Abstract
Critically ill patients with sepsis or septic shock are at an increased risk of death. Early and aggressive interventions are essential for improving clinical outcomes. There are a number of therapeutic and practical challenges in the management of antimicrobials in patients with sepsis. These include the timely selection and administration of appropriate antimicrobials, significant physiological alterations that can influence antimicrobial pharmacokinetics, and significant interpatient variability of antimicrobial concentrations using standard dosing approaches. Understanding the impact of these factors on the probability of attaining pharmacokinetic-pharmacodynamic target goals is essential to guide optimal therapy. Using rapid diagnostic technology could facilitate timely selection of antimicrobials, and therapeutic drug monitoring would provide a more individualized dosing approach. Using an interdisciplinary sepsis team would also be beneficial in coordinating efforts to overcome the challenges encountered during this critical period to ensure optimal care.
Keywords: dosing, treatment, antibiotics, physiological alterations, monitoring, pharmacokinetics
Sepsis and septic shock are common syndromes in critically ill patients in intensive care units (ICUs) as well as emergency departments (EDs); their optimal management is multifaceted and poses unique challenges to clinicians. The prevalence of sepsis in different settings and the impact on mortality rates are discussed in detail by other authors in this supplement. Antimicrobial therapy is an essential component of the overall management, and should be ideally targeted based on the underlying cause of sepsis. However, the causes can vary widely and are dependent on the demographics (eg, neonates, elderly persons) as well as the underlying medical conditions of the patients (eg, comorbid conditions, recent surgery, and immunosuppression). The purpose of the current article is to provide an overview of the underlying factors contributing to the complexity of managing patients with sepsis, with special emphasis on the selection and dosing of antimicrobial agents.
IMPACT OF TIMELY ADMINISTRATION AND APPROPRIATE ANTIMICROBIALS ON MORTALITY RATES
The initial management of sepsis and septic shock involves a number of coordinated efforts, including aggressive resuscitation, identifying causes and pursuing source control, optimizing hemodynamics, glycemic control, and timely initiation of empiric antimicrobial therapy. The Surviving Sepsis Campaign updated its 2016 guidelines with a new sepsis “hour-1 bundle,” with the recommendation to begin resuscitation and management immediately [1, 2]. Data suggest that optimal outcomes are associated with early administration of appropriate empiric intravenous antimicrobials after recognition of sepsis or septic shock [3–5]. Each hour of delay in administration of appropriate antimicrobials is associated with decreased survival. Kumar et al [3] found that initiation of appropriate antimicrobials within the first hour after onset of septic shock was associated with 79.9% survival to hospital discharge. Survival dropped an average of 7.6% with every hour of delay in appropriate administration of antimicrobials in the first 6 hours after the onset of hypotension.
A more recent retrospective study by Seymour et al [4] evaluated the impact of adhering to a 3-hour sepsis bundle in patients with severe sepsis or septic shock in the ED. The authors found an association between the time to administration of antimicrobials and in-hospital mortality rate, with an odds ratio (OR) of 1.04 per hour (95% confidence interval [CI], 1.03–1.06) (P < .001). The odds of in-hospital mortality rate was 14% higher among patients who received antimicrobials between hours 3 and 12 (OR [95% CI], 1.14 [1.06–1.22]; P = .001) [4]. Liu et al [5] found similar results among patients with sepsis who received antimicrobials within 6 hours after presentation to the ED. The adjusted ORs (95% CIs) for in-hospital mortality rate based on timing of antimicrobials after presentation were 1.09 per hour (1.00–1.19) (P = .046) for patients with sepsis, 1.07 per hour (1.01–1.24) (P = .014) for those with severe sepsis, and 1.14 per hour (1.06–1.23) (P = .001) for those with septic shock. Definitions for sepsis severity in these studies were based on prior international consensus guidelines [6].
Selection of empiric antimicrobial therapy must take into consideration suspected source of infection, prevalent pathogens within the community or hospital, prior surveillance and clinical culture data from the patient, resistance patterns of prevalent pathogens, and the patient’s comorbid conditions, including immune status. Inappropriate empiric therapy is associated with increased mortality rates in patients with septic shock [7]; thus, broad-spectrum empiric antimicrobial therapy is often warranted to ensure coverage against likely pathogen(s).
Numerous studies have found that prompt administration of appropriate empiric antimicrobials is critical in achieving favorable outcomes [8–10]. In studies evaluating infections with Pseudomonas and extended-spectrum β-lactamase–producing Enterobacteriales, an appropriate empiric antimicrobial was defined as administration of an antimicrobial regimen, within 24 hours of culture collection, that was active against the identified pathogen based on in vitro susceptibility testing [11]. In contrast, the Surviving Sepsis Campaign’s hour-1 bundle recommendations promote the timely administration of empiric antimicrobials within 1 hour of presentation of sepsis or septic shock [2]. In patients at risk for infection with a multidrug-resistant pathogen, empiric combination therapy using antimicrobials from ≥2 different classes (eg, β-lactam with an aminoglycoside) is recommended [11, 12]. Rapid molecular diagnostics can assist in the rapid identification of pathogens as well as important mechanisms of resistance, which may aid in selecting of empiric antimicrobial therapy. Early de-escalation of combination therapy is recommended based on clinical response.
