Abstract
Background
Hospital admissions for non-coronavirus disease 2019 (COVID-19) pathology have decreased significantly. It is believed that this may be due to public anxiety about acquiring COVID-19 infection in hospital and the subsequent risk of mortality.
Aim
To identify patients who acquire COVID-19 in hospital (nosocomial COVID-19 infection (NC)) and their risk of mortality compared to those with community-acquired COVID-19 (CAC) infection.
Methods
The COPE-Nosocomial Study was an observational cohort study. The primary outcome was the time to all-cause mortality (estimated with an adjusted hazard ratio (aHR)), and secondary outcomes were day 7 mortality and the time-to-discharge. A mixed-effects multivariable Cox's proportional hazards model was used, adjusted for demographics and comorbidities.
Findings
The study included 1564 patients from 10 hospital sites throughout the UK, and one in Italy, and collected outcomes on patients admitted up to April 28th, 2020. In all, 12.5% of COVID-19 infections were acquired in hospital; 425 (27.2%) patients with COVID died. The median survival time in NC patients was 14 days compared with 10 days in CAC patients. In the primary analysis, NC infection was associated with lower mortality rate (aHR: 0.71; 95% confidence interval (CI): 0.51–0.98). Secondary outcomes found no difference in day 7 mortality (adjusted odds ratio: 0.79; 95% CI: 0.47–1.31), but NC patients required longer time in hospital during convalescence (aHR: 0.49, 95% CI: 0.37–0.66).
Conclusion
The minority of COVID-19 cases were the result of NC transmission. No COVID-19 infection comes without risk, but patients with NC had a lower risk of mortality compared to CAC infection; however, caution should be taken when interpreting this finding.
Keywords: COVID-19, Nosocomial infection, Community-acquired infection
Introduction
The novel coronavirus SARS-CoV-2 is implicated in causing the disease COVID-19 and its associated complications. Whereas most infected people develop mild flu-like symptoms, some have significant respiratory complications and go on to develop multi-organ failure and death [1,2]. Despite robust infection control efforts, hospital-acquired (herein described as nosocomial) COVID-19 infections have been reported [[3], [4], [5]]. Heightened anxiety among the general public has resulted in individuals' reluctance to attend hospital for diagnostic tests or treatments. This may account for the significant reduction in acute hospital attendances and possibly contributed to the high excess mortality toll [6].
The hallmark of SARS-CoV-2 is its highly contagious nature; it remains viable and infectious on surfaces for up to three days [7]. Its main mode of transmission is through droplets and close contact with people with the disease [8]. Incubation is estimated at 5–7 days, but this can take up to 14 days [9]. Nosocomial infection is defined as an infection that is acquired in hospital by a patient who was admitted for a reason other than that infection (at least 15 days prior to a positive COVID-19 diagnosis), and in whom the pathogen was not incubating at the time of admission. Risk factors for developing a nosocomial infection include: age >70 years, immunosuppression, admission to intensive care, history of trauma, antibiotic use, and use of an indwelling catheter [10]. Prior to the current COVID-19 pandemic, nosocomial infections (most commonly from respiratory and urinary tracts and surgical wounds) already posed significant healthcare and economic burdens in both developed and resource-poor countries, with an average estimated prevalence of 8.7% worldwide [11].
In general, nosocomial infections are not life-threatening. However, a large study in the USA reported that non-ventilator-associated nosocomial pneumonia occurred in 2.1% of all hospital admissions, with a mortality rate of 13.1% [12]. In addition, patients diagnosed with a nosocomial infection are likely to spend 2.5 times longer in hospital [13]. SARS (severe acute respiratory syndrome, 2003) and MERS (Middle East respiratory syndrome, 2012) had estimated nosocomial infection prevalence of 36% and 56%, respectively [14]. By comparison, Chinese estimates of the prevalence of nosocomial COVID-19 are as high as 41% [14,15]. There are currently no published data for nosocomial versus community-acquired COVID-19 in UK hospitals, leaving uncertainty around morbidity or mortality and heightened public anxiety. A robust evidence base will help to direct policy-makers and aid the dissemination of public health advice.
The COPE (COVID-19 in Older PEople study) study was designed to assess a number of clinical parameters and biomarkers as prognostic tools for patients with COVID-19. The aim of this secondary study was to assess the burden of nosocomial COVID-19 (NC) infection and determine whether patients with NC exhibited poorer outcomes than those who experienced community-acquired COVID-19 (CAC) infection.
