Abstract
Background:
The optimal management of high-risk patients with differentiated thyroid cancer (DTC) consists of thyroidectomy followed by radioiodine (131I) therapy. The prescribed activity of 131I can be determined using two approaches: 1) empiric prescribed activity of 131I (E-Rx); and 2) dosimetry-based prescribed activity of 131I (D-Rx).
Aim:
The aim of the study was to compare the relative treatment efficacy and side effects of D-Rx vs. E-Rx.
Methods:
A retrospective analysis was performed of patients with distant metastases and/or locoregionally advanced radioiodine-avid DTC who were treated with either D-Rx or E-Rx. Response to treatment was based on RECIST (Response Evaluation Criteria in Solid Tumors) 1.1 criteria.
Results:
The study group consisted of 87 patients followed for 51 ± 35 months, of whom 43 were treated with D-Rx and 44 with E-Rx. Multivariate analysis, controlling for age, gender, and status of metastases revealed that the D-Rx group tended to be 70% less likely to progress (odds ratio, 0.29; 95% confidence interval, 0.087–1.02; P = 0.052) and more likely to obtain complete response (CR) compared to the E-Rx group (odds ratio, 8.2; 95% confidence interval, 1.2–53.5; P = 0.029). There was an association in the D-Rx group between the observed CR and percentage of maximum tolerable activity given as a first treatment of 131I (P = 0.030). The advantage of D-Rx was specifically apparent in the locoregionally advanced group because CR was significantly higher in D-Rx vs. E-Rx in this group of patients (35.7 vs. 3.3%; P = 0.009). The rates of partial response, stable disease, and progression-free survival, as well as the frequency of side effects, were not significantly different between the two groups.
Conclusion:
Higher efficacy of D-Rx with a similar safety profile compared to E-Rx supports the rationale for employing individually prescribed activity in high-risk patients with DTC.
The incidence of differentiated thyroid cancer (DTC) in the United States is increasing, with 44,670 new cases and 1,690 deaths due to thyroid cancer in 2010 (1). Indeed, DTC in women has the fifth highest incidence of all cancers, and from 1992 to 2000 the percentage change in thyroid cancer mortality in men increased by 2.4%, the largest increase of any type of cancer (2–6). Tumors presenting with locoregional extension, distant metastases (DM), or more aggressive variants are considered at higher risk for recurrence and death. The optimal management of patients with high risk DTC consists initially of total thyroidectomy with therapeutic neck dissection for patients with clinically involved central or lateral neck lymph nodes and prophylactic central compartment neck dissection in patients with papillary thyroid cancer (PTC) with advanced tumors. Surgical treatment is followed by 131I therapy. Depending on the risk stratification of the individual patient, the primary goal of the first dose of radioactive iodine (RAI) after surgery may be: 1) remnant ablation; 2) adjuvant therapy (to decrease risk of recurrence and disease-specific mortality by destroying suspected, but unproven metastatic disease); or 3) RAI therapy (to treat known persistent disease) (7). RAI may be administered by one of two possible methods: 1) use of fixed, arbitrary, or empiric prescribed activity of 131I (E-Rx) as is presently employed at most treatment centers; and 2) use of dosimetry-based prescribed activity of 131I (D-Rx) (8).
The rationale for the first approach (i.e. fixed prescribed activity) is based on its relatively lower cost, ease of administration, and a long history of use showing high efficacy and an acceptable rate and severity of side effects. However, recent analyses have indicated that therapies with fixed prescribed activity may be associated with a risk of either overtreatment, exceeding the safe radiation-absorbed dose to the blood (bone marrow), or undertreatment in specific individuals (9, 10). We report a dosimetric approach that is based on calculation of maximum tolerated activity (MTA) of 131I that would deliver a radiation dose to blood (considered a surrogate for the bone marrow) of 200 rad (2 Gy) or less, thus diminishing the likelihood of an adverse bone marrow effect (11). The theoretical rationale for administration of the MTA is based on the concept that a maximal safe prescribed activity should have greater therapeutic benefit than multiple smaller empiric activities that may induce nonlethal changes in the cancer tissue with subsequent cellular repair (12, 13).
Calculation of MTA is based on measurements of kinetics of a tracer-prescribed activity of 131I [usually 1–2 mCi (37–74 MBq) to minimize a possible “stunning effect”], administered 1–2 wk before anticipated therapy. This analysis is based on two compartments: 1) the contribution of the radiation-absorbed dose to the blood from the electron or β-component due to activity in the blood; and 2) the γ-ray contribution from activity throughout the whole body. After administration of the tracer activity of 131I, heparinized blood samples are obtained at 2, 24, 48, 72, 96, and 144 h to assess β-radiation and whole body scans (WBS) are performed at approximately the same time points to measure γ-radiation (8, 10).