IMPACT OF PHYSIOLOGICAL ALTERATIONS ON PHARMACOKINETICS IN CRITICALLY ILL PATIENTS
Critically ill patients undergo significant physiological alterations that affect the pharmacokinetics (PK) of antimicrobials based on their physiochemical properties (Table 1). These alterations generally affect different PK processes, including absorption, distribution, metabolism, and elimination. In septic shock, blood flow is preferentially shunted to vital organs, including the brain and heart, whereas flow to other organs, including the gastrointestinal tract or subcutaneous tissue, may be reduced [13]. As a result, drug absorption may be altered in a hemodynamically compromised patient (with hypotension and shock) when administered via the gastrointestinal tract or subcutaneously. The literature evaluating the impact of various vasopressors on splanchnic perfusion is conflicting [14]. Intravenously administered drugs are recommended during the acute phase of sepsis or septic shock to avoid these concerns.
Table 1.
Physicochemical and Pharmacokinetic Characteristics of Antimicrobials Commonly Used to Treat Sepsis
| Antimicrobial | Hydrophilic | Lipophilic | Highly Protein Bound (>70%) | Hepatic Metabolism | Renal Elimination |
|---|---|---|---|---|---|
| Amikacin | √ | √ | |||
| Azithromycin | √ | √ | |||
| Aztreonam | √ | √ | |||
| Cefazolin | √ | √ | |||
| Cefepime | √ | √ | |||
| Ceftazidime | √ | √ | |||
| Ceftriaxone | √ | √ | |||
| Ciprofloxacin | √ | ||||
| Clindamycin | √ | √ | √ | ||
| Daptomycin | √ | √ | √ | ||
| Doripenem | √ | √ | |||
| Doxycycline | √ | √ | √ | ||
| Ertapenem | √ | √ | √ | ||
| Gentamicin | √ | √ | |||
| Levofloxacin | √ | ||||
| Linezolid | √ | ||||
| Meropenem | √ | √ | |||
| Metronidazole | √ | √ | |||
| Minocycline | √ | √ | √ | ||
| Nafcillin | √ | √ | |||
| Oxacillin | √ | √ | |||
| Piperacillin-tazobactam | √ | √ | |||
| Tigecycline | √ | √ | √ | ||
| Tobramycin | √ | √ | |||
| Vancomycin | √ | √ |
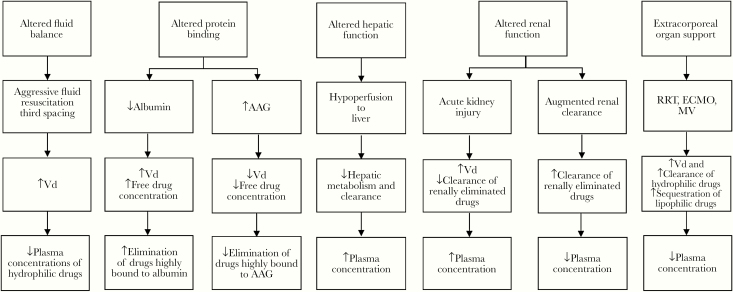
Several factors may influence the volume of distribution (Vd) in a critically ill patient (Figure 1). Fluid balance is altered in critically ill patients owing to aggressive fluid resuscitation in response to hypotension and/or third spacing. These factors lead to an increase in Vd, which in turn decrease plasma drug concentrations with standard dosing. This effect is more pronounced with hydrophilic antimicrobials, including aminoglycosides, β-lactams, glycopeptides, and lipopeptides [15, 16]. The influence of altered protein binding may be clinically important on antimicrobial PK, particularly in highly protein-bound antimicrobials (eg, cefazolin, ceftriaxone, ertapenem, and daptomycin) [17]. Hypoalbuminemia is common in critically ill patients and may lead to an increase in both Vd and elimination of unbound acidic antimicrobials. In addition, as part of the stress response during critical illness, patients may have increased expression of another plasma protein, α1-acid glycoprotein. This acute-phase reactant may bind to basic drugs and decrease free drug concentrations [13].
Figure 1.
Physiological alterations and the effects on antimicrobial pharmacokinetics. Abbreviations: AAG, α1-acid glycoprotein; ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation; RRT, renal replacement therapy; Vd, volume of distribution.
In sepsis or septic shock, hypoperfusion may lead to “shock liver” and significant hepatic dysfunction. This may lead to alterations in hepatic enzyme activity as well as hepatic blood flow, which influence drug metabolism and clearance, respectively [13, 15].