Methods
Study design
Data were obtained as part of a multi-centre international cohort study: the COPE study, which assessed clinical and biomarkers as prognostic indicators of mortality. In the UK, authority to conduct the study was granted by the Health Research Authority (20/HRA/1898), and in Italy by the Ethics Committee of Policlinico Hospital Modena (Reference 369/2020/OSS/AOUMO). Cardiff University was the study sponsor. This article follows the STROBE statement for reporting of cohort studies (https://www.strobe-statement.org/index.php?id = strobe-home). Investigators at each site collated electronic and manual patient records. Prior to participating, all study personnel completed specific data collection training. Local policies on data protection were followed in order to record data securely at each site. Full study details can be found within the COPE protocol (A. Price et al., unpublished data).
Setting
An established network of clinical teams with an interest in frailty from ten UK sites and one Italian site (www.opsoc.eu) was used. The UK centres were Ysbyty Ystrad Fawr in Caerphilly, Royal Gwent Hospital in Newport, Nevill Hall Hospital in Abergavenny, University Hospital of Wales in Cardiff, Southmead Hospital in Bristol, Aberdeen Royal Infirmary, Royal Alexandra Hospital in Paisley, Inverclyde Royal Hospital, Salford Royal Hospital, and Glasgow Royal Infirmary. The Italian centre was the University Hospital of Modena Policlinico in Italy. All hospitals adhered to infection control guidelines with the application of appropriate personal protective equipment, isolation of suspected and confirmed cases, and had a policy of having no outside visitors during the period of data collection [16]. All hospitals deliver urgent and emergency care to patients diagnosed with COVID-19. Data were collected from patients admitted with COVID-19 from February 27th and April 28th, 2020. Further details of the study design are found within the protocol, and the main study findings are reported in the COPE study report [17]. In the original protocol we estimated a 30% mortality in the frail, and 20% in those not frail (hazard ratio (HR) of 0.60). In order to detect this difference with 80% power and with 5% significance, at least 500 patients were to be included. The sample size was increased to assess CFS cat-egorized into four groups (rather than frail vs not frail) (A. Price et al., unpublished data).
Participants
We attempted to include all consecutive patients admitted to hospital aged ≥18 years with a diagnosis of COVID-19. Diagnostic criteria were swabs confirming the presence of SARS-CoV-2, or a clinical diagnosis (made by the site clinical team and based on signs, symptoms and/or radiology) consistent with COVID-19. Patients were screened and excluded due to: not having a clinical (or laboratory) diagnosis; clinical documentation not available; or no available clinical resource for data capture. Clinical teams at each site screened inpatient admission lists for eligibility and had access to infection control records of positive COVID-19 laboratory testing. Screening logs of eligible participants were retained at each site.
Outcomes
The primary outcome was the time-to-mortality from the date of admission (or date of diagnosis, if diagnosis was five or more days after admission). For example, all 196 NC patients were diagnosed 15 or more days after admission, and were analysed as the time from diagnosis to outcome (death or discharge). The 169 CAC patients were analysed from the date of diagnosis to outcome (since they had a positive diagnosis between five and 14 days after admission they could not be confirmed as true NC), with the remaining 1199 CAC patients analysed as the difference from admission to outcome. The time-to-event was censored at death or discharge.
Secondary outcomes
Day 7 mortality and the time-to-discharge (herein described as the length of stay).
Variables with prognostic utility were collected, including: patient age and sex; C-reactive protein (CRP) on admission; estimated glomerular filtration rate (eGFR) on admission; smoking status (never, previous, or current); frailty; and previous or current history of: coronary artery disease, diabetes mellitus, and hypertension [1,[18], [19], [20]]. Frailty was measured using the pre-admission Clinical Frailty Scale (CFS) representing a patient's frailty two weeks prior to admission. The CFS is widely used within the UK to aid clinical management and is an ordinal hierarchical scale that numerically ranks frailty from 1 to 9: 1, very fit; 2, well; 3, managing well; 4, vulnerable; 5, mildly frail; 6, moderately frail; 7, severely frail; 8, very severely frail; 9, terminally ill. For the purposes of the analyses, scores were grouped into clinically meaningful groups: 1–2, 3–4, 5–6, and 7–9 [21].
Data analysis
Baseline demographic and clinical characteristics were partitioned by mortality, and location of infection to describe the included participants.
Time to mortality (primary outcome) and length of stay (secondary outcome) were analysed with mixed-effects multivariable Cox's proportional baseline hazards regression models. The analyses were fitted with a random effect to account for hospital variation, and adjusted for the base model of: patient age group; sex; smoking status; CRP; diabetes; hypertension; coronary artery disease; and the CFS [22]. The adjusted hazard ratio (aHR) was estimated with associated 95% confidence interval (95% CI). The baseline proportionality assumption was tested visually with log10–log10 residuals. Each time-to-event analysis was reported with a Kaplan–Meier survival plot.
