Abstract
The structure and optical properties of polyethylene oxide (PEO) doped with tin titanate (SnTiO3) nano-filler were studied by X-ray diffraction (XRD) and UV-Vis spectroscopy as non-destructive techniques. PEO-based composed polymer electrolytes inserted with SnTiO3 nano-particles (NPs) were synthesized through the solution cast technique. The change from crystalline phase to amorphous phase of the host polymer was established by the lowering of the intensity and broadening of the crystalline peaks. The optical constants of PEO/SnTiO3 nano-composite (NC), such as, refractive index (n), optical absorption coefficient (α), dielectric loss (εi), as well as dielectric constant (εr) were determined for pure PEO and PEO/SnTiO3 NC. From these findings, the value of n of PEO altered from 2.13 to 2.47 upon the addition of 4 wt.% SnTiO3NPs. The value of εr also increased from 4.5 to 6.3, with addition of 4 wt.% SnTiO3. The fundamental optical absorption edge of the PEO shifted toward lower photon energy upon the addition of the SnTiO3 NPs, confirming a decrement in the optical band gap energy of PEO. The band gap shifted from 4.78 eV to 4.612 eV for PEO-doped with 4 wt.% SnTiO3. The nature of electronic transitions in the pure and the composite material were studied on the basis of Tauc’s model, while optical εi examination was also carried out to calculate the optical band gap.
Keywords: PEO polymer nano-composite, SnTiO3 nano-particles, XRD, UV-vis analysis, optical properties
1. Introduction
Over the last three decades, polymer materials have been studied extensively for their potential applications, with a focus on developing various polymers to replace the utilization of metals [1]. For this purpose, several strategies have been established for the synthesis of many advanced polymer nano-composites (NCs) using semiconductors and metals [2]. During these decades, several common types of polymers have been explored, for instance, poly (vinyl chloride) (PVC), poly (methyl methacrylate) (PMMA), poly (ethylene oxide) (PEO), and poly (vinylidene fluoride) (PVDF) [3]. Modification of the optical, electronic, and structural properties of inorganic-organic nano-particles (NPs) can be carried out via manipulation of the particle dimensions without changing the chemical composition. Enhancement of properties of the host polymer via NP synthesis relies on the shape, size, composition, and structure of the NPs [4,5]. These enhancements have particular impact on the optical properties, namely, light absorption, reflection, antireflection, and polarization [6,7,8,9]. The superiorities of polymer materials derive from their transparency, cost-effectiveness, ease of processing, light weight, and satisfactory mechanical properties. It is also well established that the polymer of PEO is one of the most appropriate candidates for application in the optics field. However, these materials normally exhibit relatively low refractive indices [10,11].
A polymer that encompasses more than one desired property as a result of various modifications can perform multiple functions. These modifications, especially of the polymer’s structure and optical properties involve addition of nano-size materials. Addition of dopants reduces the energy gap as a consequence of changing the mechanism of electron transitions [12]. PEO-based materials are promising polymer candidates because of their relatively high thermal stability. PEO is a semi-crystalline material that contains a crystalline phase as well as amorphous phase at ambient temperature [12,13]. Several studies have confirmed that PEO has unique properties. The outstanding properties are sufficient dimensional stability, high capacity to encompass salts, relatively high ionic conductivity in the amorphous region, resistance to corrosion, relatively low cost, convincing mechanical flexibility, and chemical stability [14,15,16]. In material design, to gain insight into the band gap structure and band gap energy in non-crystalline as well as crystalline materials, it is critical to take into consideration the optical absorption spectra [17,18]. On the one hand, absorption spectra examination in the low energy region provides details regarding atomic assignment of the fundamental vibrations; on the other hand, analysis of the spectra in the high energy region offers insight into the electronic levels in atoms [19]. Modification of the optical properties is achieved by insertion of dopants into polymer matrices [20]. As a principle, when UV and visible photons interact with materials, three phenomena occur: absorption, transmission, and reflection. Based on theoretical and practical considerations, a direct relation between these phenomena has been exhibited where the material’s absorption coefficient (α) and refractive index (n) are decisive [18].
Much research effort has been devoted to inspect the optical characteristics of doped host polymer matrices [21], for instance, PVA polymer incorporated with Cu(II)-complex [22], PEO polymer treated with TiO2nano-filler [23], and doped with ZnO NPs [24]. In a previous work, the optical properties of solid polymer doped with PbTiO3 and Pb(ZrTi)O3 NPs have been examined. However, due to the health-hazardous nature of Pb and its detrimental impact on the environment, there is a strong motivation to replace Pb with environmentally friendly elements [25]. One of the promising Pb-free materials that has recently been studied is SnTiO3 that is theoretically indicated to possess a large dielectric constant (εr) as computed via the study of first-principle [25,26,27]. Therefore, Sn-based materials were recognized as a promising replacement for Pb-based materials in future use in devices such as piezoelectric transducers, power harvesters, non-volatile memories, and optical waveguides [28,29,30]. The replacement of the PbTiO3 A-site with Sn allows us to obtain analogous material which is more eco-friendly.
In this report the optical characteristics of PEO doped with SnTiO3 NPs are investigated. Environmental concern regarding hazardous elements has encouraged researchers to discover more efficient and eco-friendly materials. This material should have big electric polarization which is reliant on the structure of the perovskite and relatively high n [11,31]. Furthermore, SnTiO3 material possesses elastic properties which are vital for basic familiarization with inter-atomic potentials (inter-atomic bonding). As a consequence, this material exhibits relatively high εr [32,33]. The goal of this study is to examine the optical and structural characteristics of a NC solid polymer. In the current study, enhancements of optical characteristics of the PEO/SnTiO3nano-composite have been achieved, namely, relatively high n, εr, and transparency. It is also shown that the current methodology might offer an alternative for the band gap energy and precisely determining the kind of electronic transitions.
2. Experimental Detail
2.1. Polymer Composite Preparation
Polyethylene oxide (PEO) was employed as the host polymer in this study. The PEO (molecular weight> 5 × 106 g/mol) powder material was provided by Sigma-Aldrich. Solution-casting method was used in the films’ preparation. The PEO solution was prepared by adding one gram of PEO powder to distilled water (50 mL) and stirring through a magnetic stirrer for 5 h at room temperature. Once the polymer solution was obtained in the form of a clear viscous solution, a portion of 4 wt.% of SnTiO3, supplied by Sigma-Aldrich, was added to it. Then, the solution was stirred continuously to prepare the PEO/SnTiO3 polymer NC. The pure PEO and PEO/4 wt.% of SnTiO3 were labeled as PESNT0 and PESNNT1, respectively. Ultimately, the homogenous solutions were cast into plastic Petri dishes and permitted to dry at room temperature. Later, the films were placed in a desiccator containing blue silica gel for additional drying prior to characterizations.
2.2. Characterization Techniques
2.2.1. X-ray Diffraction
The structural characterizations and the impact of the nano-size filler for all the samples were assessed by means of X-ray diffraction (XRD) and UV-Vis spectroscopy. An X-ray diffractometer (PANalytical, Almelo, The Netherlands) with a working current of 40 mA and a working voltage of 40 kV was used for acquiring the patterns of XRD. The samples were examined with a monochromatic CuKα X-ray radiation beam with a wavelength (λ) of 1.5406 Å plus a glancing angle (2θ) in the range of10° to 80° with 0.1° step size at room temperature.