Acute kidney injury (AKI) is a common manifestation in critically ill patients, which leads to a decrease in glomerular filtration rate (GFR). Because renal drug clearance is often proportionally linked to GFR for hydrophilic drugs, modifications to dosing regimen are warranted, depending on the degree of renal impairment. However, it is important to note that dose modifications should take into consideration not only the risk of drug accumulation in AKI, but also the potential need for increased loading doses in the setting of increased Vd. Given that AKI may be transient during the initial phase of sepsis or septic shock, deferring renal adjustment of antimicrobials with a wide therapeutic index has been recommended [18]. In addition, treatment interventions with fluid resuscitation and vasopressors can lead to an early increase in cardiac output and enhance renal blood flow, thus augmenting renal clearance in some patients [19]. Augmented renal clearance is defined as a GFR of ≥130 mL/min and can increase the clearance of antimicrobials primarily eliminated by the kidneys (eg, β-lactams, aminoglycosides, and glycopeptides). Thus, patients with augmented renal clearance may have suboptimal antimicrobial exposure at standard doses and require dose modifications [20].
During critical illness, using equations such as the Cockcroft-Gault or the Modification of Diet in Renal Disease equation to estimate renal function may be inaccurate given that they use a single serum creatinine concentration. Critically ill patients tend to have rapidly changing renal function; thus, alternative approaches for assessment may be warranted. These include the following: measuring urine creatinine and urine output; measuring novel renal biomarkers, such as cystatin C; performing therapeutic drug monitoring (TDM) for β-lactams or other agents to assess clearance; and using a kinetic estimated GFR equation, which takes into account the magnitude of changes in serum creatinine relative to baseline [21, 22].
IMPACT OF MEDICAL (DEVICE) INTERVENTIONS ON PK IN CRITICALLY ILL PATIENTS
Critically ill patients require prompt interventions which include not only administration of life-saving drugs, but often mechanical support for failing organs. Some of these mechanical support devices alter the PK of antimicrobials and must be taken into account. Patients with sepsis or septic shock often have severe AKI, requiring the initiation of renal replacement therapy (RRT). Several RRT modalities are available, with the most common including continuous RRT, intermittent hemodialysis, or sustained low-efficiency dialysis. The use of these RRT modalities help support renal function, but can also augment drug clearance. Drug clearance can be highly variable owing to many factors, including mode of dialysis, drug properties, degree of protein binding, type of membrane, dialysate flow rate, and duration of dialysis. An understanding of these factors is necessary to optimize antimicrobial dosing, particularly because patients receiving continuous RRT will often require higher doses due to extracorporeal drug clearance [23, 24].
Mechanical ventilation may be necessary in patients with acute respiratory failure and may alter PK of antimicrobials. Positive pressure ventilation may increase intrathoracic pressure which in turn decreases venous return to the heart. As a result, there is a decrease in cardiac output and increase in Vd [25]. In addition, there is a subsequent decrease in renal and hepatic blood flow, which may affect antimicrobial clearance via these routes. Furthermore, atrial compression by distended lungs may lead to a decreased release of α-atrial natriuretic peptide. A decline in α-atrial natriuretic peptide levels contributes to fluid retention, which may in turn increase Vd [26, 27]. Conflicting data have been reported on the effect of positive end-expiratory pressure. The use of positive end-expiratory pressure in mechanical ventilation may be associated with an increase in antidiuretic hormone secretion and subsequent fluid retention [28, 29]; Vd may consequently be increased in these patients.
The use of extracorporeal membrane oxygenation (ECMO) has become more common in patients with severe respiratory and/or cardiac failure. Consequently, ECMO can further increase the PK alterations of antimicrobials in critically ill patients. The Vd may be increased in ECMO owing to hemodilution or drug sequestration. Hydrophilic drugs are more affected by fluid shifts and hemodilution when ECMO is initiated; this may result in decreased systemic drug concentrations and therapeutic failure [30]. Lipophilic and highly protein-bound drugs are more likely to be sequestered by the ECMO circuits and oxygenator membrane [31]. Understanding the physicochemical properties of antimicrobials can help determine the influence of ECMO on their PK and, in turn, help with designing an appropriate antimicrobial dosing regimen in different clinical scenarios (Table 1).
ANTIMICROBIAL PK/PD PARAMETERS FOR OPTIMAL EFFICACY
To optimize antimicrobial dosing, it is important to understand the 3 most common PK–pharmacodynamic (PK/PD) indices that best describe optimal efficacy. The 3 PK/PD indices include fT > MIC, where fT indicates the time the free (unbound) antimicrobial concentration remains above the minimum inhibitory concentration (MIC) of the organism; Cmax/MIC, the ratio between the maximum concentration of free drug and the MIC; and AUC0–24/MIC, the ratio between the total area under the concentration-time curve (AUC) over a 24-hour period and the MIC. Patients are more likely to have positive outcomes when PK/PD targets associated with efficacy are achieved. PK/PD target goals for different antimicrobial classes have been proposed [32]. However, many factors may influence the probability of attaining these PK/PD targets during critical illness (eg, significant pathophysiological changes affecting PK, pathogen with MIC at or near breakpoint). Given the considerable variation in PK among critically ill patients, using a standard dosing regimen in this population may lead to suboptimal dosing and increase the risk for clinical failure or adverse effects.
