Abstract
The conclusions of the EFSA following the peer review of the initial risk assessments carried out by the competent authority of the rapporteur Member State, France, for the pesticide active substance prosulfuron are reported. The context of the peer review was that required by Regulation (EC) No 1107/2009 of the European Parliament and of the Council. The conclusions were reached on the basis of the evaluation of the representative uses of prosulfuron as a herbicide on maize and sweet corn. Following the renewal of approval, prosulfuron has been renewed with the restriction that the use shall be limited to one application every 3 years. This assessment follows the request of the applicant to lift the restriction of the approval conditions. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: prosulfuron, peer review, risk assessment, pesticide, herbicide
Summary
Regulation (EC) No 1107/2009 lays down, inter alia, the detailed rules as regards the procedure for the assessment of applications for amendment to the conditions of approval of active substances.
Prosulfuron was renewed on 1 May 2017 by Commission Implementing Regulation (EU) No 2017/375, in accordance with the approval criteria laid down in Regulation 1107/2009 following a peer review of the risk assessment as set out in the EFSA conclusion on prosulfuron, finalised on 18 August 2014. It was a specific provision of the renewal of approval that use should be limited to one application every 3 years on the same field at a maximum dose of 20 g active substance per hectare may be authorised. In accordance with Article 7 of the Regulation, the rapporteur Member State (RMS), France, received an application from Syngenta Crop Protection AG on 12 October 2016 for amendment to the conditions of approval of the active substance prosulfuron to lift the restriction and to allow uses as herbicide on maize and sweet corn to be authorised.
The assessment of the amendment dossier was carried out against the background that any changes to data requirements will be addressed in the context of any further renewal of approval and that Article 56 of Regulation (EC) No 1107/2009 requires that holders of authorisations for plant protection products, at any moment, notify Member States immediately if there are indications that the approval criteria are no longer satisfied.
An initial evaluation of the dossier on prosulfuron was provided by the RMS in a revised renewal assessment report (RAR) (Volume 1, Volume 2, Volume 3 B6, Volume 3 B8 and list of end points), and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 12 of Regulation (EC) No 1107/2009. The following conclusions are derived.
The uses of prosulfuron according to the representative uses as herbicide on maize and sweet corn, as proposed at EU level, result in sufficient herbicidal efficacy against the target weeds.
The data gaps identified on the physical–chemical properties in the renewal conclusion have been addressed.
Based on the available assessments under this application, genotoxic potential cannot be concluded for the metabolite triazine amine (CGA150829) and CGA325025. They can therefore be considered relevant groundwater metabolites according to European Commission, 2003.1 Metabolites CGA159902, CGA349707 and SYN547308 are unlikely to be genotoxic. Pending further assessment in the residue section, the toxicological profile of CGA159902 and SYN547308 and groundwater relevance assessment might need to be further considered (issue that could not be finalised). CGA349707 is considered a non‐relevant groundwater metabolite according to European Commission, 2003 for the representative uses assessed.
The groundwater relevance assessment for prosulfuron metabolites triazine amine (CGA150829), CGA159902, CGA325025 and SYN547308 could not be finalised whilst residue levels in plants for these metabolites needed further consideration to characterise the total consumer intake from food and water (data gap). The RMS made the assessment of the occurrence of metabolite residues in food commodities and of their dietary exposure potentials conditional on the finalisation of the toxicology assessment for all groundwater metabolites. The consumer risk assessment was only updated for metabolite CGA349707.
The data available on environmental fate and behaviour are sufficient to carry out the required groundwater exposure assessments at the EU level for the representative uses, with the notable exception that the groundwater exposure assessment for soil metabolite M17 (unidentified) was not available leading to an assessment not finalised. Furthermore, it was identified that the consumer risk assessment could not be finalised whilst satisfactory information to address the effect of water treatment processes on the nature of residues in surface water and groundwater, that might be abstracted to use for the production of drinking water was not available. At a dose rate of 20 g/ha, prosulfuron was predicted to be present in annual average recharge concentrations leaving the top 1 m soil layers above the parametric drinking water limit that is applied for groundwater of 0.1 μg/L, in geoclimatic regions represented by the Hamburg and Okehampton FOCUS groundwater scenarios. At a dose rate of 15 g/ha, these concentrations were below this regulatory trigger at all eight pertinent FOCUS groundwater scenarios. The soil metabolite triazine amine (CGA150829) was indicated to exceed this limit in six out of eight FOCUS scenarios. As with the available plant residues assessment and toxicological data, it was not possible to conclude that this was not a relevant groundwater metabolite, this led to the identification of an assessment not finalised. For CGA325025 which is concluded as relevant due to insufficient data to conclude that it is unlikely to be genotoxic, it was indicated to exceed this limit in one out of eight FOCUS scenarios. As discussed above, the groundwater non‐relevance assessment for CGA159902 and SYN547308 could not be finalised whilst residue levels in plants for these metabolites needed further consideration to characterise the total consumer intake from food and water. This led to the identification of an assessment not finalised for seven out of eight and five out of eight FOCUS groundwater scenarios, respectively, for all the representative uses, as this represented the situations where these metabolites were predicted to be above the parametric drinking water limit.
In the area of ecotoxicology, no new data or assessment have been provided in the context of the amendment to the conditions of approval of prosulfuron. Therefore, the data gaps and issues identified in the conclusion on renewal of approval of the active substance remained unchanged.
Background
Regulation (EC) No 1107/2009 of the European Parliament and of the Council2 (hereinafter referred to as ‘the Regulation’) lays down, inter alia, the detailed rules as regards the procedure for the assessment for an amendment to the conditions of an approval of active substances. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States and the applicant(s) for comments on the initial evaluation in the renewal assessment report (RAR), provided by the rapporteur Member State (RMS), and the organisation of an expert consultation, where appropriate.
