Planet earth has been witness to at least 5 major mass extinctions (Ordovician, late Devonian, Permian, Triassic–Jurassic, and Cretaceous–Tertiary) over the past 450 million years. Depressing evidence suggests that Homo sapiens (i.e., “wise man”) is responsible for the ongoing sixth major mass extinction, which has led to a multitude of urgent external environmental problems, such as global warming, deforestation, habitat loss, pollution, and a shortage of clean water. Global temperatures have risen 1 °C since 1880 and continue to increase at an accelerated rate. Global temperature rise is currently responsible for extreme wheather conditions, such as heat waves, hurricanes, mega wildfires, and droughts. The rapidity of temperature change has challenged our ability to adapt and respond, but international efforts are ongoing to reduce CO2 emissions and slow this ever-looming threat.
The kidney, more than any other organ, has a critical role in protecting the body from rising temperatures, through its ability to control water and electrolyte balance. Nephrologists should take a special interest in the effects of global warming on health and its potential to cause acute and chronic kidney injury, severe dehydration, and electrolyte disturbances. Special interest should be given to those with impaired kidney function who may have particular difficulty adapting to rapidly changing environmental conditions, a trait that could be both more common and accompanied by worse outcomes among populations in which organ capacity has not reached its full development potential, mainly in low- and middle-income settings.1 This issue alone may be found in up to 10%–15% of the world population, with vulnerable groups including the aged and the 3.4 million people on dialysis. It can estimated that with about 3 million hemodialysis patients on the planet, >230 billion liters of clean water are used, and 1.3 billion tons of mostly plastic waste are generated every year. Thus, dialysis services and industry must urgently start efforts to advance sustainable kidney-treatment technologies that minimize water usage and generation of waste.2
Climate change is also expected to have negative effects on the current epidemic of “lifestyle diseases,” which comprise a diseasome of aging, such as chronic kidney disease (CKD), type 2 diabetes, obesity, fatty liver disease, cardiovascular disease, cancer, depression, osteoporosis, and Alzheimer’s disease, with some of these conditions beginning at younger chronological ages during an individual’s lifetime. It is likely that the current high burden of lifestyle diseases represents a by-product of past genetic and functional adaptions to various climatic, dietary, and pathogen-selection pressures.3 Increasing heat may have multiple effects, including stimulating sedentary behavior and driving intake of sugary beverages, and potentially direct effects on inflammatory and oxidative pathways that facilitate development and progression of the diseasome of aging, including the impairment of mitochondrial function and integrity, which is central to metabolic and kidney disease. As the uremic phenotype is characterized by persistent inflammation, tissue hypoxia, and decreased mitochondrial biogenesis with oxidative stress, it is not surprising that impaired kidney function (i.e., an internal environmental problem) is a major risk factor and component of the “burden of lifestyle diseases.”4
Epidemics of CKD are emerging in various regions of the world where the common denominator appears to be working under extreme heat conditions. Although toxins may play a role in these outbreaks,5 the evidence that recurrent heat stress is playing a role is substantial.6 The stress on the local populations, in terms of both availability of health care and resources, such as safe drinking water and adequate nutrition, and exposure to novel pathogens, could be extreme. Furthermore, the effects of climate change may have dire effects on many regions of the world, and lack of clean water and extreme weather conditions, such as hurricanes and increasing sea levels owing to melting of glaciers in polar regions, may lead to massive migration of “environmental refugees,” with subsequent increased risk of conflicts and epidemics, adding more pressing challenges for the global nephrology community. We have already seen that increases in intense weather events, such as hurricanes, owing to climate change, have damaging effects on the provision of care to dialysis patients.7
The effects of global warming, water shortage,6 and air pollution8 on the risk of CKD is already evident. In 2015, the United Nations adopted an agenda for sustainable development that includes 17 sustainable development goals (SDGs) as the blueprint to transform our world into a better place by 2030 (Figure 1 ). The 17 SDGs are integrated—that is, they recognize that action in one area will affect outcomes in others. Thus, in order to accomplish these SDGs, the expertise of the global renal community should be used, not only for SDG no. 3 (good health and well-being) but also for others, such as SDG no. 6 (clean water and sanitation) and SDG no. 13 (climate action).