DOSING STRATEGIES TO OPTIMIZE PK/PD OF ANTIMICROBIALS
β-Lactams are commonly used in sepsis or septic shock, given their broad spectrum of activity and wide therapeutic index. As time-dependent antimicrobials, they exhibit improved PK/PD profiles when the concentration is maintained above a threshold (eg, MIC or multiples of MIC) for a longer duration. This can be accomplished by more frequent dosing or extended or continuous infusions. However, the clinical impact of extended or continuous infusions on clinical outcome is less clear.
A systematic review by Roberts and colleagues [33] evaluated the clinical benefits of extended or continuous infusion β-lactams. The authors included randomized controlled trials along with relevant observational studies, and that found that continuous- or extended-infusion β-lactams were not associated with significant improvement in clinical cure or mortality rates in these studies [33]. However, they did note significant benefits in 2 observational studies, particularly in critically ill patients with Pseudomonas aeruginosa infections [34, 35]. A study by Arnold and colleagues [36] did not find a significant benefit of prolonged versus intermittent infusion for the empiric treatment of gram-negative infections in the ICU. The authors postulated that perhaps there was no benefit due to very few isolates with elevated MICs in their study. They also acknowledged that an initial loading dose was not used before beginning the prolonged infusion, which may delay the time to target attainment [36]. These are important considerations when determining who would most benefit from prolonged infusion.
A 2018 systematic review and meta-analysis [37] compared prolonged versus short-term infusion of antipseudomonal β-lactams in patients with sepsis. The review included only randomized, controlled trials and evaluated all-cause mortality rate as the primary end point. Overall, prolonged infusion was associated with a significantly lower all-cause mortality risk compared to short-term infusion. Furthermore, a subgroup analysis found that studies using a loading dose in the prolonged arm had a significant reduction in mortality rate [37]. Despite inconsistent findings among available studies, there may be an overall benefit of prolonged infusion of β-lactams in a select population including critically ill patients or patients with infections due to pathogens associated with high MICs [38, 39].
A multicenter, randomized, controlled trial (BLING III) is currently underway comparing continuous versus intermittent infusion of β-lactams in the ICU and may definitively answer whether prolonged infusion is associated with decreased mortality rates compared with intermittent infusion [40]. Because prolonged infusion is being implemented more widely, it is important to emphasize the need for a loading dose to rapidly achieve target concentrations, which should be recommended irrespective of renal function. Without a loading dose, β-lactams administered by prolonged infusion may take hours to reach adequate concentrations [41]. In critically ill patients, this is particularly worrisome because timely administration of appropriate antimicrobials and achievement of target PK/PD is essential for favorable outcomes.
Vancomycin continues to be widely used for the treatment of infections due to gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA). The PK/PD parameter that best predicts its efficacy is AUC24/MIC, with a target of ≥400 mg • h/L associated with improved outcomes [42]. Widespread availability and routine use of TDM for vancomycin allows for individualization of vancomycin dosing to achieve target AUC24/MIC. Furthermore, several bayesian dose-optimizing software are commercially available to aid in estimating AUC with single or multiple vancomycin levels [43]. An update to the 2009 vancomycin guidelines is pending release and is expected to provide more refined PK/PD targets and potential dosing strategies to achieve them.
RENAL FUNCTION DURING EMPIRIC MANAGEMENT
Patients with sepsis or septic shock may present with significant physiological changes, including AKI. Antimicrobial dosing may be considerably influenced by renal function, and any adjustments should be made thoughtfully. Dose adjustment protocols for renal dysfunction are recommended per the manufacturer labeling for many antimicrobials. However, these dose adjustment recommendations are based on data from patients enrolled in clinical trials with chronic kidney disease and may not reflect the transient nature of AKI experienced during critical illness.
Crass and colleagues [18] evaluated patients with AKI on admission, as defined by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [44]. They found that >50% of patients with AKI on admission had resolution (defined as resolution of KDIGO criteria) within 48 hours. In patients with moderate renal impairment on admission (creatinine clearance or estimated GFR 30–50 mL/min/1.73 m2), 42.8%–45.9% of patients had improvement of renal function greater than 50 mL/min/1.73 m2) by 48 hours. Based on these findings, the authors suggested deferring dose adjustments of antimicrobials with a wide therapeutic index (ie, β-lactams) until >48 hours after initiation of therapy, when renal function is better characterized [18]. Given the importance of achieving optimal antimicrobial therapy within the first 48 hours in sepsis or septic shock, premature renal dose adjustment of antimicrobials may increase the risk of suboptimal concentrations and poor outcomes [45].
INTERSUBJECT PK VARIATIONS AND THE ROLE OF THERAPEUTIC DRUG MONITORING
During critical illness, there may be considerable variation in antimicrobial concentrations from patient to patient and a standard dosing approach may not fit all. The DALI study was a prospective, multinational PK study that assessed whether β-lactam antibiotic dosing in critically ill patients achieved concentrations necessary for maximal activity and whether this impacted patient outcomes. The primary PK/PD targets in this study were 50% fT > MIC and 100% fT > MIC of the dosing interval. The authors of this study found large variations in β-lactam concentrations, with 16% of patients being treated for infections not achieving the conservative PK/PD target of ≥50% fT > MIC. Of note, they found that a positive clinical outcome was associated with a higher PK/PD ratio, which was consistent with findings of other studies [46–48]. The findings of this study reinforce the need to refine antimicrobial dosing strategies in critically ill patients. Given the large variability in these patients, a more individualized dosing approach may be warranted to ensure optimal target PK/PD exposures.
