This meta-analysis evaluates which factors are associated with diffusion-weighted imaging lesions and whether these lesions are associated with functional outcomes among patients with intracerebral hemorrhage.
Key Points
Question
What are the factors associated with diffusion-weighted imaging lesions after intracerebral hemorrhage, and are the lesions associated with functional outcomes?
Findings
In this individual patient–level study of 1752 patients with intracerebral hemorrhage, factors associated with diffusion-weighted imaging lesions were age, hematoma volume, admission systolic blood pressure, and neuroimaging markers of cerebral small-vessel disease, which did not differ by hematoma location. The lesions were associated with poor outcome in deep but not in lobar intracerebral hemorrhage.
Meaning
Diffusion-weighted imaging lesions likely represent acute sequelae of prevailing chronic hypertensive cerebrovascular disease and may be a prognostic marker for poor outcomes after intracerebral hemorrhage.
Abstract
Importance
The etiology and significance of diffusion-weighted imaging (DWI) lesions in patients with acute intracerebral hemorrhage (ICH) remain unclear.
Objective
To evaluate which factors are associated with DWI lesions, whether associated factors differ by ICH location, and whether DWI lesions are associated with functional outcomes.
Design, Setting, and Participants
This analysis pooled individual patient data from 3 randomized clinical trials (Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation phase 3 trial, Antihypertensive Treatment of Acute Cerebral Hemorrhage trial, and Intracerebral Hemorrhage Deferoxamine phase 2 trial) and 1 multicenter prospective study (Ethnic/Racial Variations of Intracerebral Hemorrhage). Patients were enrolled from August 1, 2010, to September 30, 2018. Of the 4782 patients, 1788 who underwent magnetic resonance imaging scans of the brain were included. Data were analyzed from July 1 to December 31, 2019.
Main Outcomes and Measures
The primary outcome consisted of factors associated with DWI lesions. Secondary outcomes were poor functional outcome, defined as a modified Rankin score (mRS) of 4 to 6, and mortality, both assessed at 3 months. Mixed-effects logistic regression was used to evaluate the association between exposures and outcomes. Subgroup analyses stratified by hematoma location were performed.
Results
After exclusion of 36 patients with missing data on DWI lesions, 1752 patients were included in the analysis (1019 men [58.2%]; mean [SD] age, 60.8 [13.3] years). Diffusion-weighted imaging lesions occurred in 549 patients (31.3%). In mixed-effects regression models, factors associated with DWI lesions included younger age (odds ratio [OR] per year, 0.98; 95% CI, 0.97-0.99), black race (OR, 1.64; 95% CI, 1.17-2.30), admission systolic blood pressure (OR per 10–mm Hg increase, 1.13; 95% CI, 1.08-1.18), baseline hematoma volume (OR per 10-mL increase, 1.12; 95% CI, 1.02-1.22), cerebral microbleeds (OR, 1.85; 95% CI, 1.39-2.46), and leukoaraiosis (OR, 1.59; 95% CI, 1.67-2.17). Diffusion-weighted imaging lesions were independently associated with poor mRS (OR, 1.50; 95% CI, 1.13-2.00), but not with mortality (OR, 1.11; 95% CI, 0.72-1.71). In subgroup analyses, similar factors were associated with DWI lesions in lobar and deep ICH. Diffusion-weighted imaging lesions were associated with poor mRS in deep but not lobar ICH.
Conclusions and Relevance
In a large, heterogeneous cohort of prospectively identified patients with ICH, results were consistent with the hypothesis that DWI lesions represent acute sequelae of chronic cerebral small vessel disease, particularly hypertensive vasculopathy. Diffusion-weighted imaging lesions portend a worse prognosis after ICH, mainly deep hemorrhages.
Introduction
Among patients with acute intracerebral hemorrhage (ICH), rates of potentially covert brain infarcts as observed on diffusion-weighted imaging (DWI) range from 15% to 41%,1,2 considerably more frequent than clinically apparent ischemic strokes.3 Diffusion-weighted imaging lesions are typically punctate lesions, and their location appears to have no clear spatial relationship to the index hematoma.4 These lesions are associated with microangiopathy, particularly white matter hyperintensities and cerebral microbleeds, ICH characteristics such as baseline hematoma volumes and intraventricular hemorrhage, and aggressive blood pressure reduction.5,6
Of these potential mechanisms, blood pressure control has been studied extensively, but 2 large cohort studies have yielded conflicting results. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study found a significant association between acute blood pressure lowering in patients with ICH and DWI lesions,5 whereas data from the Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH-ADAPT) did not support a hemodynamic mechanism of DWI lesions.7 In a meta-analysis of summary data from prior published studies,8 the frequency of DWI lesions was similar regardless of hematoma location or ICH etiology, but the study lacked granular data on ICH severity, clinical parameters such as blood pressure control, and outcomes. Therefore, the etiology of DWI lesions represents a major knowledge gap in our understanding of the downstream effects of acute ICH. Biological pathways that lead to ICH differ by location of the hemorrhage, yet prior studies on DWI lesions have assessed all ICHs together.9 We therefore sought to explore factors associated with DWI lesions and hypothesized that biological pathways determining DWI lesions may differ between deep and lobar ICHs and that DWI lesions in turn have a location-specific influence on ICH outcomes. To test these hypotheses, we pooled data from several prospective patient cohorts with neuroimaging to study DWI lesions after ICH.
