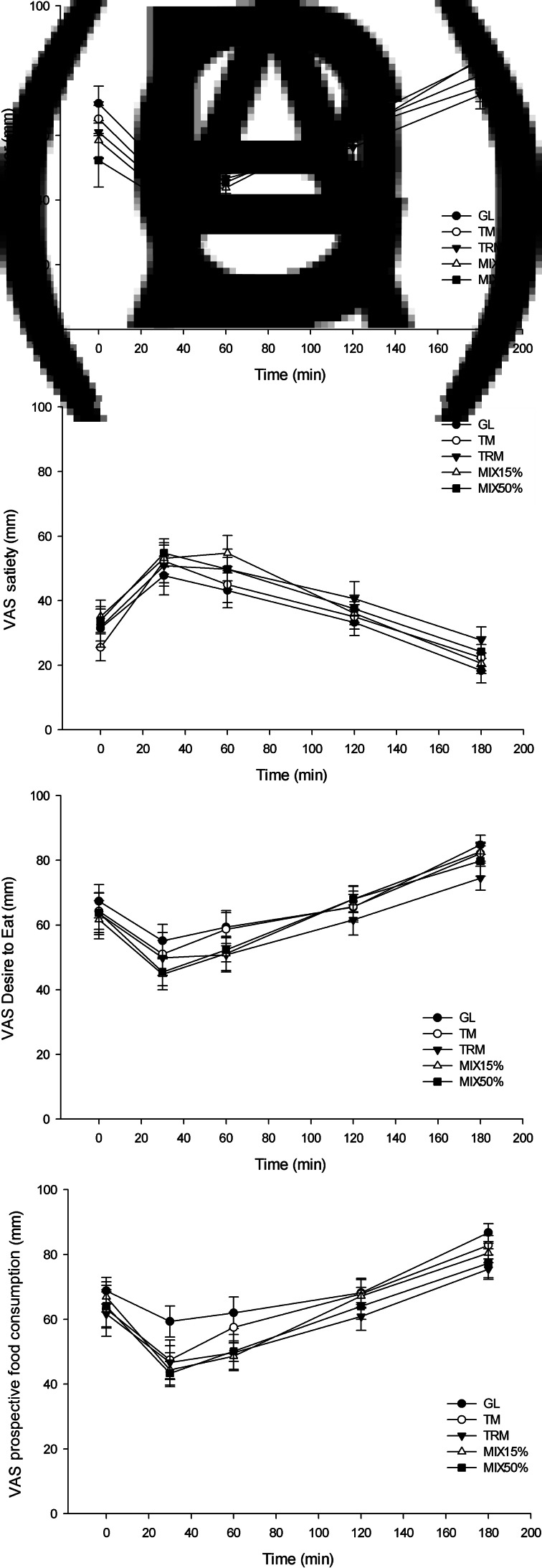
There was no significant effect of TRM on subjective appetite, including hunger, satiety, desire to eat and prospective food consumption compared with GL, TM, MIX15% and MIX50% when observed during 180 min (P > 0⋅05), as shown in Fig. 4.
Fig. 4.
Visual analogue scale (VAS; mm) subjective appetite(35) from 0 to 180 min, including: (A) hunger, (B) satiety, (C) desire to eat and (D) prospective food consumption, in healthy adults (n 16). Values are means, with their standard errors represented by vertical bars. GL, 50 g of glucose; TM, 50 g tapioca maltodextrin; TRM, 50 g tapioca resistant maltodextrin; MIX15%, 42⋅5 g TM (85 %) + 7⋅5 g TRM (15 %); MIX50%, 25 g TM (50 %) + 25 g TRM (50 %).
Gastrointestinal symptoms
The intensity of flatulence increased from 0⋅19 (sem 0⋅10) up until 0⋅88 (sem 0⋅22) (P = 0⋅005) after 24 h of 50 g TRM consumption. However, the intensity of abdominal pain and bloating insignificantly increased from 0⋅06 (sem 0⋅06 to 0⋅19 (sem 0⋅14) (P = 0⋅317) and 0⋅19 (sem 0⋅10) to 0⋅63 (sem 0⋅24) (P = 0⋅068), respectively, after 24 h of 50 g TRM consumption. No significant changes of stool form were observed before and after consumption of every treatment drink (P = 0⋅749).
