Abstract
Two new hypothetical zirconium diboride (ZrB) polymorphs: (hP6-P6/mmc-space group, no. 194) and (oP6-Pmmn-space group, no. 59), were thoroughly studied under the first-principles density functional theory calculations from the structural, mechanical and thermodynamic properties point of view. The proposed phases are thermodynamically stable (negative formation enthalpy). Studies of mechanical properties indicate that new polymorphs are less hard than the known phase (hP3-P6/mmm-space group, no. 191) and are not brittle. Analysis of phonon band structure and density of states (DOS) also show that the phonon modes have positive frequencies everywhere and the new ZrB phases are not only mechanically but also dynamically stable. The estimated acoustic Debye temperature, , for the two new proposed ZrB phases is about 760 K. The thermodynamic properties such as internal energy, free energy, entropy and constant-volume specific heat are also presented.
Keywords: zirconium diboride, ab initio calculations, mechanical properties, elastic properties, phonons
1. Introduction
During latest years, the transition metal borides have attracted attention among materials researchers due to the combination of their outstanding physical properties such as electric and thermal conductivity comparable with metals, low compressibility, high shear strength and exceptionally high hardness [1,2]. Even in the form of thin films, they possess extraordinary properties such as very high hardness with increased flexibility, great thermal properties and very good corrosion and wear resistance [3,4,5]. Among borides, zirconium diboride (ZrB) deserves special attention. ZrB, with melting temperature 3245 C [6], is a member of a family of materials known as ultra-high temperature ceramics (UHTCs). In addition to high melting temperatures, ZrB has a unique combination of chemical stability, high electrical and thermal conductivities and resistance to erosion and corrosion that makes its suitable for the extreme chemical and thermal environments associated with, for example, hypersonic flight, atmospheric re-entry and rocket propulsion [7]. According to the Zr–B phase diagram [8,9] there are three phases, namely, ZrB, ZrB, and ZrB, which have been reported and widely studied for this system. Theoretical investigations show that ZrB can create different crystallographic structures. The basic phase is NaCl-type face-centered cubic ZrB (Fm-3m-space group, no. 225) with lattice constant a = 4.900 Å [10]. Furthermore, ZrB also can crystallize in a FeB-type structure with a primitive orthorhombic (Pnma) crystal structure [9,11], CrB-type orthorhombic structure with Cmcm-space group [11,12] and hexagonal Pmmm [13]. A literature review shows that ZrB is only stable in one structure type. The cubic LuB structure (Fm3m-space group, no. 225) with lattice constant a = 7.4085 Å was studied theoretically and experimentally for example in Reference [14]. They compared electric-field gradient measurements at the B sites and first-principles calculations in order to analyse the chemical bonding properties. Both experimental and theoretical results were in good agreement. In Reference [15], however, the mechanical properties of ZrB were calculated. The Vickers hardness of zirconium boride was 32.9 GPa, which is in good agreement with other theoretical results [14,16,17].
In contrast to ZrB, depending on the calculation method, different hardness values have been determined for zirconium diboride ZrB. The calculated values of hardness range from 12.82–55.53 GPa [18], whereas experimentally measured hardness reached 23 ± 0.9 GPa [6,19]. All mentioned structures were assigned as ZrB with the crystal hexagonal structure of AlB-type with the P6/mmm-space group, no. 191. Such large differences in the values of the analysed properties may, however, come not only from differences in calculation methods but also from the possibility of the existence of other stable forms of ZrB, which may form nanocomposites of different polymorphs of ZrB. A similar conclusion about the possibility of the existence of other ZrB crystal types can be drawn on the basis of other studies on possible forms of transition metal diborides, for example, WB [20,21] or ReB [22,23]. For comparison, in Reference [21] the authors proposed six different phases of WB. In the case of ZrB, it is hard to find such a study.
It should also be noted that in addition to the phases appearing in the Zr-B equilibrium diagram [8,24], other zirconium and boron compounds have been theoretically determined. There are hypothetical Zr-B phases such as: ZrB, ZrB, ZrB [16], ZrB [25], ZrB [26] and ZrB [13]. All polymorphs are both mechanically and dynamically stable but have not been confirmed experimentally yet. In this work, structural, mechanical and thermodynamic properties of stable ZrB polymorphs from density functional calculations will be studied.
2. Computational Methods
First-principle calculations based on density functional theory (DFT) [27,28] within the pseudopotential plane-wave approximation (PP-PW) implemented in ABINIT [29,30] code were performed in this work. Projector augmented-wave formulation (PAW) pseudopotentials [31] were used to describe the interactions of ionic core and non-valence electrons.
To enhance the confidence of the calculations as an exchange-correlation (XC) functional, three approximations were used: local density approximation (LDA) [32,33], classical Perdew-Burke-Ernzerhof (PBE) generalised gradient approximation (GGA) [34] and modified Perdew-Burke-Ernzerhof GGA for solids (PBEsol) [35]. There is a strong view that the PBEsol is the overall best performing XC functional for identifying the structure and elastic properties [36,37,38].
Projector augmented wave method (PAW) pseudopotentials used for LDA and PBE XC functionals were taken from PseudoDojo project [39]. PAW pseudopotentials for PBEsol exchange-correlation functional [35] were generated using ATOMPAW software [40] and a library of exchange-correlation functionals for density functional theory, LibXC [41].