In accordance with Article 12 of the Regulation, EFSA is required to adopt a conclusion on whether an active substance can be expected to meet the approval criteria provided for in Article 4 of the Regulation (also taking into consideration recital (10) of the Regulation) within 120 days from the end of the period provided for the submission of written comments, subject to an extension of 30 days where an expert consultation is necessary, and a further extension of up to 150 days where additional information is required to be submitted by the applicant(s) in accordance with Article 12(3).
Prosulfuron was renewed on 1 May 2017 by Commission Implementing Regulation (EU) No 2017/3753, in accordance with the approval criteria laid down in Regulation 1107/2009 following a peer review of the risk assessment as set out in the EFSA conclusion on prosulfuron, finalised on 18 August 2014 (EFSA, 2014b). It was a specific provision of the renewal of approval that use should be limited to one application every 3 years on the same field at a maximum dose of 20 g active substance per hectare may be authorised. In accordance with Article 7 of the Regulation, the rapporteur Member State (RMS), France, received an application from Syngenta Crop Protection AG on 12 October 2016 for amendment to the conditions of approval of the active substance prosulfuron to lift the restriction and to allow uses as herbicide on maize and sweet corn to be authorised.
The assessment of the amendment dossier was carried out against the background that any changes to data requirements will be addressed in the context of any further renewal of approval and that Article 56 of Regulation (EC) No 1107/2009 requires that holders of authorisations for plant protection products, at any moment, notify Member States immediately if there are indications that the approval criteria are no longer satisfied.
The RMS provided its initial evaluation of the dossier on prosulfuron in the form of a revised RAR (Volume 1, Volume 2, Volume 3 B6, Volume 3 B8 and list of end points), which was received by EFSA on 5 April 2018 (France, 2018). The peer review was initiated on 5 June 2018 by dispatching the revised RAR to Member States and the applicant, Syngenta Crop Protection AG, for consultation and comments. EFSA also provided comments. In addition, EFSA conducted a public consultation on the revised parts of the RAR. The comments received were collated by EFSA and forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 12(3) of the Regulation were considered in a telephone conference between EFSA, the RMS on 28 September 2018. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant and that EFSA should conduct an expert consultation in the area of mammalian toxicology.
The outcome of the telephone conference together with EFSA's further consideration of the comments is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation where this took place, were reported in the final column of the evaluation table.
In accordance with Article 12 of the Regulation, EFSA should adopt a conclusion on whether prosulfuron can be expected to meet the approval criteria provided for in Article 4 of the Regulation, taking into consideration recital (10) of the Regulation. A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in May 2019.
This conclusion report summarises the outcome of the peer review of the risk assessment on the active substance and the representative formulation evaluated on the basis of the representative use of prosulfuron as a herbicide on maize and sweet corn as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review are presented in the conclusion. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2020), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views where applicable, can be found:
the comments received on the revised RAR;
the reporting table (28 September 2018);
the evaluation table (5 June 2020);
the report of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the revised RAR including its further revisions (France, 2019) and the peer review report, both documents are considered as background documents to this conclusion.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Prosulfuron is the ISO common name for N‐[(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)carbamoyl]‐2‐(3,3,3‐trifluoropropyl)benzenesulfonamide or 1‐(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)‐3‐[2‐(3,3,3‐trifluoropropyl)phenylsulfonyl]urea (IUPAC).
The representative formulated product for the evaluation was ‘A8714C (PEAK 75 WG)’, a water dispersible granule (WG) containing 750 g/kg prosulfuron.
The representative uses evaluated comprise applications by foliar spraying to control broadleaved weeds in maize and sweet corn. Full details of the Good Agricultural Practices (GAPs) can be found in the list of end points in Appendix A.
A data gap has been identified for a search of the scientific peer‐reviewed open literature on the groundwater metabolites, dealing with side effects on human health and published within the 10 years before the date of submission of the dossier, to be conducted and reported in EFSA, 2011.
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance document was followed in the production of this conclusion: SANCO/825/00 rev. 8.1 (European Commission, 2010).
In the section on identity, physical/chemical/technical properties and methods of analysis, a data gap was identified for the determination of the boiling point and the decomposition temperature of the active substance in the renewal conclusion (EFSA, 2014b).This data gap was addressed in this conclusion.
The main data regarding the identity of prosulfuron and its physical and chemical properties are given in Appendix A.
An appropriate LC‐MS/MS method exists for monitoring prosulfuron in surface water and drinking water with a limit of quantification (LOQ) of 0.05 μg/L.
2. Mammalian toxicity
Following the assessment of the proposed amendment of the approval conditions, the toxicological assessment was triggered for several metabolites exceeding 0.1 μg/L in groundwater, in order to conclude on their relevance according to European Commission, 2003.
Metabolite CGA159902 is unlikely to be genotoxic and, although not exceeding 0.75 μg/L, a consumer risk assessment is triggered since it is also a plant metabolite (see Section 3).
Based on the available assessment under this application4 and in the framework of confirmatory data request to address the triazine amine genotoxic potential for several sulfonylureas active substances, the genotoxic potential cannot be concluded for triazine amine (CGA150829) (EFSA, 2018). It could therefore be considered a relevant groundwater metabolite according to European Commission (2003). It is also noted that, being also a plant metabolite, a consumer risk assessment is triggered5 (see Section 3).
