Abstract
Juvenile osteochondritis dissecans (OCD) of the knee is a rare condition of subchondral bone that has secondary effects on articular cartilage as the condition advances. Traditional treatment for early-stage OCD involves different types of drilling procedures that work to stimulate healthy bone formation via creeping substitution. This article describes a technique that involves a complete removal, or decompression of an early-stage OCD, while preserving the overlying articular cartilage that is augmented with bone grafting and bone marrow aspirate concentrate. This allows for quicker and more reliable healing of early-stage OCD and can minimize the chance for reoperation.
Osteochondritis dissecans (OCD) of the knee is a local avascular process of subchondral bone and immature epiphyseal cartilage that can ultimately result in fragmentation and eventual separation of the unhealthy bone and cartilage to produce a loose body and an associated osteochondral defect.1 This has the potential to lead to early osteoarthritis and the associated morbidity that comes with it.2,3
The fragmentation process occurs very gradually over many years and often patients present with pain while the OCD is still in its early stage and the overlying cartilage is healthy and stable. Most patients who present at this stage are preadolescent.4 Conservative and operative treatments have been described for early-stage OCD. The core of conservative treatment is activity restriction with adjuncts such as an unloader brace or crutches. Conservative measures are often instituted for a minimum of 3 months before determining some level of success or treatment failure.1,5, 6, 7 If conservative treatments fail, or if patients do not or will not tolerate conservative treatment, then operative interventions are entertained.
The standard treatment for an early-stage juvenile OCD lesion of the knee is either a transarticular or retroarticular drilling procedure. The philosophy of these procedures is to create vascular channels that will allow blood and marrow elements to egress into the diseased bone and gradually replace it with healthy bone.8 Both procedures are minimally invasive with equivalent results. Reported success rates for these procedures vary between 80% and 90% and healing time can average 11 months.9, 10, 11, 12 In general, the larger the OCD, the longer it takes to heal from a drilling procedure.3 In addition, as many as 25% of radiographs never fully normalize, and it is unclear what happens to these patients later in life when they transition care to adult providers.3,13
In an effort to accelerate healing and obtain more reliable and complete normalization of radiographs, a procedure called retroarticular core decompression (RCD) has been developed by the senior authors. This procedure uses known orthopaedic techniques in combination with orthobiologic augmentation with bone marrow aspirate concentrate (BMAC) to accelerate and stimulate healing. It represents a minimally invasive technique that allows for complete decompression of the OCD lesion.
Indications for the RCD Procedure
A patient is considered appropriate for the RCD with BMAC procedure if the OCD is deemed early stage via magnetic resonance imaging (MRI) and confirmed by diagnostic arthroscopy and the patient has failed conservative management. Typically, the MRI will show normal or thickened cartilage without fissuring. MRI characteristics of the involved bone often show an absence of a high T2 signal line traversing deep to the lesion (to suggest instability), but cysts or ovoid bodies may be present.14 The presence of progeny bone on imaging is variable and has not yet indicated the onset of instability.
Technique
Video 1 demonstrates an overview of the technique for this procedure. The patient is placed under general anesthesia in the supine position and a tourniquet is placed high on the operative thigh. Both the operative knee as well as the ipsilateral iliac crest (for harvest of bone marrow from the iliac crest and BMAC procurement) are prepped and draped. The iliac crest is squared off with towels and covered with Ioban (3M, Maplewood, MN). An extremity drape is then placed and a hole is cut in the drapes to expose the prepped iliac crest. A second Ioban is placed over this hole to allow for access to both the iliac crest as well as the operative lower extremity (Fig 1). The bone marrow should not be harvested until after diagnostic arthroscopy has confirmed that the OCD lesion is stable and that the RCD can be performed.
Fig 1.
When draping, the left (operative) leg and ipsilateral iliac crest (marked with arrow) are both prepped and draped with the patient supine. This allows for arthroscopic and fluoroscopic access to the operative knee and for bone marrow harvest from the ipsilateral iliac crest.