All calculations were made by tuning the precision of the calculations, which was done by automatically setting the variables at accuracy level 4 (accuracy = 4 corresponds to the default tuning of ABINIT). The cut-off energy consistent with PAW pseudopotentials of the plane-wave basis set was 15 Ha with valence electrons for Zr and valence electrons for B. K-PoinTs grids were generated with kptrlen = 30.0 (grids that give a length of smallest vector LARGER than kptrlen). Metallic occupation of levels with the Fermi-Dirac smearing occupation scheme and tsmear (Ha) = 0.02 was used in all ABINIT calculations.
2.1. Optimization of Structures
As mentioned earlier, tungsten diboride [20] and rhenium diboride [23] crystallise in various space groups. Searching for new structures of ZrB, we started with basic cells of hP6-P6/mmc-WB and oP6-Pmmn-WB and replaced tungsten atoms with zirconium atoms, wherein the designations mean: Pearson symbol, Space group and Chemical formula [20]. Then, all structures were relaxed by using the Broyden-Fletcher-Goldfarb-Shanno minimisation scheme (BFGS) with full optimisation of cell geometry and atomic coordinates. Maximal stress tolerance (GPa) was set to 1 × 10.
2.2. Formation Enthalpy and Cohesive Energy
The formation enthalpy and cohesive energy were determined as follows [23,42]:
| (1) |
| (2) |
where is the formation enthalpy of the ZrB; is the cohesive energy of the ZrB; is the cohesive energy of Zr; is the cohesive energy of B; is the total energy of the ZrB; is the total energy of a Zr atom and is the total energy of a B atom.
The cohesive energy of the is calculated, taking into account stoichiometry, as the total energy difference of zirconium crystal (hP2-P6/mmc-space group, no.194) and single Zr atom in the box, whereas is the total energy difference of -boron crystal (hR12-R-3m-space group, no. 166) and single B atom in a sufficiently large box [23].
2.3. Mechanical Properties Calculations
The theoretical ground state elastic constants of all structures were established with the metric tensor formulation of strain in density functional perturbation theory (DFPT) [43]. Isotropised bulk modulus B, shear modulus G, Young’s modulus E and Poisson’s ratio were estimated by means of a Voigt–Reuss–Hill average [44,45].
In order to verify the elastic stability of all the structures, positive definiteness of the stiffness tensor was checked [46] by calculating Kelvin moduli, that is, eigenvalues of stiffness tensor represented in second-rank tensor notation [47].
Hardness of ZrB polymorphs in the present paper was calculated with the use of semi-empirical relation proposed in Reference [48]. The equation is defined as follow:
| (3) |
G/B ratio appearing in the above formula named Pugh’s modulus ratio [49] is commonly used as a universal ductile-to-brittle criterion.
2.4. Phonon and Thermodynamic Properties Calculations
To calculate phonons, DFPT was utilised [29,30]. The phonon dispersion curves [50] of the analysed structures were then used to determine their dynamical stability [46,51], complementary to elastic stability. Acoustic Debye temperature was calculated from the phonon densities of states (DOS).
Using the calculated phonons under the harmonic approximation, that is, in the range up to Debye temperature, thermal quantities: phonon internal energy, free energy, entropy and constant-volume heat capacity as a function of the temperature were determined [24,52].
3. Results
Using the approach described in Section 2.1, the first step in our calculations was the geometry optimisation of the two new hypothetical (ZrB) polymorphs—(hP6-P6/mmc-space group, no.194) and (oP6-Pmmn-space group, no. 59), and the formerly known polymorph (hP3-P6/mmm-space group, no. 191).
3.1. Structural Properties
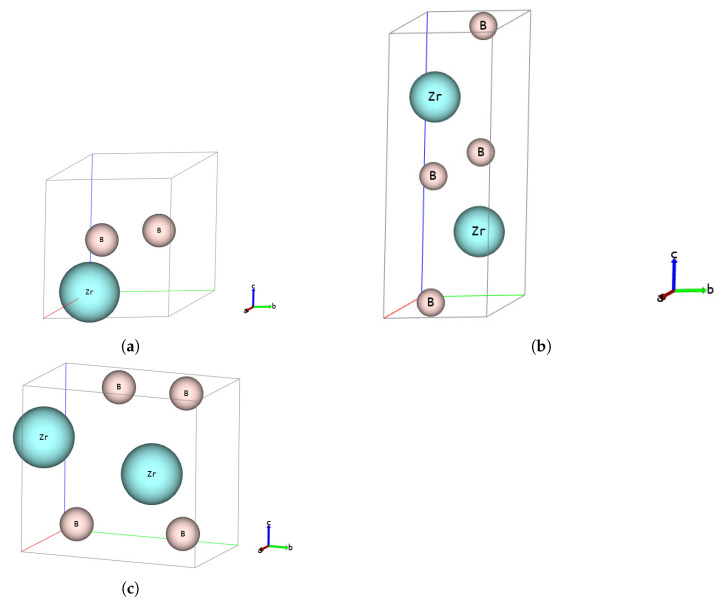
The basic cells for all three analysed polymorphs are depicted in Figure 1, whereas the crystallographic data, calculated with three different XC functionals (LDA, PBE and PBEsol), are stored in crystallographic information files (CIFs) in Supplementary Materials.
Figure 1.
ZB-Basic cells: (a) hP3-P6/mmm, (b) hP6-P6/mmc and (c) oP6-Pmmn.
Determined lattice parameters, formation enthalpy and cohesive energy for known hP3-P6/mmm polymorph (Figure 1a) are comparable to those of other authors [18] (see Table 1). We treat this as a verification of the correctness of the methodology used.
Table 1.
