Abstract
There are >12 million patients with peripheral artery disease in the United States. The most severe form of peripheral artery disease is critical limb ischemia (CLI). The diagnosis and management of CLI is often challenging. Ethnic differences in comorbidities and presentation of CLI exist. Compared with white patients, black and Hispanic patients have higher prevalence rates of diabetes mellitus and chronic renal disease and are more likely to present with gangrene, whereas white patients are more likely to present with ulcers and rest pain. A thorough evaluation of limb perfusion is important in the diagnosis of CLI because it can not only enable timely diagnosis but also reduce unnecessary invasive procedures in patients with adequate blood flow or among those with other causes for ulcers, including venous, neuropathic, or pressure changes. This scientific statement discusses the current tests and technologies for noninvasive assessment of limb perfusion, including the ankle-brachial index, toe-brachial index, and other perfusion technologies. In addition, limitations of the current technologies along with opportunities for improvement, research, and reducing disparities in health care for patients with CLI are discussed.
Keywords: AHA Scientific Statement; ischemia, lower extremity; perfusion imaging; peripheral arterial disease
It is estimated that >12 million patients have peripheral artery disease (PAD) in the United States.1 Patients with PAD can progress to critical limb ischemia (CLI). Patients with CLI have higher mortality rates than patients with symptomatic coronary artery disease.2 In patients with coronary artery disease, there have been significant improvements in reducing mortality rates; however, this has not been true of patients with PAD. Moreover, those with both PAD and coronary artery disease have higher mortality rates than patients with coronary artery disease alone.
Disparities in detection and treatment for patients with PAD still exist. There are ethnic differences in comorbidities and presentation of CLI.3,4 Compared with white patients, black and Hispanic patients have higher prevalence rates of diabetes mellitus and chronic renal disease and are more likely to present with gangrene, whereas white patients are more likely to present with ulcers and rest pain.5
Accurate, prompt diagnosis of CLI can help facilitate treatment options for these patients. One challenge in managing these patients is having technologies available that can detect perfusion abnormalities in the lower extremities. The purpose of the present scientific statement is to discuss the current state of perfusion technology and opportunities for future improvements.
DEFINITION OF CLI
The American Heart Association (AHA)/American College of Cardiology (ACC) guidelines for lower-extremity PAD6 define CLI as the presence of ischemic rest pain, nonhealing wound/ulcer, or gangrene for >2 weeks with associated evidence of hypoperfusion as measured by ankle-brachial index (ABI), ankle pressure, toe-brachial index (TBI), toe systolic pressure, transcutaneous oximetry (TcPo2), or skin perfusion pressure (SPP). Because timely revascularization for CLI is a Class I indication in the AHA/ACC guidelines, accurate perfusion assessment is critically important.6 The goal of an ideal perfusion assessment test for CLI, unlike claudication, is to identify whether adequate blood is supplying the extremity to prompt timely wound healing and reduce major and minor amputations.7 Accurate noninvasive limb perfusion assessment will likely allow timely diagnosis but also reduce unnecessary invasive procedures in patients with adequate blood flow or among those with venous, neuropathic, or pressure ulcers.
THE ABI
The ABI is calculated by dividing the higher of the posterior tibial and dorsalis pedis artery systolic blood pressures in a given limb by the higher of the brachial artery systolic blood pressures obtained from either arm. Values ≤0.90 are suggestive of PAD, whereas those >1.4 are considered to be due to noncompressible arteries and therefore nondiagnostic. The higher of the dorsalis pedis or posterior tibial ABI is normally reported. However, the 2011 AHA/ACC guidelines noted the use of the lower of the dorsalis pedis or posterior tibial ABI to assess limb perfusion.8,9 The ABI should be measured with the patient lying flat. There are several other technical considerations when measuring the ABI that need to be considered, including the cuff size, placement of the cuff, use of the Doppler method for systolic blood pressure measurement in each arm and each leg, and others as described elsewhere.8 Typically, the patients will rest for 5 to 10 minutes before the ABI is performed. Despite the diagnostic and prognostic utility afforded by the ABI and the ankle pressures from which it is derived, and in spite of its favorable operating characteristics when used in patients with stable PAD,10 its utility may be limited in patients with CLI. In particular, the ABI may be less useful in the setting of noncompressible vessels and below-knee tibial disease.7,11 Additionally, the ABI may correlate poorly with the Rutherford classification and angiographic runoff and may not accurately predict wound healing.7,11 Furthermore, the ABI provides summative data at the level of the ankle, where more granular perfusion data (eg, by angiosome) may be needed to assess disease severity and predict treatment response in CLI.
Data in Support of the ABI
Of the perfusion methods noted in the guidelines,8 ABI is the most widely used method. Although ABI was first described to diagnose PAD, it has not been shown to be an accurate predictor of wound healing or major adverse limb events. Clearly, the ABI provides important prognostic information, including the risk of death, myocardial infarction, and stroke, beyond the Framingham risk score or other traditional prognostic measures, and should be performed in all patients suspected of having PAD.8 However, in ≈30% of patients with angiographically documented CLI, the ABI is normal or noncompressible.11,12 Moreover, a recent large study from Michigan Blue Cross Blue Shield revealed that ≈50% of patients with suspected CLI who underwent lower-extremity angiography and revascularization never had a preprocedure ABI assessment.13 Recent data have shown that toe pressure may be a better predictor of major adverse limb events and tibial disease in patients with CLI, especially among those with isolated below-knee disease.12 However, to date, there is no solid evidence with core laboratory–adjudicated wound healing data for ABI or TBI to assess the sensitivity and specificity of these tests as perfusion tools to assess wound healing or limb salvage.
Other more advanced perfusion tools such as TcPo2 and SPP have single-center data with limited sample size as predictors of perfusion for wound healing.14-16 Furthermore, these techniques are not widely available and have many limitations.
Pathophysiological Challenges of the ABI
There are a number of challenges in measuring limb perfusion in the setting of CLI. Many patients have diabetes mellitus and chronic kidney disease, resulting in arterial calcinosis and noncompressible vessels. A recent analysis showed that >70% of the tibial vessels that were considered to be noncompressible were actually occluded or severely stenotic by angiography.17 The current noninvasive modalities for limb perfusion assessment measure different aspects of perfusion, and each modality has unique limitations. For example, TcPo2 measures oxygen tension, but SPP measures the capillary opening pressure. On the other hand, ABI and TBI measure tibial or toe pressure, respectively, but cannot quantify oxygenation. Thus, the current devices only address flow or pressure and fail to provide an answer to the most important question: does the wound have enough perfusion to heal? Unfortunately, perfusion can be affected by blood flow, edema, nutritional status, medications, inflammation, infection, and other factors.
LIMITATIONS OF ABI AND ANKLE SYSTOLIC PRESSURE
Although most patients with PAD, including those with diabetes mellitus, have compressible tibial vessels, a significant proportion of patients referred to noninvasive vascular laboratories for arterial evaluation do not. As a result, an accurate ABI cannot be obtained. In a large series of consecutive outpatients (n=17 485) who underwent noninvasive lower-extremity arterial testing at the Mayo Clinic over a decade, 2781 (16%) had non-compressible vessels.18 Interestingly, abnormal Doppler waveforms suggestive of occlusive PAD were found in 71% of those with noncompressible vessels, and CLI was more prevalent in those with noncompressible vessels than among those who had ABIs ≤0.90 (37% versus 18.5%, respectively). Noncompressible vessels were associated with a lower likelihood of survival over a mean follow-up of 5.8 years compared with both those with normal and abnormal ABIs. In a smaller cohort of patients with diabetes mellitus at a tertiary teaching hospital referred for Doppler assessment of their lower extremities (n=403), noncompressible vessels were identified in 150 (37.2%). Of these 150 patients, 84 (56%) had abnormal Doppler assessments suggestive of occlusive PAD.19 Over 6.4 years of follow-up, noncompressible vessels were associated with major adverse cardiovascular events among those who also had abnormal Doppler findings. Finally, among 284 patients with CLI, the presence of noncompressible vessels was associated with a greater likelihood of amputation (hazard ratio, 1.75 [95% CI, 1.12–2.78]) and major adverse cardiovascular events (hazard ratio, 2.04 [95% CI, 1.35–3.03]) over 3 years of follow-up than in those with CLI and compressible vessels.20 It is noteworthy that the ABI may be partially compressible, with resultant values that underestimate the severity of disease; in a series of 5984 patients with CLI who were undergoing revascularization in a large statewide registry, 21% had normal ABI and 53% had only mildly reduced ABI.13
Infrapopliteal PAD is present among a large proportion of patients presenting with CLI.21 In a subgroup analysis (n=237) from the IN.PACT DEEP Trial (Randomized Amphirion DEEP DEB vs Standard PTA for the Treatment of Below the Knee Critical Limb Ischemia), only 6% of patients with CLI and isolated infrapopliteal artery disease had an ABI <0.4 and only 16% had an abnormal ankle pressure according to commonly accepted societal guideline thresholds.12 There was no apparent correlation between the ABI or ankle pressures. Furthermore, the number of diseased below-knee vessels, the ABI, and ankle pressure were inversely associated with Rutherford classification. In another study of patients with CLI, increasing ABI was paradoxically associated with abnormal runoff.11
Ankle pressures are poorly correlated with toe pressure; in a study of 237 patients with CLI who met diagnostic criteria for abnormal toe pressure, only 58% had abnormal ABIs.12 Although the change in ABI measured before and after endovascular limb salvage procedures independently predicts the likelihood of wound healing, static postprocedure ABI is not predictive.22 Perhaps because the ABI is a more summative measure of leg perfusion, it is less useful than one specific to the revascularized target tibial artery. A number of studies support this concept and have shown that direct angiosome-guided revascularization is associated with superior wound healing and limb salvage rates compared with indirect revascularization.23 Furthermore, hemodynamic cutoffs for wound healing and limb preservation vary from one patient to another because wound healing is a complex process that is dependent on several factors, such as the presence of superimposed infection and wound size. The above observations notwithstanding, the ABI and ankle pressures remain an important component of the WIfI classification (wound, ischemia, and foot infection), which has been shown to predict wound healing in CLI and with diabetic foot ulcers.24,25 Lastly, recent data indicate that using the lower of the systolic ankle pressures led to better prediction of angiographically documented PAD; whether this approach would have a stronger predictive value for CLI is unknown.26
LIMITATIONS OF TBI AND TOE PRESSURE
Toe pressure and TBI are currently recommended tests of forefoot perfusion appropriate to diagnose and manage CLI.6,24,27,28 Toe pressure assessment offers the primary advantage over ABI of improved accuracy in detecting limb ischemia in the presence of heavily calcified and poorly compressible vessels, which are particularly common among patients with diabetes mellitus and chronic kidney disease.11,12,29,30 Nevertheless, important limitations exist for toe perfusion assessment that require further consideration.