The secondary outcome of day 7 was analysed using a mixed-effects multivariable logistic model, fitting each hospital as a random intercept effect, and adjusted with covariates consistent with the primary outcome. The adjusted odds ratio (aOR) was estimated and presented with associated 95% CI. Missing data were explored for patterns of missingness. The primary outcome analysis was repeated within each of the comorbidity subgroups to assess the impact of NC within each subgroup. Analysis was carried out using Stata version 15 (StataCorp., College Station, TX, USA). Kaplan–Meier survival plots were generated in R (R Foundation, Vienna, Austria).
Results
The COPE study screened 1687 participants from general medical, surgical, geriatric, respiratory, and infectious diseases wards, as well as intensive care units where applicable. These wards solely managed suspected or confirmed COVID-19 patients. A total of 143 patients were excluded from the study after screening, with the remaining 1564 participants included. There were 1410 (90.2%) patients from the UK, and 154 (9.8%) from Italy (Table I ). Most were diagnosed by laboratory testing (95.1%) and 64 (4.9%) by clinical diagnosis. Data quality was high and a complete case dataset was obtained for >97% of included patients. There were 25 cases of missing smoking status, which were imputed as never smokers, and 32 cases of missing CRP, which were median imputed. Other missing covariates occurred in no more than 14 patients. Since there were so few missing data, the complete case population was used within each analysis, and the number included shown as the population under investigation.
Table I.
Demographics, frailty and nosocomial infection, by mortality
| Variable | Deceased | Alive | Total |
|---|---|---|---|
| Hospital sitesa | 425 (27.2%) | 1139 (72.8%) | 1564 |
| A | 15 (13.0%) | 100 (87.0%) | 115 (7.4%) |
| B | 14 (28.0%) | 36 (72.0%) | 50 (3.2%) |
| C | 34 (22.2%) | 119 (77.8%) | 153 (9.8%) |
| D | 10 (23.3%) | 33 (76.7%) | 43 (2.8%) |
| E | 15 (12.2%) | 108 (87.8%) | 123 (7.9%) |
| F | 23 (14.9%) | 131 (85.1%) | 154 (9.9%) |
| G | 36 (32.1%) | 76 (67.9%) | 112 (7.2%) |
| H | 108 (43.9%) | 138 (56.1%) | 246 (15.7%) |
| I | 126 (33.2%) | 254 (66.8%) | 380 (24.3%) |
| J | 43 (24.0%) | 136 (76.0%) | 179 (11.5%) |
| K | 1 (11.1%) | 8 (88.9%) | 9 (0.6%) |
| Age (years) | |||
| <65 | 55 (11.3%) | 433 (88.7%) | 488 (31.2%) |
| 65–79 | 168 (31.4%) | 367 (68.6%) | 535 (34.2%) |
| ≥80 | 202 (37.3%) | 339 (62.7%) | 541 (34.6%) |
| Sex | |||
| Female | 170 (25.7%) | 491 (74.3%) | 661 (42.3%) |
| Male | 255 (28.2%) | 648 (71.8%) | 903 (57.7%) |
| Smoking status | |||
| Never smokers | 205 (25.2%) | 609 (74.8%) | 814 (52.9%) |
| Ex-smokers | 185 (30.7%) | 418 (69.3%) | 603 (39.2%) |
| Current smokers | 26 (21.5%) | 95 (78.5%) | 121 (7.9%) |
| Missing | 9 | 17 | 26 |
| CRP (mg/L), median (IQR) | 113 (64–185) | 71 (30–137) | 83 (37–153) |