The genotoxic potential of metabolite CGA325025 was discussed during the Pesticides Peer Review Meeting 01 – session 1 (April 2019). All experts agreed that CGA325025 did not induce gene mutation; however, the chromosome aberration test showed equivocal results and an in vitro micronucleus assay is missing to allow conclusion on the genotoxic potential (clastogenicity and aneugenicity potential) of the metabolite leading to a data gap. Based on the currently available assessment, CGA325025 is considered a relevant groundwater according to European Commission (2003). It is also noted that, although not exceeding 0.75 μg/L, a consumer risk assessment might be triggered if it is finally identified as being present as a plant residue (see Section 3).
CGA349707 is unlikely to be genotoxic and an acceptable daily intake (ADI) of 0.001 mg/kg body weight (bw) per day was already set during the renewal peer review (EFSA, 2014b).
SYN547308 is unlikely to be genotoxic and, although not exceeding 0.75 μg/L, a consumer risk assessment might be triggered if it is finally identified being present as a plant residue (see Section 3).
Although an assessment of the groundwater relevance is not triggered for metabolites CGA300406 and SYN542604 (see Section 4), new genotoxicity studies have been submitted by the applicant in the context of the application for amendment to the conditions of approval of prosulfuron. On the basis of these studies, CGA300406 and SYN542604 are considered unlikely to be genotoxic.
3. Residues
Following the assessment of the proposed amendment of the approval conditions, for several metabolites exceeding 0.1 μg/L in groundwater, consumer exposure considerations were triggered to conclude on their relevance according to European Commission (2003). The RMS was requested to assess the potential occurrence of these metabolites in other sources such as primary and rotational crops and the resulting total consumer exposure from food and groundwater when the current restrictions to the approval would be lifted. The RMS made the assessment of the occurrence of metabolites in food commodities and of their dietary exposure potential conditional on the finalisation of the toxicology assessment for groundwater metabolites. The inconclusive hazard assessment for CGA325025 and for triazine amine (CGA150829) is noted in this context (see Section 2). Specifically, for triazine amine (CGA150829) that is a major plant metabolite and moreover a common residue of other active substances in food, a data gap and an issue that could not be finalised was identified with regard to consumer exposure. An assessment to characterise the total consumer intake from food and water was also not provided for the metabolite CGA159902 that occurs in plant matrices (France, 2013) and groundwater while reliable information is currently not available whether or not metabolites CGA325025 and SYN547308 could as well contribute to consumer exposure via food, leading to a data gap and an issue that could not be finalised.
The previously conducted consumer risk assessment for CGA349707 was updated in line with the amended groundwater exposure assessment (see Section 4). The consumer exposure with regard to residues of metabolite CGA349707 occurring at levels up to 1.07 μg/L in groundwater that could be used as drinking water was assessed. The estimates are based on the default assumptions laid down in the WHO Guidelines for drinking water quality (WHO, 2011) for the consumer groups of adults (weighing 60 kg), toddlers (10 kg) and bottle‐fed infants (5 kg) with a daily per capita consumption of 2 L, 1 L and 0.75 L, respectively. The potential intake through drinking water of CGA349707 corresponds to 3.6%, 10.7% and 16.1%, respectively, of the ADI of 0.001 mg/kg bw per day for CGA349707 for the above‐mentioned consumer groups.
Due to missing data to address the effect of water treatments processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water, the consumer risk assessment could not be finalised in this regard (see also Section 4).
4. Environmental fate and behaviour
Kinetic end points (DT values) for the soil metabolite triazine amine (CGA150829) were discussed at the Pesticides Peer Review Meeting TC 139 in March 2017, in the context of the active substance tribenuron‐methyl (EFSA, 2017).
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, prosulfuron exhibited moderate to high persistence forming six major (> 10% applied radioactivity (AR)) metabolites: prosulfuron phenyl sulfonamide (CGA159902; (max. 47.4% AR, exhibiting moderate to very high persistence), triazine amine (CGA150829, max. 40.6% AR, exhibiting moderate to very high persistence), O‐desmethyl‐prosulfuron (CGA300406, max. 24.0% AR, exhibiting low to moderate persistence), demethoxy amino‐prosulfosulfuron (CGA 325025, max. 17.4% AR, exhibiting moderate to high persistence), CGA349707 (max. 22.6% AR, exhibiting medium to very high persistence) and SYN542604 (M5, max. 30.8% AR, exhibiting moderate to high persistence). Two additional metabolites, SYN547308 (M18, max 9.9% AR, exhibiting moderate to high persistence) and unidentified M17 (max 6.1% AR), triggered the need for a groundwater exposure assessment. Mineralisation (as CO2) ranged from negligible (phenyl labelled) to 21.5% AR (triazine labelled). Non‐extractable residues accounted for up to 34.5% AR (triazine labelled) and 44% AR (phenyl labelled) after 90 days. For metabolite O‐desmethyl‐prosulfuron (CGA300406) degradation showed pH dependence with longer DT50 being observed in soils with alkaline pH.
The degradation of prosulfuron was investigated in one soil under anaerobic conditions. In this experiment, prosulfuron exhibited medium to high persistence in soil and the metabolites identified were common to the aerobic route of degradation.
Field dissipation of prosulfuron (soil samples only analysed for prosulfuron) was investigated at 15 European sites where reliable kinetic end points representing dissipation end points could be estimated following FOCUS (2006) kinetics guidance. The dissipation kinetics indicate that prosulfuron exhibited low to moderate persistence at these sites. At six field trial study sites the study design followed that described to determine DegT50matrix in EFSA (2014a) guidance. Kinetic end points at FOCUS reference conditions (20°C and pF 2 field capacity soil moisture) were appropriately determined at these six trial sites where prosulfuron exhibited moderate persistence at these FOCUS reference conditions. These FOCUS reference condition end points for prosulfuron were used in FOCUS groundwater exposure calculations. According to the laboratory soil DT values determined for metabolites CGA 159902, triazine amine (CGA150829), CGA325025, CGA349707, SYN542604 and SYN547308, these metabolites needed to have been investigated in field dissipation studies. Trials where metabolites had been analysed were only available from two sites (in Georgia and Iowa, USA) and then only CGA 159902, triazine amine (CGA150829) and CGA300406 (for which investigation was not triggered based on its laboratory DT values) had been investigated. Therefore, a data gap for field investigations for the metabolites triggering assessment was identified. The assessment for the representative uses applied for at European level has been completed using the available laboratory DT values for these soil metabolites.