Methods
Study Design
We performed an individual patient–level analysis of patients with ICH enrolled in the Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation phase 3 trial (MISTIE III),10 Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH-2) trial,11 Intracerebral Hemorrhage Deferoxamine (i-DEF) phase 2 trial,12 and the multicenter prospective ERICH study.13 The study and trial protocols were approved by an ethics committee at each enrolling site, and written informed consent was obtained from each participant or their legal surrogate. This study was approved by the institutional review board of Weill Cornell Medicine, New York, New York, and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (eAppendix in the Supplement).
Study Source and Patient Population
Details about the 3 randomized clinical trials and the ERICH study are highlighted in the eMethods in the Supplement. All patients were enrolled from August 1, 2010, to September 30, 2018. A prior study from the ERICH cohort evaluated DWI lesions after ICH analyzed 600 patients.5 For this study, we obtained data on an additional 601 patients with ICH enrolled subsequently in ERICH who had magnetic resonance imaging (MRI) scans of the brain.5
We included all patients with ICH who had an MRI scan performed during hospitalization for ICH. The MRI scans were obtained at the discretion of the treating physicians in ATACH-2 and i-DEF trials, whereas every fifth patient recruited in the ERICH trial received an MRI.13,14,15 In the MISTIE III trial, MRI scans were obtained on day 7 as part of the study protocol, with the exception of patient instability, withdrawal of care, death, or contraindications for MRI.16 We only included patients with MRI scans performed before cerebral angiography or surgical intervention (ie, hemicraniectomy or surgical hematoma evacuation).
Neuroimaging Measurements
In each study, a neuroimaging core of board-certified neurologists and radiologists evaluated the computed tomographic scans of the head and MRI scans of the brain while blinded to treatment assignment and outcome. Hematoma volumes were assessed using semiautomated planimetry. Lobar ICH was defined as selective involvement of cerebral cortex, underlying white matter, or both. Deep ICH was defined as selective involvement of thalami, basal ganglia, or both. Magnetic resonance imaging scans were interpreted for the following: presence of DWI lesions, location of DWI lesions, presence of microbleeds, number of microbleeds, location of microbleeds, and presence and severity of white matter hyperintensities. All MRI scans were performed on 1.5-T to 3.0-T scanners with gradient recalled echo or susceptibility-weighted imaging, DWI with apparent diffusion coefficient mapping, and fluid-attenuated inversion recovery sequences. Our variable of interest was a DWI lesion, defined as a lesion of high signal intensity on DWI, accompanying low signal intensity on apparent diffusion coefficient mapping (eFigure 1 in the Supplement). The lesions with high signal intensity in the area of hematoma or within a 10-mm rim from the hematoma were excluded.5
Severity of white matter hyperintensities was quantified using the Fazekas score, which ranges from 0 to 3 in each of the periventricular white matter and deep white matter regions.17 For this analysis, we defined leukoaraiosis as a combined periventricular and deep white matter score of greater than 2 in the MISTIE III, ATACH-2, and ERICH cohorts, similar to prior studies.5,6 In the i-DEF cohort, a white matter hyperintensity volume greater than the median value in the trial (>5000 mm3) was considered leukoaraiosis. Despite differences in ascertainment of white matter hyperintensity burden, visual rating scales and volume assessments have been shown to have near-equivalent estimates.18 Diffusion-weighted imaging lesions were not included in the assessment of leukoaraiosis. Blood pressure measurements were obtained on arrival in the emergency department and subsequently at 24 hours. The delta systolic blood pressure (SBP) was calculated using the difference between the highest and lowest SBP measurements. Our primary outcome was an MRI DWI lesion after ICH. Our secondary outcomes were poor outcome, defined as a modified Rankin score (mRS) of at least 3 and all-cause mortality, both assessed at 3 months.
Statistical Analysis
Data were analyzed from July 1 to December 31, 2019. We summarized normally distributed continuous variables as means with corresponding SDs, whereas nonnormally distributed variables were reported as medians with corresponding interquartile ranges (IQRs). All categorical variables were presented as absolute numbers with corresponding percentages. We used an unpaired t test or the Wilcoxon rank sum test for continuous variables, depending on the normality of distribution, and the χ2 test for categorical variables.
We conducted 2 different analyses. The first evaluated factors associated with DWI lesions. The second studied the association between DWI lesions and ICH outcomes. We used mixed-effects logistic regression to evaluate factors associated with a DWI lesion with the individual study included as a random effect. To build our regression models, we chose covariates for the models based on bivariate regression with a significance of 2-sided P < .20 for the outcome of interest. Collinear covariates, defined by a variance inflation factor of greater than 4, were subsequently identified and removed from the model. We additionally checked for interaction between covariates for each outcome.
For the analysis on ICH outcomes, we performed mixed-effects logistic regression to assess the association between a DWI lesion and ICH outcome. The covariates for the models included known risk factors for poor ICH outcomes: baseline hematoma volume, hematoma location, presence of intraventricular hemorrhage, age, anticoagulant use, and Glasgow Coma Scale score.19 We checked for interaction and multicollinearity as mentioned above. We subsequently performed prespecified subgroup analyses, where we studied factors associated with DWI lesions and the association between DWI lesions and outcomes in lobar and deep ICH separately.