Lattice parameters (Å); formation enthalpy (eV/Atom); cohesive energy (eV/Atom); elastic constants (GPa); Kelvin moduli (GPa); bulk modulus B (GPa); shear modulus G (GPa); Young’s modulus E (GPa); Poisson’s ratio ; Pugh’s modulus ratio; Debye temperature (K); hardness (GPa), of ZrB phases: ZrB (hP3-P6/mmm-space group, no. 191; hP6-P6/mmc-space group, no.194; and oP6-P6/mmm-space group, no.59). Experimental and calculated data for hP3-P6/mmm phase are taken from Reference [18].
| Phase | hP3-P6/mmm-No.191 | hP6-P6/mmc-No. 194 | oP6-Pmmn-No. 59 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Exp. | Calc. | LDA | PBE | PBEsol | LDA | PBE | PBEsol | LDA | PBE | PBEsol |
| a | 3.165–3.169 | 3.127–3.197 | 3.135 | 3.173 | 3.156 | 3.025 | 3.076 | 3.050 | 3.057 | 3.100 | 3.071 |
| b | 4.931 | 5.029 | 4.981 | ||||||||
| c | 3.523—3.547 | 3.490–3.561 | 3.477 | 3.527 | 3.495 | 8.515 | 8.624 | 8.565 | 4.541 | 4.604 | 4.578 |
| 1.113 | 0.985–1.099 | 1.145 | 1.078 | 1.141 | 0.211 | 0.195 | 0.218 | 0.164 | 0.158 | 0.178 | |
| 5.67–8.648 | 8.769 | 8.072 | 8.411 | 7.834 | 7.187 | 7.488 | 7.187 | 7.150 | 7.448 | ||
| 581 | 551–606 | 618 | 591 | 597 | 224 | 214 | 217 | 333 | 325 | 336 | |
| 581 | 551–606 | 618 | 591 | 597 | 224 | 214 | 217 | 331 | 316 | 334 | |
| 445 | 436–482 | 477 | 481 | 456 | 495 | 447 | 479 | 436 | 380 | 439 | |
| 240 | 240–281 | 278 | 253 | 269 | 81 | 73 | 80 | 136 | 134 | 144 | |
| 240 | 240–281 | 278 | 253 | 269 | 81 | 73 | 80 | 43 | 48 | 35 | |
| 263 | 252–268 | 283 | 272 | 274 | 1 | 9 | 2 | 122 | 145 | 126 | |
| 55 | 48–71 | 52 | 47 | 49 | 222 | 196 | 213 | 111 | 80 | 109 | |
| 121 | 118–169 | 135 | 105 | 126 | 70 | 63 | 69 | 97 | 70 | 88 | |
| 121 | 118–169 | 135 | 105 | 126 | 70 | 63 | 69 | 99 | 85 | 92 | |
| 787 | 727 | 753 | 572 | 519 | 555 | 578 | 500 | 569 | |||
| 566 | 544 | 548 | 162 | 146 | 160 | 301 | 290 | 314 | |||
| 566 | 544 | 548 | 162 | 146 | 160 | 272 | 283 | 288 | |||
| 556 | 506 | 538 | 2 | 18 | 4 | 244 | 268 | 252 | |||
| 556 | 506 | 538 | 2 | 18 | 4 | 221 | 238 | 226 | |||
| 360 | 392 | 349 | 369 | 338 | 354 | 86 | 96 | 70 | |||
| B | 220–245 | 239–260 | 262 | 242 | 250 | 184 | 168 | 178 | 189 | 165 | 187 |
| G | 225–243 | 229–243 | 256 | 247 | 248 | 37 | 44 | 37 | 102 | 108 | 100 |
| E | 502–554 | 520–555 | 580 | 554 | 560 | 104 | 121 | 105 | 260 | 267 | 256 |
| 0.109–0.13 | 0.137–0.144 | 0.13 | 0.118 | 0.126 | 0.406 | 0.38 | 0.402 | 0.271 | 0.231 | 0.272 | |
| 0.99–1.023 | 0.935–0.958 | 0.981 | 1.024 | 0.995 | 0.200 | 0.261 | 0.21 | 0.541 | 0.655 | 0.539 | |
| 910 | 921–950 | 1007 | 973 | 971 | 794 | 754 | 779 | 787 | 752 | 774 | |
| 23±0.9 | 23–56 | 46 | 47 | 45 | 2 | 3 | 2 | 12 | 16 | 12 | |
The first new hypothetical phase, hP6-P6/mmc (Figure 1b), also crystallises in the hexagonal system but has 6 atoms in the cell, whereas the second new hypothetical phase, oP6-Pmmn (Figure 1c), crystallises in the orthorhombic system and also has 6 atoms in the cell. There is little sense to compare lattice parameters for phases in different systems, but it is worth comparing the formation enthalpy and the cohesive energy. It can be seen that the formation enthalpy, , for new phases is significantly lower than for the known hP3-P6/mmm phase and comparable between the new phases (see Table 1). The calculated cohesive energy, , is a little higher than for the known hP3-P6/mmm phase and again comparable between the two new phases. These two facts suggest that the new phases are comparably thermodynamically stable but less stable than the known hP3-P6/mmm phase.
3.2. Mechanical Properties
Computed elastic constants, Kelvin moduli, isotropised bulk, shear and Young’s modulus, Poisson’s ratio, G/B Pugh’s modulus ratio, Debye temperature and estimated hardness of all analysed zirconium diboride structures are listed in Table 1. For known hP3-P6/mmm phase (Figure 1a), these quantities are comparable to those of other authors [18], which is a further verification of the validity of the methodology used.