Expert consensus statements consistently recommend the use of toe perfusion assessment to diagnose CLI, although a universally accepted threshold for TBI or toe pressure to confirm the diagnosis does not exist. Most guideline statements use separate toe pressure criteria for CLI, according to the presence or absence of tissue loss.24,27,28,31,32 In the presence of a pedal ulcer, CLI is indicated by a toe pressure <50 mm Hg, whereas ischemic foot pain is suggested by a toe pressure <30 mm Hg. Furthermore, a toe pressure ≥45 mm Hg has been correlated with ulcer healing.30 Toe pressure offers stronger correlation with ulcer healing and major amputation than ankle pressure or ABI, especially among patients with diabetes mellitus. An absolute increase in TBI ≥0.21 after endovascular revascularization is similarly associated with wound healing and reduced major adverse limb events.22 Overall, toe pressure assessment across studies demonstrates variable diagnostic accuracy and requires additional study to confirm its utility in CLI.33
Although toe perfusion assessment is quick and inexpensive, toe pressure and TBI are not consistently available in many clinical settings, and current guidelines do not give preference for one test over the other. Toe pressure testing requires careful technique and specialty cuffs fitted for the toes to provide reliable results. Photoplethysmographic tracings can be added to the toe pressure to provide qualitative information of forefoot perfusion. Toe plethysmography offers assessment of toe pulse-wave amplitude, which can supplement toe pressure results by indicating the risk of amputation.34 Patients with an overlying toe wound or those with prior forefoot amputation may not be amenable to toe pressure assessment. In such circumstances, TcPo2, SPP, and pulse-volume recordings are acceptable alternatives. Because toe pressure does not provide localization of arterial disease, these tests of local perfusion might have an additional advantage in angiosome-based revascularization.35
PERFUSION ASSESSMENT: UNMET NEEDS
No single vascular test has been identified as the most important predictor of wound healing or major amputation for the threatened limb. Furthermore, the management of PAD based on these results is inconsistent, largely because of a lack of consensus-driven high-quality clinical data. Although expert consensus and guideline statements that provide a framework for assessment of ischemia are a positive step forward,6,24 intraprocedural perfusion guidance would also allow more efficient procedures, including revascularization of the angiosome-related artery, pedal arch, and vessels, and multivessel intervention. At the present time, only angiographic end points are available to subjectively evaluate the completeness and adequacy of perfusion intraprocedurally. There are still unmet needs for adding sophistication to the use of noninvasive diagnostic vascular testing in the management of the threatened limb.36 A key target area is the spatial and quantitative assessment of tissue perfusion or oxygenation.
PERFUSION IMAGING IN CLINICAL PRACTICE: UNMET NEEDS
Oxygen delivery to the tissues of the lower limb is determined by many biological factors, including multi-scale blood flow distribution, oxygen-carrying capacity (ie, hemoglobin concentration), hemoglobin oxygen saturation, and efficiency of hemoglobin-oxygen dissociation. There are potential roles in clinical care for direct assessment of tissue oxygenation or the primary determinant of oxygen delivery, microvascular perfusion. Some of these techniques have been introduced into clinical practice (Table 1), whereas others remain investigational (Table 2). In addition, the discordance of tissue perfusion and tissue oxygenation could potentially be used to determine the relative contribution of flow-dependent and flow-independent contributions to ischemic complications, which could have an impact on treatment strategy.
Table 1.
Technologies Capable of Assessing Limb Tissue Oxygenation or Perfusion in Humans
| Technology | Type of Measurement |
Indications | Sensitivity, % | Specificity, % | Limitations | Reference |
|---|---|---|---|---|---|---|
| Ankle-brachial index | Surrogate | Evaluate for PAD | 69–79* | 83–99* | Calcified arteries, poor predictor for wound healing | 8, 11 |
| TcPo2 | Surrogate | Nonhealing wounds or gangrene | 98† | 44† | Time, open wounds, amputation | 1, 37 |
| Skin perfusion pressure | Surrogate | Below-ankle lesions | 72‡ | 88‡ | Reproducibility, pain, artifacts from motion, bony prominences, and veins | 16 |
| Phosphorescence biosensors | Biomarker | Monitor PAD progression and treatment response | NA | NA | Limited efficacy data, relatively invasive | 38, 39 |
| Indocyanine green | Biomarker | Nonhealing wounds or gangrene | 85§ | 100§ | Limited tissue depth penetration, iodine or sulfa allergy | 40 |
NA indicates not available; PAD, peripheral arterial disease; and TcPo2, transcutaneous oximetry.
Sensitivity and specificity for predicting PAD.
Sensitivity and specificity for predicting resolution of limb salvage problems.
Sensitivity and specificity for wound healing.
Sensitivity and specificity correlated to ankle-brachial index.
Table 2.
Experimental Technologies for Lower-Extremity Perfusion Assessment
| Research Applications Only |
Indications | Limitations |
|---|---|---|
| Indigo carmine angiography | Evaluate microcirculation and angiosomal revascularization | Invasive |
| CT perfusion | Quantify perfusion, monitor treatment response | Cost, radiation, iodinated contrast use |
| MRI (gadolinium kinetics, BOLD, ASL) | Quantify perfusion, monitor treatment response |
Cost, time, potential use of gadolinium contrast |
| Contrast-enhanced ultrasound | Calf muscle perfusion assessment | Operator dependent, limited data below the ankle |
| Hyperspectral imaging | Tissue oxygenation assessment | Potential influence from respiratory disease |
ASL indicates arterial spin labeling; BOLD, blood oxygen level dependent; CT, computed tomography; and MRI, magnetic resonance imaging.
Methods such as TcPo2 and the measurement of oxygenated versus deoxygenated hemoglobin with standard near-infrared spectroscopy or hyperspectral imaging provide regional information on tissue oxygen content or delivery.41-43 In the clinical setting, they are able to quantify the net effect of disorders that involve any or all of the physiological determinants of oxygen delivery. They are particularly helpful for evaluating combined effects of PAD together with diabetic microvascular dysfunction, anemia, or abnormalities in hemoglobin oxygen-carrying capacity or dissociation associated with sickle cell disease.44-47 However, given the spectral dependence of these light-based techniques, they are best suited to assessing dermal and immediate subdermal status, although depths in excess of 10 to 15 mm are also possible with near-infrared spectroscopy, enabling measurement of muscle oxygenation as well.48 More data are needed to determine the sensitivity and specificity of near-infrared spectroscopy measurements to predict wound healing.
Measurement of tissue blood flow rather than oxygenation is also possible. Although these approaches do not directly measure oxygen or nutrient delivery, they do provide vital information on the reduction of tissue perfusion, which is the primary pathophysiological problem and treatment target in CLI. Direct assessment of skin perfusion can be performed with fluorescent imaging of indocyanine green (ICG) and application of various transit rate functions, as well as indirectly by postocclusive skin perfusion pressure.15,40 Techniques have also been developed that are able to quantify limb skeletal muscle perfusion with kinetic modeling of contrast-enhanced magnetic resonance, contrast-enhanced ultrasound, or radionuclide imaging.49-52 Perfusion imaging techniques that are capable of assessing muscle blood flow both at rest and during exercise or other metabolic stress (eg, postischemic hyperemia) could be particularly helpful in the evaluation of patients with intermittent claudication.50,53 However, there are limited data on these imaging techniques in CLI patients. Although one could argue that blood flow measurements in the conduit artery could be used to assess the severity or impact of arterial stenosis, regional perfusion imaging is of added value because it provides information on (1) the adequacy of collateral blood flow, (2) the combined effects of arterial stenosis and microvascular dysfunction, and (3) the ability to “redistribute” perfusion from one limb tissue to another according to flow requirements.
There are several clinical scenarios in which perfusion imaging can provide incremental information to conventional evaluation methods. Skin perfusion and oxygen delivery is the primary determinant of whether traumatic wounds or chronic ischemic ulcers will heal and whether tissue infection will resolve. Normal TcPo2 is >55 mm Hg. Although there is a continuous relationship between oxygen concentration and ulcer healing, a TcPo2 level <30 mm Hg has been identified as a threshold below which the chance of wound healing is considered doubtful.54,55 Ballard and colleagues37 demonstrated that 100% of patients who underwent bypass or angioplasty and achieved a TcPo2 of ≥30 mm Hg had complete wound healing. Furthermore, a postprocedure TcPo2 of ≥30 mm Hg was 96% accurate in predicting wound healing or resolution of rest pain.37 Wound healing is also unlikely when SPPs are <30 mm Hg, serving as a validation that adequate microvascular driving pressure is critical for capillary perfusion where oxygen delivery occurs.56 A key issue is how this information can be integrated into decision making to enhance clinical care, particularly in regard to revascularization strategy. Although still largely untested, critical reductions in tissue perfusion or oxygenation could be used as a strategy for selecting low- to medium-risk individuals for early revascularization based on futility of conservative management and to prevent further tissue loss.