2.2.2. UV-Vis Measurement
UV-VIS spectroscopy is employed to measure the light absorption of liquid and solid samples through the wavelength range of ultraviolet and optical. It is an influential technique to find the optical properties of samples such as transmittance, absorbance, and reflectance. An ultraviolet-visible (UV-Vis) spectrometer (V-570, Jasco, Japan) with the wavelength in the range between 190 and 790 nm was employed to obtain the UV-Vis absorption spectra of the fabricated samples. For this purpose, the prepared samples were cut with dimensions of (1 × 2 cm2) in order to fit into the sample holder. Then, the spectrometer was calibrated to subtract the air absorption. The samples’ thicknesses were measured and found to be in the range of119 to 122 µm.
3. Results and Discussion
3.1. X-Ray Diffraction (XRD) Analysis
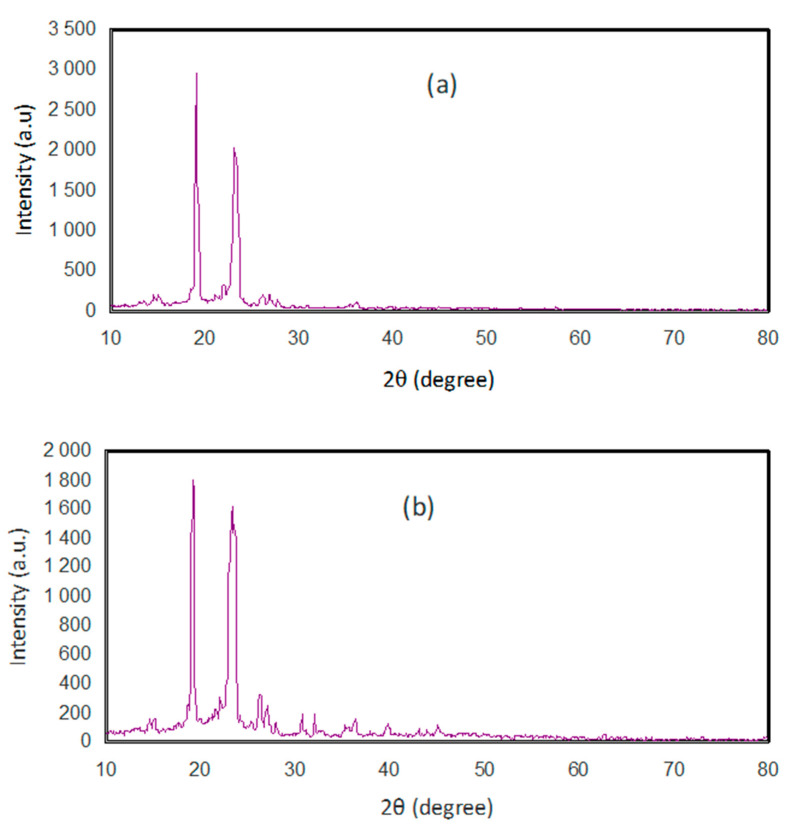
Figure 1 indicates the XRD spectra for the pure PEO and the composite film. Figure 1a exhibits the pure PEO XRD pattern. The appearance of two narrow peaks illustrates the dominance of the crystalline phase [34]. The two characteristic peaks centered at 18° and 24° and weak peaks (i.e., low intensities) at higher-angle degrees. It is fascinating to perceive that the intensity of the peaks decreases and they become broad in the presence of the nano-size SnTiO3 as shown in Figure 1b. This implies that the amorphous region in PEO increases at the expense of the crystalline phase. This could be a result of the interaction that occurred between the SnTiO3 and PEO. It also reveals the dominance of the amorphous phase facilitated by polymer chain segmental motion [35].
Figure 1.
XRD pattern of for (a) PESNT0 and (b) PESNT1 composite films.
These results emphasize that the decrease in the crystalline phase is due to the lowering of the compact nature of the polymer body [36,37]. It was found that the PEO building units such as C–H bond, C–C bond, and C–O bond provide a good crystalline and electrochemical stability nature [36,38]. The PEO is found to exhibit as a linear and semi-crystalline polymer with distinguishable diffraction peaks [38,39]. It is well known that the structural properties of the pure PEO can be altered through both blending and adding additives. The literature documented that the decrease in crystalline nature of PEO after blending and adding NPs are ascribed to a strong interaction between the host polymer and the additives [40,41,42,43,44]. This supports the results obtained in this study and confirms the interaction occurred between the PEO polymer and the nano-size SnTiO3.
3.2. Optical Properties
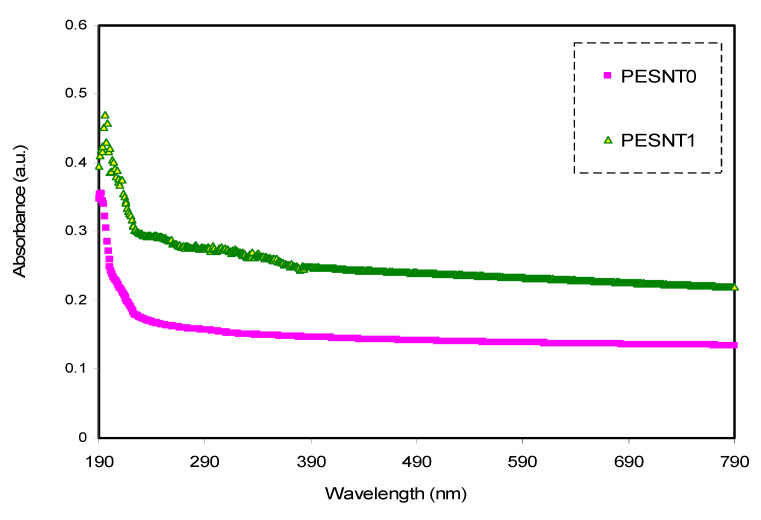
Examinations of optical absorption for pure PEO as well as PEO doped with SnTiO3 films were conducted to find out more concerning the band structure alterations and the optical energy band gap determination at room temperature. Analysis of optical absorption spectra was employed to determine the optically induced transitions and gain details regarding the films’ band structures [45,46]. The absorption spectra of UV-Vis for pure PEO as well as PEO/SnTiO3 films are shown in Figure 2. It can be seen that the pure PEO film has no noticeable absorption peak in the 200 and 380 nm wavelength. However, in the UV region there is a broad absorption band for the addition of 4 wt.% SnTiO3 into the PEO sample. It is also perceived that there is a small change of the peak in the PEO/SnTiO3NC sample to a higher wavelength with peak broadening (i.e., low intensity) compared to pure PEO.
Figure 2.
Pure PEO (PESNT0) and PESNT1 composite samples’ optical absorption spectra.
It is well-known that when the energy of the emitted photons is lower than the energy difference between the two levels, the photons’ energy is not absorbed and the material is transparent to the photons. Conversely, in the case of absorption of photons with enough high energy, the valence electron transport between the two energy states are observed [47]. The absorption spectrum plateau region in the optical ranges indicates that the PEO composite is transparent to the visible photons while electrons are incapable of jumping within the band structure [48].
