Abstract
Background and aim
The pandemic of COVID-19 has put forward the public health system across countries to prepare themselves for the unprecedented outbreak of the present time. Recognition of the associated risks of morbidity and mortality becomes not only imperative but also fundamental to determine the prevention strategies as well as targeting the high-risk populations for appropriate therapies.
Methods
We reviewed, collated and analysed the online database i.e. Pubmed, Google scholar, Researchgate to highlight the demographic and mechanistic link between obesity and associated risks of severity in COVID-19.
Results
We observed a changing dynamic in the reporting from the time of initial pandemic in China to currently reported research. While, initially body mass index (BMI) did not find a mention in the data, it is now clearly emerging that obesity is one of the profound risk factors for complications of COVID-19.
Conclusion
Our review will help clinicians and health policy makers in considering the importance of obesity in making the prevention and therapeutic strategies of COVID-19. An extra attention and precaution for patients with obesity in COVID-19 pandemic is recommended.
Keywords: Obesity, COVID-19, SARS-CoV-2
Highlights
-
•
Identification of high risk group becomes fundamental to COVID-19 till the availability of potent vaccine/therapy.
-
•
Reports from USA and Europe show notable morbidity and mortality in obese individuals with COVID-19.
-
•
Extra attention and precaution for patients of COVID-19 with obesity is recommended.
1. Background
On December 31, 2019 several cases of pneumonia with an unidentified origin emerged from Wuhan, China which were reported to World Health Organisation (WHO) [1].The cause of these cases was confirmed to be severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after a week [2]. Considering its spread; the outbreak was declared a Public Health Emergency of International Concern on January 30, 2020 and later WHO announced a name for the new coronavirus disease as COVID-19 [3].
As of July 14, 2020 there have been 1,29,64,809cases and 45,70,288 deaths globally due to COVID-19 spanning across all countries with USA, Brazil and Europe reporting the maximum load [4].
While most people with COVID-19 develop no symptoms or have only mild illnesses, the evidence from China indicate that approximately 14% develop severe disease that requires hospitalisation and oxygen support, while 5% require admission to an Intensive Care Unit (ICU) [5].
Due to the mounting public health concerns about COVID 19 worldwide, scientists have been poring over data about the spectrum of clinical manifestations of COVID-19. Certain factors such as age and co-morbidities like hypertension, diabetes and cardiovascular disorders have mostly been found to be associated with severe illnesses requiring robust measures and support from health care system [6]. Such an association with obesity and high BMI has been insufficiently reported and considering it as the forerunner of many of these co-morbidities, it becomes inevitable to investigate an association of obesity with severe outcomes of COVID-19.
Nonetheless, the exact mortality rate varies greatly between regions and countries which can partly be due to the varying extent of testing and also possibly be due to differing trajectories based on population demographics and quality of health care availability.
Keeping in mind that there is lack of herd immunity and absence of an effective vaccine and antiviral therapy, countries are bound to take stringent measures to flatten the transmission curve in order to cope with the demands of health care systems. Thus, taking into account evidence and lessons from the ongoing situation of COVID-19 from different parts of the world, it becomes imperative to identify which patients are most at risk for hospitalisation. It can assist emergency care providers in making triage decisions. Further, it can help inform policymakers about highest risk populations, who may need particular protection through policy decisions. Finally, it can help epidemiologists to improve the accuracy of projections about the likely need for hospital beds and staffing in a region by knowing its demographic characteristics. Hence, we put forth the detailed evidence and changing clinical demographics related to obesity being an important risk factor for complicated outcomes in COVID-19, thereby also ascertaining the putative reasons behind it.
2. Early data on COVID-19 in obesity: evidence from China
After a scrutiny of published literature, we found a paucity of information about the BMI of cases of COVID-19 because majority of the studies from China [7] which have reported co-morbidities, did not provide any data on BMI of such patients. However, the findings from a retrospective analysis on 112 patients with COVID-19 infection admitted in a Hospital in Wuhan, between 20 January 2020 to 15 February 2020 are noteworthy. In this study, the BMI of the critical group was significantly higher than that of the general group. Patients were further divided into two groups, survivors (84.8%) and non-survivors (15.18%). Among the non-survivors, 88.2% of patients had a BMI >25 kg/m2, which was in a significantly higher proportion (P < 0.001) than in survivors (18.8%) [8]. The authors concluded that a higher BMI was more often seen in critical cases and non-survivors.