Diagnostic arthroscopy, with or without tourniquet insufflation, is commenced in standard fashion. Once the area of interest has been probed and found to be stable, the arthroscope is removed and the bone marrow harvest kit (Angel; Arthrex, Naples, FL) tubing and harvesting needles are heparinized before harvest per manufacturer instructions. The bone marrow is harvested percutaneously approximately 2 cm posterior to the anterior superior iliac spine. The needle is angled medial and distal so that it stays between the inner and outer tables of the ilium. It is advanced 2 to 3 cm and approximately 60 mL (2 30-mL syringes) of bone marrow is aspirated. The marrow is then handed off and the stem cells are concentrated in a centrifuge (Angel). The BMAC is then placed back on the sterile field for later use. Typically, 60 mL of bone marrow aspiration yields 2 to 3 mL of BMAC in this age group.
The leg is then exsanguinated with an Esmarch dressing and the tourniquet is inflated. Although only small incisions are used, tourniquet control is helpful to minimize bony bleeding that may dilute the BMAC preparation later in the case. The knee is typically placed over a radiolucent tibial triangle or other apparatus that allows the knee to be flexed 40° to 50°. This serves 2 purposes: it moves the surgical knee out of the way of the nonsurgical limb for easy lateral fluoroscopy imaging and most OCDs are best visualized radiographically in the anteroposterior (AP) plane with the knee in flexion.
The OCD lesion is then localized under AP and lateral fluoroscopy and, with fluoroscopic guidance, an anterior cruciate ligament tibial guide pin is placed percutaneously into the OCD. Pin placement begins distal to the physis on the outer cortex of the condyle with the OCD lesion (i.e., medial cortex for medial OCD). The guide pin is placed as close as possible to the osteochondral junction and appropriate placement is confirmed on AP and lateral views (Fig 2). A small incision is made on the skin around the pin and dissection is taken down the femoral cortex (Fig 3). The articular surface is exposed to ensure that pin placement and subsequent reaming will not violate the cartilage. Care is taken to ensure the pin is anterior to the collateral ligaments. A 7-mm anterior cruciate ligament acorn reamer is placed over the guide pin and the reamer is advanced under fluoroscopic control to the tip of the guide pin, which is at the subchondral plate (Fig 4). The drill and guide pin are removed and the guide pin is repositioned into another part of the OCD. Typically, the guide pin is repositioned through the same cortical aperture to minimize the amount of bone removal from the femoral cortex. This process is repeated until the OCD is completely or nearly completed decompressed in both anterior to posterior and medial to lateral directions (Fig 4). Often, this portion of the procedure is begun while the surgeon is waiting for the bone marrow harvest instruments and tubing to be heparinized and completed after bone marrow harvest. It is recommended to get the bone marrow harvest done as early as possible because there is a 15- to 20-minute spin time in the centrifuge.
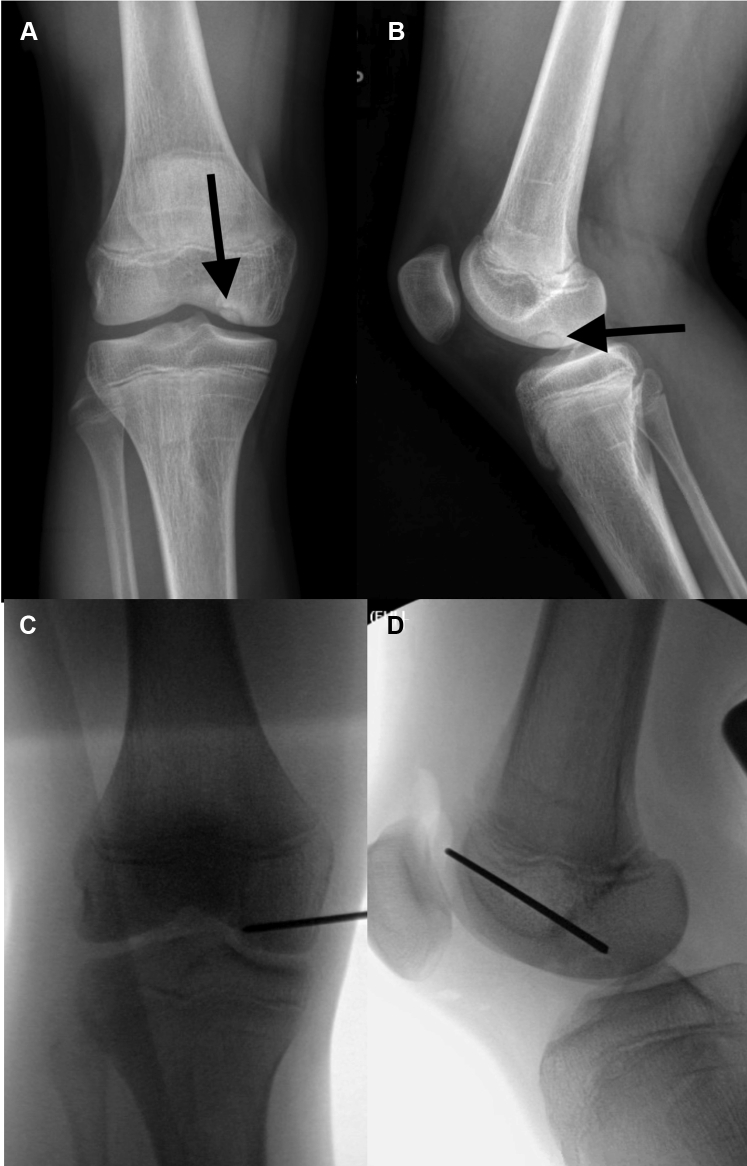
Fig 2.