Analysing the data received, it can be concluded that the new phases have lower mechanical parameters than the known hP3-P6/mmm phase, except for the Poisson’s ratio. Both new phases have a similar isotropised bulk modulus of about 170 GPa, but the shear modulus for the hP6-P6/mmc phase is much lower and is only about 40 GPa. The consequence of this is that the hardness of the hP6-P6/mmc phase is only 2 GPa, while for the oP6-Pmmn phase it is about 14 GPa. A high G/B Pugh’s modulus ratio would correspond to a more brittle than ductile character of material. The critical value, separating ductile from brittle materials, is approximately 0.571 [49,53]. It can be seen that the known hP3-P6/mmm phase is brittle, the hP6-P6/mmc phase is ductile and the oP6-Pmmn phase is somehow between brittle and ductile.
All analysed structures have a positive definite stiffness tensor and positive Kelvin moduli, that is, eigenvalues of stiffness tensor represented in second-rank tensor notation, so there are mechanically stable (see Table 1).
3.3. Phonon and Thermodynamic Properties
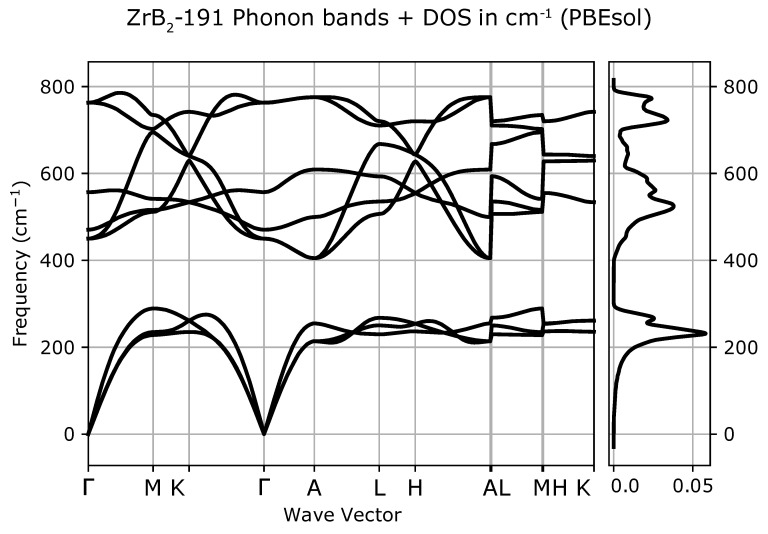
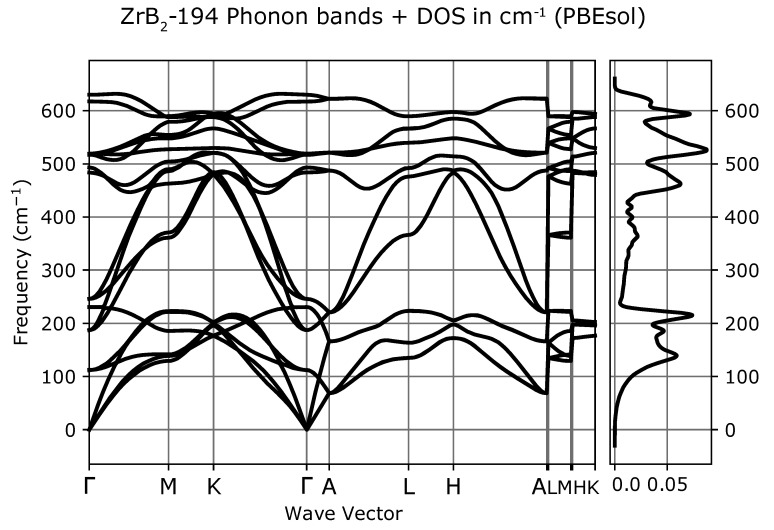
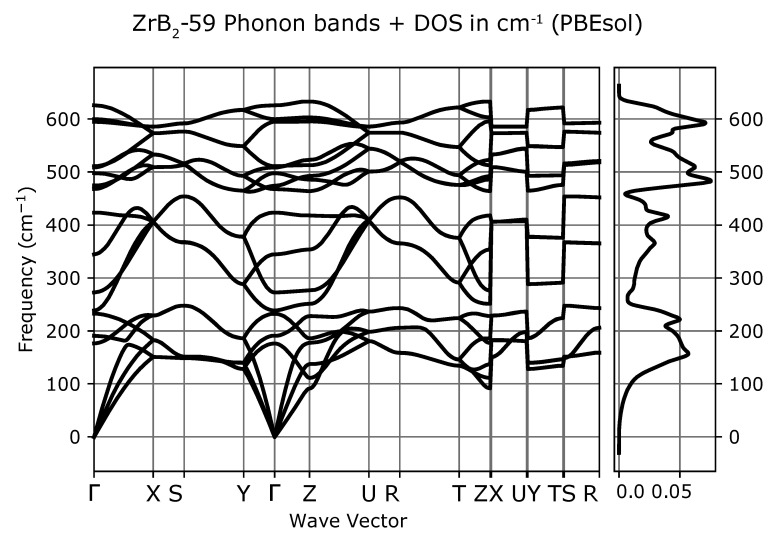
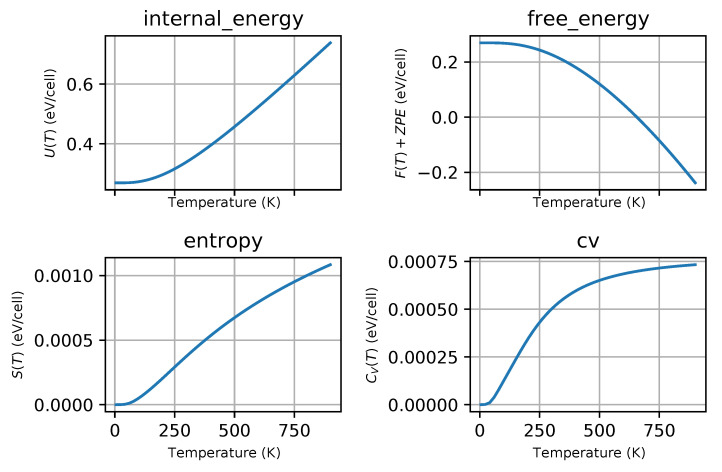
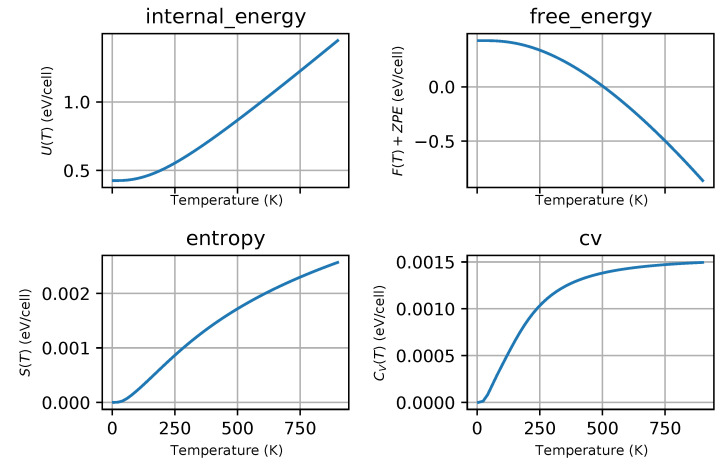
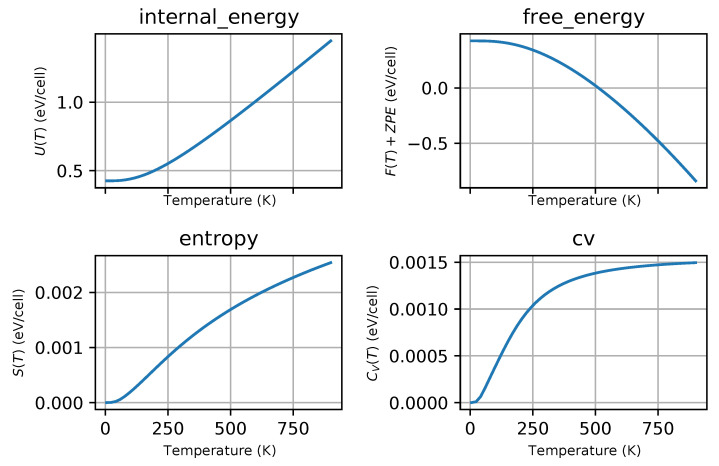
Phonon dispersion curves along the high symmetry q-points [50] and phonon densities of states (DOS) calculated with the use of PBEsol exchange-correlation (XC) functional for known hP3-P6/mmm phase, Figure 1a), are depicted in Figure 2, for the phase hP6-P6/mmc, Figure 1b), in Figure 3 and for the phase oP6-Pmmn, Figure 1c), in Figure 4, respectively. Phonon results for all exchange-correlation (XC) functionals are stored in Supplementary Materials. Analysis of the calculated curves allows us to state that phonon modes everywhere have positive frequencies and the new ZrB phases are not only mechanically but also dynamically stable. The estimated acoustic Debye temperature for the two new proposed ZrB phases is about 760 K and is about 200 K lower