In addition, we conducted 2 sensitivity analyses. First, we analyzed factors associated with DWI lesions in the randomized clinical trials (ATACH-2, i-DEF, and MISTIE III) and separately in the ERICH study. In the analysis of the clinical trials cohort, we additionally included treatment arm assignment as a covariate. Second, we evaluated factors that are associated with DWI lesions by excluding patients from the i-DEF trial to minimize possible heterogeneity, because white matter hyperintensities were quantified differently in this cohort. The threshold of statistical significance was set at 2-sided α = .05. All analyses were performed using STATA/MP, version 13 (StataCorp LLC).
Results
Primary Analyses
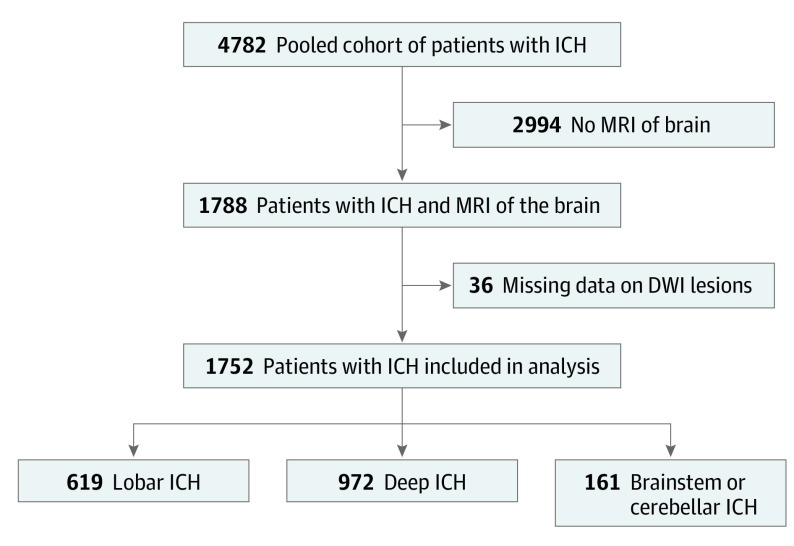
The pooled cohort consisted of 4782 patients with ICH, of whom 1752 (36.6%) were included in the main analysis (1019 men [58.2%]; mean [SD] age, 60.8 [13.3] years) (Figure). Of these ICHs, 619 (35.3%) were lobar, 972 (55.5%) were deep, and 161 (9.2%) were brainstem or cerebellar. Baseline demographics, comorbidities, and ICH characteristics in each of the 4 cohorts are shown in Table 1. Differences in demographics, comorbidities, and ICH characteristics between patients with and without MRI data in these cohorts are highlighted in eTable 1 in the Supplement, along with box plots showing admission SBP and delta SBP, stratified by the presence of DWI lesions (eFigures 2-5 in the Supplement). Median time to MRI scan was 1.5 (IQR, 1.0-4.0) days. Diffusion-weighted imaging lesions were identified in 549 patients (31.3%). In univariate analysis, DWI lesions were associated with male sex (351 [63.9%] vs 668 [55.1%]; P < .001), history of hypertension (480 [87.4%] vs 950 [78.4%]; P < .001), baseline hematoma volume (median, 25.1 [IQR, 7.1-49.7] vs 13.7 [IQR, 4.3-36.9] mL; P < .001), admission SBP (median, 191 [IQR, 160-220] vs 172 [IQR, 147-199] mm Hg; P < .001), reduction in SBP over 24 hours (median, 47 [IQR, 22-78] vs 35 [IQR, 13-55] mm Hg; P = .001), cerebral microbleeds (311 [56.6%] vs 515 [42.5%]; P < .001), and moderate to severe leukoaraiosis (330 [60.1%] vs 642 [52.9%]; P = .01) (Table 2).
Figure. Flowchart Showing Selection of Patients in the Study.
DWI indicates diffusion-weighted imaging; ICH, intracerebral hemorrhage; and MRI, magnetic resonance imaging.
Table 1. Baseline Characteristics of Intracerebral Hemorrhage Patients With MRI Scans, Stratified by Study Cohort.