TDM may be particularly useful in this critically ill population, given the variability in antimicrobial concentrations observed with standard dosing regimens. β-Lactams are generally regarded as well-tolerated, so TDM has historically been limited to aminoglycosides and glycopeptides for toxicity monitoring. The adoption of TDM for β-lactams is not as widespread and is not routinely available in most hospitals. However, given our understanding of altered PK in critical illness, TDM may be useful to maximize probability of target PK/PD attainment and improve clinical outcomes with β-lactams [49]. An international multicenter survey was conducted to examine the use of β-lactam TDM in ICUs. The study included responses from 9 TDM-using ICUs, predominantly in Europe, and demonstrated substantial variation in their TDM practices including the selection of β-lactams for TDM, the patient population in which TDM was performed, drug assay methods, PK/PD targets, and dose adjustment strategies [50].
To obtain optimal β-lactam concentrations, appropriate dose adjustments in response to TDM are necessary. Dose adjustment strategies will need to consider patient-specific culture data (eg, MIC of pathogen and site of infection), PK/PD targets, and initial method of drug administration (eg, intermittent vs prolonged infusion). Of note, dose adjustments based on TDM and the MIC of the infecting pathogen should be done cautiously, because MIC measurements are imperfect owing to inherent assay variation. There may be a potential risk of underdosing a patient if a single measured MIC happened to be on the lower end of a range of MIC values observed, if testing had been performed repeatedly. Thus, it may be prudent to increase the PK/PD target of β-lactams to 100% fT > MIC or multiples of MIC in critically ill patients to account for possible variation in MIC measurements [51]. Although there is currently not a formal dosing nomogram for critically ill patients, an evidence-based, pragmatic, adult dosing nomogram has been developed for this population with validation studies underway; this may serve as a useful tool until TDM becomes more readily available [45].
ROLE OF RAPID DIAGNOSTIC TESTING IN TREATMENT APPROACH
Given the critical nature of sepsis and septic shock, it is imperative that appropriate antimicrobial therapy be initiated without delay. There has been an increasing trend to adopt rapid diagnostic technology in routine clinical microbiology workups to aid in quick identification of pathogens (please also see Perez et al in this supplement). Several proprietary platforms for rapid diagnostic testing have been approved for bacteremia, each with different ranges of organisms and mechanisms of resistance they could identify [52]. The ability to rapidly identify both the infecting organism and presence of select antimicrobial resistance markers enables improved guidance in selection of empiric antimicrobial therapy; this is particularly important when escalation of therapy is warranted.
Although rapid diagnostic testing can help guide early antimicrobial selection by providing information on presence of antimicrobial resistance markers, most platforms do not routinely provide rapid antimicrobial susceptibility testing (AST) or MICs. At this time, there is a single available commercial platform with the ability to provide MIC and phenotypic AST results for several antimicrobial agents targeting gram-positive and gram-negative organisms within 7 hours after positive blood culture. Studies that have compared AST results from this platform with routine AST methods (eg, broth microdilution or disk diffusion) have found overall essential and categorical agreement of ≥95% [53, 54]. The ability to provide rapid AST and MICs is invaluable for early optimization of MIC-based PK/PD dosing of antimicrobials in critically ill patients.
PRACTICAL ISSUES IN THE ICU AND THE ED
As discussed above, initial antimicrobial dose selection and timely administration of the antimicrobial after recognition of sepsis are essential aspects of sepsis management. Using order sets in electronic medical records is a helpful method for ensuring evidence-based dosing for septic patients, particularly in the ED [55, 56]. Order sets that include both a one-time “stat” loading dose linked to a maintenance dose can ensure timely administration of an adequate initial dose regardless of renal function, but they also provide a reminder to ensure that subsequent doses are not forgotten or delayed.
A retrospective analysis [57] of 828 patients with sepsis and septic shock found that 1 in 3 patients experienced a delay in receiving their second antimicrobial dose that was ≥25% of the intended dosing interval, particularly with antimicrobials given at 6-hour or 8-hour intervals. In an exploratory multivariable analysis, major delays in administration of subsequent doses was associated with an increased hospital mortality rate (OR, 1.61; 95% CI, 1.01–2.57) and mechanical ventilation (2.44; 1.27–4.69). The risk of delay in second doses was ironically higher in patients who had initially received 3-hour sepsis bundle–compliant care [57]. This study highlights the need for interdisciplinary collaboration and coordination with pharmacists, particularly between transitions in care for patients with sepsis (eg, between the ED or the floor and the ICU) and the need for electronic means (eg, electronic medical record prompts) to ensure that optimal care continues outside the initial bundle time window.