Batch adsorption/desorption studies in soil were available for prosulfuron and all the identified soil metabolites discussed above. From the results of these experiments, it may be considered that prosulfuron and the metabolites demethoxy amino‐prosulfuron (CGA325025) and CGA349707 exhibited very high mobility in soil, metabolites triazine amine (CGA150829) and SYN542604 exhibited medium to very high mobility and metabolites O‐desmethyl‐prosulfuron (CGA300406) and prosulfuron phenyl sulfonamide (CGA159902) exhibited high to very high mobility in soil. pH dependency of adsorption was not observed for prosulfuron or any of these metabolites. SYN547308 (M18) exhibited medium to high mobility where adsorption was higher at a low soil pH. Three lysimeter studies, one in Switzerland and two in the USA, are available for prosulfuron. In the Swiss lysimeter study in sandy soil, the yearly average concentrations of total radioactivity in leachate during the 3‐year study period were 0.07–0.23 μg/L when applied only once and 0.22–0.31 μg/L when applied on two consecutive years. However, the concentrations of prosulfuron, CGA159902, CGA300406, CGA349707, SYN542604 and CGA325025 were each < 0.1 μg/L. In the USA lysimeter studies in silty loam and sandy soils, no annual average concentrations could be calculated for the individual compounds due to the approach taken. Prosulfuron was seen at trace amounts at a soil depth of 0.9 m, probably due to preferential flow at an early stage of the study. The maximum metabolite concentrations in individual samples (not annual averages) were: CGA159902 at 2.4 μg/L, CGA300406 at 0.08 μg/L, CGA325028 at 0.74 μg/L and M5 (derivative of CGA159902) at 1 μg/L.
According to the available study, prosulfuron hydrolyses under sterile conditions in aqueous buffer at acidic pH (5) and it is practically stable at neutral and alkaline pHs. The major hydrolysis metabolites were prosulfuron phenyl sulfonamide (CGA159902), triazine amine (CGA150829), prosulfuron polyimide (CGA325030) and G28533.
Potential for groundwater exposure was estimated by calculation of the 80th percentile annual average concentrations moving below the top 1 m soil depth following European Commission (2014) guidance using the models PEARL 4.4.4, PELMO 5.5.3 and MACRO 5.5.4.6 Predicted environmental concentrations (PEC) in groundwater were calculated for prosulfuron and its identified soil metabolites as identified above, that triggered consideration for groundwater exposure. Separate sets of calculations were performed to take into account the observed pH dependence of degradation of the metabolite O‐desmethyl‐prosulfuron (CGA300406). In these simulations for annual applications of 20 g prosulfuron/ha, O‐desmethyl‐prosulfuron (CGA300406) and SYN542604 were below the parametric drinking water limit of 0.1 μg/l. Parent prosulfuron was indicated to exceed this limit for two of the eight relevant FOCUS scenarios for prosulfuron (max 0.127 μg/L). This was the case for all eight FOCUS scenarios (0.393–1.07 μg/L) for CGA349707, seven out of eight FOCUS scenarios (0.139–0.373) for prosulfuron phenyl sulfonamide (CGA159902), six out of eight FOCUS scenarios (0.151–0.228 μg/L) for triazine amine (CGA150829),7 five out of eight FOCUS scenarios (0.148–0.223 μg/L) for SYN547308 (M18) and one out of eight FOCUS scenarios (0.124 μg/L) for demethoxy amino‐prosulfuron (CGA325025). CGA349707 has been concluded as groundwater non‐relevant (see Sections 2 and 3). With the available toxicological information, currently, triazine amine (CGA150829) could be considered a relevant groundwater metabolite and demethoxy amino‐prosulfuron (CGA 325025) needs to be considered a relevant groundwater metabolite. The conclusion on non‐relevance regarding prosulfuron phenyl sulfonamide (CGA159902) which is also a residue in plants and SYN547308 (M18) which may be a residue in plants remain open (see Section 3). In these simulations for annual applications of 15 g prosulfuron/ha, parent prosulfuron and demethoxy amino‐prosulfuron (CGA 325025) were indicated to be below the parametric drinking water limit of 0.1 μg/L. Triazine amine (CGA150829) was indicated to exceed this limit in six out of eight FOCUS scenarios (0.112–0.166 μg/L). Prosulfuron phenyl sulfonamide (CGA159902) was indicated to exceed this limit in seven out of eight FOCUS scenarios (0.1–0.268 μg/L) whilst for SYN547308 (M18), this was the case for five out of eight FOCUS scenarios (0.108–0.162 μg/L). Whilst unidentified M17 triggers a groundwater exposure assessment, such an assessment is not available. This results in a data gap and results in the identification of an assessment not finalised that would be applicable to any use where there would be soil exposure of the active substance.
The applicant did not provide appropriate information to address the effect of water treatments processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water. This has led to the identification of a data gap and results in the consumer risk assessment not being finalised.
5. Ecotoxicology
Neither new data nor updated assessments have been provided in the context of the amendment of approval conditions for prosulfuron. Therefore, data gaps and issues identified in the ecotoxicological area in the renewal conclusion (EFSA, 2014b) are still applicable.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1 and 2)
Table 1.