Discussion
The peak of incremental plasma glucose after 50 g TRM consumption was significantly lower when compared with other treatments. Resistant starches are known to reduce carbohydrate digestion, not only by the presence of undigested starch fractions, but also through α-amylase inhibition(23,40). In addition, Sadakiyo et al.(41) found that isomaltodextrin decreased postprandial blood glucose in a carbohydrate-loading study due to inhibition of maltase and isomaltase. In the present study, 5 and 10 mg/ml of tapioca resistant maltodextrin were able to inhibit 38–50 % of amylase activity (in vitro). However, further studies are required to investigate the detailed mechanism of amylase inhibition by tapioca resistant maltodextrin.
The peaks of postprandial plasma glucose after TRM, TM and glucose ingestion were at 30 min. This result is similar to a study by Crapo et al.(42), which showed that the peak plasma glucose following glucose drink consumption occurred at 30 min. The level of postprandial plasma glucose depends on many factors, such as meal composition, gastric emptying status, insulin release and action, glucose absorption in the small intestine, as well as the capability of glucose metabolism. Meal composition may affect gastric emptying through the actions of GLP-1 and glucose-dependent insulinotropic peptide (GIP) that influence the rate of glucose absorption in the small intestine(43). This study demonstrates the beneficial effect of the replacement of digestible carbohydrate by resistant starch, particularly TRM, on postprandial glucose and insulin. Reduction of the amounts of digestible glucose or increases in indigestible starch could be causes. The mechanism of this beneficial effect is yet to be determined. A minimum of 14 % replacement of digestible starch by resistant starch is needed to reduce postprandial glycaemia(8). However, also in this study, replacement of TM by TRM of up to 50 % did not significantly reduce the incremental plasma glucose but slightly lowered glucose iAUC from 0 to 180 min in a dose-dependent manner. The iAUC reflects the glycaemic response to food accurately; the higher available carbohydrate content in a meal, the greater the increase of iAUC glucose(44). A previous study also showed that incorporation of resistant starch type 4 into high-glycaemic index food significantly reduced glucose iAUC over 2 h(32,45). Replacement of TM by TRM decreased the amount of readily digestible carbohydrate, and, therefore, less carbohydrate available for hydrolysis by carbohydrate digestive enzymes in the small intestine. Consequently, there was less glucose absorbed, and the glycaemic response was decreased(32).
Tapioca resistant maltodextrin significantly attenuated the insulin response when compared with other test drinks. This finding is consistent with findings of Nazare et al.(19) which stated that breakfast containing 50 g maize starch RMD lowered peak and AUC of insulin by 50⋅42 and 51⋅30 %, respectively, when compared with breakfast containing only 50 g digestible maize starch maltodextrin. As TRM is partially digested in the small intestine, it reduces the available carbohydrate absorbed into the blood circulation. Consequently, it triggers less insulin secretion. In addition, a previous study showed that consumption of 48 g resistant starch significantly reduced the postprandial insulin response, without affecting C-peptide concentration. These results indicated that resistant starch did not affect insulin secretion, but probably increased hepatic insulin clearance, and thus lowered the postprandial insulin response(46). This study demonstrates the beneficial effect of replacement of digestible carbohydrate by resistant starch, particularly TRM, on postprandial glucose and insulin.
In the present study, TRM did not significantly reduce subjective appetite over 180 min. Similarly, Emilien et al.(20) did not find any significant effect of 10–20 g of maize starch RMD on hunger and satiety when observed over 150 min. Furthermore, the participants reported that hunger and desire to eat were reduced, but satiety increased after 3⋅5–8⋅5 h following 20 g RMD consumption(20).