Despite the possible advantages of D-Rx-guided therapy with 131I compared with E-Rx, no study has examined the relative merits of these two approaches.
The aim of this study was to compare a D-Rx approach to an E-Rx approach in the selection of the prescribed activity of 131I in regard to: 1) treatment efficacy; and 2) frequency of side effects.
Patients and Methods
We retrospectively analyzed medical records of patients with DTC treated and/or monitored at the Washington Hospital Center (WHC) and Georgetown University Hospital (GUH), Washington, D.C., between 1996 and 2009. High-risk patients with thyroid cancer who are admitted to WHC are treated routinely with a dosimetry-based approach, whereas patients at GUH are treated with empirically determined RAI activity.
Inclusion criteria were: 1) confirmed diagnosis of DTC after total or near-total thyroidectomy; 2) evidence of DM or locoregionally advanced (LA) disease (T4a and/or N1b) based on histopathological data and imaging studies [computer tomography (CT), magnetic resonance imaging, positron emission tomography-CT]; 3) RAI-avid disease documented by uptake on posttherapy RAI WBS; and 4) at least one complete follow-up examination after 131I therapy including CT and other imaging techniques (magnetic resonance imaging, positron emission tomography-CT, WBS) if indicated, and serum thyroglobulin (Tg) levels. All CT scans were performed without administration of iv radiocontrast using a slice thickness no greater than 5 mm. The study group was divided into two subgroups: D-Rx, patients treated at WHC exclusively with individually determined 131I activity based on calculated MTA (8, 11); and E-Rx, patients treated exclusively with empiric prescribed activity of 131I.
It is believed that assessing these separate groups of patients who only received empiric or dosimetric treatment would lead to more conclusive results than if we had considered individual patients who had received both empiric and dosimetric treatments during the course of disease. The study protocol was approved by the Institutional Review Boards of WHC and GUH.
The patients' staging was based on the American Joint Committee on Cancer guidelines (7).
The response to therapy was defined according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria (14). The study included patients with target lesions as well as exclusively nontarget lesions. Definitions of target and nontarget lesions as well as definitions of response to 131I therapy [complete response (CR), partial response (PR) for patients with target lesions, stable disease (SD), and progressive disease (PD)] are presented in Supplemental Tables 1 and 2 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). The best overall response was assessed based on CT scans performed after the end of 131I therapy (14). Progression-free survival (PFS) was assessed from the day of the last 131I therapy until the last CT scan performed during the follow-up period. The effect of RAI therapy on percentage change in Tg levels (ΔTg) was based on the difference between the 1) baseline nonstimulated Tg measured after surgery but before RAI therapy; and 2) suppressed Tg measured after the last follow-up study. During the subsequent years of follow-up, Tg measurements were performed with three immunometric assays with functional sensitivities of 0.2, 0.5, and 0.9 ng/ml and were analyzed by Quest Diagnostics (Madison, NJ), LabCorp (Burlington, NC), and the WHC Laboratory (Washington, D.C.). All patients were screened for anti-Tg antibody using the chemiluminescence immunoassay at all three laboratories.
The frequency and grade of leukopenia and thrombocytopenia were documented by complete blood counts performed approximately 4–6 wk after 131I therapy (Supplemental Table 3) (15). Frequency of xerostomia was assessed for the individuals for whom there was clinical record information regarding the presence/absence of symptoms of chronic dry mouth after 131I therapy. Occurrence of pulmonary fibrosis was assessed based on documentation of the presence/absence of respiratory signs or symptoms warranting further diagnostic procedures, including pulmonary function tests.
Statistical analysis
Continuous variables were compared by t test and Mann-Whitney test. Pearson's χ2 and Fisher's exact tests were employed for comparison of categorical variables. A multivariate logistic regression model with variables potentially affecting the treatment response was used to compare treatment efficacy. Kaplan-Meier analysis was performed to assess PFS after the last 131I treatment.
Results
The study group consisted of 87 patients, 48 women and 39 men, whose tumors were histologically classified as 60.9% PTC, 12.6% follicular variant of PTC, 5.8% follicular thyroid cancers, 6.9% Hurthle cell thyroid cancers, 8.0% insular, and 5.8% tall cell variant thyroid cancer. There was no difference in the histological breakdown between the group of patients treated with D-Rx and E-Rx (P = 0.332). Within the study group, 71.3% of patients presented with exclusively nontarget lesions, 25.3% presented with both target and nontarget lesions, and the remaining 3.4% presented with exclusively target lesions. During follow-up (mean, 52 ± 35 months), 43 patients received one to four D-Rx, and 44 individuals received one to four E-Rx-based therapies.