Soil
| Compound (name and/or code) | Persistence |
|---|---|
| prosulfuron |
Moderate to high persistence, laboratory Single first order DT50 = 15.4–229 days (at 20°C and pF2) Low to moderate persistence, European field dissipation trials Single first order and biphasic kinetics DT50 = 3.8–20.5 days (DT90 15.2–150 days) |
| CGA159902 (prosulfuron phenyl sulfonamide) |
Moderate to very high persistence Single first order and biphasic kinetics DT50 = 3.1 – > 1,000 days (DT90 140 – > 1,000 days laboratory conditions at 20°C and pF2) |
| CGA150829 (triazine amine) |
Moderate to very high persistence Single first order and biphasic kinetics DT50 = 22.5 – > 1,000 days (DT90 97 – > 1,000 days laboratory conditions at 20°C and pF2) |
| CGA300406 (O‐desmethyl‐prosulfuron) |
Low to moderate persistence Single first order DT50 = 2.6–47.5 days (laboratory conditions at 20°C and pF2 or 40% MWHC or 60% FC) |
| CGA325025 (demethoxy amino‐prosulfuron) |
Moderate to high persistence Single first order DT50 = 47.4–102 days (laboratory conditions at 20°C and pF2) |
| CGA349707 |
Medium to very high persistence Single first order DT50 = 91.9–737 days (laboratory conditions at 20°C and pF2 or 40% MWHC or 60% FC) |
| SYN542604 (M5) |
Moderate to high persistence Single first order DT50 = 25.0–184 days (laboratory conditions at 20°C and pF2 or 40% MWHC or 60% FC) |
| SYN547308 (M18) |
Moderate to high persistence Biphasic kinetics DT50 = 7.8–174 days (DT90 120–654 days, laboratory conditions at 20°C and pF2) |
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| prosulfuron | Very high (KFoc = 4–37 mL/g) | Yes at 2 of 8 FOCUS scenarios Hamburg 0.125 μg/L Okehampton 0.127 μg/L | Yes | Yes |
| CGA159902 (prosulfuron phenyl sulfonamide) | High to very high (KFoc = 44–88 mL/g) | Yes at 7 of 8 FOCUS scenarios 0.139–0.373 μg/L only Sevilla < 0.1 μg/L | No | Open unlikely to be genotoxic, though is < 0.75 μg/L, as it is a plant metabolite, further information on combined food and water consumer intake is needed to conclude on non‐relevance |
| CGA150829 (triazine amine) | Medium to very high (KFoc = 3–225.5 mL/g) | Yes in at least 6 of 8 FOCUS scenarios 0.151–0.228 μg/L only Porto and Sevilla may be < 0.1 μg/L | No | Open in the absence of a conclusive assessment on the lack of genotoxicity and consumer risk assessmentb |
| CGA300406 (O‐desmethyl‐prosulfuron) | High to very high (KFoc = 42–126 mL/g) | No | No | Assessment not triggered unlikely to be genotoxic |
| CGA325025 (demethoxy amino‐prosulfuron) | Very high (KFoc = 21–32 mL/g) | Yes at 1 of 8 FOCUS scenarios Hamburg 0.124 μg/L | No | Yes in the absence of conclusive data on the lack of genotoxicity |
| CGA349707 | Very high (KFoc = 37–52 mL/g) | Yes at all 8 FOCUS scenarios 0.393–1.07 μg/L | No | No unlikely to be genotoxic an ADI of 0.001 mg/kg bw per day was set, consumer intake was up to 16.1% of this ADI |
| SYN542604 (M5) | Medium to very high (KFoc = 58–223 mL/g) | No | No | Assessment not triggered unlikely to be genotoxic |
| SYN547308 (M18) | Medium to high (KFoc = 65–288 mL/g) potential pH dependence | Yes at 5 of 8 FOCUS scenarios 0.14–0.223 μg/L Porto, Sevilla and Thiva < 0.1 μg/L | No | Open unlikely to be genotoxic, unclear if there might be residues in food, this would need to be clarified before non‐relevance could be concluded as a combined food and water consumer intake needs to be estimated |
| Unidentified (M17) | Data gap | Data gap | Open | Open |
At least one FOCUS scenario or relevant lysimeter.
It is acknowledged that the genotoxic potential of metabolite CGA150829 (triazine amine) has been assessed by the EFSA PPR Panel (EFSA PPR Panel, 2020). According to the EFSA PPR Panel, an in vitro Micronuclei test would be needed to conclude on its aneugenic potential. EFSA noted that considering the EFSA PPR Panel Opinion and given that aneugenicity is a threshold‐mechanism the setting of reference would be possible. However, further discussion is needed on the most appropriate basis for setting the reference values for triazine amine and finalise a consumer risk assessment and groundwater relevance assessment.
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of the Regulation concerning information on potentially harmful effects).
A search of the scientific peer‐reviewed open literature on the groundwater metabolites, dealing with side effects on human health and published within the 10 years before the date of submission of the dossier, to be conducted and reported in accordance with EFSA (2011); (relevant for all representative uses evaluated).
Further toxicological assessment on metabolite CGA159902 to allow performing the consumer risk assessment (relevant for all representative uses evaluated; see Sections 2 and 3).
Further toxicological assessment on metabolite CGA150829 (triazine amine) to allow consumer risk assessment, if genotoxicity potential is excluded8 (relevant for all representative uses evaluated; see Sections 2 and 3).
In vitro micronucleus test on metabolite CGA325025 and further toxicological assessment to allow consumer risk assessment, if genotoxicity is excluded and consumer risk assessment is finally triggered (relevant for all representative uses evaluated; see Sections 2 and 3).
Further toxicological assessment on metabolite SYN547308 to allow consumer risk assessment, if consumer risk assessment is finally triggered (relevant for all representative uses evaluated; see Sections 2 and 3).