(A) AP and (B) lateral preoperative radiographs demonstrating an OCD of the medial aspect of the medial femoral condyle of the right knee in a skeletally immature patient (identified with arrows). (C) AP and (D) lateral intraoperative fluoroscopic radiographs demonstrating localization of the OCD lesion and percutaneous placement of the guide pin within the OCD lesion in preparation for utilization of the acorn reamer. Note that the guide pin is placed distal to the distal femoral physis to avoid iatrogenic injury while reaming. (AP, anteroposterior; OCD, osteochondritis dissecans.)
Fig 3.
(A) Percutaneous placement of the guide pin with the right knee over a radiolucent tibial triangle to allows for easy fluoroscopic evaluation. Placement is then confirmed with AP and lateral fluoroscopy. (B) An incision has been made around the guide pin after it has been confirmed to be within the OCD. Dissection is taken down to the medial femoral cortex. An arthrotomy is made at this time to expose the medial femoral cartilage to ensure it is protected throughout the reaming process and to be able to further examine the OCD lesion. (AP, anteroposterior; OCD, osteochondritis dissecans.)
Fig 4.
(A) The 7-mm acorn reamer as it is placed over the guide pin through the arthrotomy and against the medial femoral cortex. (B) AP fluoroscopic image of the reamer being sent to the tip of the guide pin to core out the OCD lesion. Reaming is then repeated after placing the guide pin in different positions within the OCD and by pivoting the reamer in multiple directions to more completely remove the OCD lesion and create a large enough bony window on the medial femoral cortex to allow for endoscopic decompression. (AP, anteroposterior; OCD, osteochondritis dissecans.)
The guide pin and drill bit are then removed and a curved curette is used to remove the remnant OCD lesion, debride the margins of the cavity, and confirm that the cartilage has not been penetrated. This can be performed either under direct visualization through the cortical window or by placing the arthroscope within the cavity. Utilization of the arthroscope allows for more complete visualization of the lesion and underlying cartilage and allows for concomitant use of the curved curette to more accurately remove the remainder of the OCD (Fig 5). Final radiographs are then obtained to ensure complete decompression of the OCD lesion (Fig 6).
Fig 5.
(A) Incision required to perform this procedure as well as the cortical window within the medial femoral cortex formed during reaming. (B) An intraoperative picture looking from the medial cortex of the medial femoral condyle lateral toward femoral notch during the endoscopic decompression of the remainder of the OCD lesion after initial use of the acorn reamer. Here the arthroscopic has been placed in the cortical window and a small curette is used to debride the remainder of the OCD lesion. Relevant anatomic structures are numbered. (1) The backside of the articular cartilage after curettage of the overlying diseased subchondral bone. Care must be taken not to violate this cartilage during curettage. (2) The diseased bone of the OCD lesion that is removed during curettage. (3) Healthy subchondral bone outside of the borders of the OCD lesion. (OCD, osteochondritis dissecans.)