Other means to facilitate timely, appropriately dosed antimicrobials for septic patients include interdisciplinary sepsis teams, easily accessible antimicrobials and potentially concomitant administration of compatible broad-spectrum agents in a single infusion bag. Compounded combination antimicrobial bags that contain broad-spectrum coverage of MRSA, Pseudomonas, and Enterobacteriales can facilitate timely antimicrobial administration. An evaluation of the impact of a compounded bag containing 2 g of cefepime and 1 g of vancomycin decreased the median time to administration of antimicrobials from 135 to 72 minutes (P < .001), compared with conventional administration of antimicrobials [58]. There are several limitations to this strategy, however, one being the limitation of dosing options with precompounded combinations, particularly for narrow therapeutic index drugs such as vancomycin, for which a 1-g dose may be an inadequate loading dose for many patients based on body weight.
In addition, the compatibility of other antimicrobial combinations must be considered, because certain formulations of piperacillin-tazobactam, a commonly used broad-spectrum β-lactam for empiric sepsis therapy, have mixed data on stability with vancomycin [59]. In a patient in whom empiric therapy for both MRSA and Pseudomonas is being started, the broad-spectrum β-lactam (eg, cefepime or piperacillin-tazobactam) should be administered first before the MRSA coverage (eg, vancomycin) given its broader spectrum of activity and shorter infusion times for the initial dosing. The use of a combination infusion bag may facilitate the administration time of both antimicrobials but does slow the administration of the β-lactam to accommodate the vancomycin. If such a strategy is pursued, the formulation of the combination bag requires expert input regarding drug stability and compatibility and to ensure that the chosen combination reflects local infection and drug resistance patterns.
INNOVATIVE PRACTICE MODEL
Many institutions have initiated “code sepsis” teams to rapidly respond to patients with suspected sepsis. Clinical pharmacists on these teams can assist with the selection and dosing of initial antimicrobials, initial weight-based fluid resuscitation, and prompt bedside delivery of medications. One such team demonstrated a significant reduction in time to initiation of appropriate empiric antimicrobial therapy, by 48 minutes (decrease from 126 vs 78 minutes; P < .001) [60]. Improving access to broad-spectrum antimicrobials by ensuring placement in ED and ICU automated dispensing cabinets can also improve timely administration. A retrospective evaluation of antimicrobial administration time in patients presenting with severe sepsis to a community ED found that the mean time from order to antimicrobial administration was reduced by 29 minutes (55 vs 26 minutes, respectively; 95% CI, 12.5–45.19 minutes), and the time from arrival to antimicrobial administration by 70 minutes (167 vs 97 minutes; 95% CI, 37.53–102.29 minutes) when broad-spectrum antimicrobials were added to the automated dispensing cabinets [61].
It must be emphasized that early and rapid administration of broad-spectrum antimicrobials empirically in sepsis should be balanced by prompt de-escalation of antimicrobial therapy based on culture results as well as diagnostic findings, to reduce selection pressure for resistant pathogens. A prospective, observational study conducted in ICU patients admitted to a hospital in Spain with severe sepsis and septic shock evaluated the impact of antimicrobial de-escalation in 628 patients. The initial antimicrobial regimen was de-escalated in 35% of patients, with in-hospital mortality rates 27% in patients with therapy de-escalation, 33% with no treatment change, and 43% with treatment escalation (P = .006); de-escalation was actually found to be protective of in-hospital mortality rates in the propensity-score adjusted multivariate regression analysis (OR, 0.55). When illness severity and other confounders potentially associated with in-hospital mortality rates were controlled for, de-escalation of antimicrobials was not only safe but was also beneficial for patients [62]. Antimicrobial stewardship programs should incorporate evaluation of empiric sepsis regimens to identify opportunities for de-escalation based on cultures and diagnostic findings as part of their routine practice.
CONCLUSIONS
The management of critically ill patients with sepsis or septic shock is complex and must take into consideration multiple factors in order to provide the most optimal care. Timely initiation of appropriately dosed antimicrobial therapy is crucial in the management of patients with sepsis. Using a sepsis order set and improving access to broad-spectrum antimicrobials in the ED and ICU can facilitate selection as well as prompt administration of antimicrobials. A major challenge in the management of critically ill patients involves the significant physiological alterations that take place and can affect the PK properties of antimicrobials. Standard dosing strategies in critically ill patients are unlikely to consistently achieve target PK/PD parameters for efficacy, specifically with β-lactams. This can lead to an increased risk of both clinical failure and the development of antimicrobial resistance. Implementing different dosing strategies (eg, prolonged or continuous infusion, loading doses) may help optimize the PK/PD of antimicrobials. Given the significant intersubject variability in antimicrobial concentrations, using an individualized dosing approach with β-lactam TDM would be advantageous once it is widely available.
Notes
Financial support. This work is supported in part by the National Institutes of Health (grant R01AI140287-01 to V. H. T.).