| Characteristic | Cohorta | |||
|---|---|---|---|---|
| MISTIE III (n = 288) | ATACH-2 (n = 178) | i-DEF (n = 85) | ERICH (n = 1201) | |
| Age, mean (SD), y | 60.6 (12.3) | 61.5 (12.9) | 59.9 (12.2) | 60.9 (12.3) |
| Male | 172 (60.1) | 112 (62.9) | 46 (54.1) | 691 (57.5) |
| Race/ethnicity | ||||
| White | 175 (61.2) | 63 (35.4) | 63 (74.1) | 408 (34.0) |
| Black | 54 (18.9) | 34 (19.1) | 11 (12.9) | 401 (33.4) |
| Hispanic | 40 (14.0) | 0 | 11 (12.9) | 392 (32.6) |
| Other | 19 (5.9) | 81 (45.5) | 0 | 0 |
| Hypertension | 279 (97.6) | 136 (76.4) | 58 (71.6) | 958 (79.8) |
| Type 2 diabetes | 90 (31.5) | 37 (20.7) | 18 (22.5) | 312 (25.9) |
| Hyperlipidemia | 111 (38.8) | 55 (30.9) | 34 (44.7) | 535 (44.7) |
| Atrial fibrillation | 18 (6.3) | 2 (1.1) | 2 (2.4) | 98 (8.2) |
| Prior therapy | ||||
| Antiplatelet | 95 (33.2) | NA | 31 (36.5) | 518 (43.1) |
| Anticoagulation | 19 (6.6) | NA | 0 | 100 (8.3) |
| Prior ischemic stroke | 8 (2.8) | 32 (17.9) | 5 (5.9) | 130 (10.8) |
| Baseline data, median (IQR) | ||||
| Glasgow Coma Scale scoreb | 10 (8-13) | 15 (14-15) | 14 (12-15) | 15 (13-15) |
| Hematoma volume | 41.8 (30.9-53.2) | 11.0 (5.9-22.6) | 16.1 (8.9-30.5) | 8.9 (3.0-20.4) |
| Presence of IVH | 122 (42.7) | 40 (22.4) | 28 (32.9) | 381 (32.9) |
| ICH location | ||||
| Lobar | 111 (38.8) | 42 (23.5) | 30 (35.3) | 436 (36.7) |
| Deep | 177 (61.2) | 136 (75.9) | 55 (64.7) | 604 (50.9) |
| Infratentorial | 0 | 0 | 0 | 161 (12.4) |
| INR, baseline, median (IQR) | 1.0 (0.9-1.0) | 1.0 (0.9-1.0) | 1.0 (0.9-1.1) | 1.0 (0.9-1.1) |
| Admission SBP, median (IQR), mm Hg | 181 (158-204) | 198 (182-215) | 132 (122-146) | 179 (154-210) |
| Time to MRI scan, median (IQR), d | 2 (1-7) | 1 (1-5) | 1 (1-2) | 2 (1-4) |
| Presence of DWI lesions | 168 (58.7) | 46 (25.8) | 24 (29.6) | 311 (25.9) |
| Presence of cerebral microbleeds | 118 (40.9) | 113 (63.4) | 22 (25.9) | 573 (47.7) |
| Leukoaraiosis, moderate to severec | 82 (28.7) | 163 (91.5) | 45 (52.9) | 685 (58.2) |
Abbreviations: ATACH-2, Antihypertensive Treatment of Acute Cerebral Hemorrhage Trial; DWI, diffusion-weighted imaging; ERICH, Ethnic/Racial Variations of Intracerebral Hemorrhage Study; i-DEF, Intracerebral Hemorrhage Deferoxamine phase 2 trial; INR, international normalized ratio; IQR, interquartile range; IVH, intraventricular hemorrhage; MISTIE III, Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation phase 3 trial; MRI, magnetic resonance imaging; NA, not available; SBP, systolic blood pressure.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Data were available for 286 patients in MISTIE III for some of the variables.
Scores range from 3 to 15, with lower scores indicating coma.
Defined as a combined periventricular and deep white matter score of greater than 2.
Table 2. Baseline Characteristics of Patients With Intracerebral Hemorrhage With and Without DWI Lesions.
| Characteristic | DWI lesion statusa | P value | |
|---|---|---|---|
| With (n = 549) | Without (n = 1212) | ||
| Age, mean (SD), y | 58.9 (13.2) | 61.8 (13.4) | .001 |
| Male sex | 351 (63.9) | 668 (55.1) | <.001 |
| Race/ethnicity | |||
| White | 208 (37.9) | 498 (41.1) | <.001 |
| Black | 203 (36.9) | 297 (24.5) | |
| Hispanic | 113 (20.6) | 329 (27.1) | |
| Other | 23 (4.6) | 75 (7.3) | |
| Hypertension | 480 (87.4) | 950 (78.4) | <.001 |
| Type 2 diabetes | 144 (26.2) | 313 (25.8) | .85 |
| Hyperlipidemia | 212 (38.6) | 523 (43.2) | .21 |
| Atrial fibrillation | 30 (5.5) | 90 (7.5) | .13 |
| Prior therapyb | |||
| Antiplatelet | 199 (39.6) | 445 (41.8) | .40 |
| Anticoagulation | 26 (5.2) | 93 (8.7) | .01 |
| Prior ischemic stroke | 62 (11.3) | 113 (9.4) | .25 |
| Baseline data, median (IQR) | |||
| Glasgow Coma Scale scorec | 13 (10-15) | 15 (13-15) | <.001 |
| Hematoma volume | 25.1 (7.1-49.7) | 13.7 (4.3-36.9) | <.001 |
| IVH volume, median (IQR) | 4.5 (1.0-11.8) | 3.2 (1.0-9.3) | .001 |
| Presence of IVH | 215 (39.2) | 354 (29.2) | <.001 |
| ICH locationd | |||
| Lobar | 189 (34.7) | 429 (36.1) | .79 |
| Deep | 311 (57.2) | 657 (55.3) | |
| Infratentorial | 44 (8.1) | 103 (8.7) | |
| INR, baseline, median (IQR) | 1.0 (0.9-1.1) | 1.0 (0.9-1.1) | .27 |
| Admission SBP, median (IQR), mm Hg | 191 (160-220) | 172 (147-199) | <.001 |
| Time to MRI scan, median (IQR), d | 2 (1-5) | 1 (1-4) | .31 |
| Location of DWI lesions | |||
| Lobar | 186 (33.9) | NA | NA |
| Deep | 148 (27.0) | ||
| Lobar and deep | 215 (39.2) | ||
| Cerebellar | 0 | ||
| Presence of cerebral microbleeds | 311 (56.6) | 515 (42.5) | <.001 |
| No. of microbleeds, median (IQR) | 3 (1-10) | 2 (1-5) | <.001 |
| Location of microbleedse | |||
| Lobar | 96 (30.9) | 238 (46.2) | <.001 |
| Deep | 146 (46.9) | 162 (31.4) | |
| Lobar and deep | 69 (22.2) | 115 (22.3) | |
| Leukoaraiosis, moderate to severef | 330 (60.1) | 642 (52.9) | .01 |
| Delta SBP, median (IQR), mm Hg | 47 (22-78) | 35 (13-55) | .001 |
Abbreviations: DWI, diffusion-weighted imaging; INR, international normalized ratio; IQR, interquartile range; IVH, intraventricular hemorrhage; MRI, magnetic resonance imaging; NA, not applicable; SBP, systolic blood pressure.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
Data were not available from ATACH-2, leaving 503 participants with DWI lesions and 1065 without.