Fig 6.
(A) A curette is placed within the cavity to determine the margins of resection under fluoroscopic guidance. (B) AP and (C) lateral final fluoroscopic radiographs demonstrating complete resection of the OCD lesion with the area of resection marked with ovals. (AP, anteroposterior; OCD, osteochondritis dissecans.)
After appropriate OCD decompression, the BMAC is mixed with a bone scaffold, typically demineralized bone matrix (DBX) putty (DePuy Synthes, Raynham, MA), to create a paste that is used to fill the resultant cavity (Fig 7). Before filling of the cavity, the wound undergoes final irrigation to not disrupt the BMAC paste after placement. Any extra BMAC can be injected into the base of the cavity before filling it with the paste. The paste should be of a consistency that it requires a large-bore plastic catheter to easily inject it into the bony cavity (Fig 8). To maximize the volume of BMAC paste injected, the surgeon places his or her fingers around the catheter to minimize any back flow out of the cavity.
Fig 7.
(A) Five milliliters of DBX putty mixed with 3 mL of BMAC to form a paste. (B) The paste has been loaded into a 20-mL syringe fitted with a flexible large bore catheter for easy injection into the cavity after decompression. (BMAC, bone marrow aspirate concentration; DBX, demineralized bone matrix.)
Fig 8.
(A) Demonstrating injection of the DBX-BMAC mixture using a large-bore catheter into the cavity and (B) after bone grafting is complete. Note the entirety of the cavity has been filled with bone graft. Deep tissue closure will help contain the graft within the cavity. (BMAC, bone marrow aspirate concentration; DBX, demineralized bone matrix.)
At the conclusion of the case, the aperture of the cavity is sealed with fibrin glue to prevent influx of joint fluid, the deep fascia is closed with a heavy absorbable suture, and the skin and portal incisions are closed in layer fashion per surgeon preference. Meticulous deep closure is important to help close down the space over the cortical window and assist the fibrin glue to contain the BMAC and bone graft within the defect. Sterile dressings are placed over both the incision and the bone marrow harvest site. The patient is then recovered and sent home the same day.
Postoperative Protocol
Toe-touch weightbearing is commenced for 4 weeks and then 50% partial weight bearing is allowed for 2 subsequent weeks to allow for sufficient bony healing in the cavity. Full weightbearing is allowed after 6 weeks. The patient can range the knee as tolerated immediately after surgery. Physical therapy begins 1 to 2 weeks after surgery for restoration of range of motion and isometric strengthening. The patient is allowed to ride a stationary bike immediately after surgery and can begin pool-based therapy once the incisions are fully healed. At the 6-week postoperative mark when the patient has returned to full weightbearing, nonimpact strengthening is begun. Radiographs are repeated at the 6-week and 3-month postoperative appointments. An MRI scan may be obtained at the 6-month postoperative mark as well. If appropriate healing is seen at the 3-month postoperative radiography, a return to impact activities is commenced.
Discussion
This procedure represents a paradigm shift for treatment of early stage OCD of the knee. Traditional drilling procedures, either trans or retroarticular, attempt to heal an OCD via a method of creeping substitution in which channels are created from the unhealthy bone of the OCD into the healthy surrounding bone. This allows blood and marrow elements to egress into the unhealthy bone and gradually revascularize it and replace it with healthy bone. Several studies have reported on the efficacy of traditional drilling procedures with success rates of 80% to 90%.9, 10, 11, 12 However, the process of creeping substitution is slow and the average healing time has been reported to be as long as 11 months for larger lesions and the definition of healing in these prior reports remains controversial.9, 10, 11, 12, 13 For example, Edmonds et al. reported that 25% of patients who underwent a retroarticular drilling still had incomplete resolution of the OCD on radiographs.3 With relatively short follow-up, it is unclear what will happen later as patients with incomplete radiographic healing transition to adulthood.