NEED FOR SPATIAL INFORMATION
Assessment of regional perfusion or oxygenation is a critical component of treatment algorithms based on angiosomal patterns of flow disturbance. Angiosomes are the anatomic territories that are supplied by major arterial networks that were originally mapped by arterial dissection and postmortem dye injection.57 Six separate angiosomes have been defined for the ankle and foot. Although no randomized controlled trial has been performed to compare angiosomal-directed revascularization to indirect single tibial vessel revascularization for wound healing, limb salvage, or mortality, data from meta-analyses indicate direct revascularization should be performed when feasible.23,58,59 Revascularization according to angiosomal need rather than the best target vessel approach has been proposed as a more effective prioritization strategy for procedural planning.60 However, angiosome-based assessment relies on the ability to measure anatomic perfusion patterns and possibly the impact of collateralization between angiosomes.
The ability to quantify regional limb muscle perfusion can impact revascularization strategy. It may also address the confounding problem that limb muscle perfusion during exercise can be influenced by microvascular dysfunction, particularly in patients with diabetes mellitus.61,62 At the present time, it is unknown whether muscle perfusion can be used to select patients with noncompressible vessels or intermediate ABIs who have a particularly ominous prognosis and will benefit from early revascularization. In addition, angiosomal patterns similar to that of the foot apply to muscle perfusion and could potentially be used to optimize percutaneous or surgical revascularization based on regional flow abnormalities and likelihood for symptom resolution.
Evaluation of regional perfusion and oxygenation is of potential importance for evaluating disease severity and planning revascularization strategy, as well as assessing the benefit of medical therapy or arterial revascularization (Figures 1, 2, and 3). The ability to immediately assess therapeutic response could be particularly useful for establishing the need for additional or alternative therapy in those patients at high risk for tissue loss, or to guide clinicians on the expected degree of clinical improvement. Implicit in this application is that perfusion imaging or assessment of oxygenation will be an important asset in the development of new arterial revascularization strategies (ie, new devices or selection of surgical versus endovascular approach) or new drug, gene, or cell-based therapies that are designed to improve microvascular function.
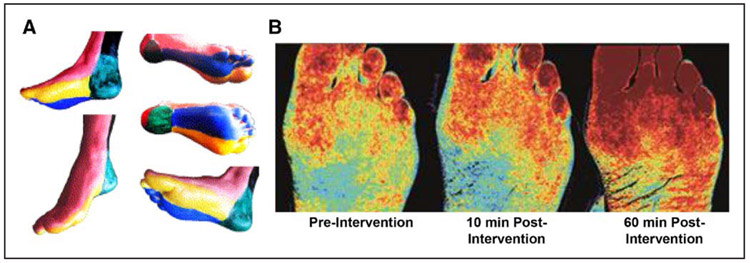
Figure 1. Examples of noninvasive techniques that have been used to assess the effect of revascularization on microvascular perfusion or oxygen content.
(A) Foot and ankle angiosomal distribution. Adapted from Sumpio et al60 with permission from the Society for Vascular Surgery. Copyright © 2013, Society for Vascular Surgery. (B) Noninvasive hyperspectral imaging of the plantar aspect of the foot illustrating progressive improvement in oxyhemoglobin (red spectrum) in 2 separate angiosomes after lower extremity revascularization. Reproduced from Sumpio63 with permission. Copyright © 2018, BIBA Medical Ltd.
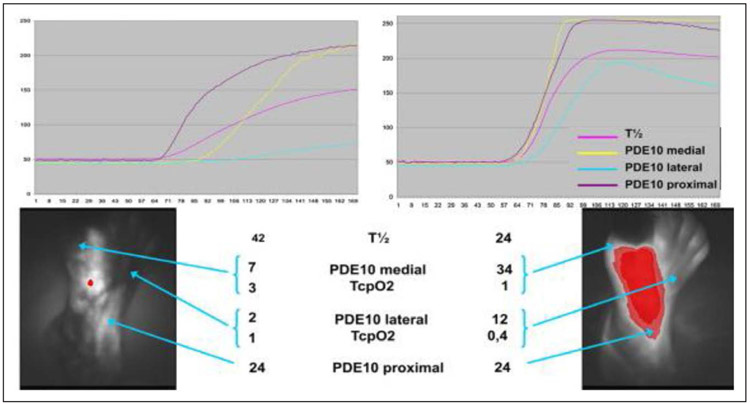
Figure 2. Noninvasive indocyanin green fluorescence imaging of the dorsal foot at rest (quantitative curves at top) illustrating shorter time-to-peak and mean transit time after multilevel percutaneous revascularization.
The corresponding transcutaneous oximetry (TcPo2) measurements are shown below. PDE10 indicates fluorescence intensity measured 10 seconds after onset of fluorescence; and T1/2, time elapsed from onset of fluorescence to half-maximum intensity. Adapted from Sumpio et al60 with permission from the Society for Vascular Surgery. Copyright © 2013, Society for Vascular Surgery.
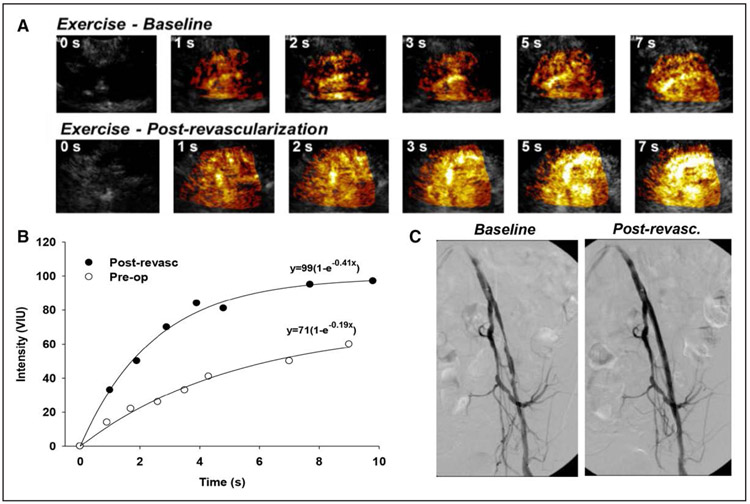
Figure 3. Quantitative perfusion imaging before and after percutaneous revascularization.
(A) Quantitative contrast-enhanced ultrasound perfusion imaging from the calf during plantar-flexion exercise illustrating background-subtracted images, and (B) corresponding time-intensity data, where improvement in muscle exercise perfusion was achieved after percutaneous revascularization. (C) Pre- and post-revascularization angiograms are also shown. Post-revasc indicates post-revascularization; Pre-op, preoperatively; and VIU, volumetric iodine uptake. Courtesy of J.R. Lindner.
LASER DOPPLER AND SPECKLE IMAGING DEVICES
There are multiple laser applications for tissue perfusion assessment. This noninvasive technology exploits the interaction between coherent light and flowing blood cells. When laser light contacts blood cells, it is reflected and scattered. The reflected or scattered light is then measured by 1 of 2 methods, the difference of which defines laser Doppler technologies and laser speckle imaging (LSI), respectively.
In laser Doppler flowmetry (LDF), light is delivered to tissue through an optical fiber. Most Doppler flow meters use a 780-nm wavelength to allow for skin penetration independent of skin color and oxygen saturation.64 When the light interacts with tissue, the backscattered portion is transmitted through a second optical fiber to a photodetector for measurement. LDF provides real-time tissue perfusion sampling, which gives it utility during revascularization procedures. This technology is limited by the relatively small area and shallow depth of measurement.65 Furthermore, the heterogeneity of skin perfusion decreases measurement reproducibility. Laser Doppler imaging relies on the same physical principles as LDF, with the advantage of producing 2-dimensional perfusion map images of the microvascular blood perfusion.64 This allows for better appreciation of the angiosome involved by disease.
Similar to laser Doppler technologies, LSI enables real-time perfusion assessment in the dermal tissues. When coherent light is scattered, it produces a random interference pattern called a speckle. Temporal fluctuations in the speckle pattern can be measured to determine blood flow, analogous to the intensity fluctuations that occur from Doppler shifts.64,66 This technology was originally used to measure blood flow in the retina and was subsequently translated to cerebral and skin perfusion assessment.67 Similar to LDF and laser Doppler imaging, LSI has high spatial resolution, which allows for measurements at the microvascular level. In contrast to LDF, LSI has the benefit of not requiring direct contact with tissue to measure blood flow.67,68
LSI tends to be hindered by motion artifact, device size, and complicated data acquisition analysis. The FlowMet-R (Laser Associated Sciences, Inc) is a novel device designed specifically for use in extremity perfusion assessment. This technology has a small profile and comes in the form of a clip-on device similar to a pulse oximeter. Speckle patterns from scattered 785-nm coherent light are sampled at a rate of 250 Hz, which allows for real-time blood flow monitoring.69 By affixing the device to the patient’s extremity, motion artifact is decreased.
Although several investigations have shown laser technologies could be effective for limb perfusion assessment, these studies are limited by small size and novelty. In current practice, laser devices have not supplanted the widely used ABI, TBI, and TcPo2 tests. In addition, there are currently no societal guidelines for their use in patients with PAD. Although LDF, laser Doppler imaging, and LSI might be useful adjuncts to current diagnostic modalities, larger prospective studies are required to demonstrate their utility as a primary tool for perfusion evaluation.