| eGFR >40 mL/min/1.73 m2 | |||
| No | 202 (20.6%) | 778 (79.4%) | 980 (63.2%) |
| Yes | 217 (38.1%) | 353 (61.9%) | 570 (36.8%) |
| Missing | 6 | 8 | 14 |
| Hypertension | |||
| No | 184 (24.4%) | 571 (75.6%) | 755 (48.4%) |
| Yes | 238 (29.6%) | 566 (70.4%) | 804 (51.6%) |
| Missing | 3 | 2 | 5 |
| Coronary artery disease | |||
| No | 290 (23.9%) | 924 (76.1%) | 1214 (77.9%) |
| Yes | 132 (38.3%) | 213 (61.7%) | 345 (22.1%) |
| Missing | 3 | 2 | 5 |
| Diabetes | |||
| No | 295 (25.8%) | 849 (74.2%) | 1144 (73.2%) |
| Yes | 128 (30.8%) | 287 (69.2%) | 415 (26.6%) |
| Missing | 2 | 3 | 5 |
| COVID-19 infection | |||
| Community-acquired | 372 (27.2%) | 996 (72.8%) | 1368 (87.5%) |
| Nosocomial | 53 (27.0%) | 143 (73.0%) | 196 (12.5%) |
| Clinical Frailty Scale (CFS) | |||
| 1. Very fit | 7 (7.7%) | 84 (92.3%) | 91 (5.8%) |
| 2. Fit | 22 (11.2%) | 175 (88.8%) | 197 (12.6%) |
| 3. Managing well | 55 (19.2%) | 232 (80.8%) | 287 (18.4%) |
| 4. Vulnerable | 52 (28.1%) | 133 (71.9%) | 185 (11.9%) |
| 5. Mildly frail | 50 (27.5%) | 132 (72.5%) | 182 (11.7%) |
| 6. Frail | 84 (33.5%) | 167 (66.5%) | 251 (16.1%) |
| 7. Severely frail | 96 (36.9%) | 164 (63.1%) | 260 (16.7%) |
| 8. Very severely frail | 44 (55.7%) | 35 (44.3%) | 79 (5.1%) |
| 9. Terminally ill | 12 (44.4%) | 15 (55.6%) | 27 (1.7%) |
| Missing | 3 | 2 | 5 |
CRP, C-reactive protein; IQR, interquartile range; eGFR, estimated glomerular filtration rate; COVID-19, coronavirus disease 2019.
Hospitals are anonymized.
Descriptive data
The median patient age was 74 years (interquartile range (IQR): 61–83), and 903 were male (57.7%). The overall in-hospital COVID-19 mortality rate was 27.2% (425/1564), and this varied throughout the 11 hospitals at between 12.2% and 43.9%. Of all hospital episodes of COVID-19 infection, 12.5% were NC (196/1564) and 87.5% were CAC (1368/1564). The median proportion of NC infections from the total number of COVID-19 cases from the 11 hospitals was 8.7% (IQR: 3.0–14.1). The median number of days between patient admission and a positive COVID-19 test for NC infection was 32.5 days (IQR: 23–54), and for CAC the median was 0 days (IQR: 0–1). The median patient age for NC infection was 80 years (IQR: 71.5–86.5), and was 73 years (IQR: 60–82) for patients with CAC infection (Supplementary Table S1). The median level of frailty was moderately frail (CFS: 6) for NC, and vulnerable (CFS: 4) for CAC. Full patient demographics and clinical characteristics are shown in Table I.
Data analysis
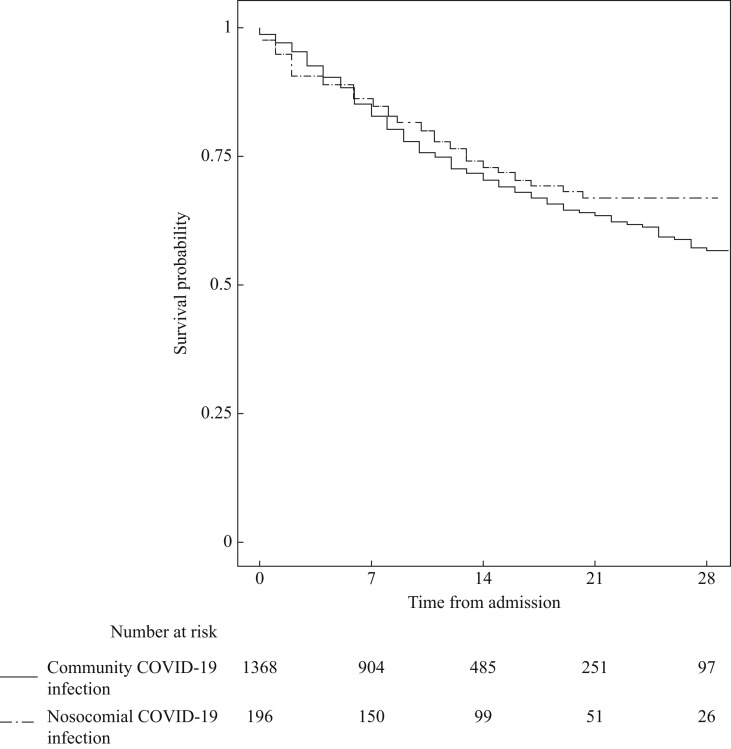
By end of the study period, 27.0% of patients with NC had died versus 27.2% CAC patients. The median time-to-mortality was 14 days in the NC group versus 10 days in the CAC group (Figure 1 ). In the multivariable analysis, NC infection was associated with lower mortality rate (Table II ). Higher mortality rate was associated with: older age, increased frailty, renal failure, and increased CRP (Table II).