Scores range from 3 to 15, with lower scores indicating coma.
Data were available for 544 participants with DWI lesions and 1189 without.
Data were available for 311 participants with DWI lesions and 515 without.
Defined as a combined periventricular and deep white matter score of greater than 2.
In multivariable regression models (Table 3), factors associated with DWI lesions included younger age (odds ratio [OR] per year, 0.98; 95% CI, 0.97-0.99), black race (OR, 1.64; 95% CI, 1.17-2.30), admission SBP (OR per 10–mm Hg increase, 1.13; 95% CI, 1.08-1.18), baseline hematoma volume (OR per 10-mL increase, 1.12; 95% CI, 1.02-1.22), cerebral microbleeds (OR, 1.85; 95% CI, 1.39-2.46), and leukoaraiosis (OR, 1.59; 95% CI, 1.67-2.17). Hematoma location and delta SBP were not associated with DWI lesions. There was no interaction between admission SBP and delta SBP (P = .65 for interaction). In mixed-effects regression models adjusted for known risk factors for poor outcome and treatment randomization, patients with DWI lesions had higher odds for a poor outcome (mRS of 4-6) compared with patients without DWI lesions (OR, 1.50; 95% CI, 1.13-2.00) (Table 4). We did not find an association between an MRI DWI lesion and mortality.
Table 3. Multivariable Logistic Regression of Factors Associated With DWI Lesionsa.
| Characteristic | OR (95% CI) | P value |
|---|---|---|
| Age, per y | 0.98 (0.97-0.99) | .004 |
| Male sex | 1.33 (1.01-1.74) | .04 |
| Race/ethnicity | ||
| White | 1 [Reference] | NA |
| Black | 1.64 (1.17-2.30) | .004 |
| Hispanic | 0.89 (0.62-1.28) | .54 |
| Other | 0.64 (0.22-1.13) | .42 |
| Prior anticoagulant therapy | 0.63 (0.35-1.13) | .12 |
| Hematoma volume, baseline (per 10-mL increase) | 1.12 (1.02-1.22) | .01 |
| Presence of IVH | 1.07 (0.79-1.43) | .66 |
| Hematoma location | ||
| Lobar | 1 [Reference] | NA |
| Deep | 0.81 (0.58-1.11) | .19 |
| Infratentorial | 1.09 (0.65-1.82) | .73 |
| Baseline SBP (per 10–mm Hg increase) | 1.13 (1.08-1.18) | <.001 |
| Delta SBP | 1.00 (0.98-1.04) | .49 |
| Presence of cerebral microbleeds | 1.85 (1.39 -2.46) | <.001 |
| Leukoaraiosis, moderate to severe | 1.59 (1.67-2.17) | .003 |
Abbreviations: DWI, diffusion-weighted imaging; IVH, intraventricular hemorrhage; NA, not applicable; OR, odds ratio; SBP, systolic blood pressure.
Covariates selected based on a significance of P < .20 in the univariable analysis.
Table 4. Multivariate Analysis of the Association Between DWI Lesions and ICH Outcomesa.
| ICH characteristic | Mortality | Modified Rankin scale score 4-6 | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| All | 1.11 (0.72-1.71) | .64 | 1.50 (1.13-2.00) | .005 |
| Lobar | 1.29 (0.63-2.63) | .48 | 1.01 (0.61-1.64) | .93 |
| Deep | 1.10 (0.62-1.95) | .74 | 1.80 (1.19-2.71) | .005 |
Abbreviations: DWI, diffusion-weighted imaging; ICH, intracerebral hemorrhage; OR, odds ratio.
Models adjusted for age, sex, baseline hematoma volume, intraventricular hemorrhage, anticoagulant use, Glasgow Coma Scale score, study clustering, and intervention assignment in the trial.
Subgroup Analyses
Among the patients with DWI lesions, 186 (33.9%) had lobar ICH. In the logistic regression models, factors associated with DWI lesions in lobar ICH included black race (OR, 2.05; 95% CI, 1.17-3.59), admission SBP (OR per 1–mm Hg increase, 1.12; 95% CI, 1.03-1.18), admission hematoma volume (OR per 10-mL increase, 1.15; 95% 1.02-1.29), presence of intraventricular hemorrhage (OR, 0.45; 95% CI, 0.25-0.81), leukoaraiosis (OR, 1.71; 95% CI, 1.01-2.89), and cerebral microbleeds (OR, 1.71; 95% CI, 1.05-2.79) (eTable 2 in the Supplement). In patients with lobar ICH, DWI lesions were not associated with mortality (OR 1.29; 95% CI, 0.63-2.63) or mRS of 4 to 6 (OR 1.01; 95% CI, 0.61-1.64) (Table 4).