NONINVASIVE IMAGING
Imaging modalities such as computed tomography (CT) angiography, magnetic resonance imaging (MRI) angiography, and duplex ultrasound can accurately measure blood flow in the lower extremities; however, these are only surrogate markers for tissue perfusion. Perfusion CT and MRI, specifically arterial spin labeling (ASL) and blood oxygen level–dependent (BOLD) imaging, can better quantify lower-extremity tissue perfusion, although these techniques are not routinely performed for clinical evaluation of PAD.
Perfusion CT has been widely used in cerebral imaging to stratify patients who may benefit from thrombolytic therapy, characterize tumors, and monitor cerebral perfusion after subarachnoid hemorrhage.70 This imaging technique may also be able to quantify perfusion in the lower extremity and monitor revascularization treatment response. In a 2016 study by Hur et al,71 perfusion CT was found to have a strong correlation with fluorescent microsphere blood flow analysis in rabbit foot models. The color-coded perfusion maps also correlated well with clinical and angiographic findings in patients with PAD who underwent revascularization.71 Perfusion CT examinations can be readily performed on modern CT scanners and can be acquired as an adjunct to CT angiography. The drawback of this technique is that it exposes patients to additional radiation and requires iodinated contrast, the latter of which is not always feasible in patients with chronic kidney disease or contrast allergy.
ASL MRI has been used since the 1990s to measure cerebral blood flow.72 In ASL, arterial blood water is magnetically labeled just upstream from the region of interest by a 180° radiofrequency inversion pulse. This pulse leads to inversion of the net magnetization of water. The inflowing inverted spins within blood water alter total tissue magnetization, and consequently, the signal intensity is decreased. The image acquired with this reduced signal is called the tagged image. Subsequently, an image is taken without blood water labeling, which is referred to as the control image. By subtracting the tagged and controlled images from the static surrounding tissue signal, information on tissue perfusion and transit time is gained.73,74 West et al75 demonstrated that ASL was a reproducible noncontrast technique that could effectively quantify peak exercise blood flow in the calf muscle in patients with claudication.
Similarly, BOLD imaging has been used for many years in functional MRI to quantify cerebral perfusion by measuring regional differences in cerebral blood flow. This technique relies on the ability of MRI to delineate the magnetic properties of oxygenated and deoxygenated hemoglobin. Deoxygenated hemoglobin is paramagnetic, whereas oxygenated blood is diamagnetic. The difference in signal is measurable with heavily T2* weighted sequences.76 A study by Bajwa and colleagues77 assessed BOLD MRI as a tool for measuring calf muscle perfusion in patients with CLI. They showed statistically significant interuser and interscan reproducibility. Furthermore, there was significant correlation between the MRI measurements and tissue vascularity found in muscle biopsy samples obtained in the region scanned.
Both ASL and BOLD imaging are performed without the use of gadolinium-based contrast agents. These techniques can be added to other noncontrast MRI sequences, namely, 3-dimensional fast spin echo time-of-flight angiography, to generate a complete evaluation of lower-extremity vascular anatomy and tissue perfusion. This is an important consideration given that many patients with PAD have concomitant chronic kidney disease.78 Imaging with MRI also provides an alternative modality for patients who have iodinated contrast allergies.
Both ASL and BOLD imaging are dependent on adequate blood flow. Patients with PAD often have sluggish arterial flow and relatively high concentrations of deoxyhemoglobin, which limits the amount of MRI signal that can be measured.79 MRI is also time intensive and costly and has limited application for real-time imaging during revascularization intervention. Generally, BOLD imaging has a high signal-to-noise ratio and faster acquisition time than ASL.77
ICG FLUORESCENCE ANGIOGRAPHY
Similar to LSI, ICG fluorescence angiography began as a technique for imaging retinal vessels before use in other applications, including detection of arteriovenous malformation and anastomotic perfusion monitoring after colorectal surgery.80,81 Fluorescent ICG dye is injected intravenously. The dye is then activated with near-infrared laser light, producing fluorescence, which can be detected and measured. Limited studies have applied ICG fluorescence angiography to patients with PAD. Zimmermann and colleagues82 demonstrated ICG angiography correlated with the degree of vascular collateralization in the lower extremities of patients with PAD. This technique may also allow for real-time flow assessment in peripheral bypass surgery.83 ICG fluorescence angiography is limited by its ability to only assess superficial tissues within 3 mm of the skin surface. Furthermore, alterations in muscle microvascular perfusion occur earlier than skin changes that tend to be observed in advanced CLI.80 Hence, ICG fluorescence angiography may have limited efficacy in patients with early PAD.
INDIGO CARMINE ANGIOGRAPHY
Indigo carmine is an organic salt commonly used as a food-coloring agent. There are limited data on the use of indigo carmine in patients with PAD; however, a prospective, multicenter study performed by Higashimori and colleagues84 demonstrated the utility of intra-arterially injected indigo carmine for limb perfusion assessment immediately after revascularization. Their study included 53 patients with Rutherford categories 5 and 6 CLI. After revascularization, indigo carmine angiography was performed by catheterization of the distal popliteal artery and injection of the dye. The dye was able to demarcate the arterial perfusion distribution and stain the wound if it was adequately perfused. As a result, the success of revascularization could be assessed, providing a prognosis for potential wound healing.84
IMPLANTABLE DEVICES
The Lumee Oxygen Platform (Profusa, Inc) is a novel tissue-integrating micro-oxygen biosensor. These injectable biosensors provide the advantage of measuring tissue oxygen concentration rather than vascular oxygenation measured by noninvasive techniques such as pulse oximetry, near-infrared spectroscopy, or laser technologies.85 The device is a phosphorescence biosensor that is implanted within the tissue of interest. The biosensors are soft, flexible, biocompatible hydrogels measuring 0.5 × 0.5 × 5.0 mm. The biosensors are injected into the subcutaneous tissues using a 16- or 18-gauge needle. Once deployed, they remain in the body permanently. A reader is then taped onto the skin above the implanted biosensor, and an LED (light-emitting diode) pulses light into the skin above the sensor. Based on the degree of local tissue oxygenation, the biosensor will produce measurable phosphorescence, which can be measured externally by a photodetector in the reader. The reader also has a thermometer, which allows for continuous temperature correction. Tissue oxygen levels can be monitored in continuous real-time.38
The Lumee Oxygen Platform is currently an investigational device in the United States but has received the CE mark in Europe.85 Injectable biosensor technology has been shown to efficaciously measure reactive hyperoxia in the hind limbs of rats subjected to a series of oxygenation challenges and may be more sensitive to fluctuations in relative tissue oxygenation than near-infrared spectroscopy.39 This device has also been shown to safely and effectively measure local tissue oxygen levels in the feet of 10 human subjects with CLI during surgical intervention, as well as postoperatively for 28 days.85 Larger studies are still needed to validate this device and its utility for perioperative limb perfusion assessment.
EVALUATION OF THERAPIES
Most devices that have obtained US Food and Drug Administration approval have undergone a 510K pre-market submission pathway. As a consequence, there are limited comparative data among devices.86 The pre-market approval pathway should in part depend on the mechanism of action of the device: blood flow mediated, measuring tissue oxygen, perfusion, or molecular imaging. In certain circumstances, a comparative study would be helpful to identify gaps where certain devices would be superior to others. In addition, understanding the strengths and weaknesses of the devices in the different disease conditions would be helpful. For example, are certain devices better in noncompressible vessels in patients with diabetes mellitus and dialysis? Are certain devices better at identifying successful revascularization using the angiosome concept in patients with planned surgery or wounds?
The hemodynamic cutoffs for various limb perfusion technologies can vary significantly from patient to patient based on wound size, infection, wound location, edema, and nutritional factors. Combining hemodynamic data with other important predictors of wound healing and limb salvage could provide better diagnostic and prognostic predictors. The WIfI classification has incorporated ABI and TBI with wound infection and size.24 When combining hemodynamic information, the WIfI classification has been shown to help segregate patients who will likely benefit from revascularization and predict wound healing in CLI and with diabetic foot ulcers.25
IMPACT OF PERFUSION ASSESSMENT ON PUBLIC HEALTH AND DISPARITIES
The AHA/ACC “Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease” lists advancement in PAD diagnostics, including “tools for more reliable noninvasive perfusion assessment in CLI,” as a critical evidence gap future direction for PAD-related research.6 Such advancement in diagnostics would result in a variety of opportunities in CLI: proper diagnosis, identification of differences related to sex and ethnicity, reduction of major amputation, identification of procedural failures or incomplete revascularization, the potential for telemedicine to reduce disparities in CLI, and potential cost savings in the care of patients with CLI.
Opportunities for Proper Diagnosis
The proper diagnosis of CLI is the first step in the prevention of major adverse cardiovascular outcomes, including mortality, and the prevention of amputation. As discussed, no noninvasive hemodynamic measures of PAD are 100% sensitive or specific for CLI.87 If the ABI is near-normal or normal (>0.90) in up to one-third of patients with CLI, and prevalence of CLI in the United States is ≈1.3%, the diagnosis of CLI would be missed in >1 million patients.11,35,88 Thus, if perfusion assessment is able to provide improved sensitivity for CLI over available hemodynamic measures (eg, ABI), a significant number of patients would be correctly diagnosed, potentially leading to a cascade of proper medical care and revascularization to preserve limb and life. A recent report by Ferraresi et al89 divided the tibial arteries into a big-artery disease and the pedal artery into a small-artery disease. They found in a retrospective analysis that small-artery disease had an independent and strong association with CLI, diabetes mellitus, and dialysis.89
Opportunities to Identify Differences Related to Sex and Ethnicity
Early population-based estimates of PAD prevalence in women have been plagued by methodological difficulties, including the use of intermittent claudication as the basis for disease categorization and failure to account for mean height differences between men and women when determining normal ABI values.90 The prevalence of PAD increases with age for both men and women; however, when calculating the “burden” of disease by age and sex from US census data, there are more women than men with PAD among US adults ≥40 years of age.91 Contemporary reviews now estimate that the global prevalence of PAD is higher in women than in men,92 and the increase in mortality and disability associated with PAD worldwide has been greater among women than men.93
Despite the high prevalence of PAD in women, women are underrepresented in PAD clinical trials.91 This is particularly troubling because women are more likely to be hospitalized emergently for PAD,94 more likely to present with CLI,95 and have higher mortality rates after lower-extremity revascularization or amputation.94,95 Finally, there may be significant anatomic differences in the distribution of atherosclerotic lesions between men and women with CLI. Women have increased risk for femoropopliteal lesions, multilevel disease, and occlusive lesions compared with men.96 Taking these factors into account, the potential earlier diagnosis of CLI or the increased sensitivity for CLI afforded by perfusion imaging could have significant potential influences on the study of sex differences in CLI.