than that for the known hP3-P6/mmm phase and it is consistent with the mechanical properties, see Table 1. Results for thermodynamic properties up to 760 K for the three zirconium diboride polymorphs calculated with the use of PBEsol exchange-correlation (XC) functional, that is, phonon internal energy, free energy, entropy, constant-volume heat capacity are depicted in Figure 5, Figure 6 and Figure 7 and it can be seen that are very similar for the two new proposed ZrB polymorphs. This additional fact suggests again that the new phases are comparably thermodynamically stable up to Debye temperature , but less stable than the known hP3-P6/mmm phase.
Figure 2.
ZrB(hP3-P6/mmm-space group, no. 191)-phonon band structure and densities of states (DOS).
Figure 3.
ZrB(hP6-P6/mmc-space group, no. 194)-phonon band structure and DOS.
Figure 4.
ZrB(oP6-P6/mmm-space group, no.59)-phonon band structure and DOS.
Figure 5.
ZrB(hP3-P6/mmm-space group, no. 191)-thermodynamic properties: internal energy, free energy, entropy and constant-volume specific heat.
Figure 6.
ZrB(hP6-P6/mmc-space group, no.194)-thermodynamic properties: internal energy, free energy, entropy and constant-volume specific heat.
Figure 7.
ZrB(oP6-P6/mmm-space group, no.59)-thermodynamic properties: internal energy, free energy, entropy and constant-volume specific heat.
4. Conclusions
In the present paper, extensive analysis of two new hypothetical and one previously known zirconium diboride (ZrB) polymorphs within the framework of density functional theory from the structural, mechanical and thermodynamic properties point of view was performed. We can conclude that:
two new hypothetical zirconium diboride (ZrB) polymorphs: (hP6-P6/mmc-space group, no. 194) and (oP6-Pmmn-space group, no. 59) are mechanically and dynamically stable;
these phases are comparably thermodynamically stable but less stable than the known hP3-P6/mmm phase;
hP6-P6/mmc phase is ductile and oP6-Pmmn phase is intermediate between brittle and ductile;
both new phases have a lower hardness than the known hP3-P6/mmm phase.