The optical absorption coefficient (α) as opposed to wavelength (λ) is described as the power portion, which is absorbed per unit length of a medium. The α is assessed from the absorption data investigation using the giving equation [6,36,49]
| (1) |
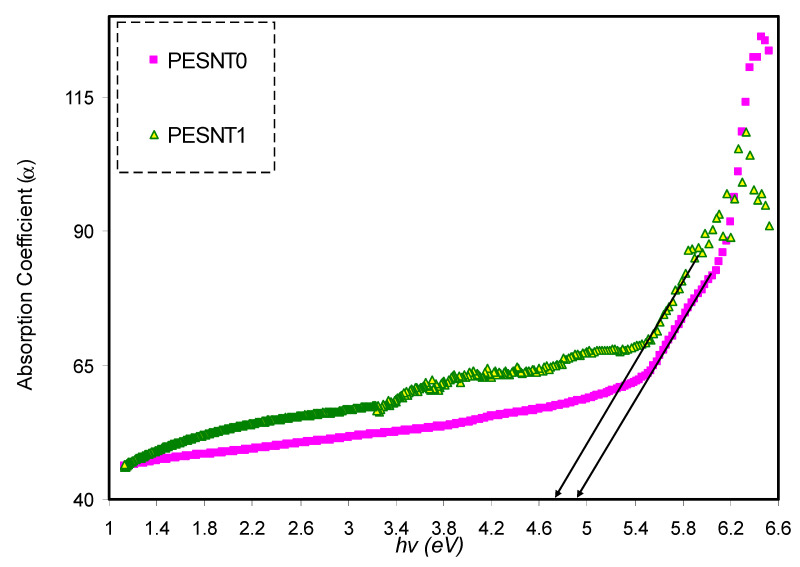
where d and A are the thickness of the samples and the absorbance, respectively; then α can also be defined as a measure of the ability of materials to absorb light photons [50]. Optical absorption examination offers insight into the solid’s band structure. The α against photon energy for pure PEO as well as PEO inserted with SnTiO3 is shown in Figure 3. It is noticeably evident that addition of nano-size SnTiO3 into the PEO causes an absorption edge shift to lower photon energy. In general, semiconductors and insulators are categorized into two main groups: direct band gap (DBG) group and indirect band gap (IBG) group. For DBG semiconductors, the top of the valance band (VB) and bottom of the conduction band (CB) coincide at similar values of k. In contrast, in IBG semiconductors, the top of VB and bottom of CB do not coincide at similar values of k. Transition from VB to CB in materials of IBG groups has to be in relation with a phonon (lattice vibration energies) of the crystal momentum [20]. To determine the absorption edge position, the α linear part as opposed to the curve of hυ can be extrapolated to zero value of absorption [6]. The trap levels development inside the optical band gap results in shifting of the optical absorption edge. As a consequence, electrons cross the VB at the top to the CB at the bottom through these new levels [51,52]. The current optical measurement results are comparable to those obtained for PEO by Kumar et al. [3].
Figure 3.
Optical absorption coefficient (α) against photon energy of pure PEO (PESNT0) and PESNT1 composite samples.
3.3. Refractive Index and Optical Dielectric Constant Study
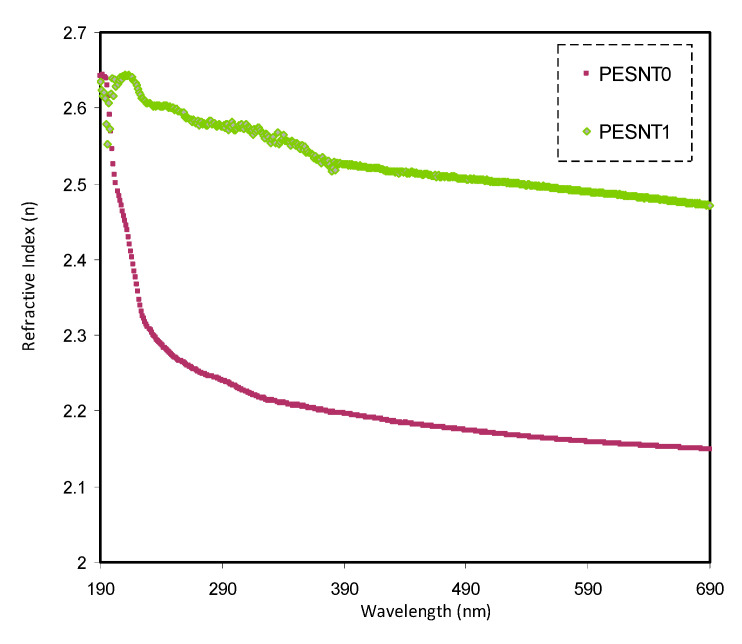
Another decisive parameter of optical phenomena is the refractive index (n) that is governed by the reflection coefficient (R) and extinction coefficient (k). The n value is dependent on the wavelength of incident photon as illustrated in Figure 4. The current study, has confirmed that the n of PEO increases with addition of SnTiO3 NPs. Aziz et al. [18] indicated that then of the polymer films is characterized by a dispersion region at the low wavelength of the incident photons and a plateau region at the high wavelength of the incident photons [53]. Figure 4 indicates that the n of the PEO is modified by addition of SnTiO3, with the n value changing from 2.18 to 2.50. These values have been obtained at the plateau region. n is the decisive optical communication factor when using a material in a specific optoelectronic application [18,54]. It is important that the optical characteristics are dependent on the electronic band structure and atomic structure of materials [18]. Herein, the electronic structure of the PEO matrix is drastically modified via addition of SnTiO3 NPs. The n of materials is formulated on the basis ofR and k by the Fresnel formulae [55,56,57]:
| (2) |
Figure 4.
Refractive index (n) of pure PEO (PESNT0) and PEO:SnTiO3 (PESNT1) samples.
The R denotes the reflectance. The k stands for the extinction coefficient. Thus, when the k is smaller than the n this means, to a large extent, transparency of the composite samples [58]. It is worth mentioning that the n depends upon both the polarizability and density of the composite. Density here refers to the extent of the electron population; when the density is high, the interaction of light with the exposed material increases. Meanwhile, polarizability refers to the strength of an external applied electric field to distort charge distribution (i.e., cause charge redistribution). For example, when light is used as an external field, the molecules undergo polarization and the velocity of the light decreases [58].
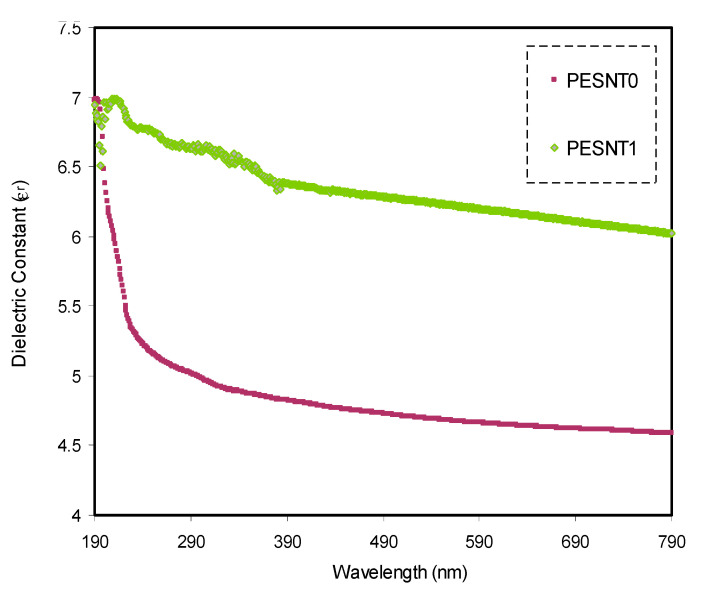
The parameter of the dielectric constant (εr) is another important parameter in the characterization of the material’s optical properties. Figure 5 allows comparison of the optical parameters of εr of PEO/SnTiO3 and pure PEO. It was established that the parameter of εr is linked not just with the values of n, but also with values of k, as shown below [59]:
| (3) |
Figure 5.