In another study from Wenzhou, China; it was found that the presence of obesity in metabolic associated fatty liver disease (MAFLD) patients was associated with a ∼6 -fold increased risk of severe COVID-19 illness (unadjusted –OR 5.77, 95% CI 1.19–27.91, p = 0.029). This association with obesity and COVID-19 severity remained significant (adjusted –OR 6.32, 95% CI 1.16–34.54, p = 0.033) even after adjusting for age, sex, smoking, diabetes, hypertension, and dyslipidaemia [9]. Moreover, Qingxian Cai et al. examined an association between obesity and severity of COVID-19 using data from only the referral hospitals in Shenzhen, China [10]. They found that overweight group showed 86% of higher odds, and obesity group showed 2·42-fold higher odds of developing severe pneumonia.
As the COVID-19 continues to spread worldwide, such an evidence from China is sufficient to provide an insight to clinicians to maintain a high level of attention for obese patients. Persons with severe obesity who become ill and require intensive care (5% of infections) present challenges in patient management like difficult intubations, difficulty in obtaining an imaging diagnosis (there are weight limits on imaging machines) and a requirement of special beds and positioning/transport equipment which might not be widely available in a majority of hospital set ups.
3. Emerging data from Europe and USA: Change in trend for risk factors of severe outcomes in COVID-19
A close survey of the available data on co-morbidity and mortality for COVID-19 across the world (Later March and April 2020); points towards the emergence of obesity as one of the major risks for complications apart from older age [[12], [13], [14], [15], [16], [17], [18]].
A descriptive study of a small sample of 24 critically ill patients diagnosed with COVID-19 in the Seattle region (USA) was among the first to report BMI data (3 patients with a BMI in the normal category, 7 with overweight, 13 with obesity and 1 with missing data). Although the numbers were too small for meaningful statistical analyses but 85% of the patients with obesity required mechanical ventilation and 62% of the patients with obesity died was a noteworthy finding. These proportions were greater than those in the patients without obesity, in which 64% required mechanical ventilation and 36% died [11].
Many recent studies are now reporting obesity as one of the risk factors for severity of COVID-19 in USA, Brazil, UK, Italy, Spain and France [12−18,67] (summarised in Table no 1 ).
Table 1.
A summary of prevalence of obesity as well as co-morbidities in Covid-19 from review of the current literature.
| First Author | City/Country | No of subjects | Obesity (%) | Adverse clinical outcomes in obese |
|---|---|---|---|---|
| Goyal P et al. [12] | New York City | 393 | 35.8 | Majority needed invasive mechanical ventilation |
| Petrilli CM et al. [13] | New York City | 4103 | 26.8 | BMI≥ 40 kg/m2 was second strongest independent predictor of hospitalisation after old age. |
| Richardson S et al. [14] | 12 Hospitals in New York City area, long Island, Westchester County. | 5700 | 41.7 | Obesity was second major co-morbidity after hypertension |
| Lighter J et al. [15] | New York City | 3615 | BMI: 30–34 kg/m2 (21%) and BMI≥35 kg/m2 (16%) | patients with BMI≥35 were 3.6 times more likely to be admitted for critical care as compared to normal BMI. |
| Garg S et al. [16] | COVID-NET USA | – | 48.3 | – |
| Simmonet A et al. [17] | Lille, France | 124 | BMI >30 kg/m2 (47.6%) and BMI >35 kg/m2 (28.2%) | Need for mechanical ventilation was associated with BMI≥35 kg/m2, independently of other comorbidities. |
| Intensive Care National Audit and Research Centre (ICNARC) report [18] | UK | 3383 | 72% were overweight or obese | 38% of patients admitted to critical care with a diagnosis of SARS-CoV-2 were obese.Out of these 57.6% died in critical care, as opposed to approximately 45% of those with a BMI<30 kg/m2 |
| Bello-Chavalla OY [19] | Mexico | 51,633 | 20.7 | Obesity mediated 49.5% of the effect of diabetes on COVID-19 lethality. Obesity also conferred an increased risk for ICU admission and intubation with five-fold increased risk of mortality in COVID-19 patients. |
| Suleyman G et al. [67] | Detroit | 463 | 57.6% obese with 19.2% severely obese | Severe obesity was significantly associated with need for mechanical ventilation (OR 3.2; 95%CI, 1.7–6.0) |
In the current scenario, since USA has become the epi-centre of the COVID-19 pandemic; the dynamics of patient characteristics in terms of associated complications is showing a difference from the initial data put out by China. This might be attributed, on one hand, to the paucity of reported BMI related information of the positive and critical cases from the data of China during the initial stages, and on the other hand, to the higher incidence and prevalence of obesity in USA as compared to other nations in the world. The prevalence of obesity in US has been 42.4% in 2017–2018 with a high burden of class III obesity i.e. 9.2% of the population with BMI>40 kg/m2 [20]. In fact, it is also listed as a risk factor for COVID-19 by the USA Centres for Disease Control and Prevention [21].