We hope that results relating to new hypothetical zirconium diboride (ZrB) polymorphs will be confirmed by other calculations, as well as through experiments. Knowledge about these new phases can be very useful when doping metal borides with zirconium.
Acknowledgments
Additional assistance was granted through the computing cluster GRAFEN at Biocentrum Ochota, the Interdisciplinary Centre for Mathematical and Computational Modelling of Warsaw University (ICM UW) and Poznań Supercomputing and Networking Center (PSNC).
Abbreviations
The following abbreviations are used in this manuscript:
| UHTCs | ultra high-temperature ceramics |
| DFT | density functional theory |
| DFPT | density functional perturbation theory |
| PAW | projector augmented-wave |
| PP-PW | pseudopotential, plane-wave |
| XC | exchange-correlation |
| LDA | local density approximation |
| GGA | generalized gradient approximation |
| PBE | Perdew-Burke-Ernzerhof |
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/13/3022/s1.
Author Contributions
M.M. contributed to the conceptualization, investigation, methodology, analysis, interpretation of results, visualization, and the writing—original draft preparation. M.T. contributed to the conceptualization, investigation, writing—review and editing, funding acquisition, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre (NCN—Poland), research project: UMO-2017/25/B/ST8/01789.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Akopov G., Yeung M.T., Kaner R.B. Rediscovering the Crystal Chemistry of Borides. Adv. Mater. 2017;29:1604506. doi: 10.1002/adma.201604506. [DOI] [PubMed] [Google Scholar]
- 2.Akopov G., Pangilinan L.E., Mohammadi R., Kaner R.B. Perspective: Superhard metal borides: A look forward. APL Mater. 2018;6:070901. doi: 10.1063/1.5040763. [DOI] [Google Scholar]
- 3.Fuger C., Moraes V., Hahn R., Bolvardi H., Polcik P., Riedl H., Mayrhofer P.H. Influence of Tantalum on phase stability and mechanical properties of WB2. MRS Commun. 2019;9:375–380. doi: 10.1557/mrc.2019.5. [DOI] [Google Scholar]
- 4.Mirzaei S., Alishahi M., Souček P., Ženíšek J., Holec D., Koutná N., Buršíková V., Stupavská M., Zábranský L., Burmeister F., et al. The effect of chemical composition on the structure, chemistry and mechanical properties of magnetron sputtered W-B-C coatings: Modeling and experiments. Surf. Coat. Technol. 2020;383:125274. doi: 10.1016/j.surfcoat.2019.125274. [DOI] [Google Scholar]
- 5.Moscicki T., Psiuk R., Słomińska H., Levintant-Zayonts N., Garbiec D., Pisarek M., Bazarnik P., Nosewicz S., Chrzanowska-Giżyńska J. Influence of overstoichiometric boron and titanium addition on the properties of RF magnetron sputtered tungsten borides. Surf. Coat. Technol. 2020;390:125689. doi: 10.1016/j.surfcoat.2020.125689. [DOI] [Google Scholar]
- 6.Fahrenholtz W.G., Hilmas G.E., Talmy I.G., Zaykoski J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007;90:1347–1364. doi: 10.1111/j.1551-2916.2007.01583.x. [DOI] [Google Scholar]
- 7.Opeka M.M., Talmy I.G., Zaykoski J.A. Oxidation-based materials selection for 2000 °C + hypersonic aerosurfaces: Theoretical considerations and historical experience. J. Mater. Sci. 2004;39:5887–5904. doi: 10.1023/B:JMSC.0000041686.21788.77. [DOI] [Google Scholar]
- 8.Chen H., Zheng F., Liu H., Liu L., Jin Z. Thermodynamic assessment of B–Zr and Si–Zr binary systems. J. Alloy Compd. 2009;468:209–216. doi: 10.1016/j.jallcom.2008.01.061. [DOI] [Google Scholar]
- 9.Tokunaga T., Terashima K., Ohtani H., Hasebe M. Thermodynamic Analysis of the Phase Equilibria in the Fe-Zr-B System. Mater. Trans. 2008;49:2534–2540. doi: 10.2320/matertrans.MB200809. [DOI] [Google Scholar]
- 10.Li H., Zhang L., Zeng Q., Wang J., Cheng L., Ren H., Guan K. Crystal structure and elastic properties of ZrB compared with ZrB2: A first-principles study. Comput. Mater. Sci. 2010;49:814–819. doi: 10.1016/j.commatsci.2010.06.027. [DOI] [Google Scholar]
- 11.Huang B., Duan Y.H., Hu W.C., Sun Y., Chen S. Structural, anisotropic elastic and thermal properties of MB (M = Ti, Zr and Hf) monoborides. Ceram. Int. 2015;41:6831–6843. doi: 10.1016/j.ceramint.2015.01.132. [DOI] [Google Scholar]
- 12.Xu X., Fu K., Li L., Lu Z., Zhang X., Fan Y., Lin J., Liu G., Luo H., Tang C. Dependence of the elastic properties of the early-transition-metal monoborides on their electronic structures: A density functional theory study. Phys. B Condens. Matter. 2013;419:105–111. doi: 10.1016/j.physb.2013.03.018. [DOI] [Google Scholar]