Multivariate analysis of the study group
The logistic regression model revealed that controlling for age, gender, and the status of metastases, patients in the D-Rx group tended to be 70% less likely to progress compared with the E-Rx group [odds ratio, 0.29; 95% confidence interval (CI), 0.087–1.02; P = 0.052). Controlling for the same confounders, the D-Rx group was found to be more likely to obtain CR compared with the E-Rx group (odds ratio, 8.2; 95% CI, 1.2–53.5; P = 0.029). Clinical data of the patients who did obtain CR after the treatment are presented in Supplemental Tables 4 and 5. There was an association in the D-Rx group between the observed CR and percentage of MTA given as a first treatment of 131I (P = 0.030). Additionally, in the D-Rx group, there was an association between total cumulative prescribed activity of 131I and disease stabilization, lasting either during the whole follow-up period (P = 0.034) or transiently (P = 0.039). This association was not observed in the E-Rx group.
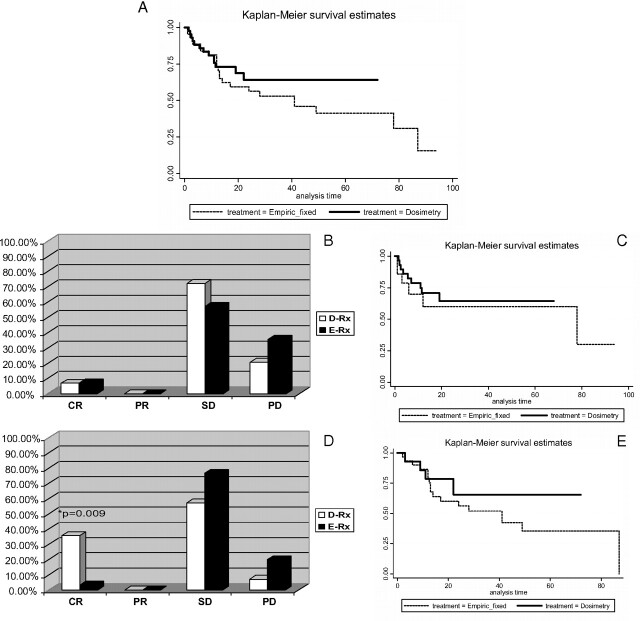
Although PFS was not significantly different between the D-Rx and E-Rx groups (P = 0.34), the Kaplan-Meier curves show a trend toward longer PFS in patients treated with D-Rx (Fig. 1A).
Fig. 1.
Objective response to the treatment with 131I. A, Whole study group. Kaplan-Meier curves of PFS for patients treated with D-Rx vs. E-Rx (P = 0.34). B and C, Patients with DM. B, No statistically significant differences in the treatment efficacy in patients with DM treated with D-Rx vs. E-Rx [CR, two of 29 (6.9%) vs. one of 14 (7.1%), P = 0.704; PR, 0 of 29 vs. 0 of 14, P = 1.0; SD, 21 of 29 (72.4%) vs. eight of 14 (57.2%), P = 0.202; and PD, six of 29 (20.7%) vs. five of 14 (35.7%), P = 0.244]. C, No differences in PFS in the DM group treated with D-Rx vs. E-Rx (P = 0.61). D and E, Patients with LA disease. D, Higher rate of CR in D-Rx group compared with E-Rx [five of 14 (35.7%) vs. one of 30 (3.3%); P = 0.009]. No differences in PR, SD, PD [PR, 0 of 14 (0%) vs. 0 of 30 (0%), P = 1.0; SD, eight of 14 (57.2%) vs. 23 of 30 (76.7%), P = 0.951; PD, one of 14 (7.1%) vs. six of 30 (20.0%), P = 0.270]. E, No difference in PFS in the LA group of patients treated with D-Rx vs. E-Rx (P = 0.422). DM, Distant metastases; LA, locally advanced.
Subgroup analysis
Baseline characteristics of the subgroups of patients with DM and LA disease are presented in Table 1.
Table 1.