Optical dielectric loss (εr) for pure PEO (PESNT0) and PEO:SnTiO3 (PESNT1) samples.
Accordingly, extrapolation of the optical εr plateau region to the axis of Y is employed to extract the optical εr of the PEO and 4 wt.% of PEO/SnTiO3 NC samples. It is apparent that the insertion of the SnTiO3 NPs produces an increment in the εr value from 4.5 to 6.3. The density of state’s increment is the cause of such an increment, as a straight connection exists between the density of states and εr through the polymer film’s forbidden gaps [46,48].
3.4. Band Gap Study
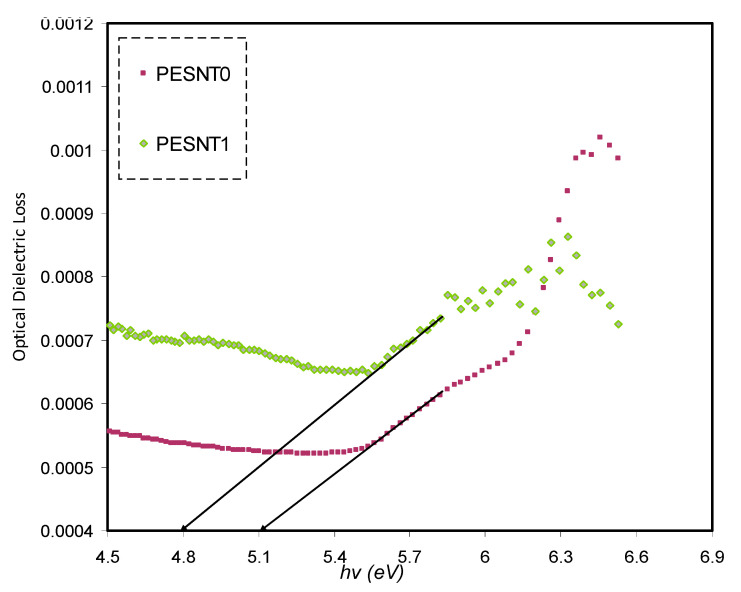
In the current work, the optical characteristics in terms of the complex optical dielectric function were plotted in order to gain a comprehensive understanding of the structure-property of samples. It is known that the complex dielectric function establishes a relationship between the applied electromagnetic field and the density of electrons [48]. Based on the quantum mechanics viewpoint, there is a substantial association between the material’s band structure and optical dielectric function. The real electronic transitions between the filled and unfilled wave functions cause photon absorption or emission and are represented by the optical dielectric loss (εi). The expression below shows the optical parameter of εi [48,60,61]:
| (4) |
In Equation (4), stands for the frequency of incident photons and stands for the volume of the crystal. The charge of an electron and permittivity in space are respectively referred to as e and . The position vector and a vector signifying the electromagnetic wave incident polarization are respectively referred to as and . The CB and VB wave functions at k are respectively referred to as and .
From a quantum perspective, the εi parameter is linked with the filled and unfilled materials’ electronic states, i.e., the optical energy band gap [62]. From the gained n and k data, the εi can be computed through the following relation [63]:
| (5) |
The εi as a function of h(υ) for pure PEO as well as PEO/SnTiO3 NC samples is shown in Figure 6. It was indicated that the optical band gap is computed on the basis of the extrapolated linear part intersection of εi in opposition to the h(υ) [63]. The electronic transition nature is precisely computed on the basis of Tauc’s method as well as εi plot.
Figure 6.
Optical εispectra of pure PEO (PESNT 0) and PESNT 1 films.
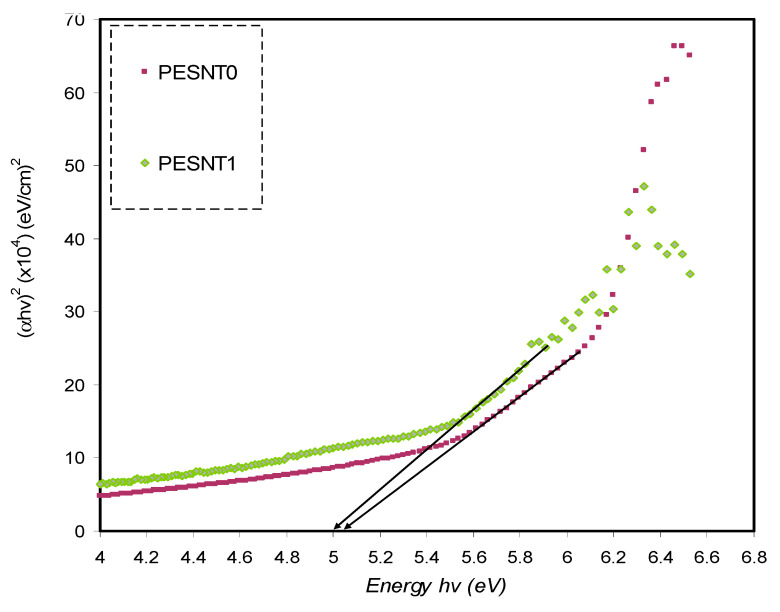
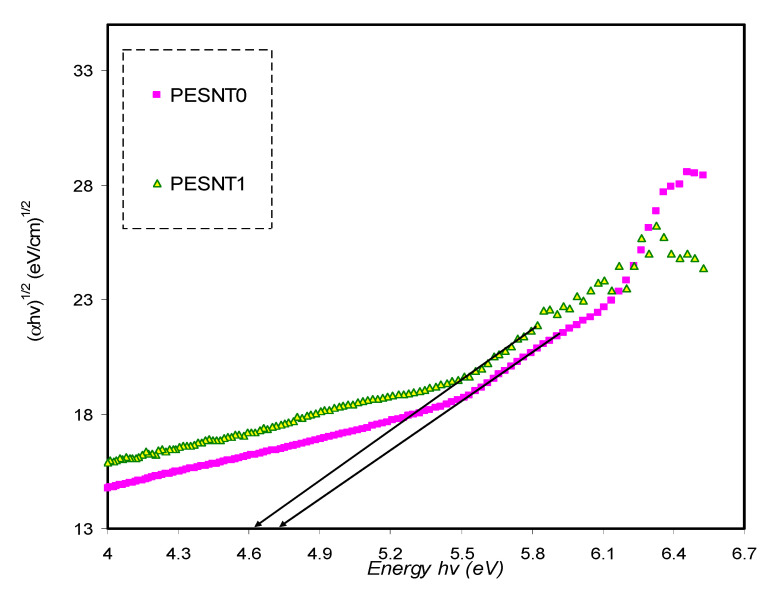
Based on the analysis of the α, it is possible to calculate the optical energy band gap (Eg) of the pure PEO as well as PEO/SnTiO3NC.To assess the energy gap of the films, Tauc’s relationship can be applied as shown below [6,50,64]:
| (6) |
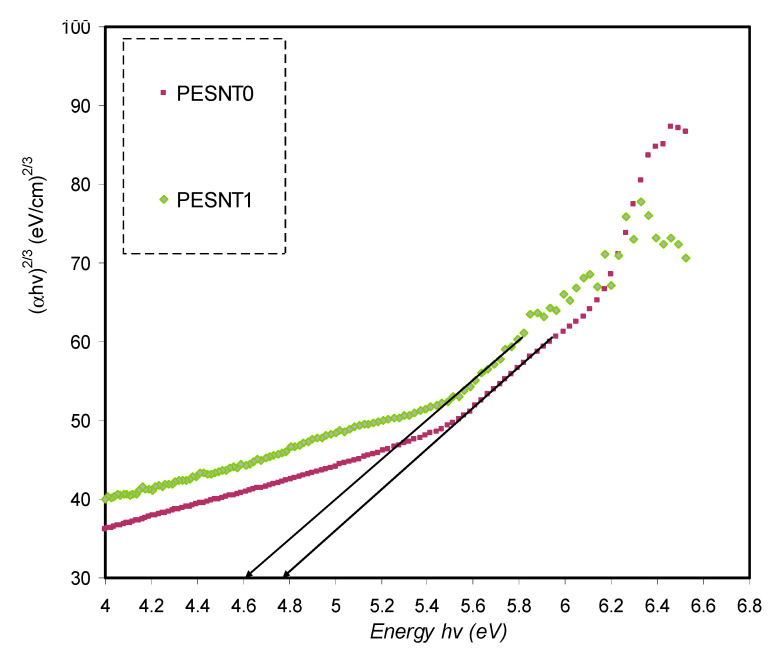
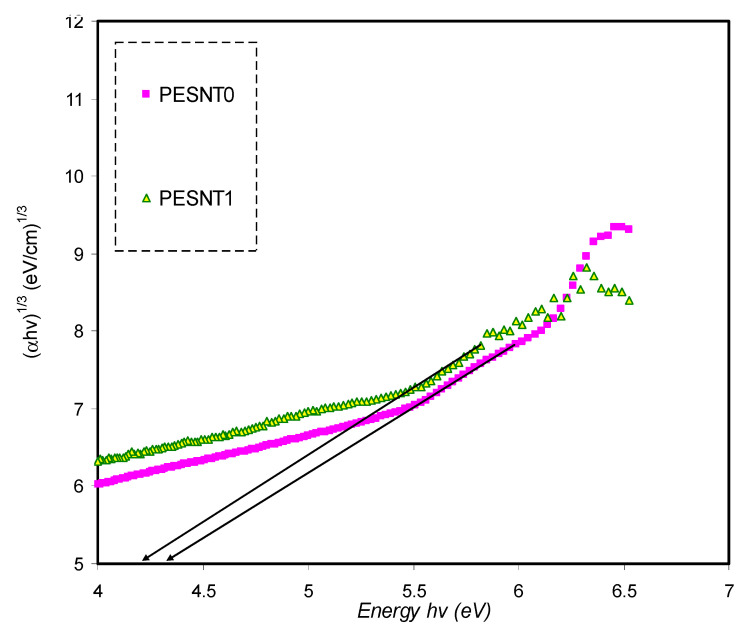
where B isthe constant inside the range of visible frequency and the γ exponent is employed in specifying the electron transitions nature and is reliant on the density of states distribution. γ = 2 or 1/2 to show indirect allowed transition and direct allowed transition, respectively. The situation is different for direct and indirect forbidden transitions, where γ is equal to 3/2 or 3, corresponding to direct forbidden transition and indirect forbidden transition, respectively [65,66]. Additionally, the (αhν)1/γ plots in opposition to hν aid in ascertaining achievable transitions through extrapolating the graph’s straight line part on the axis of hν to α = 0 and consequently obtain the optical band gap. The (hυα)1/γ plots in opposition to hυ for four values of γ, respectively, are shown in Figure 7, Figure 8, Figure 9 and Figure 10. The band gap values determined by Tauc’s method and plot of εi are tabulated in Table 1.
Figure 7.
(αhυ)2 in opposition to energy of photon for pure PEO (PESNT0) and doped (PESNT1) samples.
Figure 8.
(αhυ)1/2 in opposition to energy of photon for pure PEO (PESNT0) and doped (PESNT1) samples.
Figure 9.