Among 972 patients with deep ICH, DWI lesions were observed in 311 (32.0%). In the logistic regression models, factors associated with DWI lesions in deep ICH were younger age (OR per year, 0.97; 95% CI, 0.96-0.99), black race (OR 1.55; 95% CI, 1.04-2.53), admission SBP (OR per 1–mm Hg increase, 1.12; 95% CI, 1.06-1.20), admission hematoma volume (OR per 10-mL increase, 1.17; 95% CI, 1.03-1.33), and cerebral microbleeds (OR 1.86; 95% CI, 1.26-2.74) but not leukoaraiosis (eTable 2 in the Supplement). Diffusion-weighted imaging lesions were associated with mRS of 4 to 6 (OR, 1.80; 95% CI, 1.19-2.71) but not with mortality (OR, 1.10; 95% CI, 0.62-1.95) (Table 4).
Sensitivity Analyses
In the first sensitivity analysis limited to patients from the 3 randomized clinical trials (MISTIE III, i-DEF, and ATACH-2), DWI lesions were associated with black race (OR, 1.25; 95% CI, 0.77-2.02), admission hematoma volume (OR per 10-mL increase, 1.17; 95% CI, 1.03-1.33), admission SBP (OR per 10–mm Hg increase, 1.14; 95% CI, 1.06-1.22), and cerebral microbleeds (OR, 1.76; 95% CI, 1.18-2.84) congruent to the results of the primary analyses (eTable 3 in the Supplement). Treatment arm assignment was not associated with DWI lesions. When we studied patients from the ERICH cohort separately, we found similar results with the exception of baseline hematoma volume, which was no longer significantly associated with DWI lesions. In the second analysis, after the exclusion of patients enrolled in the i-DEF trial, the results mirrored those of the primary analysis (eTable 4 in the Supplement).
Discussion
In this individual patient data meta-analysis of more than 1700 patients with ICH and MRI scans, younger age, black race, acute blood pressure elevation, radiological markers of small vessel disease, and admission hematoma volume were associated with DWI lesions after ICH. Diffusion-weighted imaging lesions were independently associated with poor ICH outcomes in patients with deep ICH but not lobar ICH.
Prior studies3,7 have yielded conflicting results on the association between blood pressure lowering and DWI lesions. It is also unclear from prior studies what these DWI lesions represent, with previous reports4,20,21 suggesting acute decreases in blood pressure, microangiopathy, a prothrombotic state, stress-induced hyperglycemia, and enlarged perivascular spaces as possible explanations. In the context of these prior conflicting reports, our study adds novel findings suggesting that DWI lesions are most likely acute ischemic sequelae of chronic cerebral small vessel disease precipitated by blood pressure elevation in the setting of acute ICH.
The lack of an association between SBP lowering and DWI lesions in our study aligns with the findings of secondary analysis of the ICH-ADAPT trial,7 in which SBP reduction to less than 150 mm Hg compared with a liberal SBP target of less than 180 mm Hg was not associated with DWI lesions and did not result in a significant reduction in cerebral blood flow. Further support for this finding comes from the secondary analysis of the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial 2 (INTERACT-2),22 in which target-driven intensive blood pressure control in patients with moderate to severe white matter hyperintensities did not result in poor functional outcomes, compared with those with liberal blood pressure targets. These findings assume importance in light of recent evidence showing a potential protective effect of intensive, early, and sustained blood pressure treatment in the acute phase of ICH on hematoma expansion and outcomes.23,24 In the prior ERICH study,5 admission SBP and reduction in mean arterial blood pressure were both associated with DWI lesions. Our study benefited from a larger ERICH cohort that consisted of 1201 patients compared with 600 patients in the previous study.5 In addition, residual confounding of a high admission SBP or variability in SBP during a 24-hour period may have resulted in SBP reduction being significantly associated with DWI lesions in prior studies, but not ours. Regardless, incident DWI lesions have been documented to occur well beyond the acute phase of ICH,3,6 making it less likely that acute SBP lowering fully explains DWI lesions after ICH.
Our results confirm that neuroimaging markers of small vessel disease such as cerebral microbleeds and leukoaraiosis are associated with DWI lesions.6,8,25 The association between black race and DWI lesions noted in our analysis may also be attributable to a greater burden of subclinical cerebrovascular disease among black participants compared with those of other races, including a higher prevalence of vascular risk factors and differences in genetic susceptibility.26,27 Furthermore, the burden of high blood pressure alleles is associated with larger hematoma volumes and worse ICH outcomes, implying that small vessel disease and hematoma size may share a similar genetic predisposition.28 Therefore, DWI lesions may be surrogate markers for chronic hypertensive arteriopathy. A countervailing argument is that hematoma location did not influence DWI lesions. Although hypertension is a common etiology for both deep and lobar ICH, cerebral amyloid angiopathy, not adjudicated in our study, also plays an important role in lobar ICH.29 Moreover, cerebral amyloid angiopathy is independently associated with neuroimaging biomarkers of cerebral small vessel disease,30 which may partly explain our findings. However, the stringent inclusion criteria of the trials and the fact that DWI lesions were noted more frequently in younger patients in our study likely implicate hypertensive vasculopathy as a prevailing mechanism of DWI lesions.