Characterisation of the potential combined consumer intakes from food and water regarding metabolites CGA150829 (triazine amine), CGA325025, CGA159902, SYN547308 (relevant for all representative uses evaluated; see Section 3).
Investigations in field soil dissipation studies in at least four trial sites, were not available for metabolites CGA159902, CGA150829 (triazine amine), CGA325025, CGA349707, SYN542604 and SYN547308 (relevant for all representative uses evaluated, though the assessment at EU level was completed using the available soil laboratory incubation end points; see Section 4).
Satisfactory information to address the effect of water treatment processes on the nature of residues present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water was not available. Probably in the first instance, a consideration of the processes of ozonation and chlorination would appear appropriate. If an argumentation is made that concentrations at the point of abstraction for drinking water purposes will be low, this argumentation should cover metabolites predicted to be in groundwater and surface water, as well as the active substance. Should this consideration indicate that novel compounds might be expected to be formed from water treatment, the risk to human or animal health through the consumption of drinking water containing them should be addressed (relevant for all representative uses evaluated; see Section 4).
Information to address the potential for groundwater exposure by unidentified metabolite M17 was not available to finalise the groundwater exposure assessment. Should such information indicate exposure above 0.1 μg/L, consequent assessments for the relevance of this metabolite would be necessary (relevant for all representative uses evaluated; see Section 4).
Further information is required to address the risk to aquatic organisms from prosulfuron in situations which are represented by the D4 and D5 FOCUS surface water scenarios for all the representative uses (relevant for all representative uses evaluated; see Section 5 in EFSA (2014b)).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
Risk mitigation measures such as 20 m no‐spray buffer zones and 20 m vegetative filter strips to protect aquatic plants have been indicated as being necessary in situations representative of the following FOCUS surface water scenarios: D3, D6, R1, R2, R3 and R4 for all the representative uses of prosulfuron (see EFSA (2014b)).
Risk mitigation measures to protect non‐target terrestrial plants are required (e.g. such as that afforded by 20 m no‐spray buffer zone) for all the representative uses of prosulfuron (see EFSA (2014b)).
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of the Regulation and as set out in Commission Regulation (EU) No 546/20119 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of the Regulation.
The clastogenic and aneugenic potential of metabolite CGA325025 cannot be excluded. With the available information, CGA325025 is considered a relevant groundwater metabolite following the groundwater metabolite relevance guidance (this has pertinence for decision‐making for one out of eight FOCUS scenarios, for the representative use with a dose rate of 20 g a.s./ha).
The groundwater non‐relevance assessment for prosulfuron phenyl sulfonamide (CGA159902) and SYN547308 could not be finalised whilst residue levels in plants for these metabolites needed further consideration to characterise the total consumer intake from food and water (this was relevant for seven out of eight and five out of eight FOCUS scenarios, respectively, for all the representative uses).
The groundwater exposure assessment for metabolite M17 (unknown) could not be finalised (relevant for all the representative uses).
The consumer risk assessment could not be finalised whilst satisfactory information to address the effect of water treatment processes on the nature of residues in surface water and groundwater, that might be abstracted for use to produce drinking water was not available (relevant for all the representative uses).
The soil metabolite triazine amine (CGA150829) was indicated to exceed the parametric drinking water limit of 0.1 μg/L (that is applied to groundwater) in annual average recharge concentrations leaving the top 1 m soil layers, in geoclimatic conditions represented by at least six out of eight FOCUS scenarios (0.112–0.166 μg/L) for all the representative uses assessed. With the available assessment under this application, triazine amine could be considered a relevant groundwater metabolite following the groundwater metabolite relevance guidance (European Commission, 2003). However, it is acknowledged that the genotoxic potential of the triazine amine metabolite has been assessed by the EFSA PPR Panel (EFSA PPR Panel, 2020). According to the EFSA PPR Panel, an in vitro Micronuclei test would be needed to conclude on its aneugenic potential. EFSA noted that triazine amine is also a residue in plants, so a consumer risk assessment is triggered but not available. Given that aneugenicity is a threshold mechanism, the setting of reference values for triazine amine would be possible. However, further discussion would be needed on the most appropriate basis for setting the reference values for triazine amine and finalise the consumer risk assessment and relevance assessment for triazine amine as groundwater metabolite.
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of the Regulation and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of the Regulation.
None
9.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 3.)
Table 3.
Overview of concerns relevant for the amendment of the approval conditions assessment
| Representative use | Maize and sweet corn 20 g a.s./ha | Maize and sweet corn 15 g a.s./ha within BBCH 18 | Maize and sweet corn 15 g a.s./ha within BBCH 19 | |
|---|---|---|---|---|
| Consumer risk | Risk identified | |||
| Assessment not finalised | X4 | X4 | X4 | |
| Groundwater exposure to active substance | Legal parametric value breached | 2 of 8 FOCUS scenarios | ||
| Assessment not finalised | ||||
| Groundwater exposure to metabolites | Legal parametric value breached | 1 of 8 FOCUS scenarios1 | ||
| Parametric value of 10 μg/La breached | ||||
| Assessment not finalised | X2,3,5 | X2,3,5 | X2,3,5 | |
Abbreviations
- 1/n
slope of Freundlich isotherm
- λ
wavelength
- ε
decadic molar extinction coefficient
- a.s.