In this procedure, the OCD lesion is nearly or completely removed via direct retroarticular drilling and open curetting in a physeal sparing manner. Biologic stimulation with a scaffold (BMAC mixed with bone graft) is placed to fill in the cavity to stimulate bony reconstitution of the cavity. Core decompression with biologic stimulation has been performed successfully for avascular necrosis of the femoral head as well as spontaneous osteonecrosis of the knee with good results.15,16 This procedure follows the same logic as core decompression of the femoral head and condyles and allows for complete removal of the lesion rather than relying on the slow and sometimes unreliable process of creeping substitution. Utilization of BMAC to biologically stimulate and accelerate healing and bone graft integration has been shown to be safe and effective in both animal models and in clinical trials.17,18
There are some theoretical risks associated with this procedure when compared with trans or retroarticular drilling. First, this procedure requires a larger incision to access affected femoral condyle. Although the incision is small, it is still larger than those required for drilling, and thus there is the increased morbidity associated with this larger incision. Second, there is the risk of chondral perforation either with the initial reamer or while curetting. However, careful use of fluoroscopic guidance during reaming and utilization of an arthroscope during curetting can help to minimize this risk. Third, there are theoretical risks associated with iliac crest bone marrow harvest for BMAC procurement, including perforation of the inner or outer table of the pelvis and puncture injury to the intrapelvic soft-tissue structures. Finally, for larger lesions, a significant amount of bone is removed during core decompression, which can place the patient at theoretical risk for osteochondral insufficiency fracture in the postoperative phase. For this reason, weightbearing is restricted for 4 to 6 weeks after surgery until radiographic evidence of reconstitution of the lesion cavity is noted. To date, the senior authors have not had any episodes of osteochondral fracture in short-term follow-up. The advantages and disadvantages of this technique are shown in Table 1, and pearls and pitfalls are shown in Table 2.
Table 1.
Advantages and Disadvantages
| Advantages | Disadvantages |
|---|---|
| Allows for direct visualization and complete decompression of OCD lesion. | Increased surgical time and morbidity associated with open surgery. |
| More quickly and reliably normalizes postoperative radiographs. | Endoscopic decompression is technically demanding and runs the risks of large cartilage violation. |
| Uses BMAC augmentation to assist in bony reconstitution. | Increased cost associated with BMAC use. |
BMAC, bone marrow aspirate concentration; OCD, osteochondritis dissecans.
Table 2.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Have multiple small curettes (curved, ring) available for endoscopic decompression. | Ensure start site does not violate MCL and is distal to distal femoral physis. |
| Perform endoscopic decompression under tourniquet to improve visualization. | Care must be taken not to injure cartilage with reamer passage or curette use. |
| Harvest BMAC early to allow time for centrifuge to occur. | Use frequent fluoroscopy as it can be difficult to orient during endoscopic decompression. |
BMAC, bone marrow aspirate concentration; MCL, medial collateral ligament.
In conclusion, RCD with BMAC and bone graft augmentation represents an effective procedure by which to directly and completely decompress an OCD lesion. It uses techniques that have been proven effective in the hip and knee and obviates the need to rely on the slow process of creeping substitution. This provides for more complete and reliable removal of the OCD lesion and biologic augmentation allows for faster healing. To date, empiric short-term data obtained by the senior authors have been promising with a future plan to publish outcome data when appropriate long-term follow-up is achieved.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: B.M. reports personal fees from Arthrex, DePuy Mitek, and Exatech, outside the submitted work. J.P. is a consultant for Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
This video uses an example case to describe in detail the relevant pathology as well as the surgical indications and technical details of the procedure.