4. Obesity in Indian population
In India, the prevalence of obesity varies due to age, gender, geographical environment and socio-economic status. According to ICMR-INDIAB study 2015, prevalence rate of obesity and central obesity varies from 11.8% to 31.3% and 16.9%–36.3% respectively.[22] Infact more than 135 million individuals are affected by obesity [22] and the prevalence is rising fast amounting to a huge burden on healthcare infrastructure in our country, particularly, abdominal obesity which is considered to be one of the leading causes of cardiovascular diseases.
Moreover, Asians often display lower cardiorespiratory fitness and carry proportionally more fat percentage for any BMI as compared to their counterparts from the western world [23].
5. Experiences from history: Influenza pandemics and obesity
The influenza of 1956–1960 (Asian) and of 1968 (Honk Kong) have confirmed that obesity and diabetes had led to a higher mortality and a prolonged duration of illness even in the absence of other chronic conditions which increased the risk of influenza-related complications [24]. During the 2009 H1N1 pandemic, obesity was recognized as an independent risk factor for complications from influenza [25]. Moreover, it had been well established that obesity increased the duration of viral shedding in H1N1 pandemic by 42% as compared to adults who did not have obesity [25]. Worldwide, diabetes and obesity were the most frequently identified underlying conditions in fatal cases who were older than 20 years of age. However, anecdotal observations of high prevalence of obesity in severe and fatal cases were also reported from Chile, Manitoba and Mexico [25].
Several reports from around the world identified obesity and severe obesity as risk factors for hospitalisation and mechanical ventilation [26]. The distribution of obesity among hospitalized patients in California and New Mexico exceeded the 35% prevalence of obesity in US adults in 2009–2010 [25,27].
Moreover, it’s a matter of concern knowing the fact that persons with obesity have diminished protection from influenza immunization with a study showing that adult recipients of Influenza vaccine (IIV3) with obesity had two times greater incidence of influenza and/or influenza like illness despite being vaccinated [28].
Thus, prior experience of the impact of obesity on morbidity and mortality from previous influenza pandemics should definitely sensitise the clinicians in caring for patients with obesity and COVID-19 towards a need for their aggressive management. Moreover, strict precautionary measures must be advised to this group of individuals in the present pandemic. These include mandatory social distancing and home quarantine considerations with a close watch on the quality and quantity of food being consumed.
6. SARS-CoV-2- Host dynamics in obesity
6.1. Presence of other co-morbidities is common
Obesity potentiates multiple cardiovascular risk factors with premature development of cardiovascular disease and adverse cardiorenal outcomes. In individuals with diabetes, obesity and excess ectopic fat leads to impairment of insulin sensitivity and a reduced beta-cell function. Both limit the ability to evoke an appropriate metabolic response upon immunologic challenge. Overall, there is a loss of integrated regulation of metabolism required for the complex cellular interactions and for an effective host defence, further leading to the functional immunologic deficit. There is a significant worsening of the clinical profile and influenza disease severity whenever there is presence of multiple co-morbidities associated with obesity [23].
Furthermore, obesity enhances thrombosis, which is evident by the association between severe COVID-19 and pro-thrombotic disseminated intravascular coagulation and high rates of venous thromboembolism. Beyond cardiometabolic and thrombotic consequences, obesity has detrimental effects on lung function, diminishing forced expiratory volume and forced vital capacity [23]. Obesity has a well-known impact on Pulmonary functions. It is associated with decreased expiratory reserve volume, functional capacity, and respiratory system compliance. Pulmonary function is further compromised by decreased diaphragmatic excursion, making ventilation even more difficult [29].