Tapioca resistant maltodextrin is considered as dietary fibre, which is classified as resistant starch type 5(6–8). In this study, TRM is assayed to contain 90 % dietary fibre. As previously known, dietary fibre promotes satiety through several mechanisms, including increasing intraluminal viscosity; decreasing energy density and increasing mastication; and fermentation to SCFA in the colon(5,47). Viscosity of dietary fibre also plays an important role in promoting satiety through increasing intraluminal viscosity which leads to delayed gastric emptying, as well as increased distention in the gastric antrum(48). The effect of non-viscous dietary fibre, including RMD, on hunger and satiety has been inconsistent. Ye et al.(12) found significant effects of 10 g of maize starch RMD on delaying hunger and increasing satiety over 1⋅5–2 h following meal consumption. In addition, 10 g of maize starch RMD was also found to increase plasma GLP-1 and PYY from the first hour following consumption(12). Fermentation of RMD by gut bacteria in the colon produces SCFA, including acetate, propionate, and butyrate, which stimulates satiety hormones (e.g. GLP-1 and PYY) released from intestinal L-cells and sends the satiety signal to the hypothalamus(14). A previous study showed that maize starch RMD was fermented within 6–8 h following consumption, as evidenced by the increasing of breath H2(19,49). We therefore propose that TRM has a null effect on satiety within 180 min after ingestion because of its slow fermentation in the colon.
In the present study, TRM has an average molecular weight of 2516 Da. The intensity of flatulence increased after 24 h of 50 g TRM consumption. This GI discomfort may occur as a result of TRM fermentation in the colon by gut microbiota. A previous study demonstrated that TRM was fermentable and, therefore, increased SCFA production as well as increased the number of faecal lactobacilli and bifidobacterial populations(50). However, fermentation of RMD in the colon not only produces SCFA but also gases, such as CH4, H2 and CO2. These gases may cause GI discomfort, including abdominal pain, bloating, as well as flatulence(16). GI tolerability of fermentable dietary fibre would be influenced by the physical properties of the dietary fibre itself, such as the molecular weight. Therefore, different sources of RMD may have different tolerable doses. Low-molecular-weight dietary fibre is more likely to stimulate GI disturbance when compared with high-molecular-weight dietary fibre at the same amount(51). There has been a lack of studies reporting the tolerable dose of TRM, since it was recently produced. In the present study, the highest acute tolerable dose in healthy persons was observed at MIX50% which composed of 25 g TRM and 25 g TM. It was also the highest dose where the effect was comparable with TM, without TRM added. In a previous study it was demonstrated that a single dose of maize starch RMD with molecular weight of 2000 Da at the dose of 0⋅7 g/kg body weight caused gurgling sounds and flatus. In addition, a tolerable dose was up to 1⋅0 g/kg body weight in men and 1⋅1 g/kg body weight in women(51). In another study, Pasman et al.(52) showed that a daily dose of 30–45 g of wheat starch RMD (molecular weight 2480 Da) was tolerable in healthy subjects for long-term consumption. Thus, it is important to consider the tolerable dose of TRM to use it safely.
The present study used a randomised cross-over controlled trial to minimise interindividual bias. We also provided information about the effects of full and partial replacement of digestible maltodextrin by TRM on metabolic responses, satiety and GI symptoms. The results of this study will then benefit the food manufacturing sector. However, there are some limitations. First, we did not investigate the mechanism of TRM on glucose and insulin responses. Second, we only recruited healthy participants; therefore, this result cannot be generalised beyond healthy persons, and so it cannot apply to any diabetes mellitus patients.
Further studies related to the mechanism of TRM on glucose and insulin responses are necessary to describe a clear picture of TRM and its potential use in daily life. In addition, further studies comparing the efficiency of TRM and maize starch or other sources of RMD on glucose and insulin responses are needed.
In conclusion, tapioca resistant maltodextrin is beneficial to reduce postprandial plasma glucose and serum insulin in healthy persons. In addition, partial replacement of digestible maltodextrin by TRM may reduce the amount of available carbohydrate and attenuate the glycaemic and insulinaemic responses, in a dose-dependent manner. The highest acute tolerable dose in the present study was observed at 25 g of TRM. Subjective appetite during 180 min was not significantly different between treatments in this study. The study of chronic use of TRM would be interesting for future research to confirm the beneficial effect on subjective appetite. However, a high dose of tapioca resistant maltodextrin may cause GI discomfort, mainly flatulence, due to colonic fermentation by gut microbiota which produces gases.