(αhυ)2/3 in opposition to energy of photon for pure PEO (PESNT0) and PESNT1 composite samples.
Figure 10.
Plot of (αhυ)1/3 in opposition to energy of photon for pure PEO (PESNT0) and PESNT1 composite samples.
Table 1.
Optical bandgap energy using Tauc’smodel as well as optical dielectric loss (εi) plot.
| Sample Code | Eg for γ = 1/2 | Eg for γ = 2 | Eg for γ = 3/2 | Eg for γ = 3 | Eg for ɛi Plot |
|---|---|---|---|---|---|
| PESNT 0 | 5.1 | 4.74 | 4.78 | 4.33 | 5.12 |
| PESNT 1 | 5.00 | 4.61 | 4.612 | 4.21 | 4.78 |
Table 1 clearly shows the optical band gap decrement resulting from the inclusion ofSnTiO3 NPs. It is suggested from the optical band gap decrement that the electronic structure of the PEO molecules undergo adjustments when SnTiO3 NPs are inserted, and defects produced in the PEO polymer might be the determinants of such adjustments [67]. Localized energy levels at the optical band gap might be created by those defects within the new bandgap states creation, mediating the electrons transition from the VB to the CB that underpins this decrement [6]. By comparing the estimated band gap energy and the energy estimated by the parameter of εi, the nature of electronic transitions in the films can be identified [18]. By comparing the Eg acquired from Tauc’s method (Figure 7, Figure 8, Figure 9 and Figure 10) with the energy obtained from the εi plot (Figure 6), it becomes obvious that direct allowed (γ = 1/2) and forbidden (γ = 3/2) transitions are the main possible transitions for pure PEO and PEO/SnTiO3 NC, respectively. In applications such as light emitting photovoltaic diodes, as well as laser diodes, the best materials are most likely DBG materials. Previous studies established that PEO based polymer composites are significant for diverse optoelectronics applications including sensors, solar cells, transistors, diodes, capacitors, and energy storage [68,69].
4. Conclusions
The key conclusion of the current study is that the DBG polymer NC with amorphous structure is a vital material for applications in optoelectronic devices. The solution cast technique was employed for constructing PEO/SnTiO3 NC films.XRD examination exhibited that a clear interaction happened between SnTiO3 NPs and the PEO polymer. The addition of 4 wt.% SnTiO3 NPs emphasized the change of the PEO phase towards the amorphous phase. The optical band gap energy of pure PEO was modified by adding SnTiO3 NPs, as was calculated from UV-Vis spectroscopy analysis. This modification in the pure PEO’s band gap energy was evident from the interaction between PEO and SnTiO3 NPs. The bandgap reduction with increased amorphous region can significantly enhance the charge transportation within the polymer composite and make it a potential candidate for optoelectronic device applications including solar cells, optical waveguides, and LEDs. The type of electronic transitions between VB and CB was specified using the plot of εi and Tauc’s model. The n of PEO increased with addition of the SnTiO3NPs, as characterized by a plateau region at the high wavelength and a dispersion region at the low wavelength of incident photons. The optical parameter of εr also increased with addition of 4 wt.% SnTiO3NPs. This increment in εr was explained on the basis of formation of new energy levels in the middle of the VB and the CB as well as lowering of the band gap energy. The nature of electronic transitions in the pure and the composite material were identified from Tauc’s model. The optical εi examination was also carried out to calculate the optical band gap and establish the type of electronic transition.
Acknowledgments
The authors appreciatively acknowledge the financial assistance for this work by the Ministry of Higher Education & Scientific Research, Kurdish National Research Council (KNRC), Kurdistan Regional Government, Iraq. The financial support by the University of Sulaimani and Komar Research Center (KRC) and Komar University of Science and Technology are impressively respected.