active substance
- ADE
actual dermal exposure
- ADI
acceptable daily intake
- AF
assessment factor
- AP
alkaline phosphatase
- AR
applied radioactivity
- AST
aspartate aminotransferase (SGOT)
- AV
avoidance factor
- BUN
blood urea nitrogen
- bw
body weight
- CAS
Chemical Abstracts Service
- CI
confidence interval
- CL
confidence limits
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EEC
European Economic Community
- FID
flame ionisation detector
- FIR
food intake rate
- FOB
functional observation battery
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GM
geometric mean
- GS
growth stage
- Hb
haemoglobin
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- iv
intravenous
- KFoc
Freundlich organic carbon adsorption coefficient
- LC
liquid chromatography
- LC‐MS
liquid chromatography–mass spectrometry
- LC‐MS‐MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- M/L
mixing and loading
- mm
millimetre (also used for mean measured concentrations)
- mN
milli‐Newton
- MS
mass spectrometry
- MWHC
maximum water‐holding capacity
- NOEL
no observed effect level
- OECD
Organisation for Economic Co‐operation and Development
- OM
organic matter content
- Pa
Pascal
- PD
proportion of different food types
- PEC
predicted environmental concentration
- PHI
preharvest interval
- PIE
potential inhalation exposure
- PPE
personal protective equipment
- ppm
parts per million (10−6)
- PT
proportion of diet obtained in the treated area
- PTT
partial thromboplastin time
- RAR
Renewal Assessment Report
- REACH
Registration, Evaluation, Authorisation of Chemicals Regulation
- SC
suspension concentrate
- SD
standard deviation
- SMILES
simplified molecular‐input line‐entry system
- TWA
time‐weighted average
- UV
ultraviolet
- W/S
water/sediment
- w/v
weight per unit volume
- w/w
weight per unit weight
- WBC
white blood cell
- WG
water dispersible granule
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2020.6181
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| CGA 159902 (prosulfuron phenyl sulfonamide) |
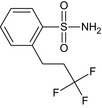
2‐(3,3,3‐trifluoropropyl)benzenesulfonamide O=S(N)(=O)c1ccccc1CCC(F)(F)F PIJUMEVHGDAQBH‐UHFFFAOYSA‐N |
|
| CGA150829 (prosulfuron triazine amine) |
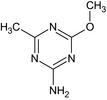
4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐amine Cc1nc(N)nc(OC)n1 NXFQWRWXEYTOTK‐UHFFFAOYSA‐N |
|
| CGA300406 (O‐desmethyl‐prosulfuron) |
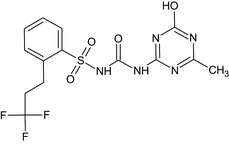
N‐[(4‐methyl‐6‐oxo‐1,6‐dihydro‐1,3,5‐triazin‐2‐yl)carbamoyl]‐2‐(3,3,3‐trifluoropropyl)benzenesulfonamide Cc2nc(NC(=O)NS(=O)(=O)c1ccccc1CCC(F)(F)F)nc(O)n2 IRTSSKZJZPPPHZ‐UHFFFAOYSA‐N |
|
| CGA325025 (demethoxy amino‐prosulfuron) |
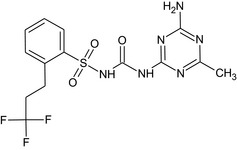
N‐[(4‐amino‐6‐methyl‐1,3,5‐triazin‐2‐yl)carbamoyl]‐2‐(3,3,3‐trifluoropropyl)benzenesulfonamide O=C(Nc1nc(C)nc(N)n1)NS(=O)(=O)c2ccccc2CCC(F)(F)F WRYWRIIVQBZZMZ‐UHFFFAOYSA‐N |
|
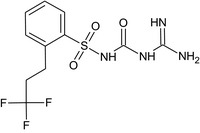
| CGA349707 |
N‐(carbamimidoylcarbamoyl)‐2‐(3,3,3‐trifluoropropyl)benzenesulfonamide O=S(=O)(NC(=O)NC(=N)N)c1ccccc1CCC(F)(F)F JWTNDJFRSJOTRB‐UHFFFAOYSA‐N |
|
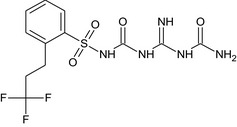
| SYN542604 (M5) |
N‐[(N‐carbamoylcarbamimidoyl)carbamoyl]‐2‐(3,3,3‐trifluoropropyl)benzenesulfonamide O=S(=O)(NC(=O)NC(=N)NC(N)=O)c1ccccc1CCC(F)(F)F LBFDCCZLYFPCDE‐UHFFFAOYSA‐N |
|
|
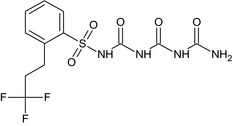
CGA325028 lysimeter metabolite M5 |
N‐[(carbamoylcarbamoyl)carbamoyl]‐2‐(3,3,3‐trifluoropropyl)benzenesulfonamide O=S(=O)(NC(=O)NC(=O)NC(N)=O)c1ccccc1CCC(F)(F)F VQUROYZSWAVIDT‐UHFFFAOYSA‐N |
|
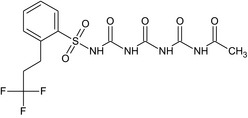
| prosulfuron polyimide (CGA325030) |
N‐{[({[2‐(3,3,3‐trifluoropropyl)phenyl]sulfonyl}carbamoyl)carbamoyl]carbamoyl}acetamide O=S(=O)(NC(=O)NC(=O)NC(=O)NC(C)=O)c1ccccc1CCC(F)(F)F ZXRDDGALQSDCAB‐UHFFFAOYSA‐N |
|
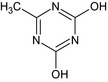
| G28533 |
6‐methyl‐1,3,5‐triazine‐2,4(1H,3H)‐dione Oc1nc(C)nc(O)n1 KZVYWMJOLANZSI‐UHFFFAOYSA‐N |
|
|
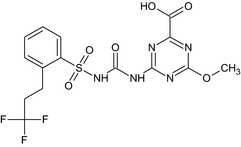
M18 (SYN547308) (peer review accepted that proposed structure 1 is correct one) |
4‐methoxy‐6‐[({[2‐(3,3,3‐trifluoropropyl)phenyl]sulfonyl}carbamoyl)amino]‐1,3,5‐triazine‐2‐carboxylic acid COc2nc(nc(NC(=O)NS(=O)(=O)c1ccccc1CCC(F)(F)F)n2)C(=O)O JKDRYDNUHMVJFG‐UHFFFAOYSA‐N |
|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2018.2.2 ACD/Labs 2018 Release (File version N50E41, Build 103230, 21 July 2018).