Acknowledgements
We would like to express our appreciation to all participants for their enthusiasm and collaboration, and our sincere gratitude to Dr Uthaiwan Suttisansanee for her support on the in vitro laboratory experiment.
The research grant funds were provided by the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship (J. A.); Thailand Research Foundation (J. A. and S. S.; grant no. RDG61l0024-37106006130205); and Banpong Novitat, Co. Ltd Foundation (J. A. and S. S.).
Both authors were involved in formulating the research questions, and designing and conducting the study. Data were analysed and interpreted by J. A. and S. S.; the article was written by J. A. and S. S.; furthermore, J. A. and S. S. read and approved the final document before submission to the editor.
The authors declare that there are no conflicts of interest.
References
- 1.Ivanovitch K, Klaewkla J, Chongsuwat R, et al. (2014) The intake of energy and selected nutrients by Thai urban sedentary workers: an evaluation of adherence to dietary recommendations. J Nutr Metab 2014, 145182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig DS, Hu FB, Tappy L, et al. (2018) Dietary carbohydrates: role of quality and quantity in chronic disease. BMJ 361, k2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidelmann SB, Claggett B, Cheng S, et al. (2018) Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 3, e419–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley SH, Hamdy O, Mohan V, et al. (2014) Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383, 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavin JL (2005) Dietary fiber and body weight. Nutrition 21, 411–418. [DOI] [PubMed] [Google Scholar]
- 6.Mermelstein NH (2009) Laboratory: analyzing for resistant starch. Food Technol (Chicago) 63, 80–84. [Google Scholar]
- 7.Astina J & Sapwarobol S (2019) Resistant maltodextrin and metabolic syndrome: a review. J Am Coll Nutr 38, 380–385. [DOI] [PubMed] [Google Scholar]
- 8.Lockyer S & Nugent AP (2017) Health effects of resistant starch. Nutr Bull 42, 10–41. [Google Scholar]
- 9.Kapusniak J & Jane J-L (2007) Preparation and characteristics of enzyme-resistant pyrodextrins from corn starch. Pol J Food Nutr Sci 57, 261–265. [Google Scholar]
- 10.Guerin-Deremaux L, Li S, Pochat M, et al. (2011) Effects of NUTRIOSE® dietary fiber supplementation on body weight, body composition, energy intake, and hunger in overweight men. Int J Food Sci Nutr 62, 628–635. [DOI] [PubMed] [Google Scholar]
- 11.Guérin-Deremaux L, Pochat M, Reifer C, et al. (2011) The soluble fiber NUTRIOSE induces a dose-dependent beneficial impact on satiety over time in humans. Nutr Res 31, 665–672. [DOI] [PubMed] [Google Scholar]
- 12.Ye Z, Arumugam V, Haugabrooks E, et al. (2015) Soluble dietary fiber (Fibersol-2) decreased hunger and increased satiety hormones in humans when ingested with a meal. Nutr Res 35, 393–400. [DOI] [PubMed] [Google Scholar]
- 13.Hira T, Ikee A, Kishimoto Y, et al. (2015) Resistant maltodextrin promotes fasting glucagon-like peptide-1 secretion and production together with glucose tolerance in rats. Br J Nutr 114, 34–42. [DOI] [PubMed] [Google Scholar]