Limitations
This study has several important limitations. First, we combined data from different trials and an epidemiological study, which likely resulted in heterogeneity in the overall pooled patient sample. To minimize this confounding effect, we accounted for study database as a random effect. Furthermore, we performed sensitivity analyses in which patients from randomized clinical trials and those from the ERICH study were analyzed separately, and we noted results similar to those of the primary analysis. Nevertheless, although stringent inclusion criteria of trials limit generalization of results, an epidemiological study, such as ERICH, is more reflective of the general population with ICH. Other restrictions on generalizability concern several imbalances between patients without MRI, who had more severe ICH, compared with those who had MRI, which may have limited feasibility of performing MRI. Second, we lacked data on location of DWI lesions in relation to the hematoma (perihematomal vs distant, and ipsilateral vs contralateral), although we excluded DWI lesions within a 1-cm outer rim of the hematoma. It remains unclear whether the pathophysiology varies by location given lack of power in prior studies.7 Third, data on blood pressure was only available at 2 time points in most cohorts, which precluded further study of the association between BP variability, particularly in the first 24 hours, and DWI lesions. Of note, change in blood pressure within the first hour was associated with worse outcomes in the combined analysis of INTERACT-2 and ATACH-2.24 Further studies on early blood pressure lowering and DWI lesions are warranted. Fourth, differences in the ascertainment of leukoaraiosis across studies is another limitation. Although ERICH, ATACH-2, and MISTIE III used the Fazekas score, i-DEF quantified white matter hyperintensity volume. However, close correlations have been reported between visual rating scales and volumetric assessments of white matter hyperintensities,18 and our results remained unchanged in a sensitivity analysis that excluded patients from the i-DEF trial (eTable 4 in the Supplement). Next, we lacked data on ICH etiology, specifically hypertensive or cerebral amyloid angiopathy, because these conditions influence cerebral microbleed occurrence and distribution. Last, obtaining early MRI scans and variability in timing of MRI scans across studies may have resulted in underreporting of the frequency of DWI lesions; however, the incidence was similar to that reported in prior studies.6,25 There is also a possibility of confounding by indication in obtaining MRI scans, although we tried to navigate this limitation by comparing patients with and without MRI in the 4 cohorts.
Conclusions
In a large, heterogeneous cohort of prospectively identified patients with ICH, our results are consistent with the hypothesis that DWI lesions represent acute sequelae of chronic cerebral small vessel disease, particularly hypertensive vasculopathy. Diffusion-weighted imaging lesions portend a worse prognosis after ICH, particularly deep hemorrhages.
eAppendix. STROBE Statement—Checklist of Items That Should Be Included in Reports of Observational Studies
eMethods. Cohorts
eFigure 1. Magnetic Resonance Imaging Scan of the Brain Showing a DWI Lesion After Acute Intracerebral Hemorrhage
eFigure 2. Box Plots Showing Distribution of Baseline SBP Among Patients With and Without DWI Lesions
eFigure 3. Box Plots Showing Distribution of Change in SBP During 24 Hours Among Patients With and Without DWI Lesions
eFigure 4. Box Plots Showing Distribution of Baseline SBP Among Patients With and Without DWI Lesions in the ERICH Cohort Only
eFigure 5. Box Plots Showing Distribution of Change in SBP Over 24 hours Among Patients With and Without DWI Lesions in the ERICH Cohort Only
eTable 1. Characteristics of ICH Patients With and Without MRI in the Pooled Cohort
eTable 2. Mixed Effects Logistic Regression of Factors Associated With DWIHLs, Stratified by Hematoma Location
eTable 3. Multivariable Analysis of Factors Associated With DWIHLs, Stratified by Study Design (Randomized Trial vs Epidemiological Study)
eTable 4. Multivariable Logistic Regression of Factors Associated With DWI Lesions Excluding Patients From the i-DEF Trial
eReferences.
References
- 1.Kimberly WT, Gilson A, Rost NS, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72(14):1230-1235. doi: 10.1212/01.wnl.0000345666.83318.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke. 2012;43(1):67-71. doi: 10.1161/STROKEAHA.111.629493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auriel E, Gurol ME, Ayres A, et al. Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology. 2012;79(24):2335-2341. doi: 10.1212/WNL.0b013e318278b66f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhakaran S, Naidech AM. Ischemic brain injury after intracerebral hemorrhage: a critical review. Stroke. 2012;43(8):2258-2263. doi: 10.1161/STROKEAHA.112.655910 [DOI] [PubMed] [Google Scholar]
- 5.Kidwell CS, Rosand J, Norato G, et al. Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology. 2017;88(8):782-788. doi: 10.1212/WNL.0000000000003630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon RS, Burgess RE, Wing JJ, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71(2):199-205. doi: 10.1002/ana.22668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gioia LC, Kate M, Choi V, et al. Ischemia in intracerebral hemorrhage is associated with leukoaraiosis and hematoma volume, not blood pressure reduction. Stroke. 2015;46(6):1541-1547. doi: 10.1161/STROKEAHA.114.008304 [DOI] [PubMed] [Google Scholar]
- 8.Boulanger M, Schneckenburger R, Join-Lambert C, et al. Diffusion-weighted imaging hyperintensities in subtypes of acute intracerebral hemorrhage. Stroke. 2018;A118021407. doi: 10.1161/STROKEAHA.118.021407 [DOI] [PubMed] [Google Scholar]