All these are possible determinants of worst clinical outcomes in COVID-19 with obesity and associated metabolic co-morbidities. During the present pandemic, till now, it has been well established that cardiovascular diseases and diabetes are the major risk factors for poor outcomes but considering a higher BMI to be a forerunner for both these co-morbidities, the inclusion of obesity and overweight individuals as candidates for poor COVID-19 outcomes becomes very important. However due to paucity of reported anthropometric characteristics from the initial outbreak in China, it is difficult to comment on obesity being independent risk factor for complicated COVID-19. But changing demographic dynamics has now pointed towards importance of noting the important anthropometric data for all the affected individuals.
6.2. Perturbations in the immune system
Obesity is considered as a chronic inflammatory state. Such individuals have a higher concentration of several pro-inflammatory cytokines like TNF-α, MCP-1 and IL-6, mainly produced by visceral and subcutaneous adipose tissue leading to a defect in the innate immunity [30]. When an antigen is presented in such an environment, a reduced macrophage activation and a blunted pro-inflammatory cytokine production occurs [31]. This microenvironment explains the emergence of antiviral-resistant and vaccine escape variants in the obese population [32,33].
Studies of cytokine dynamics in human “cytokine storm” models shows that IL6 sustains activation of multiple cytokine pathways for many days post initial immune insult [34]. Interestingly, in early COVID-19 studies IL6 was a strong independent predictor of mortality [35]. Human adipose tissue is a major source of IL6 and its receptor IL6R [36] and, thus, adipose tissue may provide a reservoir for IL6 activation and cascade signalling in viral infection. Viral spread from affected organs to adjacent adipose tissue may take days, with subsequent prolonged viral shedding contributing to the delayed cytokine storm and consequences for tissue injury in patients with COVID-19. In line with this, IL6 inhibition has already been proposed as a treatment in COVID-19 and the results of trials of Tocilizumab are awaited [37].
B and T cell responses are impaired in obese patients, and this causes an increased susceptibility and a delay of resolution of the viral infection [38,39]. The hormonal milieu is such that in obesity; that there is an adipokine dysregulation with higher leptin (pro-inflammatory adipokine) and lower adiponectin (anti-inflammatory adipokine) levels. Leptin resistance was also found to be an important factor associated with severe lung injury in 2009 H1H1 pandemic [38]. Not only this, studies in obese mouse models have suggested that both innate and adaptive immune responses to Influenza A virus and its vaccine antigens like type I interferon response, Natural Killer cell functions, antigen presentation by dendritic cells and antigen specific memory of CD8+ lymphocytes were defective [38].
Additionally, a diminished interferon response and an increased influenza virus replication were found in normal bronchial epithelial cells derived from obese subjects [[40], [41], [42]]. Shared viral tropism for lung epithelium and adipose tissue has already been shown for H5N1 virus infection [44].
Gralinski and collaborators identified the complement system as an important host mediator of SARSCoV-induced disease [43]. Notably, Eculizumab, an antibody with complement system modulatory activity, is now being studied in an FDA expanded type of trial (ClinicalTrials.gov Identifier: NCT04288713) to assess its effect on COVID-19 infected patients relative to duration of intensive care treatment and mechanical ventilation, together with mortality outcomes [45].
Currently there is no evidence for direct SARS-CoV-2 infection of adipose tissue, although ACE2 receptor expression represents a basis for viral tropism in several cells within this tissue [46] including adipocytes, smooth muscle cells and endothelial cells [47].
If we look into the past influenza pandemics also, it has been found that many Adipose tissue resident cells are proven targets for multiple viruses like adipocytes (H1N1, Type A influenza and adenovirus 36) [[48], [49], [50]], adipo-stromal cells (Adenovirus 36, [51] CMV [52]), endothelial cells (SARS-CoV) [53], macrophages (influenza A, SARS-CoV, adenovirus36, HIV) [53,54] and lymphocytes (SARS-CoV, HIV) [52,53]. Considering, large extent of adipose tissue mass in obesity and its immune dysfunctions; its role in augmenting the prolongation and consequent complications of COVID-19 seems inevitable.