- 13.Li X., Peng F. Predicted superhard phases of Zr–B compounds under pressure. Phys. Chem. Chem. Phys. 2019;21:15609–15614. doi: 10.1039/C9CP01775E. [DOI] [PubMed] [Google Scholar]
- 14.Jäger B., Paluch S., Żogał O.J., Wolf W., Herzig P., Filippov V.B., Shitsevalova N., Paderno Y. Characterization of the electronic properties of YB12, ZrB12, and LuB12 using 11B NMR and first-principles calculations. J. Phys. Condens. Matter. 2006;18:2525–2535. doi: 10.1088/0953-8984/18/8/015. [DOI] [Google Scholar]
- 15.Pan Y., Lin Y. Influence of alloying elements on the mechanical and thermodynamic properties of ZrB12 ceramics from first-principles calculations. Int. J. Quantum Chem. 2020;120:e26217. doi: 10.1002/qua.26217. [DOI] [Google Scholar]
- 16.Li J., Fan C. Novel metastable compounds in the Zr–B system: An ab initio evolutionary study. Phys. Chem. Chem. Phys. 2015;17:1180–1188. doi: 10.1039/C4CP04185B. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z.Q., Peng Y.S., Hu M., Li C.M., Luo Y.T. Elasticity, hardness, and thermal properties of ZrBn (n = 1, 2, 12) Ceram. Int. 2016;42:6624–6631. doi: 10.1016/j.ceramint.2015.12.175. [DOI] [Google Scholar]
- 18.Zhang X., Luo X., Han J., Li J., Han W. Electronic structure, elasticity and hardness of diborides of zirconium and hafnium: First principles calculations. Comput. Mater. Sci. 2008;44:411–421. doi: 10.1016/j.commatsci.2008.04.002. [DOI] [Google Scholar]
- 19.Chamberlain A., Fahrenholtz W., Hilmas G., Ellerby D. High-Strength Zirconium Diboride-Based Ceramics. J. Am. Ceram. Soc. 2008;87:1170–1172. doi: 10.1111/j.1551-2916.2004.01170.x. [DOI] [Google Scholar]
- 20.Maździarz M., Mościcki T. Structural, mechanical and optical properties of potentially superhard WBx polymorphs from first principles calculations. Mater. Chem. Phys. 2016;179:92–102. doi: 10.1016/j.matchemphys.2016.05.014. [DOI] [Google Scholar]
- 21.Cheng X.Y., Chen X.Q., Li D.Z., Li Y.Y. Computational materials discovery: The case of the W–B system. Acta Crystallogr. Sect. C. 2014;70:85–103. doi: 10.1107/S2053229613027551. [DOI] [PubMed] [Google Scholar]
- 22.Marín-Suárez M., Velez M., David J., Arroyave M. Mechanical properties study for new hypothetical crystalline phases of ReB2: A computational approach using density functional theory. Comput. Mater. Sci. 2016;122:240–248. doi: 10.1016/j.commatsci.2016.05.032. [DOI] [Google Scholar]
- 23.Maździarz M., Mościcki T. Structural, Mechanical, Optical, Thermodynamical and Phonon Properties of stable ReB2 polymorphs from Density Functional calculations. J. Alloy Compd. 2016;657:878–888. doi: 10.1016/j.jallcom.2015.10.133. [DOI] [Google Scholar]
- 24.Togo A., Tanaka I. First principles phonon calculations in materials science. Scr. Mater. 2015;108:1–5. doi: 10.1016/j.scriptamat.2015.07.021. [DOI] [Google Scholar]
- 25.Zhang G., Bai T., Zhao Y., Hu Y. A New Superhard Phase and Physical Properties of ZrB3 from First-Principles Calculations. Materials. 2016;9:703. doi: 10.3390/ma9080703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Qin J., Sun X., Xue Y., Ma M., Liu R. First-principles structural design of superhard material of ZrB4. Phys. Chem. Chem. Phys. 2013;15:20894–20899. doi: 10.1039/c3cp53893a. [DOI] [PubMed] [Google Scholar]
- 27.Hohenberg P., Kohn W. Inhomogeneous electron gas. Phys. Rev. 1964;136:B864–B871. doi: 10.1103/PhysRev.136.B864. [DOI] [Google Scholar]
- 28.Kohn W., Sham L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965;140:A1133–A1138. doi: 10.1103/PhysRev.140.A1133. [DOI] [Google Scholar]
- 29.Gonze X., Jollet F., Araujo F.A., Adams D., Amadon B., Applencourt T., Audouze C., Beuken J.M., Bieder J., Bokhanchuk A., et al. Recent developments in the ABINIT software package. Comput. Phys. Commun. 2016;205:106–131. doi: 10.1016/j.cpc.2016.04.003. [DOI] [Google Scholar]
- 30.Gonze X., Amadon B., Antonius G., Arnardi F., Baguet L., Beuken J.M., Bieder J., Bottin F., Bouchet J., Bousquet E., et al. The ABINIT project: Impact, environment and recent developments. Comput. Phys. Commun. 2020;248:107042. doi: 10.1016/j.cpc.2019.107042. [DOI] [Google Scholar]
- 31.Martin A., Torrent M., Caracas R. Projector augmented-wave formulation of response to strain and electric-field perturbation within density functional perturbation theory. Phys. Rev. B. 2019;99:094112. doi: 10.1103/PhysRevB.99.094112. [DOI] [Google Scholar]
- 32.Bloch F. Bemerkung zur Elektronentheorie des Ferromagnetismus und der elektrischen Leitfähigkeit. Z. Für Phys. 1929;57:545–555. doi: 10.1007/BF01340281. [DOI] [Google Scholar]