Figure 1.
Kaplan–Meier survival plot of nosocomial versus community infection of COVID-19 patients.
Table II.
Primary outcome: crude and adjusted time-to-mortality, from admission (or diagnosis, for patients with a diagnosis five or more days after admission)
| Variable | Crude HR (N = 1520)b |
Adjusted HRa (N = 1500)c |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | aHR (95% CI) | P-value | |
| Location infection acquired | ||||
| Community-acquired (Ref.) | Reference category | Reference category | ||
| Hospital-acquired | 0.71 (0.52–0.97) | 0.03 | 0.71 (0.51–0.98) | 0.04 |
| Age (years) | ||||
| <65 | Reference category | Reference category | ||
| 65–79 | 3.30 (2.40–4.55) | <0.001 | 2.70 (1.91–3.81) | <0.001 |
| >80 | 4.05 (2.95–5.57) | <0.001 | 3.30 (2.28–4.78) | <0.001 |
| Sex (Female) | Reference category | Reference category | ||
| Male | 0.99 (0.81–1.21) | 0.93 | 1.10 (0.89–1.37) | 0.38 |
| Smoking status (Never) | Reference category | Reference category | ||
| Ex-smokers | 1.20 (0.98–1.47) | 0.08 | 0.95 (0.76–1.17) | 0.61 |
| Current smokers | 0.84 (0.55–1.29) | 0.43 | 1.09 (0.70–1.70) | 0.71 |
| C-reactive proteind | 1.003 (1.002–1.004) | <0.001 | 1.004 (1.003–1.005) | <0.001 |
| Patients with diabetes | 1.12 (0.90–1.39) | 0.30 | 1.03 (0.82–1.30) | 0.77 |
| Patients with CAD | 1.57 (1.26–1.95) | <0.001 | 1.21 (0.96–1.53) | 0.10 |
| Patients with hypertension | 1.24 (1.01–1.51) | 0.04 | 0.98 (0.80–1.22) | 0.89 |
| Patients with reduced renal function | 1.93 (1.58–2.35) | <0.001 | 1.32 (1.07–1.63) | 0.01 |
| Clinical Frailty Scale | ||||
| 1, 2 | Reference category | Reference category | ||
| 3, 4 | 2.25 (1.47–3.45) | <0.001 | 1.67 (1.08–2.60) | 0.02 |
| 5, 6 | 3.12 (2.05–4.76) | <0.001 | 2.08 (1.31–3.32) | 0.002 |
| 7, 9 | 4.41 (2.90–6.71) | <0.001 | 2.75 (1.73–4.38) | <0.001 |
HR, crude hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; CAD, coronary artery disease.
The multivariable mixed-effects Cox regression was adjusted for: age group; sex; smoking; C-reactive protein; diabetes; CAD; hypertension; and the Clinical Frailty Scale.
Forty-four cases were not included in the analysis due to patient death on admission.
Twenty cases were not included in the analysis due to missing covariate data (see Table I).
Fitted as a slope parameter.
In multivariable analysis for day 7 mortality, there was no association between NC infection and mortality (Table III ). Important factors associated with day 7 mortality were: increased age, increased CRP; reduced renal function, coronary artery disease, and increased frailty (Table III).
Table III.