6.3. Angiotensin converting enzyme 2 (ACE2) and dipeptidyl peptidase 4 (DPP-4) in adipose tissue
Severe acute respiratory syndrome coronavirus (SARS-CoV) binds with the angiotensin converting enzyme 2 (ACE2) receptor for intracellular invasion, and the mechanism for acute lung injury during infection has been postulated to be mediated through the activation of the renin-angiotensin- system (RAS) [55]. RAS blockade has been proposed as a potential treatment for COVID-19 [56]. Remarkably, ACE2 is expressed in the human adipose tissue. Individuals with obesity have more adipose tissue and therefore an increased number of ACE2 expressing cells [57]The overall angiotensin converting enzyme (ACE)/angiotensin II (Ang II)/type 1 angiotensin 2 receptor (AT1R) RAS axis activation plays an important role in the pathophysiology of obesity and visceral adiposity-related cardiac risk [58]. Thus, the interaction between ACE2-RAS system, adipose tissue and the SARS-CoV-2 could, at least partially, explain the higher morbidity and mortality risk of COVID-19 in obese patients. Additionally, it can be stated that treatment with specific anti-hypertensive medications (ACE inhibitors and angiotensin receptor blockers, ARBs) will increase expression of ACE2 and increase patient susceptibility to viral host cell entry and propagation [59]. Thus a layer of complexity is added while managing COVID-19 infected persons who have more than one co-morbidity.
Secondly, human dipeptidyl peptidase 4 (DPP4) was also identified as a functional receptor for the spike protein of the Middle East coronavirus (MERS-Co-V) [60]. MERS-CoV binds to the receptor-binding domain and interacts with T cells and nuclear factors such as NF-kβ, involved in the pathogenesis of inflammatory disorders. DPP4 a transmembrane protein, has been found to be highly expressed in human visceral adipose tissue and is associated with insulin resistance and adipocyte inflammation. DPP-4 inhibition increases glucagon like peptide −1 (GLP-1) secretion leading to an improved insulin sensitivity and glucose metabolism within the adipocyte [61]. DPP4 inhibitors are commonly prescribed in Type 2 diabetes with obesity.Considering the immune modulatory effects of DPP4 inhibitors like suppression of T-cell proliferation and the secretion of pro inflammatory cytokines, such as IL-6 and 10,[62] it becomes logical to translate such findings in context to COVID-19 in obesity. Thus DPP4 may represent a potential target for preventing and reducing the risk and progression of the acute respiratory complications that type 2 diabetes may add to COVID-19 in obesity.
6.4. Postulated role of pulmonary lipofibroblasts
Progressive consolidation of the lung leading to severe acute respiratory syndrome is recognized as the most common complication of SARS-CoV virus [63]. This virus shares a high degree of genetic homology with SARS-CoV-2. One of the main reasons for this pulmonary consolidation is an extensive pulmonary fibrosis which was evident from CT scan and autopsy findings in SARS-CoV infections [11,63]. The pathophysiology of pulmonary fibrosis is not fully elucidated but role of myofibroblasts in modifying the lung structure and functions have been established in such conditions [64]. Such cells have also been found to be connected with adipocyte –myofibroblasts trans-differentiation in certain fibrotic lesions of skin [65]. Moreover, pathophysiological pathways have been linked with adipogenic-myogenic transition in lungs in pulmonary fibrosis where pulmonary lipofibroblasts have been proposed to be playing a central role. This population of cells carry characteristic lipid droplets and express high levels of perilipin-2 and are found adjacent to the ACE2 expressing alveolar epithelial cells [66]. Thus its logical to explore whether SARS-CoV/CoV-2 can directly influence these cells and thereby promote enhanced Lipofibroblast-myofibroblast transition [66]. It has also been postulated that adipose tissue can serve as a viral reservoir, whereas transdifferentiation of pulmonary lipofibroblasts into myofibroblasts can contribute to the development of pulmonary fibrosis and thus is likely to influence the clinical severity of COVID-19 [66]. Further, this population of cell has been proposed to increase the severity of the local response to COVID-19 in the lung [66]. This could be explored to avert the pulmonary complications of COVID-19 in obesity.