Supplement sponsorship. This supplement is sponsored by bioMérieux, the Gordon and Betty Moore Foundation and Beckman Coulter.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–377. [DOI] [PubMed] [Google Scholar]
- 2. Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med 2018; 46:997–1000. [DOI] [PubMed] [Google Scholar]
- 3. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 4. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med 2017; 196:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31:1250–6. [DOI] [PubMed] [Google Scholar]
- 7. Kumar A, Ellis P, Arabi Y, et al. ; Cooperative Antimicrobial Therapy of Septic Shock Database Research Group Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136:1237–48. [DOI] [PubMed] [Google Scholar]
- 8. Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Bilker WB, Lautenbach E. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: variability by site of infection. Arch Intern Med 2005; 165:1375–80. [DOI] [PubMed] [Google Scholar]
- 9. Kang CI, Kim SH, Kim HB, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis 2003; 37:745–51. [DOI] [PubMed] [Google Scholar]
- 10. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 2005; 49:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother 2010; 54:1742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45:486–552. [DOI] [PubMed] [Google Scholar]
- 13. Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012; 141:1327–36. [DOI] [PubMed] [Google Scholar]
- 14. Beale RJ, Hollenberg SM, Vincent JL, Parrillo JE. Vasopressor and inotropic support in septic shock: an evidence-based review. Crit Care Med 2004; 32:S455–65. [DOI] [PubMed] [Google Scholar]
- 15. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014; 77:3–11. [DOI] [PubMed] [Google Scholar]
- 16. Roberts JA, Abdul-Aziz MH, Lipman J, et al. ; International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014; 14:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 2011; 50:99–110. [DOI] [PubMed] [Google Scholar]
- 18. Crass RL, Rodvold KA, Mueller BA, Pai MP. Renal dosing of antibiotics: are we jumping the gun? Clin Infect Dis 2019; 68:1596–602. [DOI] [PubMed] [Google Scholar]
- 19. Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest 2003; 124:1053–9. [DOI] [PubMed] [Google Scholar]
- 20. Udy AA, Varghese JM, Altukroni M, et al. Subtherapeutic initial β-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 2012; 142:30–9. [DOI] [PubMed] [Google Scholar]
- 21. Bidell MR, Lodise TP. Suboptimal clinical response rates with newer antibiotics among patients with moderate renal impairment: review of the literature and potential pharmacokinetic and pharmacodynamic considerations for observed findings. Pharmacotherapy 2018; 38:1205–15. [DOI] [PubMed] [Google Scholar]
- 22. Chen S. Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J Am Soc Nephrol 2013; 24:877–88. [DOI] [PubMed] [Google Scholar]
- 23. Bogard KN, Peterson NT, Plumb TJ, Erwin MW, Fuller PD, Olsen KM. Antibiotic dosing during sustained low-efficiency dialysis: special considerations in adult critically ill patients. Crit Care Med 2011; 39:560–70. [DOI] [PubMed] [Google Scholar]
- 24. Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 2005; 41:1159–66. [DOI] [PubMed] [Google Scholar]
- 25. der Merwe FV, Wallis S, Udy A. Understanding the impact of critical illness on drug pharmacokinetics—scientifically robust study design. J Clinic Toxicol 2012; S4:1–10. [Google Scholar]
- 26. Triginer C, Izquierdo I, Fernández R, et al. Changes in gentamicin pharmacokinetic profiles induced by mechanical ventilation. Eur J Clin Pharmacol 1991; 40:297–302. [DOI] [PubMed] [Google Scholar]
- 27. Perkins MW, Dasta JF, DeHaven B. Physiologic implications of mechanical ventilation on pharmacokinetics. DICP 1989; 23:316–23. [DOI] [PubMed] [Google Scholar]
- 28. Kumar A, Pontoppidan H, Baratz RA, Laver MB. Inappropriate response to increased plasma ADH during mechanical ventilation in acute respiratory failure. Anesthesiology 1974; 40:215–21. [DOI] [PubMed] [Google Scholar]
- 29. Kuiper JW, Groeneveld AB, Slutsky AS, Plötz FB. Mechanical ventilation and acute renal failure. Crit Care Med 2005; 33:1408–15. [DOI] [PubMed] [Google Scholar]
- 30. Dzierba AL, Abrams D, Brodie D. Medicating patients during extracorporeal membrane oxygenation: the evidence is building. Crit Care 2017; 21:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shekar K, Roberts JA, Mcdonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 2012; 16:R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ambrose PG, Bhavnani SM, Rubino CM, et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 2007; 44:79–86. [DOI] [PubMed] [Google Scholar]
- 33. Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med 2009; 37:2071–8. [DOI] [PubMed] [Google Scholar]
- 34. Lodise TP Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 2007; 44:357–63. [DOI] [PubMed] [Google Scholar]
- 35. Lorente L, Lorenzo L, Martín MM, Jiménez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother 2006; 40:219–23. [DOI] [PubMed] [Google Scholar]