Secondary outcomes
| Variable | Day 7 mortality |
Length of hospital staya |
||
|---|---|---|---|---|
| (N = 1494)b |
(N = 1500)c |
|||
| HR (95% CI) | P-value | aHR (95% CI) | P-value | |
| Location infection acquired | ||||
| Community-acquired (Ref.) | Reference category | Reference category | ||
| Nosocomial | 0.79 (0.47–1.31) | 0.35 | 0.49 (0.37–0.66) | <0.001 |
| Age (years) | ||||
| <65 | Reference category | Reference category | ||
| 65–79 | 3.12 (1.83–5.33) | <0.001 | 0.80 (0.66–0.97) | 0.03 |
| >80 | 3.99 (2.25–7.08) | <0.001 | 0.61 (0.48–0.78) | <0.001 |
| Sex (Female) | Reference category | Reference category | ||
| Male | 1.13 (0.80–1.58) | 0.50 | 093 (0.79–1.09) | 0.36 |
| Smoking status (Never) | Reference category | Reference category | ||
| Ex-smokers | 1.09 (0.78–1.53) | 0.61 | 0.97 (0.82–1.14) | 0.70 |
| Current smokers | 0.98 (0.49–1.99) | 0.96 | 1.03 (0.76–1.41) | 0.83 |
| C-reactive proteind | 1.01 (1.005–1.008) | <0.001 | 0.997 (0.996–0.998) | <0.001 |
| Patients with diabetes | 1.00 (0.69–1.44) | 0.99 | 0.94 (0.78–1.13) | 0.50 |
| Patients with CAD | 1.59 (1.11–2.28) | 0.01 | 1.09 (0.89–1.35) | 0.39 |
| Patients with hypertension | 0.86 (0.61–1.21) | 0.38 | 0.91 (0.77–1.07) | 0.24 |
| Patients with reduced renal function | 1.95 (1.39–2.73) | <0.001 | 0.91 (0.76–1.09) | 0.32 |
| Clinical Frailty Scale | ||||
| 1, 2 | Reference category | Reference category | ||
| 3, 4 | 1.28 (0.65–2.52) | 0.48 | 0.94 (0.77–1.16) | 0.58 |
| 5, 6 | 1.86 (0.91–3.79) | 0.09 | 0.73 (0.56–0.96) | 0.02 |
| 7[en-rule]9 | 3.62 (1.78–7.34) | <0.001 | 0.70 (0.53–0.94) | 0.02 |
HR, crude hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; CAD, coronary artery disease.
The multivariable mixed-effects logistic and cox regressions were adjusted for: age group; sex; smoking; C-reactive protein; diabetes; CAD, coronary artery disease; hypertension; and the Clinical Frailty Scale.
Six cases were excluded from the analysis as the patient was followed up for less than 7 days and alive and in hospital.
Twenty cases were not included in the analysis due to missing covariate data (see Table I).
Fitted as a slope parameter.
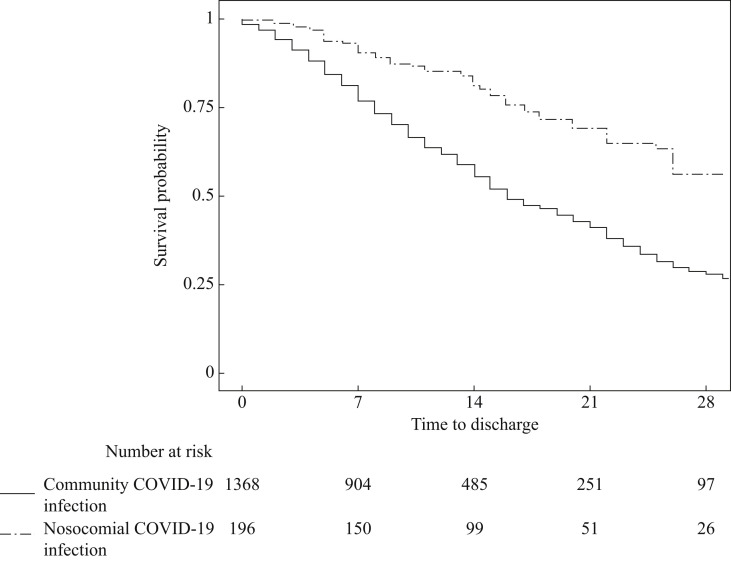
Median length of stay for CAC patients was half that of NC patients (16 days versus 33 days (Table III, Figure 2 ). Covariates associated with an increased length of stay for all patients were: increased age, worsening frailty, and elevated CRP (Table III).
Figure 2.
Kaplan–Meier survivor plot for time-to-discharge for nosocomial versus community infection of COVID-19 patients.
The multivariable mixed-effects Cox regression exploratory analyses of the time-to-mortality show consistent findings for NC versus CAC within each of the demographic and comorbidity subgroup analyses (Supplementary Figure S1).
Discussion
This study is the first to report outcomes for patients with NC infection. Of all COVID-19 cases included, 12.5% of infections were due to transmission in hospital. Overall mortality rate was 27.2% with a lower mortality rate with an NC infection. Patients with NC infection experienced a longer length of stay in hospital.
The proportion of nosocomial infections with COVID-19 found within this study was lower than the 41% previously reported by Wang et al. [15]. Although direct comparisons are difficult, Wang had a small sample size (total 138 patients) which included healthcare worker infections. Excluding these, the rate of patient NC infection was similar to that of our study (12.3% vs 12.5%). Compared to other reported rates of NC infection during historical global pandemics, it appears that NC infection rates are much lower during the COVID-19 pandemic, with the majority of in-hospital COVID cases originating from the community.