7. Conclusion
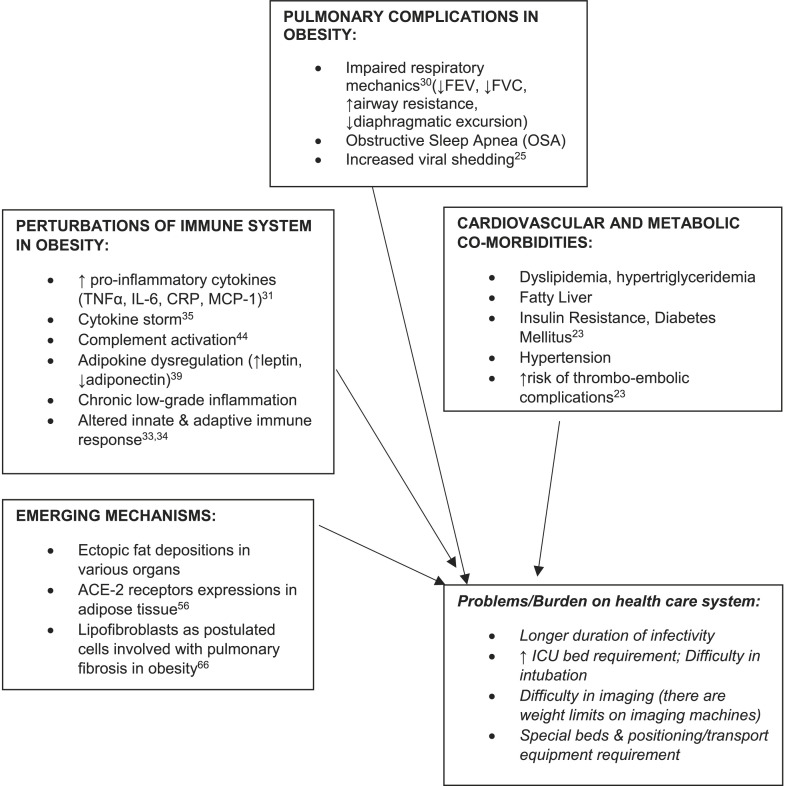
The pandemic of COVID-19 has put the public health system under immense strain to tackle the load of its high transmission and critical complications with the available limitations of the present health-care infrastructure. Putting together, our data has brought up some important insights towards considering BMI and obesity as one of the risk factors for severity and critical admissions for COVID-19 (as summarised in Fig. 1 ). However, whether obesity, over and above its cardio-metabolic co-morbidities might independently contribute to COVID-19 risk; needs more robust and detailed prospective survey.
Fig. 1.
Schematic summary of the possible mechanisms and challenging problems which could complicate COVID-19 course in obesity.
Declaration of competing interest
Rakhee Yadav: Conceptualization, Data curation, Formal analysis, Writing - original draft, review & editing, Design, Literature search, Data acquisition, Data analysis, Manuscript Preparation, Manuscript editing, Manuscript review, Guarantor, Sandeep Aggarwal: Conceptualization, Writing - review & editing, Concepts, Contribution to intellectual content, Literature search, Manuscript editing, Manuscript review, Archna Singh: Formal analysis, Writing - review & editing, Contribution to intellectual content, Data analysis, Manuscript editing, Manuscript review.