Ethnic differences in comorbidities and presentation of CLI exist.3,4 Compared with white patients, black and Hispanic patients have higher prevalence rates of diabetes mellitus and chronic renal disease and are more likely to present with gangrene, whereas white patients are more likely to present with ulcers and rest pain.5 Alarmingly, black patients are 78% more likely to receive lower-extremity amputation for CLI than their white counterparts, even after adjustment for comorbidities, socioeconomic status, and access to facilities with revascularization capacity.5 These findings have been replicated when adjusting for disease severity.97 Black patients also experience the lowest rates of revascularization.98 Although implicit provider bias has been implicated as a potential cause of these disparities,97 the study of perfusion differences in the limbs of white and nonwhite patients with CLI would provide further insight into potential differences in the anatomic distribution and severity of presentation among races. Perfusion imaging would also serve as another unbiased covariate by which amputation rates among black patients could be adjusted to understand the contributors to these apparent disparities.
Opportunities to Identify Procedural Failures or Incomplete Revascularization
CLI represents the most severe clinical expression of diagnosed PAD.99 Regardless of the type of procedure performed or pharmacological therapies prescribed, assessing for and maintaining limb patency after an intervention is an ongoing challenge. A review by Martini100 of 14 CLI studies revealed an overall 1-year limb survival rate of 75.4%, including endovascular, surgical, and conservative therapies. Iida et al101 reported that target-vessel revascularization was required in 48% of limbs with infrapopliteal angioplasty within 12 months of the primary procedure. The 3-year primary patency rate after infrainguinal surgical revascularization when polytetrafluoroethylene bypass grafting was used in patients lacking a saphenous vein was 39% in a study by Brumberg et al.102
The AHA/ACC guideline on the management of patients with lower-extremity PAD specifies in algorithms the use of noninvasive perfusion assessment techniques in the evaluation of symptomatic PAD.6 Although these same techniques are frequently used in follow-up postrevascularization visits, criteria for the type of assessment and frequency are not specified. General recommendations for perfusion follow-up after lower-extremity endovascular or open surgical intervention were recently published by the Society for Vascular Surgery.103 These include the use of ABI with or without duplex ultrasound in the early postoperative period (surgical) or within 1 month (endovascular). After the initial postprocedure perfusion assessment, recommendations for ongoing ABIs with or without duplex ultrasound were made based on the approach used.104 With the exception of prosthetic infrainguinal bypass grafts, these recommendations are based on a low quality of evidence.
There were no randomized controlled trials and several small cohort studies supporting the use of noninvasive perfusion techniques such as ABI, toe pressure, TBI, duplex ultrasound, SPP, and TcPo2 in identification of CLI postprocedure revascularization failure or impaired wound healing.22,86,105 An increase in ABI, toe pressure, TBI, or TcPo2 post procedure supported revascularization and wound healing.22,86,105 An SPP >30 mm Hg also predicted wound healing.106
Concerns with these studies include small sample size, type of study (observational), and lack of standardized or incomplete follow-up. When TcPo2 is used to assess limb perfusion, multiple issues impact the accuracy of the measurement, including site selection, electrode equilibration, concomitant use of oxygen, and patient position.106 Cold ischemic feet can impact SPP values. False ABI measurements can occur in diseases such as diabetes mellitus. More than 1 perfusion assessment technique could be needed to determine the revascularized extremity status. The literature on CLI clearly portrays the difficulties in sustaining limb patency and ultimately limb salvage after revascularization. Randomized clinical trials studying the impact of these noninvasive perfusion techniques in reducing procedural failure or incomplete revascularization are needed. These will assist in providing stronger levels of evidence to support the recommendations for their use after revascularization.
Potential Cost Savings
Symptomatic patients with PAD have considerably higher inpatient admissions, provider visits, total costs, and cardiovascular risk factors than non-PAD matched patients.107 Patients with CLI, especially if tissue loss is present, have an increased use of healthcare resources, including prolonged length of stay, frequent readmissions, and complications.108 There are no studies that directly or indirectly include costs with the analysis of CLI perfusion assessments. Incorporating noninvasive perfusion techniques as a component of ongoing CLI patient examinations could potentially improve patient outcomes and reduce costs by proactively monitoring foot macrocirculation and microcirculation on a regular basis.
Because many patients with CLI are elderly, the Centers for Medicare and Medicaid Services (CMS) is the primary payer source for such testing. Current CMS-approved reimbursed perfusion assessments include volume plethysmography, TcPo2, and ultrasound with or without provocative functional maneuvers such as reactive hyperemia or postural tests.109 If ABI or toe pressure measurements are obtained without corresponding waveforms, these are not reimbursed. Restrictions by CMS limit the frequency or indications for such testing. However, with the increasing aging population and incidence of CLI, these restrictions may be modified.
Although technologies such as fluorescence angiography, 2-dimensional perfusion angiography, and tissue oxygen saturation mapping are promising additions to the CLI noninvasive perfusion assessment options, there are concerns over inconsistent or absent reimbursement. Currently, CMS does not reimburse the following noninvasive PAD studies: thermography, mechanical oscillometry, inductance or capacitance plethysmography, photoelectric plethysmography, differential plethysmography, and light reflective rheography.109 From a clinical perspective, these new technologies allow for a more detailed assessment of the limb status and provide additional data required for a more accurate determination of flow. However, their impact on cost savings cannot be determined at this time until reimbursement issues are resolved.
Randomized controlled clinical trials are needed to determine and quantify the impact of noninvasive perfusion techniques on CLI monitoring and costs. The current CLI cost studies target the revascularization therapy differences or hospital data without specifically reporting data on the perfusion techniques used. Potential use of these techniques early on in the course of the disease and at scheduled intervals during and after revascularization could result in cost savings by preventing readmissions or by a well-timed adjustment of the treatment plan.
CONCLUSIONS
CLI is a complex disease process with great morbidity. This statement highlights the importance of incorporating perfusion assessment into the care of CLI patients. Despite the high prevalence of CLI, strategies for perfusion assessment remain limited. New technologies offer potential opportunities to improve the precision and quality of CLI management. Furthermore, technology through telemedicine provides better access to care, which could help to decrease the healthcare disparities among patients with PAD.
Appendix
Disclosures
Writing Group Disclosures
| Writing Group Member |
Employment | Research Grant | Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/ Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Sanjay Misra | Mayo Clinic | NIH (funding from HL098967 and DK 107870)† | None | None | None | None | None | None |
| Mehdi H. Shishehbor | University Hospitals Cleveland Medical Center, Case Western Reserve University Harrington Heart and Vascular Institute | AstraZeneca† | None | None | None | None | Abbott*; BSC*; MDT†; Phillips*; Terumo* | None |
| Herbert D. Aronow | Warren Alpert Medical School of Brown University Lifespan Cardiovascular Institute | None | None | None | None | None | None | None |
| Luke P. Brewster | Emory University School of Medicine | NIH (R01 basic science funding in PAD mechanisms)† | None | None | Law firm; 2018; defense; acute limb ischemia* | None | None | None |
| Matthew C. Bunte | Saint Luke’s Mid America Heart Institute | None | None | None | None | None | Medtronic PLC* | None |
| Esther S.H. Kim | Vanderbilt University | None | None | None | None | None | None | None |
| Jonathan R. Lindner | Oregon Health and Science University, Knight Cardiovascular Institute | Pfizer (investigator-initiated grant)* | None | None | None | None | None | None |
| Kathleen Rich | Franciscan Health Michigan City | None | None | None | None | None | None | None |
| Edwin A. Takahashi | Mayo Clinic | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant |
Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/ Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Heather L. Gornik | University Hospitals | CVR Global* | None | None | None | None | None | None |
| Bruce H. Gray | Greenville Hospital Systems | None | None | None | None | None | None | None |
| Tarek Hammad | Cleveland Clinic | None | None | None | None | None | None | None |
| William R. Hiatt | University of Colorado School of Medicine and CPC Clinical Research | Bayer (clinical trial in PAD)* | NIH (clinical trial in thrombosis)* | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Significant.