Author Contributions
Conceptualization, M.M.N. and S.B.A.; formal analysis, S.A.H.; investigation, D.S.M., M.A.B., and S.A.H.; methodology, D.S.M.; project administration, S.B.A.; supervision, S.B.A.; validation, M.A.B., M.M.N., and R.T.A.; visualization, S.B.A.; writing—original draft, D.S.M. and M.A.B.; writing—review and editing, M.M.N., S.B.A., S.A.H., and R.T.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support for this study from the Ministry of Higher Education and Scientific Research-Kurdish National Research Council (KNRC), Kurdistan Regional Government/Iraq. The financial support from the University of Sulaimani and Prince Sultan University is greatly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Prasher S., Kumar M., Singh S. Electrical and Optical Properties of O 6+ Ion Beam–Irradiated Polymers. Int. J. Polym. Anal. Charact. 2014;19:204–211. doi: 10.1080/1023666X.2014.879418. [DOI] [Google Scholar]
- 2.Liu Y., Yin P., Chen J., Cui B., Zhang C., Wu F. Review Article Conducting Polymer-Based Composite Materials for Therapeutic Implantations: From Advanced Drug Delivery System to Minimally Invasive Electronics. Int. J. Polym. Sci. 2020;2020:5659682. doi: 10.1155/2020/5659682. [DOI] [Google Scholar]
- 3.Kumar K.K., Ravi M., Pavani Y., Bhavani S., Sharma A., Rao V.N. Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Phys. B Condens. Matter. 2011;406:1706–1712. doi: 10.1016/j.physb.2011.02.010. [DOI] [Google Scholar]
- 4.Aziz S.B., Abdullah O.G., Saber D.R., Rasheed M.A., Ahmed H.M. Investigation of Metallic Silver Nanoparticles through UV-Vis and Optical Micrograph Techniques. Int. J. Electrochem. Sci. 2017;12:363–373. doi: 10.20964/2017.01.22. [DOI] [Google Scholar]
- 5.Lu L., Sevonkaev I., Kumar A., Goia D.V. Strategies for tailoring the properties of chemically precipitated metal powders. Powder Technol. 2014;261:87–97. doi: 10.1016/j.powtec.2014.04.015. [DOI] [Google Scholar]
- 6.Aziz S.B., Hussein S., Hussein A.M., Saeed S.R. Optical Characteristics of Polystyrene Based Solid Polymer Composites: Effect of Metallic Copper Powder. Int. J. Met. 2013;2013:123657. doi: 10.1155/2013/123657. [DOI] [Google Scholar]
- 7.Mohan K.R., Achari V., Rao V., Sharma A. Electrical and optical properties of (PEMA/PVC) polymer blend electrolyte doped with NaClO4. Polym. Test. 2011;30:881–886. doi: 10.1016/j.polymertesting.2011.08.010. [DOI] [Google Scholar]
- 8.Mohan V.M., Raja V., Bhargav P.B., Sharma A.K., Rao V.V.R.N. Structural, electrical and optical properties of pure and NaLaF4 doped PEO polymer electrolyte films. J. Polym. Res. 2007;14:283–290. doi: 10.1007/s10965-007-9108-8. [DOI] [Google Scholar]
- 9.Mohan V.M., Bhargav P.B., Raja V., Sharma A.K., Rao V.V.R.N. Optical and Electrical Properties of Pure and Doped PEO Polymer Electrolyte Films. Soft Mater. 2007;5:33–46. doi: 10.1080/15394450701405291. [DOI] [Google Scholar]
- 10.Al-Faleh R., Zihlif A. A study on optical absorption and constants of doped poly(ethylene oxide) Phys. B Condens. Matter. 2011;406:1919–1925. doi: 10.1016/j.physb.2011.01.076. [DOI] [Google Scholar]
- 11.Jin J., Qi R., Su Y., Tong M., Zhu J. Preparation of high-refractive-index PMMA/TiO2 nanocomposites by one-step in situ solvothermal method. Iran. Polym. J. 2013;22:767–774. doi: 10.1007/s13726-013-0175-x. [DOI] [Google Scholar]
- 12.Kumar K.N., Rao J.L., Ratnakaram Y. Optical, magnetic and electrical properties of multifunctional Cr3+: Polyethylene oxide (PEO) + polyvinylpyrrolidone (PVP) polymer composites. J. Mol. Struct. 2015;1100:546–554. doi: 10.1016/j.molstruc.2015.07.066. [DOI] [Google Scholar]
- 13.Peppas N.A., Argade A., Bhargava S. Preparation and properties of poly(ethylene oxide) star polymers. J. Appl. Polym. Sci. 2002;87:322–327. doi: 10.1002/app.11444. [DOI] [Google Scholar]
- 14.Ngai K.S., Ramesh S., Ramesh K., Juan J.C. A review of polymer electrolytes: fundamental, approaches and applications. Ionics. 2016;22:1259–1279. doi: 10.1007/s11581-016-1756-4. [DOI] [Google Scholar]
- 15.Poosapati A., Negrete K., Jang N., Hu L., Lan Y., Madan D. Wood cellulose-based thin gel electrolyte with enhanced ionic conductivity. MRS Commun. 2019;9:1015–1021. doi: 10.1557/mrc.2019.79. [DOI] [Google Scholar]
- 16.Armand M.B., Bruce P.G., Forsyth M., Scrosati B., Wieczorek W. Polymer Electrolytes. In: Bruce D.W., O’Hare D., Walton R.I., editors. Energy Materials. John Wiley & Sons; New York, NY, USA: 2011. pp. 1–31. [DOI] [Google Scholar]
- 17.Deshmukh S.H., Burghate D.K., Shilaskar S.N., Chaudhari G.N., Deshmukh P.T. Optical properties of polyaniline doped PVC-PMMA thin films. Indian J. Pure Appl. Phys. 2008;46:344–348. [Google Scholar]
- 18.Aziz S.B. Morphological and Optical Characteristics of Chitosan(1−x): Cuox (4 ≤ x ≤ 12) Based Polymer Nano-Composites: Optical Dielectric Loss as an Alternative Method for Tauc’s Model. Nanomaterials. 2017;7:444. doi: 10.3390/nano7120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim H.K. The surface and volume energy loss of Safranin O thin film prepared by spin coating method. APTA. 2016;53:63–73. [Google Scholar]
- 20.Reddy C.S., Sharma A., Rao V.N. Electrical and optical properties of a polyblend electrolyte. Polymer. 2006;47:1318–1323. doi: 10.1016/j.polymer.2005.12.052. [DOI] [Google Scholar]
- 21.Kumar K.N., Sivaiah K., Buddhudu S. Structural, thermal and optical properties of Tb3+, Eu3+ and co-doped (Tb3++Eu3+): PEO+PVP polymer films. J. Lumin. 2014;147:316–323. doi: 10.1016/j.jlumin.2013.11.027. [DOI] [Google Scholar]
- 22.Brza M.A., Aziz S.B., Anuar H., Al Hazza M.H.F. From Green Remediation to Polymer Hybrid Fabrication with Improved Optical Band Gaps. Int. J. Mol. Sci. 2019;20:3910. doi: 10.3390/ijms20163910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignarooban K., Dissanayake M., Albinsson I., Mellander B.-E. Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide) (PEO) based solid polymer electrolytes. Solid State Ionics. 2014;266:25–28. doi: 10.1016/j.ssi.2014.08.002. [DOI] [Google Scholar]