ACD/ChemSketch 2018.2.2 ACD/Labs 2018 Release (File version C60H41, Build 106041, 7 December 2018).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Arena M, Auteri D, Brancato A, Bura L, Carrasco Cabrera L, Chaideftou E, Chiusolo A, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Santos M, Sharp R, Stanek A, Sturma J, Szentes C, Terron A, Tiramani M, Vagenende B and Villamar‐Bouza L, 2020. Conclusion on the peer review of the pesticide risk assessment of the active substance prosulfuron. EFSA Journal 2020;18(7):6181, 20 pp. 10.2903/j.efsa.2020.6181
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00214
Approved: 11 June 2020
Notes
It is acknowledged that the genotoxic potential of metabolite triazine amine (CGA150829) has been assessed by the EFSA PPR Panel (EFSA PPR Panel, 2020). According to the EFSA PPR Panel, an in vitro Micronuclei test would be needed to conclude on its aneugenic potential. EFSA noted that considering the EFSA PPR Panel Opinion and given that aneugenicity is a threshold mechanism the setting of reference would be possible. However, further discussion is needed on the most appropriate basis for setting the reference values for triazine amine and finalise a consumer risk assessment and groundwater relevance assessment.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2017/375 of 2 March 2017 renewing the approval of the active substance prosulfuron, as a candidate for substitution, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 58, 4.3.2017, p. 3–7.
It is acknowledged that the genotoxic potential of metabolite CGA150829 (triazine amine) has been assessed by the EFSA PPR Panel (EFSA PPR Panel, 2020). According to the EFSA PPR Panel, an in vitro Micronuclei test would be needed to conclude on its aneugenic potential.
EFSA noted that considering the EFSA PPR Panel Opinion and given that aneugenicity is a threshold mechanism the setting of reference would be possible. However, further discussion is needed on the most appropriate basis for setting the reference values for triazine amine and finalise a consumer risk assessment and groundwater relevance assessment.
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
The triazine amine (CGA150829) DT50 used in simulations was 196 days though 216 days would be the correct value. With the longer DT50, it cannot be excluded that more scenarios would be above the drinking water limit.
It is acknowledged that the genotoxic potential of metabolite CGA150829 (triazine amine) has been assessed by the EFSA PPR Panel (2020). According to the EFSA PPR Panel, an in vitro Micronuclei test would be needed to conclude on its aneugenic potential. EFSA noted that considering the EFSA PPR Panel Opinion and given that aneugenicity is a threshold‐mechanism the setting of reference would be possible. However, further discussion is needed on the most appropriate basis for setting the reference values for triazine amine and finalise a consumer risk assessment and groundwater relevance assessment.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food Safety Authority), 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32 pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal 2011;9(2):2092, 49 pp. 10.2903/j.efsa.2011.2092 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Conclusion on the peer review of the pesticide risk assessment of the active substance prosulfuron. EFSA Journal 2014;12(9):3815, 94 pp. 10.2903/j.efsa.2014.3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Conclusion on the peer review of the pesticide risk assessment of the active substance tribenuron‐methyl. EFSA Journal 2017;15(7):4912, 32 pp. 10.2903/j.efsa.2017.4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for iodosulfuron and prosulfuron in light of confirmatory data. EFSA supporting publication 2018;EN‐1470, 56 pp. 10.2903/sp.efsa.2018.EN-1470 [DOI]
- EFSA (European Food Safety Authority), 2020. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance prosulfuron. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), Hernandez‐Jerez AF, Adriaanse P, Aldrich A, Berny P, Coja T, Duquesne S, Gimsing AL, Marinovich M, Millet M, Pelkonen O, Pieper S, Tiktak A, Topping CJ, Tzoulaki I, Widenfalk A, Wolterink G, Benford D, Aquilina G, Bignam i M, Bolognesi C, Crebelli R, Guertler R, Marcon F, Nielsen E, Schlatter JR, Vleminckx C, Maurici C, and Parra Morte JM, 2020. Scientific Opinion of the Scientific Panel on Plant Protection Products and their Residues (PPR Panel) on the genotoxic potential of triazine amine (metabolite common to several sulfonylurea active substances). EFSA Journal 2020;18(3):6053, 24 pp. 10.2903/j.efsa.2020.6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2014. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp., as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1, December 2014.
- France , 2013. Renewal Assessment Report (RAR) on the active substance prosulfuron. prepared by the rapporteur Member State RMS in the framework of Commission Regulation (EU) No 1141/2010, July 2013. Available at www.efsa.europa.eu
- France , 2018. Revised Renewal Assessment Report (RAR) on the active substance prosulfuron prepared by the rapporteur Member State France in the framework of Regulation (EC) No 1107/2009, April 2018. Available online: www.efsa.europa.eu
- France , 2019. Further revisions to the revised Renewal Assessment Report (RAR) on prosulfuron prepared by the rapporteur Member State France in the framework of Regulation (EC) No 1107/2009, February 2019. Available online: www.efsa.europa.eu
- WHO (World Health Organization), 2011. Guidelines for drinking‐water quality, 4th Edition, Geneva, WHO, 541 pp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