- 33.Perdew J.P., Wang Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B. 1992;45:13244–13249. doi: 10.1103/PhysRevB.45.13244. [DOI] [PubMed] [Google Scholar]
- 34.Perdew J.P., Burke K., Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 35.Perdew J.P., Ruzsinszky A., Csonka G.I., Vydrov O.A., Scuseria G.E., Constantin L.A., Zhou X., Burke K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008;100:136406. doi: 10.1103/PhysRevLett.100.136406. [DOI] [PubMed] [Google Scholar]
- 36.Råsander M., Moram M.A. On the accuracy of commonly used density functional approximations in determining the elastic constants of insulators and semiconductors. J. Chem. Phys. 2015;143 doi: 10.1063/1.4932334. [DOI] [PubMed] [Google Scholar]
- 37.Maździarz M., Mrozek A., Kuś W., Burczyński T. First-principles study of new X-graphene and Y-graphene polymorphs generated by the two stage strategy. Mater. Chem. Phys. 2017;202:7–14. doi: 10.1016/j.matchemphys.2017.08.066. [DOI] [Google Scholar]
- 38.Maździarz M., Mrozek A., Kuś W., Burczyński T. Anisotropic-Cyclicgraphene: A New Two-Dimensional Semiconducting Carbon Allotrope. Materials. 2018;11:432. doi: 10.3390/ma11030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jollet F., Torrent M., Holzwarth N. Generation of Projector Augmented-Wave atomic data: A 71 element validated table in the XML format. Comput. Phys. Commun. 2014;185:1246–1254. doi: 10.1016/j.cpc.2013.12.023. [DOI] [Google Scholar]
- 40.Holzwarth N., Tackett A., Matthews G. A Projector Augmented Wave (PAW) code for electronic structure calculations, Part I: Atompaw for generating atom-centered functions. Comput. Phys. Commun. 2001;135:329–347. doi: 10.1016/S0010-4655(00)00244-7. [DOI] [Google Scholar]
- 41.Lehtola S., Steigemann C., Oliveira M.J., Marques M.A. Recent developments in LIBXC—A comprehensive library of functionals for density functional theory. SoftwareX. 2018;7:1–5. doi: 10.1016/j.softx.2017.11.002. [DOI] [Google Scholar]
- 42.Qi C., Jiang Y., Liu Y., Zhou R. Elastic and electronic properties of XB2 (X = V, Nb, Ta, Cr, Mo, and W) with AlB2 structure from first principles calculations. Ceram. Int. 2014;40:5843–5851. doi: 10.1016/j.ceramint.2013.11.026. [DOI] [Google Scholar]
- 43.Hamann D.R., Wu X., Rabe K.M., Vanderbilt D. Metric tensor formulation of strain in density-functional perturbation theory. Phys. Rev. B. 2005;71:035117. doi: 10.1103/PhysRevB.71.035117. [DOI] [Google Scholar]
- 44.Hill R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Society. Sect. A. 1952;65:349–354. doi: 10.1088/0370-1298/65/5/307. [DOI] [Google Scholar]
- 45.Maździarz M., Gajewski M. Estimation of Isotropic Hyperelasticity Constitutive Models to Approximate the Atomistic Simulation Data for Aluminium and Tungsten Monocrystals. Comput. Model. Eng. Sci. 2015;105:123–150. doi: 10.3970/cmes.2015.105.123. [DOI] [Google Scholar]
- 46.Grimvall G., Magyari-Köpe B., Ozoliņš V., Persson K.A. Lattice instabilities in metallic elements. Rev. Mod. Phys. 2012;84:945–986. doi: 10.1103/RevModPhys.84.945. [DOI] [Google Scholar]
- 47.Maździarz M. Comment on ‘The Computational 2D Materials Database: High-throughput modeling and discovery of atomically thin crystals’. 2D Mater. 2019;6:048001. doi: 10.1088/2053-1583/ab2ef3. [DOI] [Google Scholar]
- 48.Tian Y., Xu B., Zhao Z. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 2012;33:93–106. doi: 10.1016/j.ijrmhm.2012.02.021. [DOI] [Google Scholar]
- 49.Pugh S. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. J. Sci. 1954;45:823–843. doi: 10.1080/14786440808520496. [DOI] [Google Scholar]
- 50.Hinuma Y., Pizzi G., Kumagai Y., Oba F., Tanaka I. Band structure diagram paths based on crystallography. Comput. Mater. Sci. 2017;128:140–184. doi: 10.1016/j.commatsci.2016.10.015. [DOI] [Google Scholar]
- 51.Řehák P., Černý M., Pokluda J. Dynamic stability of fcc crystals under isotropic loading from first principles. J. Phys. Condens. Matter. 2012;24:215403. doi: 10.1088/0953-8984/24/21/215403. [DOI] [PubMed] [Google Scholar]
- 52.Bottin F., Bieder J., Bouchet J. A-TDEP: Temperature Dependent Effective Potential for ABINIT—Lattice dynamic properties including anharmonicity. Comput. Phys. Commun. 2020:107301. doi: 10.1016/j.cpc.2020.107301. [DOI] [Google Scholar]
- 53.Rached H., Rached D., Benalia S., Reshak A., Rabah M., Khenata R., Omran S.B. First-principles study of structural stabilities, elastic and electronic properties of transition metal monocarbides (TMCs) and mononitrides (TMNs) Mater. Chem. Phys. 2013;143:93–108. doi: 10.1016/j.matchemphys.2013.08.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.