- 24.Suwanboon S., Amornpitoksuk P., Sukolrat A., Muensit N. Optical and photocatalytic properties of La-doped ZnO nanoparticles prepared via precipitation and mechanical milling method. Ceram. Int. 2013;39:2811–2819. doi: 10.1016/j.ceramint.2012.09.050. [DOI] [Google Scholar]
- 25.Uratani Y., Shishidou T., Oguchi T. First-Principles Study of Lead-Free Piezoelectric SnTiO3. Jpn. J. Appl. Phys. 2008;47:7735–7739. doi: 10.1143/JJAP.47.7735. [DOI] [Google Scholar]
- 26.Konishi Y., Ohsawa M., Tanimura Y., Chikyow T., Wakisaka T., Miyamoto A., Kubo M., Sasata K., Yonezawa Y., Koinuma H. Possible Ferroelectricity in SnTiO3 by First-Principles Calculations. MRS Online Proc. Libr. Arch. 2002;748:u3.13.1–u3.13.6. doi: 10.1557/PROC-748-U3.13. [DOI] [Google Scholar]
- 27.Matar S.F., Baraille I., Subramanian M. First principles studies of SnTiO3 perovskite as potential environmentally benign ferroelectric material. Chem. Phys. 2009;355:43–49. doi: 10.1016/j.chemphys.2008.11.002. [DOI] [Google Scholar]
- 28.Scott J.F., De Araujo C.A.P. Ferroelectric Memories. Science. 1989;246:1400–1405. doi: 10.1126/science.246.4936.1400. [DOI] [PubMed] [Google Scholar]
- 29.Jiwei Z., Xi Y., Liangying Z. The optical waveguide characteristics of highly orientated sol–gel derived polycrystalline ferroelectric PZT thin films. Ceram. Int. 2001;27:585–589. doi: 10.1016/S0272-8842(01)00005-0. [DOI] [Google Scholar]
- 30.Zheng Y., Wang B., Woo C. Piezoelectric bending response and switching behavior of ferroelectric/paraelectric bilayers. Acta Mater. 2008;56:479–488. doi: 10.1016/j.actamat.2007.10.011. [DOI] [Google Scholar]
- 31.Fix T., Sahonta S.-L., Garcia V., MacManus-Driscoll J.L., Blamire M.G. Structural and Dielectric Properties of SnTiO3, a Putative Ferroelectric. Cryst. Growth Des. 2011;11:1422–1426. doi: 10.1021/cg200333q. [DOI] [Google Scholar]
- 32.Taib M.F.M., Yaakob M., Chandra A., Arof A.K.M., Yahya M.Z.A. Effect of Pressure on Structural, Electronic and Elastic Properties of Cubic (Pm3m) SnTiO3 Using First Principle Calculation. Adv. Mater. Res. 2012;501:342–346. doi: 10.4028/www.scientific.net/AMR.501.342. [DOI] [Google Scholar]
- 33.Taib M.F.M., Yaakob M.K., Hassan O.H., Yahya M.Z.A. Structural, Electronic, and Lattice Dynamics of PbTiO3, SnTiO3, and SnZrO3: A Comparative First-Principles Study. Integr. Ferroelectr. 2013;142:119–127. doi: 10.1080/10584587.2013.780528. [DOI] [Google Scholar]
- 34.Ibrahim S., Yassin M.M., Ahmad R., Johan M.R. Effects of various LiPF6 salt concentrations on PEO-based solid polymer electrolytes. Ionics. 2011;17:399–405. doi: 10.1007/s11581-011-0524-8. [DOI] [Google Scholar]
- 35.Bhatt C., Swaroop R., Arya A., Sharma A.L. Effect of Nano-Filler on the Properties of Polymer Nanocomposite Films of PEO/PAN Complexed with NaPF6. J. Mater. Sci. Eng. B. 2015;5:418–434. doi: 10.17265/2161-6221/2015.11-12.003. [DOI] [Google Scholar]
- 36.Devendrappa H., Chapi S. Influence of Cobalt (II) Chloride Catalysed on the Thermal and Optical Characterization of PEO Based Solid Polymer Electrolytes. J. Res. Updat. Polym. Sci. 2015;3:205–215. doi: 10.6000/1929-5995.2014.03.04.2. [DOI] [Google Scholar]
- 37.Marzantowicz M., Dygas J., Krok F., Florjańczyk Z., Zygadło-Monikowska E. Influence of crystalline complexes on electrical properties of PEO:LiTFSI electrolyte. Electrochim. Acta. 2007;53:1518–1526. doi: 10.1016/j.electacta.2007.03.032. [DOI] [Google Scholar]
- 38.Aziz S.B., Marif R.B., Brza M., Hassan A.N., Ahmad H.A., Faidhalla Y.A., Kadir M. Structural, thermal, morphological and optical properties of PEO filled with biosynthesized Ag nanoparticles: New insights to band gap study. Results Phys. 2019;13:102220. doi: 10.1016/j.rinp.2019.102220. [DOI] [Google Scholar]
- 39.Aziz S.B., Abdullah O.G., Hussein A.M., Abdulwahid R.T., Rasheed M.A., Ahmed H.M., Abdal Qadir S.W., Mohammed A.R. Optical properties of pure and doped PVA:PEO based solid polymer blend electrolytes: two methods for band gap study. J. Mater. Sci. Mater. Electron. 2017;28:7473–7479. doi: 10.1007/s10854-017-6437-1. [DOI] [Google Scholar]
- 40.Abdelrazek E.M., Abdelghany A., Badr S.I., Morsi M.A. Structural, optical, morphological and thermal properties of PEO/PVP blend containing different concentrations of biosynthesized Au nanoparticles. J. Mater. Res. Technol. 2018;7:419–431. doi: 10.1016/j.jmrt.2017.06.009. [DOI] [Google Scholar]
- 41.Aziz S.B., Abdullah R.M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS:AgNt]x:PEO(x-1) (10 ≤ x ≤ 50) Electrochim. Acta. 2018;285:30–46. doi: 10.1016/j.electacta.2018.07.233. [DOI] [Google Scholar]
- 42.Padmaja S., Jayakumar S., Balaji R., Sudakar C., Kumaravel M., Rajendran V., Rajkumar M., Radhamani A. Structural and optical properties of CdS/PEO nanocomposite solid films. Mater. Sci. Semicond. Process. 2013;16:1502–1507. doi: 10.1016/j.mssp.2013.06.002. [DOI] [Google Scholar]
- 43.Aziz S.B., Hamsan M., Brza M., Kadir M., Abdulwahid R.T., Ghareeb H.O., Woo H. Fabrication of energy storage EDLC device based on CS:PEO polymer blend electrolytes with high Li+ ion transference number. Results Phys. 2019;15:102584. doi: 10.1016/j.rinp.2019.102584. [DOI] [Google Scholar]
- 44.Abdullah R.M., Aziz S.B., Mamand S.M., Hassan A.Q., Hussein S.A., Kadir M. Reducing the Crystallite Size of Spherulites in PEO-Based Polymer Nanocomposites Mediated by Carbon Nanodots and Ag Nanoparticles. Nanomaterials. 2019;9:874. doi: 10.3390/nano9060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aziz S.B., Abdulwahid R.T., Rsaul H.A., Ahmed H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci. Mater. Electron. 2016;27:4163–4171. doi: 10.1007/s10854-016-4278-y. [DOI] [Google Scholar]
- 46.Aziz S.B. Modifying Poly(Vinyl Alcohol) (PVA) from Insulator to Small-Bandgap Polymer: A Novel Approach for Organic Solar Cells and Optoelectronic Devices. J. Electron. Mater. 2015;45:736–745. doi: 10.1007/s11664-015-4191-9. [DOI] [Google Scholar]