References
- 1.World Health Organisation website Novel coronavirus (2019-nCoV) SITUATION REPORT – 1. 21 January 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf
- 2.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; 2020. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-atthemedia-briefing-on-2019-ncov-on-11-february-2020 [Google Scholar]
- 4.World Health Organisation website Coronavirus disease (COVID-19) situation report – 103. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200714-covid-19-sitrep-176.pdf
- 5.Xing Z.H., Za-Zhi B.X. The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19) in China. Novel Coronavirus Pneumonia Emerg. Response Epidemiol. Team. 2020;41:145–151. [Google Scholar]
- 6.Chatterjee P., Nagi N., Agarwal A., Das B., Banerjee S., Sarkar S., Gupta N., Gangakhedkar R.R. The 2019 novel coronavirus disease (COVID-19) pandemic: a review of the current evidence. Indian J Med Res. 2020;151:147–159. doi: 10.4103/ijmr.IJMR_519_20. February & March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Peng Y.D., Meng K., Guan H.Q. 2020. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV; p. 48. Mar 2. E004. [DOI] [PubMed] [Google Scholar]
- 9.Zheng K.I., Gao F., Wang X.B. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q, Fengjuan C, Fang L, Xiaohui L, Qikai Q, Zhaoqin H. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China (3/13/2020). 10.2139/ssrn.3556658. [DOI] [PubMed]
- 11.Bhatraju P.K. Covid-19 in critically ill patients in the Seattle region-case series. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal P. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrilli C.M. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. 2020. Preprint at medRxiv. [DOI]
- 14.Richardson Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.6775. Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., Stachel A. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, March 1–30, 2020. MMWR. April 17, 2020:69. doi: 10.15585/mmwr.mm6915e3. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonnet A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ICNARC report on COVID-19 in critical care. 2020. www.icnarc.og
- 19.Bello-Chavolla O.Y., Bahena-Lopez J.P., Antonio-Villa N.E., Vargas-Vazquez A., Gonzales-Diaz A., Marquez-Salinas A. Predicting Mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020 May 31:dgaa346. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centres for disease Control and prevention. Overweight Obes. 2020 https://www.cdc.gov/obesity/data/adult.html February 27. [Google Scholar]
- 21.Flint S.W., Tahrani A.A. COVID-19 and Obesity- lack of clarity, guidance, and implications for care. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahirwar R., Mondal P.R. Prevalence of Obesity in India: a systematic review. Diabetes Metab Syndr. 2019;13(1):318–332. doi: 10.1016/j.dsx.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Sattar N., McInnes I.B., McMurray J.V.J. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 24.Moser J.A.S., Galindo-Fraga A., Ortiz-Hernández A.A. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from infuenza and other respiratory viruses. Infuenza Other Respir Viruses. 2019;13:3–9. doi: 10.1111/irv.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louie J.K., Acosta M., Winter K. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. J Am Med Assoc. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 26.Venkata C., Sampathkumar P., Afessa B. Hospitalized patients with 2009 H1N1 influenza infection: the Mayo Clinic experience. Mayo Clin Proc. 2010;85:798–805. doi: 10.4065/mcp.2010.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson D.L., Jungk J., Hancock E. Risk factors for 2009 pandemic influenza A (H1N1)-related hospitalization and death among racial/ethnic groups in New Mexico. Am J Publ Health. 2011;101:1776–1784. doi: 10.2105/AJPH.2011.300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neidich S.D., Green W.D., Rebeles J. Increased risk of influenza among vaccinated adults who are obese. Int J Obes. 2017;41:1324-1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietz W., Bugoa S. Obesity and its implications for COVID-19 mortality. Obesity. 2020 Apr 1 doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 30.Richard C., Wadowski M., Goruk S. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res Care. 2017;5(1) doi: 10.1136/bmjdrc-2016-000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn S.Y., Sohn S.H., Lee S.Y. The effect of lipopolysaccharide-induced obesity and its chronic infammation on infuenza virus-related pathology. Environ Toxicol Pharmacol. 2015;40(3):924–930. doi: 10.1016/j.etap.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson E.A., Hertz T., Johnson C., Mehle A., Krammer F., SchultzCherry S. Obesity outweighs protection conferred by adjuvanted infuenza vaccination. mBio. 2016;7(4):1–12. doi: 10.1128/mBio.01144-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue K., Stevens-Ayers T., Campbell A.P. Parallel evolution of infuenza across multiple spatiotemporal scales. eLife. 2017;6 doi: 10.7554/eLife.26875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yiu H.H., Graham A.L., Stengel R.F. Dynamics of a cytokine storm. PloS One. 2012;7 doi: 10.1371/journal.pone.0045027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(6):1294–1297. doi: 10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sindhu S., Thomas R., Shihab P., Sriraman D., Behbehani K., Ahmad R. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PloS One. 2015;10 doi: 10.1371/journal.pone.0133494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinese Clinical Trial Registry ChiCTR2000029765 - a multicentre, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19) 2020. http://www.chictr.org.cn/showprojen.aspx?proj=49409 Available from.