- 14.Hira T, Suto R, Kishimoto Y, et al. (2018) Resistant maltodextrin or fructooligosaccharides promotes GLP-1 production in male rats fed a high-fat and high-sucrose diet, and partially reduces energy intake and adiposity. Eur J Nutr 57, 965–979. [DOI] [PubMed] [Google Scholar]
- 15.Canfora EE, Jocken JW & Blaak EE (2015) Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11, 577–591. [DOI] [PubMed] [Google Scholar]
- 16.Eswaran S, Muir J & Chey WD (2013) Fiber and functional gastrointestinal disorders. Am J Gastroenterol 108, 718–727. [DOI] [PubMed] [Google Scholar]
- 17.Livesey G & Tagami H (2009) Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): meta-analysis of randomized controlled trials. Am J Clin Nutr 89, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakabayashi S, Kishimoto Y, Nanbu S, et al. (1999) Effects of indigestible dextrin on postprandial rise in blood glucose levels in man. J Jpn Assoc Dietary Fiber Res 3, 13–19. [Google Scholar]
- 19.Nazare J-A, Sauvinet V, Normand S, et al. (2011) Impact of a resistant dextrin with a prolonged oxidation pattern on day-long ghrelin profile. J Am Coll Nutr 30, 63–72. [DOI] [PubMed] [Google Scholar]
- 20.Emilien CH, Zhu Y, Hsu WH, et al. (2018) The effect of soluble fiber dextrin on postprandial appetite and subsequent food intake in healthy adults. Nutrition 47, 6–12. [DOI] [PubMed] [Google Scholar]
- 21.Wolf BW, Wolever TM, Bolognesi C, et al. (2001) Glycemic response to a rapidly digested starch is not affected by the addition of an indigestible dextrin in humans. Nutr Res 21, 1099–1106. [Google Scholar]
- 22.Slavin JL, Savarino V, Paredes-Diaz A, et al. (2009) A review of the role of soluble fiber in health with specific reference to wheat dextrin. J Int Med Res 37, 1–17. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y-C & Maningat CC (2013) Resistant Starch: Sources, Applications and Health Benefits. Chichester: John Wiley & Sons. [Google Scholar]
- 24.Singh J, Dartois A & Kaur L (2010) Starch digestibility in food matrix: a review. Trends Food Sci Technol 21, 168–180. [Google Scholar]
- 25.Toraya-Avilés R, Segura-Campos M, Chel-Guerrero L, et al. (2017) Some nutritional characteristics of enzymatically resistant maltodextrin from cassava (Manihot esculenta Crantz) starch. Plant Foods Hum Nutr 72, 149–155. [DOI] [PubMed] [Google Scholar]
- 26.Srichuwong S, Sunarti TC, Mishima T, et al. (2005) Starches from different botanical sources I: contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydr Polym 60, 529–538. [Google Scholar]
- 27.Trithavisup K, Krusong K & Tananuwong K (2019) In-depth study of the changes in properties and molecular structure of cassava starch during resistant dextrin preparation. Food Chem 297, 124996. [DOI] [PubMed] [Google Scholar]
- 28.Ohkuma K, Hanno Y, Inada K, et al. (1995) Indigestible dextrin. https://patentimages.storage.googleapis.com/e8/fe/62/7ddbc3fa7efcd5/US5472732.pdf (accessed December 2018).