References
- 1.Kocher M.S., Tucker R., Ganley T.J., Flynn J.M. Management of osteochondritis dissecans of the knee: Current concepts review. Am J Sports Med. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 2.Hefti F., Beguiristain J., Krauspe R. Osteochondritis dissecans: A multicenter study of the European Pediatric Orthopedic Society. J Pediatr Orthop B. 1999;8:231–245. [PubMed] [Google Scholar]
- 3.Edmonds E.W., Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471:1118–1126. doi: 10.1007/s11999-012-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obedian R.S., Grelsamer R.P. Osteochondritis dissecans of the distal femur and patella. Clin Sports Med. 1997;16:157–174. doi: 10.1016/s0278-5919(05)70012-0. [DOI] [PubMed] [Google Scholar]
- 5.Jones M.H., Williams A.M. Osteochondritis dissecans of the knee: A practical guide for surgeons. Bone Joint J. 2016;98-B:723–729. doi: 10.1302/0301-620X.98B6.36816. [DOI] [PubMed] [Google Scholar]
- 6.Wall E.J., Vourazeris J., Myer G.D. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am. 2008;90:2655–2664. doi: 10.2106/JBJS.G.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detterline A.J., Goldstein J.L., Rue J.P., Bach B.R. Evaluation and treatment of osteochondritis dissecans lesions of the knee. J Knee Surg. 2008;21:106–115. doi: 10.1055/s-0030-1247804. [DOI] [PubMed] [Google Scholar]
- 8.Heyworth B.E., Edmonds E.W., Murnaghan M.L., Kocher M.S. Drilling techniques for osteochondritis dissecans. Clin Sports Med. 2014;33:305–312. doi: 10.1016/j.csm.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Anderson A.F., Richards D.B., Pagnani M.J., Hovis W.D. Antegrade drilling for osteochondritis dissecans of the knee. Arthroscopy. 1997;13:319–324. doi: 10.1016/s0749-8063(97)90028-1. [DOI] [PubMed] [Google Scholar]
- 10.Boughanem J., Riaz R., Patel R.M., Sarwark J.F. Functional and radiographic outcomes of juvenile osteochondritis dissecans of the knee treated with extra-articular retrograde drilling. Am J Sports Med. 2011;39:2212–2217. doi: 10.1177/0363546511416594. [DOI] [PubMed] [Google Scholar]
- 11.Edmonds E.W., Albright J., Bastrom T., Chambers H.G. Outcomes of extra-articular, intra-epiphyseal drilling for osteochondritis dissecans of the knee. J Pediatr Orthop. 2010;30:870–878. doi: 10.1097/BPO.0b013e3181f5a216. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson L.D., Wojtys E.M. Extraarticular drilling for stable osteochondritis dissecans in the skeletally immature knee. J Pediatr Orthop. 2008;28:831–835. doi: 10.1097/BPO.0b013e31818ee248. [DOI] [PubMed] [Google Scholar]
- 13.Wall E.J., Milewski M.D., Carey J.L. The reliability of assessing radiographic healing of osteochondritis dissecans of the knee. Am J Sports Med. 2017;45:1370–1375. doi: 10.1177/0363546517698933. [DOI] [PubMed] [Google Scholar]
- 14.Haeri hendy S., De sa D., Ainsworth K., Ayeni O.R., Simunovic N., Peterson D. Juvenile osteochondritis dissecans of the knee: Does magnetic resonance imaging instability correlate with the need for surgical intervention? Orthop J Sports Med. 2017;5 doi: 10.1177/2325967117738516. 2325967117738516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah S.N., Kapoor C.S., Jhaveri M.R., Golwala P.P., Patel S. Analysis of outcome of avascular necrosis of femoral head treated by core decompression and bone grafting. J Clin Orthop Trauma. 2015;6:160–166. doi: 10.1016/j.jcot.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duany N.G., Zywiel M.G., Mcgrath M.S. Joint-preserving surgical treatment of spontaneous osteonecrosis of the knee. Arch Orthop Trauma Surg. 2010;130:11–16. doi: 10.1007/s00402-009-0872-2. [DOI] [PubMed] [Google Scholar]
- 17.Patterson T.E., Boehm C., Nakamoto C. The efficiency of bone marrow aspiration for the harvest of connective tissue progenitors from the human iliac crest. J Bone Joint Surg Am. 2017;99:1673–1682. doi: 10.2106/JBJS.17.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jäger M., Herten M., Fochtmann U. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res. 2011;29:173–180. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video uses an example case to describe in detail the relevant pathology as well as the surgical indications and technical details of the procedure.