- 9.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720-731. doi: 10.1016/S1474-4422(12)70104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley DF, Thompson RE, Rosenblum M, et al. ; MISTIE III Investigators . Efficacy and safety of Minimally Invasive Surgery With Thrombolysis in Intracerebral Haemorrhage Evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021-1032. doi: 10.1016/S0140-6736(19)30195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi AI, Palesch YY, Barsan WG, et al. ; ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network . Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375(11):1033-1043. doi: 10.1056/NEJMoa1603460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selim M, Foster LD, Moy CS, et al. ; i-DEF Investigators . Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18(5):428-438. doi: 10.1016/S1474-4422(19)30069-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke. 2013;44(10):e120-e125. doi: 10.1161/STROKEAHA.113.002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeatts SD, Palesch YY, Moy CS, Selim M. High dose deferoxamine in intracerebral hemorrhage (HI-DEF) trial: rationale, design, and methods. Neurocrit Care. 2013;19(2):257-266. doi: 10.1007/s12028-013-9861-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559-576. doi: 10.1007/s12028-011-9538-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MISTIE-III Study Protocol and Statistical Analysis Plan Posted April 14, 2015. Accessed July 10, 2019. http://braininjuryoutcomes. com/images/MISTIE3/MISTIE_ III_Protocol_SAP.pdf
- 17.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351-356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 18.Valdés Hernández MdelC, Morris Z, Dickie DA, et al. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology. 2013;40(1):13-22. doi: 10.1159/000341859 [DOI] [PubMed] [Google Scholar]
- 19.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2):158-164. doi: 10.1001/jamaneurol.2013.5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu B, Yao X, Lei C, Liu M, Selim MH. Enlarged perivascular spaces and small diffusion-weighted lesions in intracerebral hemorrhage. Neurology. 2015;85(23):2045-2052. doi: 10.1212/WNL.0000000000002169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye XH, Cai XL, Nie DL, et al. Stress-induced hyperglycemia and remote diffusion-weighted imaging lesions in primary intracerebral hemorrhage. Neurocrit Care. 2020;32(2):427-436. doi: 10.1007/s12028-019-00747-y [DOI] [PubMed] [Google Scholar]
- 22.Sato S, Delcourt C, Heeley E, et al. ; INTERACT2 Investigators . Significance of cerebral small-vessel disease in acute intracerebral hemorrhage. Stroke. 2016;47(3):701-707. doi: 10.1161/STROKEAHA.115.012147 [DOI] [PubMed] [Google Scholar]
- 23.Leasure AC, Qureshi AI, Murthy SB, et al. Association of intensive blood pressure reduction with risk of hematoma expansion in patients with deep intracerebral hemorrhage. JAMA Neurol. 2019. doi: 10.1001/jamaneurol.2019.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moullaali TJ, Wang X, Martin RH, et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. Lancet Neurol. 2019;18(9):857-864. doi: 10.1016/S1474-4422(19)30196-6 [DOI] [PubMed] [Google Scholar]
- 25.Buletko AB, Thacker T, Cho SM, et al. Cerebral ischemia and deterioration with lower blood pressure target in intracerebral hemorrhage. Neurology. 2018;91(11):e1058-e1066. doi: 10.1212/WNL.0000000000006156 [DOI] [PubMed] [Google Scholar]
- 26.Gottesman RF, Fornage M, Knopman DS, Mosley TH. Brain aging in African-Americans: the Atherosclerosis Risk in Communities (ARIC) experience. Curr Alzheimer Res. 2015;12(7):607-613. doi: 10.2174/1567205012666150701102445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copenhaver BR, Hsia AW, Merino JG, et al. Racial differences in microbleed prevalence in primary intracerebral hemorrhage. Neurology. 2008;71(15):1176-1182. doi: 10.1212/01.wnl.0000327524.16575.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcone GJ, Biffi A, Devan WJ, et al. ; GOCHA Investigators . Burden of blood pressure-related alleles is associated with larger hematoma volume and worse outcome in intracerebral hemorrhage. Stroke. 2013;44(2):321-326. doi: 10.1161/STROKEAHA.112.675181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biffi A, Sonni A, Anderson CD, et al. ; International Stroke Genetics Consortium . Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68(6):934-943. doi: 10.1002/ana.22134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulouis G, Charidimou A, Jessel MJ, et al. Small vessel disease burden in cerebral amyloid angiopathy without symptomatic hemorrhage. Neurology. 2017;88(9):878-884. doi: 10.1212/WNL.0000000000003655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. STROBE Statement—Checklist of Items That Should Be Included in Reports of Observational Studies
eMethods. Cohorts
eFigure 1. Magnetic Resonance Imaging Scan of the Brain Showing a DWI Lesion After Acute Intracerebral Hemorrhage
eFigure 2. Box Plots Showing Distribution of Baseline SBP Among Patients With and Without DWI Lesions
eFigure 3. Box Plots Showing Distribution of Change in SBP During 24 Hours Among Patients With and Without DWI Lesions
eFigure 4. Box Plots Showing Distribution of Baseline SBP Among Patients With and Without DWI Lesions in the ERICH Cohort Only
eFigure 5. Box Plots Showing Distribution of Change in SBP Over 24 hours Among Patients With and Without DWI Lesions in the ERICH Cohort Only
eTable 1. Characteristics of ICH Patients With and Without MRI in the Pooled Cohort
eTable 2. Mixed Effects Logistic Regression of Factors Associated With DWIHLs, Stratified by Hematoma Location
eTable 3. Multivariable Analysis of Factors Associated With DWIHLs, Stratified by Study Design (Randomized Trial vs Epidemiological Study)
eTable 4. Multivariable Logistic Regression of Factors Associated With DWI Lesions Excluding Patients From the i-DEF Trial
eReferences.