- 29.Oku T, Tanabe K, Morita S, et al. (2015) Digestibility of new dietary fibre materials, resistant glucan and hydrogenated resistant glucan in rats and humans, and the physical effects in rats. Br J Nutr 114, 1550–1559. [DOI] [PubMed] [Google Scholar]
- 30.Adam-Perrot A, Gutton L, Sanders L, et al. (2009) Resistant starch and starch-derived oligosaccharides as prebiotics In Prebiotics and Probiotics Science and Technology, pp. 259–291 [Dimitris C and Robert AR, editors]. New York: Springer. [Google Scholar]
- 31.Lefranc-Millot C (2008) NUTRIOSE® 06: a useful soluble dietary fibre for added nutritional value. Nutr Bull 33, 234–239. [Google Scholar]
- 32.Wong THT & Louie JCY (2017) The relationship between resistant starch and glycemic control: a review on current evidence and possible mechanisms. Starch-Stärke 69, 1600205. [Google Scholar]
- 33.Watcharachaisoponsiri T, Sornchan P, Charoenkiatkul S, et al. (2016) The α-glucosidase and α-amylase inhibitory activity from different chili pepper extracts. Int Food Res J 23, 1439–1445. [Google Scholar]
- 34.Padwal MK, Murshid M, Nirmale P, et al. (2015) Association of serum ferritin levels with metabolic syndrome and insulin resistance. J Clin Diagn Res 9, BC11–BC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blundell J, De Graaf C, Hulshof T, et al. (2010) Appetite control: methodological aspects of the evaluation of foods. Obes Rev 11, 251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacqz-Aigrain E, Kassai B, Cornu C, et al. (2015) Gastrointestinal tolerance of erythritol-containing beverage in young children: a double-blind, randomised controlled trial. Eur J Clin Nutr 69, 746–751. [DOI] [PubMed] [Google Scholar]
- 37.Lewis SJ & Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32, 920–924. [DOI] [PubMed] [Google Scholar]
- 38.Brouns F, Bjorck I, Frayn K, et al. (2005) Glycaemic index methodology. Nutr Res Rev 18, 145–171. [DOI] [PubMed] [Google Scholar]
- 39.Devitt AA, Williams JA, Choe YS, et al. (2013) Glycemic responses to glycemia-targeted specialized-nutrition beverages with varying carbohydrates compared to a standard nutritional beverage in adults with type 2 diabetes. Adv Biosci Biotechnol 4, 1–10.24883227 [Google Scholar]
- 40.Patel H, Royall PG, Gaisford S, et al. (2017) Structural and enzyme kinetic studies of retrograded starch: inhibition of α-amylase and consequences for intestinal digestion of starch. Carbohydr Polym 164, 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadakiyo T, Ishida Y, Inoue SI, et al. (2017) Attenuation of postprandial blood glucose in humans consuming isomaltodextrin: carbohydrate loading studies. Food Nutr Res 61, 1325306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crapo PA, Reaven G & Olefsky J (1976) Plasma glucose and insulin responses to orally administered simple and complex carbohydrates. Diabetes 25, 741–747. [PubMed] [Google Scholar]
- 43.Marathe CS, Rayner CK, Jones KL, et al. (2013) Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 36, 1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Floch J-P, Escuyer P, Baudin E, et al. (1990) Blood glucose area under the curve: methodological aspects. Diabetes Care 13, 172–175. [DOI] [PubMed] [Google Scholar]
- 45.Al-Tamimi EK, Seib PA, Snyder BS, et al. (2009) Consumption of cross-linked resistant starch (RS4XL) on glucose and insulin responses in humans. J Nutr Metab 2010, 651063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodinham CL, Frost GS & Robertson MD (2010) Acute ingestion of resistant starch reduces food intake in healthy adults. Br J Nutr 103, 917–922. [DOI] [PubMed] [Google Scholar]
- 47.Slavin JL (2008) Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 108, 1716–1731. [DOI] [PubMed] [Google Scholar]
- 48.Slavin J & Green H (2007) Dietary fibre and satiety. Nutr Bull 32, 32–42. [Google Scholar]
- 49.Goda T, Kajiya Y, Suruga K, et al. (2006) Availability, fermentability, and energy value of resistant maltodextrin: modeling of short-term indirect calorimetric measurements in healthy adults. Am J Clin Nutr 83, 1321–1330. [DOI] [PubMed] [Google Scholar]
- 50.Sorndech W, Rodtong S, Blennow A, et al. (2019) Impact of resistant maltodextrins and resistant starch on human gut microbiota and organic acids production. Starch-Stärke 71, 1800231. [Google Scholar]
- 51.Kishimoto Y, Kanahori S, Sakano K, et al. (2013) The maximum single dose of resistant maltodextrin that does not cause diarrhea in humans. J Nutr Sci Vitaminol (Tokyo) 59, 352–357. [DOI] [PubMed] [Google Scholar]
- 52.Pasman W, Wils D, Saniez M, et al. (2006) Long-term gastrointestinal tolerance of NUTRIOSE® FB in healthy men. Eur J Clin Nutr 60, 1024–1034. [DOI] [PubMed] [Google Scholar]