Footnotes
ARTICLE INFORMATION
The devices listed here serve only to illustrate examples of these types of devices. This is not intended to be an endorsement of any commercial product, process, service, or enterprise by the American Heart Association.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on April 25, 2019, and the American Heart Association Executive Committee on June 4, 2019. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or kelle.ramsay@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Misra S, Shishehbor MH, Takahashi EA, Aronow HD, Brewster LP, Bunte MC, Kim ESH, Lindner JR, Rich K; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; and Council on Cardiovascular and Stroke Nursing. Perfusion assessment in critical limb ischemia: principles for understanding and the development of evidence and evaluation of devices: a scientific statement from the American Heart Association. Circulation. 2019; 140:e000–e000. doi: 10.1161/CIR.0000000000000708.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
REFERENCES
- 1.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. 2017;135:e790]. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teraa M, Conte MS, Moll FL, Verhaar MC. Critical limb ischemia: current trends and future directions. J Am Heart Assoc. 2016;5:e002938. doi: 10.1161/JAHA.115.002938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaranto DJ, Abbas F, Krantz S, Pearce WH, Wang E, Kibbe MR. An evaluation of gender and racial disparity in the decision to treat surgically arterial disease. J Vasc Surg. 2009;50:1340–1347. doi: 10.1016/j.jvs.2009.07.089 [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell TFX, Powell C, Deery SE, Darling JD, Hughes K, Giles KA, Wang GJ, Schermerhorn ML. Regional variation in racial disparities among patients with peripheral artery disease. J Vasc Surg. 2018;68:519–526. doi: 10.1016/j.jvs.2017.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durazzo TS, Frencher S, Gusberg R. Influence of race on the management of lower extremity ischemia: revascularization vs amputation. JAMA Surg. 2013;148:617–623. doi: 10.1001/jamasurg.2013.1436 [DOI] [PubMed] [Google Scholar]
- 6.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. 2017;135:e791–e792]. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shishehbor MH, Bunte MC. Time to redefine critical limb ischemia. JACC Cardiovasc Interv. 2017;10:2317–2319. doi: 10.1016/j.jcin.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 8.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association [published correction appears in Circulation. 2013;127:e264]. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb [DOI] [PubMed] [Google Scholar]
- 9.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–2045. doi: 10.1161/CIR.0b013e31822e80c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D, Zou L, Xing Y, Hou L, Wei Y, Zhang J, Qiao Y, Hu D, Xu Y, Li J, Ma Y. Diagnostic value of ankle-brachial index in peripheral arterial disease: a meta-analysis. Can J Cardiol. 2013;29:492–498. doi: 10.1016/j.cjca.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 11.Bunte MC, Jacob J, Nudelman B, Shishehbor MH. Validation of the relationship between ankle-brachial and toe-brachial indices and infragenicular arterial patency in critical limb ischemia. Vasc Med. 2015;20:23–29. doi: 10.1177/1358863X14565372 [DOI] [PubMed] [Google Scholar]
- 12.Shishehbor MH, Hammad TA, Zeller T, Baumgartner I, Scheinert D, Rocha-Singh KJ. An analysis of IN.PACT DEEP randomized trial on the limitations of the societal guidelines-recommended hemodynamic parameters to diagnose critical limb ischemia. J Vasc Surg. 2016;63:1311–1317. doi: 10.1016/j.jvs.2015.11.042 [DOI] [PubMed] [Google Scholar]
- 13.Sukul D, Grey SF, Henke PK, Gurm HS, Grossman PM. Heterogeneity of ankle-brachial indices in patients undergoing revascularization for critical limb ischemia. JACC Cardiovasc Interv. 2017;10:2307–2316. doi: 10.1016/j.jcin.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo T, Sample R, Moore P, Gold P Prediction of wound healing outcome using skin perfusion pressure and transcutaneous oximetry: a single-center experience in 100 patients. Wounds. 2009;21:310–316. [PubMed] [Google Scholar]
- 15.Tsai FW, Tulsyan N, Jones DN, Abdel-Al N, Castronuovo JJ Jr, Carter SA. Skin perfusion pressure of the foot is a good substitute for toe pressure in the assessment of limb ischemia. J Vasc Surg. 2000;32:32–36. doi: 10.1067/mva.2000.107310 [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, Kawanishi J. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs: comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318–323. doi: 10.1016/j.jvs.2007.10.045 [DOI] [PubMed] [Google Scholar]
- 17.Randhawa MS, Reed GW, Grafmiller K, Gornik HL, Shishehbor MH. Prevalence of tibial artery and pedal arch patency by angiography in patients with critical limb ischemia and noncompressible ankle brachial index. Circ Cardiovasc Interv. 2017;10:e004605. doi: 10.1161/CIRCINTERVENTIONS.116.004605 [DOI] [PubMed] [Google Scholar]
- 18.Arain FA, Ye Z, Bailey KR, Chen Q, Liu G, Leibson CL, Kullo IJ. Survival in patients with poorly compressible leg arteries. J Am Coll Cardiol. 2012;59:400–407. doi: 10.1016/j.jacc.2011.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aboyans V, Lacroix P, Tran MH, Salamagne C, Galinat S, Archambeaud F, Criqui MH, Laskar M. The prognosis of diabetic patients with high ankle-brachial index depends on the coexistence of occlusive peripheral artery disease. J Vasc Surg. 2011;53:984–991. doi: 10.1016/j.jvs.2010.10.054 [DOI] [PubMed] [Google Scholar]
- 20.Singh GD, Armstrong EJ, Waldo SW, Alvandi B, Brinza E, Hildebrand J, Amsterdam EA, Humphries MD, Laird JR. Non-compressible ABIs are associated with an increased risk of major amputation and major adverse cardiovascular events in patients with critical limb ischemia. Vasc Med. 2017;22:210–217. doi: 10.1177/1358863X16689831 [DOI] [PubMed] [Google Scholar]
- 21.Graziani L, Silvestro A, Bertone V, Manara E, Andreini R, Sigala A, Mingardi R, De Giglio R. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453–460. doi: 10.1016/j.ejvs.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 22.Reed GW, Young L, Bagh I, Maier M, Shishehbor MH. Hemodynamic assessment before and after endovascular therapy for critical limb ischemia and association with clinical outcomes. JACC Cardiovasc Interv. 2017;10:2451–2457. doi: 10.1016/j.jcin.2017.06.063 [DOI] [PubMed] [Google Scholar]
- 23.Huang TY, Huang TS, Wang YC, Huang PF, Yu HC, Yeh CH. Direct revascularization with the angiosome concept for lower limb ischemia: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94:e1427. doi: 10.1097/MD.0000000000001427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G; on behalf of the Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220–234.e1-e2. doi: 10.1016/j.jvs.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 25.Hicks CW, Canner JK, Mathioudakis N, Sherman R, Malas MB, Black JH 3rd, Abularrage CJ. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification independently predicts wound healing in diabetic foot ulcers. J Vasc Surg. 2018;68:1096–1103. doi: 10.1016/j.jvs.2017.12.079 [DOI] [PubMed] [Google Scholar]
- 26.Jeevanantham V, Chehab B, Austria E, Shrivastava R, Wiley M, Tadros P, Dawn B, Vacek JL, Gupta K. Comparison of accuracy of two different methods to determine ankle-brachial index to predict peripheral arterial disease severity confirmed by angiography. Am J Cardiol. 2014;114:1105–1110. doi: 10.1016/j.amjcard.2014.07.023 [DOI] [PubMed] [Google Scholar]
- 27.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K; on behalf of the TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(suppl 1):S1–S75. doi: 10.1016/j.ejvs.2006.09.0240 [DOI] [PubMed] [Google Scholar]
- 28.Setacci C, Ricco JB; European Society for Vascular Surgery. Guidelines for critical limb ischaemia and diabetic foot: introduction. Eur J Vasc Endovasc Surg. 2011;42(suppl 2):S1–S3. doi: 10.1016/S1078-5884(11)00715-5 [DOI] [PubMed] [Google Scholar]
- 29.Brooks B, Dean R, Patel S, Wu B, Molyneaux L, Yue DK. TBI or not TBI: that is the question: is it better to measure toe pressure than ankle pressure in diabetic patients? Diabet Med. 2001;18:528–532. [DOI] [PubMed] [Google Scholar]
- 30.Brownrigg JR, Hinchliffe RJ, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC; on behalf of the International Working Group on the Diabetic Foot. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(suppl 1):128–135. doi: 10.1002/dmrr.2704 [DOI] [PubMed] [Google Scholar]
- 31.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version [published correction appears in J Vasc Surg. 2001;33:805]. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 32.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, Nehler MR, Powell RJ, Sidawy AN. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–1473. e1-e3. doi: 10.1016/j.jvs.2009.09.044 [DOI] [PubMed] [Google Scholar]
- 33.Tehan PE, Santos D, Chuter VH. A systematic review of the sensitivity and specificity of the toe-brachial index for detecting peripheral artery disease. Vasc Med. 2016;21:382–389. doi: 10.1177/1358863X16645854 [DOI] [PubMed] [Google Scholar]
- 34.Carter SA, Tate RB. The value of toe pulse waves in determination of risks for limb amputation and death in patients with peripheral arterial disease and skin ulcers or gangrene. J Vasc Surg. 2001;33:708–714. doi: 10.1067/mva.2001.112329 [DOI] [PubMed] [Google Scholar]