- 36. Arnold HM, Hollands JM, Skrupky LP, et al. Prolonged infusion antibiotics for suspected gram-negative infections in the ICU: a before-after study. Ann Pharmacother 2013; 47:170–80. [DOI] [PubMed] [Google Scholar]
- 37. Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis 2018; 18:108–20. [DOI] [PubMed] [Google Scholar]
- 38. Lee YR, Miller PD, Alzghari SK, Blanco DD, Hager JD, Kuntz KS. Continuous Infusion versus intermittent bolus of beta-lactams in critically Ill patients with respiratory infections: a systematic review and meta-analysis. Eur J Drug Metab Pharmacokinet 2018; 43:155–70. [DOI] [PubMed] [Google Scholar]
- 39. Rizk NA, Kanafani ZA, Tabaja HZ, Kanj SS. Extended infusion of beta-lactam antibiotics: optimizing therapy in critically-ill patients in the era of antimicrobial resistance. Expert Rev Anti Infect Ther 2017; 15:645–52. [DOI] [PubMed] [Google Scholar]
- 40. Lipman J, Brett SJ, De Waele JJ, et al. A protocol for a phase 3 multicentre randomised controlled trial of continuous versus intermittent β-lactam antibiotic infusion in critically ill patients with sepsis: BLING III. Crit Care Resusc 2019; 21:63–8. [PubMed] [Google Scholar]
- 41. De Waele JJ, Lipman J, Carlier M, Roberts JA. Subtleties in practical application of prolonged infusion of β-lactam antibiotics. Int J Antimicrob Agents 2015; 45:461–3. [DOI] [PubMed] [Google Scholar]
- 42. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009; 49:325–7. [DOI] [PubMed] [Google Scholar]
- 43. Turner RB, Kojiro K, Shephard EA, et al. Review and validation of bayesian dose-optimizing software and equations for calculation of the vancomycin area under the curve in critically ill patients. Pharmacotherapy 2018; 38:1174–83. [DOI] [PubMed] [Google Scholar]
- 44. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–84. [DOI] [PubMed] [Google Scholar]
- 45. Williams P, Beall G, Cotta MO, Roberts JA. Antimicrobial dosing in critical care: a pragmatic adult dosing nomogram. Int J Antimicrob Agents 2020; 55:105837. [DOI] [PubMed] [Google Scholar]
- 46. Roberts JA, Paul SK, Akova M, et al. DALI Study DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58:1072–83. [DOI] [PubMed] [Google Scholar]
- 47. Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 2007; 51:1725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 2008; 31:345–51. [DOI] [PubMed] [Google Scholar]
- 49. Hayashi Y, Lipman J, Udy AA, et al. β-Lactam therapeutic drug monitoring in the critically ill: optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int J Antimicrob Agents 2013; 41:162–6. [DOI] [PubMed] [Google Scholar]
- 50. Wong G, Brinkman A, Benefield RJ, et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother 2014; 69:1416–23. [DOI] [PubMed] [Google Scholar]
- 51. Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J. MIC-based dose adjustment: facts and fables. J Antimicrob Chemother 2018; 73:564–8. [DOI] [PubMed] [Google Scholar]
- 52. Wolk DM, Johnson JK. Rapid diagnostics for blood cultures: supporting decisions for antimicrobial therapy and value-based care. J Appl Lab Med 2019; 3:686–97. [DOI] [PubMed] [Google Scholar]
- 53. Charnot-Katsikas A, Tesic V, Love N, et al. Use of the accelerate pheno system for identification and antimicrobial susceptibility testing of pathogens in positive blood cultures and impact on time to results and workflow. J Clin Microbiol 2018; 56:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pancholi P, Carroll KC, Buchan BW, et al. Multicenter evaluation of the accelerate phenotest BC kit for rapid identification and phenotypic antimicrobial susceptibility testing using morphokinetic cellular analysis. J Clin Microbiol 2018; 56:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frankel KC, Rosini JM, Levine BJ, Papas MA, Jasani NB. Computerized provider order entry improves compliance of vancomycin dosing guidelines in the emergency department. Am J Emerg Med 2013; 31:1715–6. [DOI] [PubMed] [Google Scholar]
- 56. Hall AB, Montero J, Cobian J, Regan T. The effects of an electronic order set on vancomycin dosing in the ED. Am J Emerg Med 2015; 33:92–4. [DOI] [PubMed] [Google Scholar]
- 57. Leisman D, Huang V, Zhou Q, et al. Delayed second dose antibiotics for patients admitted from the emergency department with sepsis: prevalence, risk factors, and outcomes. Crit Care Med 2017; 45:956–65. [DOI] [PubMed] [Google Scholar]
- 58. Lorenzo MP, MacConaghy L, Miller CD, et al. Impact of a combination antibiotic bag on compliance with Surviving Sepsis Campaign goals in emergency department patients with severe sepsis and septic shock. Ann Pharmacother 2018; 52:240–5. [DOI] [PubMed] [Google Scholar]
- 59. Trissel LA. Handbook on injectable drugs. 20th ed. Bethesda, MD: Oxford University Press, 2018. [Google Scholar]
- 60. Whitfield PL, Ratliff PD, Lockhart LL, et al. Implementation of an adult code sepsis protocol and its impact on SEP-1 core measure perfect score attainment in the ED. Am J Emerg Med 2019. [DOI] [PubMed] [Google Scholar]
- 61. Hitti EA, Lewin JJ 3rd, Lopez J, et al. Improving door-to-antibiotic time in severely septic emergency department patients. J Emerg Med 2012; 42:462–9. [DOI] [PubMed] [Google Scholar]
- 62. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40:32–40. [DOI] [PubMed] [Google Scholar]