Figure 1.
The 2030 Agenda for Sustainable Development, adopted by all United Nations member states in 2015, provides a shared blueprint for peace and prosperity for people and the planet, now and into the future. United Nations Sustainable Development Goal no. 3 commits the planet to reducing premature mortality due to lifestyle diseases by one-third.
We advocate investigation of novel solutions to the challenges humans face, via a study of nature’s solutions. The broad diversity in biology contained within circa 8.7 million species that currently inhabit the planet is one of the most conspicuous aspects of life on earth. Indeed, since the time that animal life emerged on our planet about 650 million years ago, animals have survived (or not) based on whether their adaptations to the enviroment and environmental changes have been beneficial or not. Failure to evolve adaptations that aid survival has led to species extinction. Unfortunately, nature has been framed inappropriately by humans, with overexploitation and destruction of ecosystems and animal habitats. It will take millions of years for the planet to recuperate from the loss of mammal diversity that is occurring during the present-day biodiversity crisis.9 Instead of exploiting and consequently devastating natural environmental balance, the “wise man” should learn from ingenious natural solutions that have evolved over time, and mimic them (i.e., use biomimetics).
Innovation-based solutions found in nature have proven successful in a wide variety of fields, but medical science has been lagging behind in recognizing and uptaking such clues.10 As a biomimetic approach builds on evolution through natural selection to develop ideal solutions to conquer diseases and aid survival in extreme environments, it may be more cost effective and efficient than the current pharma drug-discovery approach to tackling human health problems. Such an approach can complement current biological approaches that largely focus on research in laboratory mice and rats. Indeed, it should be emphasized that nature is never careless and never cheats in its evolutionary “experiments.”
There are many examples in nature from which we can learn about mechanisms to escape one or several lifestyle diseases that bedevil humans.4 For example, hibernating bears, despite months of anuria and decreased renal function during winter sleep, do not develop osteoporosis, inflammation, muscle wasting, or atherosclerosis.11 Hibernating bears also provide a natural model of reversible, healthy obesity with favorable seasonal changes in insulin resistance.11 Another example is the giraffe, which is protected from kidney disease and stroke despite alarmingly high blood pressure.10 The superior longevity and resistance against cancer and vascular aging in naked mole rats (exposed to chronic hypoxia in underground burrows) have immediate implications for several lifestyle diseases, including CKD.10 The links between fat mass accumulation and hydration status in species exposed to a shortage of fresh water, such as camels and blue whales, hint at a survival pathway involving fructose metabolism that may have gone awry in modern society, due to a sedentary lifestyle and overconsumption of calorie-dense nutrients.12 Other animals have evolved unique mechanisms for organ regeneration (such as sharks, lizards, and spiders), rapid organ growth (pythons), rapid wound healing (nonhuman primates), cancer protection (elephants and naked mole rats), protection against the toxic effects of chronic alcohol consumption (Malaysian pen-tailed tree shrews), blood glucose control (Gila monsters), protection against bacterial pathogen resistance (cockroaches), UV-ray protection (hippopotamuses), and protection against renal hypoxia (harbor seals) that could inspire us to find novel solutions for human diseases.11 To date, the best example of a successful biomimetic application in nephrology is the development of captopril (a biomimetic of a bradykinin-potentiating peptide) from the poisonous Brazilian viper Bothrops jararaca; its effects on blood pressure mechanisms mimic those of the snake’s venom. Although venom from snakes is a natural pharmacopeia, <0.01% of the planet’s snake venom toxins have yet been identified and characterized.13
The maladaptation of other species to the environment may also provide insights. For example, the susceptibility to CKD among felids provides valuable information on risk factors for deteriorating kidney health related to nutritional habits (intake of red meat) and dehydration.11 Some studies, based on this approach of looking for cross-species solutions, have led to insights into key underlying mechanisms of protection and injury. For example, central to many of these diseases is the importance of mitochondrial integrity, and evidence suggests that loss of the cytoprotective nuclear factor erythroid 2–related factor 2 may represent a common pathway for many metabolic diseases, suggesting potential targets for therapy.4 Studies from the animal kingdom suggest that nuclear factor erythroid 2–related factor 2 plays a role in protection against heat stress and dehydration,14 as well as air pollution–mediated disease.15 Critically, the beneficial effects of nuclear factor erythroid 2–related factor 2 agonism can be modulated by natural food stuffs (e.g., sulforophane, fisetin, quercetin, curcumin), offering a route to evidence-based nonpharmacologic interventions to improve health.4 It is ironic that as the evidence base rapidly grows for nutritional interventions in medicine using “food as medicine,” global warming, pollution, and environment loss threaten its development. Moreover, since about 35% of the medicines we use today have originated from nature, the development of future drugs is dependent on the preservation of nature. Thus, given that Brazil presents the largest range of biodiversity in the world (>50,000 species of higher plants), deforestation of the broader Amazonas region should be prevented for health as well as other reasons.