- 47.Parola S., Julián-López B., Carlos L.D., Sanchez C. Optical Properties of Hybrid Organic-Inorganic Materials and their Applications. Adv. Funct. Mater. 2016;26:6506–6544. doi: 10.1002/adfm.201602730. [DOI] [Google Scholar]
- 48.Aziz S.B., Rasheed M.A., Hussein A.M., Ahmed H.M. Fabrication of polymer blend composites based on [PVA-PVP] (1−x):(Ag2S) x (0.01 ≤ x ≤ 0.03) with small optical band gaps: Structural and optical properties. Mater. Sci. Semicond. Process. 2017;71:197–203. doi: 10.1016/j.mssp.2017.05.035. [DOI] [Google Scholar]
- 49.Aziz S.B., Ahmed H.M., Hussein A.M., Fathulla A.B., Wsw R.M., Hussein R.T. Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J. Mater. Sci. Mater. Electron. 2015;26:8022–8028. doi: 10.1007/s10854-015-3457-6. [DOI] [Google Scholar]
- 50.Edukondalu A., Ahmmad S.K., Kumar K.S., Rahman S., Gupta A. Optical properties of amorphous Li2O–WO3–B2O3 thin films deposited by electron beam evaporation. J. Taibah Univ. Sci. 2016;10:363–368. doi: 10.1016/j.jtusci.2015.03.012. [DOI] [Google Scholar]
- 51.Aziz S.B., Hassan A.Q., Mohammed S.J., Karim W.O., Kadir M., Tajuddin H.A., Chan N.N.M.Y. Structural and Optical Characteristics of PVA:C-Dot Composites: Tuning the Absorption of Ultra Violet (UV) Region. Nanomaterials. 2019;9:216. doi: 10.3390/nano9020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aziz S.B., Rasheed M.A., Abidin Z.H.Z. Optical and Electrical Characteristics of Silver Ion Conducting Nanocomposite Solid Polymer Electrolytes Based on Chitosan. J. Electron. Mater. 2017;46:6119–6130. doi: 10.1007/s11664-017-5515-8. [DOI] [Google Scholar]
- 53.Rahman A., Khan M.K.R. Effect of annealing temperature on structural, electrical and optical properties of spray pyrolytic nanocrystalline CdO thin films. Mater. Sci. Semicond. Process. 2014;24:26–33. doi: 10.1016/j.mssp.2014.03.002. [DOI] [Google Scholar]
- 54.Arslan M., Duymuş H., Yakuphanoglu F. Optical Properties of the Poly(N-benzylaniline) Thin Film. J. Phys. Chem. B. 2006;110:276–280. doi: 10.1021/jp054844m. [DOI] [PubMed] [Google Scholar]
- 55.Kymakis E., Amaratunga G.A. Optical properties of polymer-nanotube composites. Synth. Met. 2004;142:161–167. doi: 10.1016/j.synthmet.2003.08.011. [DOI] [Google Scholar]
- 56.Nemade K.R., Waghuley P.F.S. Synthesis of MgO Nanoparticles by Solvent Mixed Spray Pyrolysis Technique for Optical Investigation. Int. J. Met. 2014;2014:389416. doi: 10.1155/2014/389416. [DOI] [Google Scholar]
- 57.Yakuphanoglu F., Kandaz M., Yarasir M.N., Şenkal F., Yarasir M. Electrical transport and optical properties of an organic semiconductor based on phthalocyanine. Phys. B Condens. Matter. 2007;393:235–238. doi: 10.1016/j.physb.2007.01.007. [DOI] [Google Scholar]
- 58.Costner E.A., Long B.K., Navar C., Jockusch S., Lei X., Zimmerman P., Campion A., Turro N.J., Willson C.G. Fundamental Optical Properties of Linear and Cyclic Alkanes: VUV Absorbance and Index of Refraction. J. Phys. Chem. A. 2009;113:9337–9347. doi: 10.1021/jp903435c. [DOI] [PubMed] [Google Scholar]
- 59.Saini I., Rozra J., Chandak N., Aggarwal S., Sharma A., Sharma A. Tailoring of electrical, optical and structural properties of PVA by addition of Ag nanoparticles. Mater. Chem. Phys. 2013;139:802–810. doi: 10.1016/j.matchemphys.2013.02.035. [DOI] [Google Scholar]
- 60.Bouzidi C., Horchani-Naifer K., Khadraoui Z., Elhouichet H., Ferid M. Synthesis, characterization and DFT calculations of electronic and optical properties of CaMoO4. Phys. B Condens. Matter. 2016;497:34–38. doi: 10.1016/j.physb.2016.06.009. [DOI] [Google Scholar]
- 61.Li L., Wang W., Liu H., Liu X., Song Q., Ren S. First Principles Calculations of Electronic Band Structure and Optical Properties of Cr-Doped ZnO. J. Phys. Chem. C. 2009;113:8460–8464. doi: 10.1021/jp811507r. [DOI] [Google Scholar]
- 62.Biskri Z.E., Rached H., Bouchear M., Rached D., Aida M.S. A Comparative Study of Structural Stability and Mechanical and Optical Properties of Fluorapatite (Ca5(PO4)3F) and Lithium Disilicate (Li2Si2O5) Components Forming Dental Glass–Ceramics: First Principles Study. J. Electron. Mater. 2016;45:5082–5095. doi: 10.1007/s11664-016-4681-4. [DOI] [Google Scholar]
- 63.Aziz S.B., Mamand S.M., Saed S.R., Abdullah R.M., Hussein S.A. New Method for the Development of Plasmonic Metal-Semiconductor Interface Layer: Polymer Composites with Reduced Energy Band Gap. J. Nanomaterials. 2017;2017:8140693. doi: 10.1155/2017/8140693. [DOI] [Google Scholar]
- 64.Reddeppa N., Sharma A., Rao V.N., Chen W. Preparation and characterization of pure and KBr doped polymer blend (PVC/PEO) electrolyte thin films. Microelectron. Eng. 2013;112:57–62. doi: 10.1016/j.mee.2013.05.015. [DOI] [Google Scholar]
- 65.Davis E.A., Mott N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970;22:0903–0922. doi: 10.1080/14786437008221061. [DOI] [Google Scholar]
- 66.Aziz S.B., Rasheed M.A., Ahmed H.M. Synthesis of Polymer Nanocomposites Based on [Methyl Cellulose](1-x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with Desired Optical Band Gaps. Polymers. 2017;9:194. doi: 10.3390/polym9060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hemalatha K.S., Rukmani K., Suriyamurthy N., Nagabhushana B.M. Synthesis, characterization and optical properties of hybrid PVA-ZnOnanocomposite: A composition dependent study. Mater. Res. Bull. 2014;51:438–446. doi: 10.1016/j.materresbull.2013.12.055. [DOI] [Google Scholar]
- 68.Hadi A., Hashim A., Al-Khafaji Y. Structural, Optical and Electrical Properties of PVA/PEO/SnO2 New Nanocomposites for Flexible Devices. Trans. Electr. Electron. Mater. 2020;21:283–292. doi: 10.1007/s42341-020-00189-w. [DOI] [Google Scholar]
- 69.Choudhary S. Structural, optical, dielectric and electrical properties of (PEO–PVP)–ZnO nanocomposites. J. Phys. Chem. Solids. 2018;121:196–209. doi: 10.1016/j.jpcs.2018.05.017. [DOI] [Google Scholar]