In western healthcare, infection control policies have been developed for many years that have positively impacted the response to the rapidly evolving pandemic situation. This multi-centre study is predominantly UK-based and it is important to recognize that data from eastern populations may not be applicable to western populations based upon individual genetic differences, available healthcare resources, and preparedness of healthcare providers to respond to overwhelming demands on services. The first COVID-19-positive patient was reported to the World Health Organization on December 31st, 2019, in Wuhan, China. The UK and Italy reported their first cases on January 31st, 2020. It is possible that countries affected later were able to anticipate resources required and recognized the importance of being able to implement those plans quickly and have a different NC rate. This allowed patients who were diagnosed or suspected to have COVID-19 to be isolated, managed with an increased awareness of cross-infection, with preventative measures such as personal protective equipment, in dedicated ‘COVID-19’ wards.
The public health message during the UK's lockdown was to stay at home, leaving home only for essential travel, in order to maintain social distancing measures. Understandably there is much anxiety among the general public, especially among those with pre-existing healthcare conditions. This has led to 29% fewer emergency department attendances reported in March 2020 compared to March 2019 in England alone [23]. Furthermore, the Office for National Statistics reported the highest death rate in England and Wales since 2000 (week ending April 3rd, 2020), 6082 more than the five-year average. Only 3475 of these are attributed to COVID-19, raising the concern that these additional deaths may have been related to a public reluctance to seek medical attention [24]. Our findings have demonstrated that mortality rates were no worse if COVID-19 was acquired in hospital, compared with those who have acquired the disease in the community, highlighting that patients should be reassured when seeking medical attention for non-COVID-19 conditions.
This NC group of patients was older and frailer, with a non-COVID-19 pre-existing reason for hospital admission, all leading to a median hospital stay in excess of one month. With daily inpatient assessment it is likely that prompt recognition of COVID-19-like symptoms occurred, leading to prompt laboratory and clinical diagnosis of COVID-19 infection. By contrast, the CAC patients may have tolerated their symptoms at home for a period of time before requiring hospital admission. There is also a possibility that reluctance to seek medical attention may have compounded their potentially delayed presentation to hospital. This difference in clinical management may have led to the NC patients having timely supportive treatment, whereas those admitted from home may have presented late with more severe illness leading to a reduced mortality in the CAC patients. It is possible that normal targeted and individualized care for longer-term patients was reconfigured to focus on acute admission assessment and critical care. Although not assessed in this study and difficult to assess objectively, the influence of nursing in isolation and prohibition of hospital visitors is likely to have had a negative psychological impact for this patient group.
This is a large multi-centre observational cohort study including >1500 adult inpatients. Our definition of NC was conservative which only included patients in hospital for over 14 days, whereas the true proportion may be closer to 23% (196 + 169), so the infection rate should be considered ≥12.5%, since hospital workers or patient visitors with COVID-19 were not included in the definition of NC infection, or were patients with a positive diagnosis less than 15 days prior to their admission, or asymptomatic patients were discharged without a positive diagnosis (most likely in younger or less severely affected patients). A further limitation of this observational study is that we could not allow for case-mix differences between the NC and CAC groups, including mildly symptomatic or asymptomatic patients diagnosed COVID-19 as part of hospital screening programmes. Furthermore, we did not assess the cause of death for patients from both NC and CAC groups, although it is assumed that COVID-19 formed at least part of the cause of death for all those who died.
With low hospital-acquired infection rates, this study demonstrates that effective infection control policies are in place in western hospitals. It is now the responsibility of public and professional bodies to actively encourage patients to seek acute medical attention when required and to consider the risks and benefits of reintroducing elective services. Organizational response to emerging evidence should be proactive, considered and continuous. It is imperative that complacency is avoided in response to reduced published daily mortality statistics in order to prevent a second wave.
In conclusion, we found that the minority of COVID-19 hospital episodes were the result of nosocomial transmission. Although no COVID-19 infection comes without risk, those patients with NC infection had no greater risk of mortality, and potentially lower risk than people admitted to hospital with COVID-19.
Conflict of interest statement
None declared.
Funding sources
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.07.013.