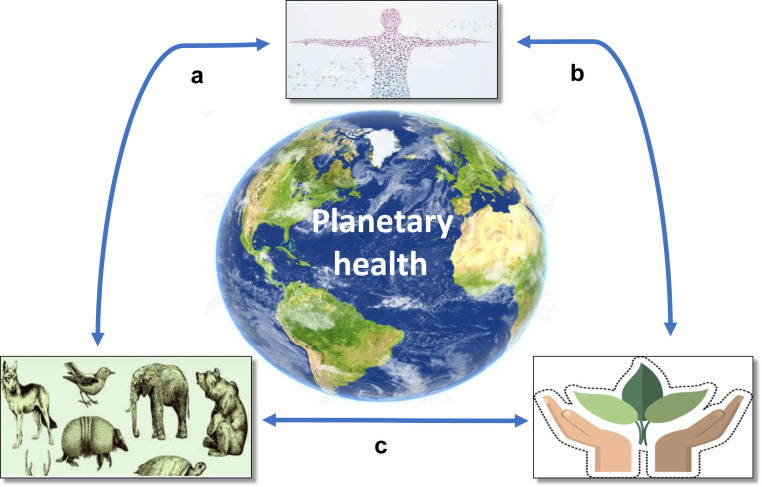
The “One Health” approach (i.e., designing and implementing programs, policies, legislation, and research to achieve better outcomes in public health) has attracted recent interest. However, it has mainly focused on the understanding of disease transmission between humans and animals, and food safety, and has not addressed the possibility of using biological biomimetics to find novel treatment strategies for lifestyle diseases. We therefore propose a “planetary health” approach (i.e., the health of human civilization is dependent on the state of the natural systems) that includes a focus on lifestyle diseases in relation to animal and environmental health, thus ensuring the well-being of the whole planet (Figure 2 ). The “planetary health” approach could also be used for comparative studies of health in different human populations (i.e., developed vs. underdeveloped regions, regions exposed to heat stress and/or water shortage, and starving vs. affluent regions), relating these to studies of how various animals cope with heat stress, water shortage, pollution, prolonged periods of fasting, etc. Detailed studies of survival mechanisms in wild animals to identify insights for human diseases, and the impact of environmental cues, requires multidisciplinary collaboration among medical doctors, veterinarians, zoologists, climate researchers, ecologists, biologists, anthropologists, and social scientists.11 A “biomimetic alliance for better health” with collaborative research among different disciplines could contribute to not only better health and a lower burden of lifestyle diseases, such as CKD, but also a better and more sustainable environment. Such an approach also supports the notion of better “planetary health,” and its benefits would be substantial. The current pandemic and the cross-species transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)16 is the best proof of the need for sustainable human and ecosystem health. Since emerging evidence suggests that a robust interferon system may be the key to understanding why bats are protected from the harmful effects of SARS-CoV-2,17 lessons from the animal kingdom suggest that the interferon plays an important role in the treatment of severe coronavirus disease 2019 infection.18
Figure 2.
Lifestyle diseases associated with inflammation, tissue hypoxia, mitochondrial dysfunction, and oxidative stress are intimately linked to the health of animals and the environment. (a) Contained within adaptations in certain species that evolved to survive extreme environmental factors, such as long, cold winters, water shortages, and hypoxia, are survival strategies that could provide clues for human health. (b) Lack of clean water, pollution, and heat stress cause human disease, creating a need for climate medicine. (c) Lack of clean water, pollution, and heat stress cause loss of animal and plant habitat. Habitat loss, illegal trade, natural selection, and evolution affect animal health. Animals that have occupied niches and developed mechanisms to protect themselves against environmental factors, such as lack of water, infections, oxygen deprivation, and heat stress, could provide valuable clues for the protection of humans from environmental changes.