- 35.Bunte MC, Shishehbor MH. Treatment of infrapopliteal critical limb ischemia in 2013: the wound perfusion approach. Curr Cardiol Rep. 2013;15:363. doi: 10.1007/s11886-013-0363-5 [DOI] [PubMed] [Google Scholar]
- 36.Menard MT, Farber A, Assmann SF, Choudhry NK, Conte MS, Creager MA, Dake MD, Jaff MR, Kaufman JA, Powell RJ, Reid DM, Siami FS, Sopko G, White CJ, Rosenfield K. Design and rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) Trial. J Am Heart Assoc. 2016;5:e003219. doi: 10.1161/JAHA.116.003219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballard JL, Eke CC, Bunt TJ, Killeen JD. A prospective evaluation of transcutaneous oxygen measurements in the management of diabetic foot problems. J Vasc Surg. 1995;22:485–490. [DOI] [PubMed] [Google Scholar]
- 38.Wisniewski NA, Nichols SP, Gamsey SJ, Pullins S, Au-Yeung KY, Klitzman B, Helton KL. Tissue-integrating oxygen sensors: continuous tracking of tissue hypoxia. Adv Exp Med Biol. 2017;977:377–383. doi: 10.1007/978-3-319-55231-6_49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chien JS, Mohammed M, Eldik H, Ibrahim MM, Martinez J, Nichols SP, Wisniewski N, Klitzman B. Injectable phosphorescence-based oxygen biosensors identify post ischemic reactive hyperoxia. Sci Rep. 2017;7:8255. doi: 10.1038/s41598-017-08490-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Igari K, Kudo T, Uchiyama H, Toyofuku T, Inoue Y. Intraarterial injection of indocyanine green for evaluation of peripheral blood circulation in patients with peripheral arterial disease. Ann Vasc Surg. 2014;28:1280–1285. doi: 10.1016/j.avsg.2013.12.036 [DOI] [PubMed] [Google Scholar]
- 41.Byrne P, Provan JL, Ameli FM, Jones DP. The use of transcutaneous oxygen tension measurements in the diagnosis of peripheral vascular insufficiency. Ann Surg. 1984;200:159–165. doi: 10.1097/00000658-198408000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin JA, Wang EC, Kibbe MR. Evaluation of hyperspectral technology for assessing the presence and severity of peripheral artery disease. J Vasc Surg. 2011;54:1679–1688. doi: 10.1016/j.jvs.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 43.Cheatle TR, Potter LA, Cope M, Delpy DT, Coleridge Smith PD, Scurr JH. Near-infrared spectroscopy in peripheral vascular disease. Br J Surg. 1991;78:405–408. [DOI] [PubMed] [Google Scholar]
- 44.Zimny S, Dessel F, Ehren M, Pfohl M, Schatz H. Early detection of microcirculatory impairment in diabetic patients with foot at risk. Diabetes Care. 2001;24:1810–1814. doi: 10.2337/diacare.24.10.1810 [DOI] [PubMed] [Google Scholar]
- 45.Sumpio BJ, Citoni G, Chin JA, Sumpio BE. Use of hyperspectral imaging to assess endothelial dysfunction in peripheral arterial disease. J Vasc Surg. 2016;64:1066–1073. doi: 10.1016/j.jvs.2016.03.463 [DOI] [PubMed] [Google Scholar]
- 46.Zuzak KJ, Gladwin MT, Cannon RO 3rd, Levin IW. Imaging hemoglobin oxygen saturation in sickle cell disease patients using noninvasive visible reflectance hyperspectral techniques: effects of nitric oxide. Am J Physiol Heart Circ Physiol. 2003;285:H1183–H1189. doi: 10.1152/ajpheart.00243.2003 [DOI] [PubMed] [Google Scholar]
- 47.Landgraf H, Grützmacher P, Garcia-Bartels C, Bergmann M, Ehrly AM, Schoeppe W. Effect of erythropoietin treatment on tissue oxygenation in patients with severe transfusion dependent renal anaemia. Eur J Med. 1993;2:393–397. [PubMed] [Google Scholar]
- 48.Malagoni AM, Felisatti M, Mandini S, Mascoli F, Manfredini R, Basaglia N, Zamboni P, Manfredini F. Resting muscle oxygen consumption by near-infrared spectroscopy in peripheral arterial disease: a parameter to be considered in a clinical setting? Angiology. 2010;61:530–536. doi: 10.1177/0003319710362975 [DOI] [PubMed] [Google Scholar]
- 49.Isbell DC, Epstein FH, Zhong X, DiMaria JM, Berr SS, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2007;25:1013–1020. doi: 10.1002/jmri.20899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindner JR, Womack L, Barrett EJ, Weltman J, Price W, Harthun NL, Kaul S, Patrie JT. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008;1:343–350. doi: 10.1016/j.jcmg.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stacy MR, Zhou W, Sinusas AJ. Radiotracer imaging of peripheral vascular disease. J Nucl Med. 2013;54:2104–2110. doi: 10.2967/jnumed.112.115105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duerschmied D, Olson L, Olschewski M, Rossknecht A, Freund G, Bode C, Hehrlein C. Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: a novel diagnostic method. Eur Heart J. 2006;27:310–315. doi: 10.1093/eurheartj/ehi636 [DOI] [PubMed] [Google Scholar]
- 53.Duerschmied D, Zhou Q, Rink E, Harder D, Freund G, Olschewski M, Bode C, Hehrlein C. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis. 2009;202:505–512. doi: 10.1016/j.atherosclerosis.2008.05.046 [DOI] [PubMed] [Google Scholar]
- 54.Padberg FT, Back TL, Thompson PN, Hobson RW 2nd. Transcutaneous oxygen (TcPO2) estimates probability of healing in the ischemic extremity. J Surg Res. 1996;60:365–369. [DOI] [PubMed] [Google Scholar]
- 55.Cina C, Katsamouris A, Megerman J, Brewster DC, Strayhorn EC, Robison JG, Abbott WM. Utility of transcutaneous oxygen tension measurements in peripheral arterial occlusive disease. J Vasc Surg. 1984;1:362–371. [DOI] [PubMed] [Google Scholar]
- 56.Castronuovo JJ Jr, Adera HM, Smiell JM, Price RM. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg. 1997;26:629–637. [DOI] [PubMed] [Google Scholar]
- 57.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40: 113–141. [DOI] [PubMed] [Google Scholar]
- 58.Biancari F, Juvonen T. Angiosome-targeted lower limb revascularization for ischemic foot wounds: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2014;47:517–522. doi: 10.1016/j.ejvs.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 59.Jongsma H, Bekken JA, Akkersdijk GP, Hoeks SE, Verhagen HJ, Fioole B. Angiosome-directed revascularization in patients with critical limb ischemia. J Vasc Surg. 2017;65:1208–1219.e1. doi: 10.1016/j.jvs.2016.10.100 [DOI] [PubMed] [Google Scholar]
- 60.Sumpio BE, Forsythe RO, Ziegler KR,van Baal JG, Lepantalo MJ, Hinchliffe RJ. Clinical implications of the angiosome model in peripheral vascular disease. J Vasc Surg. 2013;58:814–826. doi: 10.1016/j.jvs.2013.06.056 [DOI] [PubMed] [Google Scholar]
- 61.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53:2175–2183. doi: 10.1016/j.jacc.2009.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care. 2003;26:899–904. doi: 10.2337/diacare.26.3.899 [DOI] [PubMed] [Google Scholar]
- 63.Sumpio BE. Hyperspectral imaging for assessing regional foot perfusion. Vascular News. 2018. https://vascularnews.com/hyperspectral-imaging-assessing-regional-foot-perfusion/. Accessed December 5, 2018. [Google Scholar]
- 64.Humeau-Heurtier A, Guerreschi E, Abraham P, Mahé G. Relevance of laser Doppler and laser speckle techniques for assessing vascular function: state of the art and future trends. IEEE Trans Biomed Eng. 2013;60:659–666. doi: 10.1109/TBME.2013.2243449 [DOI] [PubMed] [Google Scholar]
- 65.Fredriksson I, Larsson M, Strömberg T. Measurement depth and volume in laser Doppler flowmetry. Microvasc Res. 2009;78:4–13. doi: 10.1016/j.mvr.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 66.Hultman M, Fredriksson I, Larsson M, Alvandpour A, Strömberg T. A 15.6 frames per second 1-megapixel multiple exposure laser speckle contrast imaging setup. J Biophotonics. 2018; 11 :e201700069. doi: 10.1002/jbio.201800012 [DOI] [PubMed] [Google Scholar]
- 67.Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22:R35–R66. [DOI] [PubMed] [Google Scholar]
- 68.Katsui S, Inoue Y, Igari K, Toyofuku T, Kudo T, Uetake H. Novel assessment tool based on laser speckle contrast imaging to diagnose severe ischemia in the lower limb for patients with peripheral arterial disease. Lasers Surg Med. 2017;49:645–651. doi: 10.1002/lsm.22669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laser Associated Sciences. FlowMet [brochure]. Irvine, CA: Laser Associated Sciences, Inc;2018:1–6. [Google Scholar]
- 70.Hoeffner EG, Case I, Jain R, Gujar SK, Shah GV, Deveikis JP, Carlos RC, Thompson BG, Harrigan MR, Mukherji SK. Cerebral perfusion CT: technique and clinical applications. Radiology. 2004;231:632–644. doi: 10.1148/radiol.2313021488 [DOI] [PubMed] [Google Scholar]
- 71.Hur S, Jae HJ, Jang Y, Min SK, Min SI, Lee DY, Seo SG, Kim HC, Chung JW, Kim KG, Park EA, Lee W. Quantitative assessment of foot blood flow by using dynamic volume perfusion CT technique: a feasibility study. Radiology. 2016;279:195–206. doi: 10.1148/radiol.2015150560 [DOI] [PubMed] [Google Scholar]
- 72.Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 2016;281:337–356. doi: 10.1148/radiol.2016150789 [DOI] [PubMed] [Google Scholar]