- 38.Zhang A.J., To K.K., Li C. Leptin mediates the pathogenesis of severe 2009 pandemic infuenza A (H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–1280. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 39.Park S., Jeon J.-H., Min B.-K. Role of the pyruvate dehydrogenase complex in metabolic remodeling: diferential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab J. 2018;42:270–281. doi: 10.4093/dmj.2018.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien K.B., Vogel P., Duan S. Impaired wound healing predisposes obese mice to severe infuenza virus infection. J Infect Dis. 2012;205:252–261. doi: 10.1093/infdis/jir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klinkhammer J., Schnepf D., Ye L. INF-lambda prevents infuenza virus spread from the upper airways to the lungs and limits virus transmission. elife. 2018;7 doi: 10.7554/eLife.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honce R., Karlsson E.A., Wohlgemuth N. Obesity related microenvironment promotes emergence of virulent infuenza virus strains. mBio. 2020;11(2):1–16. doi: 10.1128/mBio.03341-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gralinski L.E., Sheahan T.P., Morrison T.E. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5) doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanbe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 21 April 2020 doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura H., Itamura S., Iwasaki T., Kurata T., Tashiro M. Characterization of human influenza A (H5N1) virus infection in mice: neuro-, pneumo- and adipotropic infection. J Gen Virol. 2000;81:2503–2510. doi: 10.1099/0022-1317-81-10-2503. [DOI] [PubMed] [Google Scholar]
- 46.Uhlen M., Oksvold P., Fagerberg L., Lundberg E., Jonasson K., Forsberg M. Towards a knowledge-based human protein atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 47.Gupte M., Thatcher S.E., Boustany-Kari C.M., Shoemaker R., Yiannikouris F., Zhang X. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsatsanis C., Margioris A.N., Kontoyiannis D.P. Association between H1N1 infection severity and obesity-adiponectin as a potential etiologic factor. J Infect Dis. 2010;202:459–460. doi: 10.1086/653842. [DOI] [PubMed] [Google Scholar]
- 49.Maier H.E., Lopez R., Sanchez N., Ng S., Gresh L., Ojeda S. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218:1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouwman J.J., Visseren F.L., Bouter K.P., Diepersloot R.J. Infection-induced inflammatory response of adipocytes in vitro. Int J Obes. 2008;32:892–901. doi: 10.1038/ijo.2008.36. [DOI] [PubMed] [Google Scholar]
- 51.Ponterio E., Cangemi R., Mariani S., Casella G., De Cesare A., Trovato F.M. Adenovirus 36 DNA in human adipose tissue. Int J Obes. 2015;39:1761–1764. doi: 10.1038/ijo.2015.163. [DOI] [PubMed] [Google Scholar]
- 52.Zwezdaryk K.J., Ferris M.B., Strong A.L., Morris C.A., Bunnell B.A., Dhurandhar N.V. Human cytomegalovirus infection of human adipose-derived stromal/stem cells restricts differentiation along the adipogenic lineage. Adipocyte. 2016;5:53–64. doi: 10.1080/21623945.2015.1119957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouwman J.J., Diepersloot R.J., Visseren F.L. Intracellular infections enhance interleukin-6 and plasminogen activator inhibitor 1 production by cocultivated human adipocytes and THP-1 monocytes. Clin Vaccine Immunol. 2009;16:1222–1227. doi: 10.1128/CVI.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia X., Yin C., Lu S. Two things about COVID-10 might need attention. 2020. Preprints. 2020020315. [DOI]
- 58.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin angiotensin-aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMsr2005760. Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 60.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iacobellis G. COVID-19 and Diabetes: can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020 Mar 26:108125. doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reinhold D., Brocke S. DPP4-directed therapeutic strategies for MERS-CoV. Lancet Infect Dis. 2014;14:100–101. doi: 10.1016/S1473-3099(13)70696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuo W., Zhao X., Chen Y.G. Molecular biology of the SARS-coronavirus. Springer; Berlin, Heidelberg: 2010. SARS coronavirus and lung fibrosis; pp. 247–258. [Google Scholar]
- 64.Habiel D.M., Hogaboam C.M. Heterogeniety of fibroblasts and myofibroblasts in pulmonary fibrosis. Curr Pathobiol Rep. 2017;5:101–110. doi: 10.1007/s40139-017-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marangoni R.G., Korman B.D., Wei J. Myofibroblast in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rhematol. 2015;67:1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kruglikov I.L., Scherer P.E. The role of adipocytes and adipocyte like cells in the severity of covid-19 infections. Obesity. 2020;27(Apr) doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020 June;3(6) doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]