Disclosure
PSt serves on a Scientific Advisory Board for REATA. RJJ has equity in XORTX Therapeutics and Colorado Research Partners, LLC. PGS has equity options in Pathfinder Cell Therapy and has received research awards from 4D Pharma and Constant Pharma. All the other authors declared no competing interests.
References
- 1.Miranda J.J., Barrientos-Gutiérrez T., Corvalan C. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nature Med. 2019;25:1667–1679. doi: 10.1038/s41591-019-0644-7. [DOI] [PubMed] [Google Scholar]
- 2.Barraclough K.A., Agar J.W.M. Green nephrology. Nat Rev Nephrol. 2020; Feb. 7 doi: 10.1038/s41581-019-0245-1. [DOI] [PubMed] [Google Scholar]
- 3.Vasseur E., Quintana-Murci L. The impact of natural selection on health and disease: uses of the population genetics approach in humans. Evol Appl. 2013;6:596–607. doi: 10.1111/eva.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenvinkel P., Meyer C.J., Block G.A. Understanding the role of the cytoprotective transcription factor NRF2—lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol Dial Transpl. 2019:gfz120. doi: 10.1093/ndt/gfz120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vervaet B.A., Nast C.C., Jayasumana C. Chronic interstitial nephritis in agricultural communities is a toxin-induced proximal tubular nephropathy. Kidney Int. 2020;97:350–369. doi: 10.1016/j.kint.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Johnson R.J., Stenvinkel P., Jensen T. Metabolic and kidney diseases in the setting of climate change, water shortage, and survival factors. J Am Soc Nephrol. 2016;27:2247–2256. doi: 10.1681/ASN.2015121314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanholder R.C., Van Biesen W.A., Sever M.S. Hurricane Katrina and chronic dialysis patients: better tidings than originally feared? Kidney Int. 2009;76:687–689. doi: 10.1038/ki.2009.279. [DOI] [PubMed] [Google Scholar]
- 8.Wu M.Y., Lo W.C., Chao C.T. Association between air pollutants and development of chronic kidney disease: a systematic review and meta-analysis. Sci Total Environ. 2020;706:135522. doi: 10.1016/j.scitotenv.2019.135522. [DOI] [PubMed] [Google Scholar]
- 9.Davis M., Faurby S., Svenning J. Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proc Natl Acad Sci USA. 2018;115:11262–11267. doi: 10.1073/pnas.1804906115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenvinkel P., Painer J., Johnson R.J., Natterson-Horowitz B. Biomimetics—nature's roadmap to insights and solutions for burden of lifestyle diseases. J Intern Med. 2020;287:238–251. doi: 10.1111/joim.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenvinkel P., Painer J., Kuro -O.M. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol. 2018;14:265–284. doi: 10.1038/nrneph.2017.169. [DOI] [PubMed] [Google Scholar]
- 12.Johnson R.J., Stenvinkel P., Andrews P. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med. 2020;287:252–262. doi: 10.1111/joim.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abd El-Aziz T.M., Soares G., Stockland J.D. Snake venoms in drug discovery: valuable therapeutic tools for life saving. Toxins. 2019;11:564. doi: 10.3390/toxins11100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C., Zhou Y.L., Zhu Q. Effects of heat stress on the liver of the Chinese giant salamander Andrias davidianus: histopathological changes and expression characterization of Nrf2-mediated antioxidant pathway genes. J Therm Biol. 2018;76:115–125. doi: 10.1016/j.jtherbio.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Lawal A.O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: the role of Nrf2 and AhR-mediated pathways. Toxicol Lett. 2017;270:88–95. doi: 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Ji W., Wang W., Zhao X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook C.E., Boots M., Chandran K. Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence. Elife. 2020;9:e48401. doi: 10.7554/eLife.48401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung I.F.-N., Lung K.-C., Tso E.Y.-K. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]