- 73.Detre JA, Rao H, Wang DJ, Chen YF, Wang Z. Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging. 2012;35:1026–1037. doi: 10.1002/jmri.23581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pollak AW, Kramer CM. MRI in lower extremity peripheral arterial disease: recent advancements. Curr Cardiovasc Imaging Rep. 2013;6:55–60. doi: 10.1007/s12410-012-9175-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.West AM, Meyer CH, Epstein FH, Jiji R, Hunter JR, DiMaria JM, Christopher JM, Kramer CM. Arterial spin labeling MRI reproducibly measures peak-exercise calf muscle perfusion in healthy volunteers and patients with peripheral arterial disease. JACC Cardiovasc Imaging. 2012;5:1224–1230. doi: 10.1016/j.jcmg.2012.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stacy MR, Qiu M, Papademetris X, Caracciolo CM, Constable RT, Sinusas AJ. Application of BOLD magnetic resonance imaging for evaluating regional volumetric foot tissue oxygenation: a feasibility study in healthy volunteers. Eur J Vasc Endovasc Surg. 2016;51:743–749. doi: 10.1016/j.ejvs.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bajwa A, Wesolowski R, Patel A, Saha P, Ludwinski F, Ikram M, Albayati M, Smith A, Nagel E, Modarai B. Blood oxygenation level-dependent CMR-derived measures in critical limb ischemia and changes with revascularization. J Am Coll Cardiol. 2016;67:420–431. doi: 10.1016/j.jacc.2015.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heideman PP, Rajebi MR, McKusick MA, Bjarnason H, Oderich GS, Friese JL, Fleming MD, Stockland AH, Harmsen WS, Mandrekar J, Misra S. Impact of chronic kidney disease on clinical outcomes of endovascular treatment for femoropopliteal arterial disease. J Vasc Interv Radiol. 2016;27:1204–1214. doi: 10.1016/j.jvir.2016.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rajagopalan S, Shin T. Being BOLD in critical limb ischemia. J Am Coll Cardiol. 2016;67:432–34. doi: 10.1016/j.jacc.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 80.Bajwa A, Wesolowski R, Patel A, Saha P, Ludwinski F, Smith A, Nagel E, Modarai B. Assessment of tissue perfusion in the lower limb: current methods and techniques under development. Circ Cardiovasc Imaging. 2014;7:836–843. doi: 10.1161/CIRCIMAGING.114.002123 [DOI] [PubMed] [Google Scholar]
- 81.Protyniak B, Dinallo AM, Boyan WP Jr, Dressner RM, Arvanitis ML. Intraoperative indocyanine green fluorescence angiography–an objective evaluation of anastomotic perfusion in colorectal surgery. Am Surg. 2015;81:580–584. [DOI] [PubMed] [Google Scholar]
- 82.Zimmermann A, Roenneberg C, Reeps C, Wendorff H, Holzbach T, Eckstein HH. The determination of tissue perfusion and collateralization in peripheral arterial disease with indocyanine green fluorescence angiography. Clin Hemorheol Microcirc. 2012;50:157–166. doi: 10.3233/CH-2011-1408 [DOI] [PubMed] [Google Scholar]
- 83.Unno N, Suzuki M, Yamamoto N, Inuzuka K, Sagara D, Nishiyama M, Tanaka H, Konno H. Indocyanine green fluorescence angiography for intraoperative assessment of blood flow: a feasibility study. Eur J Vasc Endovasc Surg. 2008;35:205–207. doi: 10.1016/j.ejvs.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 84.Higashimori A, Takahara M, Utsunomiya M, Fukunaga M, Kawasaki D, Mori S, Takimura H, Hirano K, Tsubakimoto Y, Nakama T, Yokoi Y. Utility of indigo carmine angiography in patients with critical limb ischemia: Prospective multi-center intervention study (DIESEL-study). Catheter Cardiovasc Interv 2019;93:108–112. doi: 10.1002/ccd.27813 [DOI] [PubMed] [Google Scholar]
- 85.Montero-Baker MF, Au-Yeung KY, Wisniewski NA, Gamsey S, Morelli-Alvarez L, Mills JL Sr, Campos M, Helton KL. The first-in-man “Si Se Puede” study for the use of micro-oxygen sensors (MOXYs) to determine dynamic relative oxygen indices in the feet of patients with limb-threatening ischemia during endovascular therapy. J Vasc Surg. 2015;61:1501–9.e1. doi: 10.1016/j.jvs.2014.12.060 [DOI] [PubMed] [Google Scholar]
- 86.Pardo M, Alcaraz M, Bernal FL, Felices JM, Achel GD, Canteras M. Transcutaneous oxygen tension measurements following peripheral transluminal angioplasty procedure has more specificity and sensitivity than ankle brachial index. Br J Radiol. 2015;88:20140571. doi: 10.1259/bjr.20140571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shishehbor MH, White CJ, Gray BH, Menard MT, Lookstein R, Rosenfield K, Jaff MR. Critical limb ischemia: an expert statement. J Am Coll Cardiol. 2016;68:2002–2015. doi: 10.1016/j.jacc.2016.04.071 [DOI] [PubMed] [Google Scholar]
- 88.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–95.e2. doi: 10.1016/j.jvs.2014.03.290 [DOI] [PubMed] [Google Scholar]
- 89.Ferraresi R, Mauri G, Losurdo F, Troisi N, Brancaccio D, Caravaggi C, Neri L. BAD transmission and SAD distribution: a new scenario for critical limb ischemia. J Cardiovasc Surg (Torino). 2018;59:655–664. doi: 10.23736/S0021-9509.18.10572-6 [DOI] [PubMed] [Google Scholar]
- 90.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies: statement from ATVB Council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. doi: 10.1161/ATVBAHA.117.309524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat-Jacobson D; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology; Council on Epidemiology and Prevention. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2012;125:1449–1472. doi: 10.1161/CIR.0b013e31824c39ba [DOI] [PubMed] [Google Scholar]
- 92.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 93.Sampson UK, Fowkes FG, McDermott MM, Criqui MH, Aboyans V, Norman PE, Forouzanfar MH, Naghavi M, Song Y, Harrell FE Jr, Denenberg JO, Mensah GA, Ezzati M, Murray C. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart. 2014;9:145–158.e21. doi: 10.1016/j.gheart.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 94.Egorova N, Vouyouka AG, Quin J, Guillerme S, Moskowitz A, Marin M, Faries PL. Analysis of gender-related differences in lower extremity peripheral arterial disease. J Vasc Surg. 2010;51:372–378.e1. doi: 10.1016/j.jvs.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 95.Lo RC, Bensley RP, Dahlberg SE, Matyal R, Hamdan AD, Wyers M, Chaikof EL, Schermerhorn ML. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–418.e3. doi: 10.1016/j.jvs.2013.07.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ortmann J, Nüesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012;55:98–104. doi: 10.1016/j.jvs.2011.07.074 [DOI] [PubMed] [Google Scholar]
- 97.Stapleton SM, Bababekov YJ, Perez NP, Fong ZV, Hashimoto DA, Lillemoe KD, Watkins MT, Chang DC. Variation in amputation risk for black patients: uncovering potential sources of bias and opportunities for intervention. J Am Coll Surg. 2018;226:641–649.e1. doi: 10.1016/j.jamcollsurg.2017.12.038 [DOI] [PubMed] [Google Scholar]
- 98.Hughes K, Boyd C, Oyetunji T, Tran D, Chang D, Rose D, Siram S, Cornwell E 3rd, Obisesan T. Racial/ethnic disparities in revascularization for limb salvage: an analysis of the National Surgical Quality Improvement Program database. Vasc Endovascular Surg. 2014;48:402–405. doi: 10.1177/1538574414543276 [DOI] [PubMed] [Google Scholar]
- 99.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073 [DOI] [PubMed] [Google Scholar]
- 100.Martini R Trends of the treatment of critical limb ischemia during the last two decades. Clin Hemorheol Microcirc. 2018;69:447–456. doi: 10.3233/CH-170352 [DOI] [PubMed] [Google Scholar]
- 101.lida O, Soga Y, Kawasaki D, Hirano K, Yamaoka T, Suzuki K, Miyashita Y, Yokoi H, Takahara M, Uematsu M. Angiographic restenosis and its clinical impact after infrapopliteal angioplasty. Eur J Vasc Endovasc Surg. 2012;44:425–431. doi: 10.1016/j.ejvs.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 102.Brumberg RS, Back MR, Armstrong PA, Cuthbertson D, Shames ML, Johnson BL, Bandyk DF. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure. J Vasc Surg. 2007;46:1160–1166. doi: 10.1016/j.jvs.2007.07.046 [DOI] [PubMed] [Google Scholar]
- 103.Zierler RE, Jordan WD, Lal BK, Mussa F, Leers S, Fulton J, Pevec W, Hill A, Murad MH. The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures [published correction appears in J Vasc Surg 2018;68:1623]. J Vasc Surg. 2018;68:256–284. doi: 10.1016/j.jvs.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 104.Saarinen E, Laukontaus SJ, Albäck A, Venermo M. Duplex surveillance after endovascular revascularisation for critical limb ischaemia. Eur J Vasc Endovasc Surg. 2014;47:418–421. doi: 10.1016/j.ejvs.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 105.Utsunomiya M, Nakamura M, Nagashima Y, Sugi K. Predictive value of skin perfusion pressure after endovascular therapy for wound healing in critical limb ischemia. J Endovasc Ther. 2014;21:662–670. doi: 10.1583/14-4675MR.1 [DOI] [PubMed] [Google Scholar]
- 106.Larson-Lohr V, Norvell H. Role of transcutaneous oxygen monitoring for the chronic wound patient In: Hyperbaric Nursing and Wound Care. North Palm Beach, FL: Best Publishing Company; 2010:157. [Google Scholar]
- 107.Chase MR, Friedman HS, Navaratnam P, Heithoff K, Simpson RJ Jr. Comparative assessment of medical resource use and costs associated with patients with symptomatic peripheral artery disease in the United States. J Manag Care Spec Pharm. 2016;22:667–675. doi: 10.18553/jmcp.2016.15010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen LL, Lipsitz SR, Bandyk DF, Clowes AW, Moneta GL, Belkin M, Conte MS. Resource utilization in the treatment of critical limb ischemia: the effect of tissue loss, comorbidities, and graft-related events. J Vasc Surg. 2006;44:971–975. [DOI] [PubMed] [Google Scholar]
- 109.Billing and coding guidelines for non-invasive peripheral arterial vascular studies. Revised November 1, 2017. Centers for Medicare & Medicaid Services; website. https://downloads.cms.gov/medicare-coverage-database/lcd_attachments/35761_2/L35761_CV046_BCG.pdf. Accessed May 29, 2019. [Google Scholar]