Contributor Information
COPE Study Collaborators:
C. Davey, S. Jones, K. Lunstone, A. Cavenagh, C. Silver, T. Telford, R. Simmons, M. Holloway, J. Hesford, T. El Jichi Mutasem, S. Singh, D. Paxton, W. Harris, N. Galbraith, E. Bhatti, J. Edwards, S. Duffy, J. Kelly, C. Murphy, C. Bisset, R. Alexander, M. Garcia, S. Sangani, T. Kneen, T. Lee, A. McGovern, G. Guaraldi, and E. Clini
Appendix A. COPE-Nosocomial Study Collaborators
Ysbyty Ystrad Fawr: Dr C. Davey, Ms S. Jones, K. Lunstone, A. Cavenagh, C. Silver, T. Telford, R. Simmons; North Bristol Trust: Dr M. Holloway, Dr J. Hesford, T. El Jichi Mutasem, S. Singh, D. Paxton, W. Harris; Royal Alexandra Hospital, Paisley: N. Galbraith, E. Bhatti, J. Edwards, S. Duffy; King's College London: J. Kelly; C. Murphy; Inverclyde Royal Infirmary: C. Bisset, R. Alexander; Salford Royal Infirmary: M. Garcia, S. Sangani, T. Kneen, T. Lee; Glasgow Royal Infirmary: Dr A. McGovern, A. Fleck; University Hospital of Modena Policlinico, Italy: Professor G. Guaraldi, Professor E. Clini.
The Patient and Public Involvement statement
The Older Person's Surgical Outcomes Collaboration (OPSOC) over the last seven years has engaged with patients regarding study design, interventions, and dissemination. The COPE study was discussed by patients at the study set-up about the outcomes to be collected at the protocol stage. This particular study was set up, and conducted in a timely manner, to determine whether attending hospital during the first (or subsequent) waves of the epidemic was likely to lead to infection, and whether that infection would have an increased risk of experiencing a clinical event.
Data-sharing statement
Data is available to researchers to address pre-planned hypotheses by request from the COPE-Nosocomial Study investigators.
Appendix B. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . May 5 2020. Coronavirus disease 2019 (COVID-19) situation report 105.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200504-covid-19-sitrep-105.pdf?sfvrsn=4cdda8af_2 Available at: [last accessed May 2020] [Google Scholar]
- 3.Wee L.E., Conceicao E.P., Sim X.Y.J., Aung M.K., Tan K.Y., Wong H.M. Minimising intra-hospital transmission of COVID-19: the role of social distancing. J Hosp Infect. 2020 Apr 12;(20):30191–30192. doi: 10.1016/j.jhin.2020.04.016. pii: S0195-6701, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong S.C., Kwong R.T.S., Wu T.C., Chan J.W.M., Chu M.Y., Lee S.Y. Risk of nosocomial infection of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105(2):119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NHS England A&E attendances and emergency admissions. 2020–2021. Available at: https://www.england.nhs.uk/statistics/statistical-work-areas/ae-waiting-times-and-activity/[last accessed July 2020].
- 7.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . 2020 Jan 28. Clinical management of severe acute respiratory infection when patients novel coronavirus (2019-nCoV) infection is suspected: interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at: [last accessed April 2020] [Google Scholar]
- 10.Li Y., Ren L., Zou J. Risk factors and prevention strategies of nosocomial infection in geriatric patients. Can J Infect Dis Med Microbiol. 2019;2019:6417959. doi: 10.1155/2019/6417959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Department of Communicable Disease Surveillance and Response. Prevention of hospital-acquired infections. A practical guide. 2nd edn. WHO/CDS/CSR/EPH/2002.12.
- 12.Giuliano K.K., Baker D., Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46:322–327. doi: 10.1016/j.ajic.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Reed D., Kemmerly S.A. Infection control and prevention: a review of hospital-acquired infections and the economic implications. Ochsner J. 2009;9:27–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q., Gao Y., Wang X., Liu R., Du P., Wang X. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8:629. doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Public Health England . April 27 2020. COVID-19: infection prevention and control guidance.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881489/COVID-19_Infection_prevention_and_control_guidance_complete.pdf Available at: [last accessed July 2020] [Google Scholar]
- 17.Hewitt J., Carter B., Vilches-Morago A., Quinn T.J., Braude P., Verduri A. The effect of frailty on survival in patients with COVID-19 (COPE): a multi-centre, European, observational cohort study. Lancet Public Health. 2020 June 30;S2468–2667(20):30146–30148. doi: 10.1016/S2468-2667(20)30146-8. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moug S., Carter B., Myint P.K., Hewitt J., McCarthy K., Pearce L. Decision-making in COVID-19 and frailty. Geriatrics. 2020;5:30. doi: 10.3390/geriatrics5020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez R.G. Parametric frailty and shared frailty survival models. Stata J. 2002;2:22–44. [Google Scholar]
- 23.NHS England. Monthly A&E attendances & emergency admissions. Available at: https://www.england.nhs.uk/statistics/statistical-work-areas/ae-waiting-times-and-activity/ [last accessed July 2020].
- 24.Office for National Statistics . April 3 2020. Deaths registered weekly in England & Wales, provisional. Week ending.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredweeklyinenglandandwalesprovisional/weekending3april2020 Available at: [last accessed July 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.