Baseline characteristics of the study groups
| D-Rx | E-Rx | P value | |
|---|---|---|---|
| Patients with distant metastases | |||
| n | 29 | 14 | |
| Age (yr) | 55 ± 14.9 | 52.7 ± 19.2 | 0.67 |
| Sex | 48% females, 52% males | 43% females, 57% males | 0.739 |
| Tumor size (cm) | 4.4 ± 2.8 | 2.7 ± 1.4 | 0.068 |
| % of patients with LN metastases at diagnosis | 63.6 | 69.2 | 0.736 |
| % of patients with extrathyroidal extension | 40.7 | 30.8 | 0.54 |
| % of patients with pulmonary metastases countable micronodular | 20.7 | 21.4 | 0.955 |
| % of patients with pulmonary metastases countable macronodular | 17.2 | 14.3 | 0.806 |
| % of patients with micronodular multiple pulmonary metastases | 69.0 | 42.9 | 0.101 |
| % of patients with bone metastases | 41.4 | 35.7 | 0.722 |
| % of patients with atypical metastases (liver, esophagus, kidney, subcutaneous) | 17.2 | 7.1 | 0.371 |
| Sum of maximum diameter of target lesions (cm) | 5.8 ± 3.6 | 3.2 ± 1.9 | 0.200 |
| Baseline nonstimulated Tg (ng/ml) before the treatment | 4851.3 ± 2445 | 1010 ± 476 | 0.351 |
| Patients with locally advanced disease, without distant metastases | |||
| n | 14 | 30 | |
| Age (yr) | 51.3 ± 15.9 | 40.9 ± 12.7 | 0.025a |
| Sex | 36% females 64% males | 77% females 23% males | 0.009a |
| Tumor size (cm) | 3.4 ± 1.5 | 2.3 ± 1.2 | 0.048a |
| T4N0/N1aM0 | 21.4% | 20% | 0.922 |
| T4N1bM0 | 57.2% | 56.7% | |
| T1-3N1bM0 | 21.4% | 23.3% | |
| No. of positive LN | 6 (95% CI, 1–12) | 7 (95% CI, 4–10) | 0.652 |
| Positive surgical margins | 35.7% | 43.3% | 0.444 |
| Median nonstimulated Tg (ng/ml) before the treatment | 13.9 | 9.7 | 0.372 |
Patients with distant metastases: No differences in terms of age, sex, tumor size, distribution of patients with countable and multiple micro- and macronodular pulmonary metastases, bone lesions, atypical metastases, sum of dimension of the target lesions, or in the baseline nonstimulated Tg levels between patients treated with D-Rx compared to the E-Rx.
Patients with locally advanced disease: Patients treated with D-Rx were older compared to the E-Rx group and were characterized by a higher percentage of males and larger thyroid tumor size. Completeness of surgical excision of the tumor, number of metastatic lymph nodes, and baseline nonstimulated Tg levels did not differ between the D-Rx and E-Rx groups. LN, Lymph node.
Statistically significant difference.
Patients with distant metastases
Treatment modalities applied to the DM group are characterized in Table 2. The first prescribed activity was significantly higher for the D-Rx group than for the E-Rx group (mean, 251 vs. 164 mCi; P < 0.05, respectively). The total cumulative prescribed activity received by the patients during the follow-up period was also higher for the D-Rx group, but the difference was only of marginal statistical significance [mean, 393.4 mCi (14.6 GBq) vs. 347.7 mCi (12.9 GBq); P = 0.055, respectively]. Adjunctive treatments such as surgical excision of metastatic lesions, radiotherapy, or treatment with zoledronic acid for bone metastases were similar in both the D-Rx and E-Rx groups. The rates of CR, PR, SD, and PD were similar between the D-Rx and E-Rx patients (Fig. 1B). CR once obtained was present during the subsequent follow-up period (Supplemental Tables 4 and 5), whereas SD was transient in 10.3% of D-Rx patients and 7.1% of E-Rx patients. These patients developed PD documented by imaging studies performed on average every 5 months over 23.5 months (95% CI, 16.1–30.8) in the D-Rx group and every 6 months over 38.2 months (95% CI, 19.6–56.8) in the E-Rx group. PFS was not different between the D-Rx and E-Rx groups (P = 0.61; Fig. 1C). Four patients (13.8%) treated with D-Rx and two patients (14.3%) treated with E-Rx died, but the direct causes of death in these cases were not available for analysis.
Table 2.
Therapy applied to the study groups
| Patients with distant metastases |
Patients with locally advanced disease |
|||||
|---|---|---|---|---|---|---|
| D-Rx | E-Rx | P value | D-Rx | E-Rx | P value | |
| n | 29 | 14 | 14 | 30 | ||
| First dose activity | 251.3 ± 96.5 | 164 ± 34.9 | 0.002a | 303.5 ± 75.4 | 148.9 ± 27.9 | 0.000a |
| Median % of MTA given as a first dose | 97% | n/a | 79.1% | n/a | ||
| rhTSH aided 131I treatment (% of patients) | 40.7% | 21.4% | 0.305 | 35.7% | 3.3% | 0.009a |
| No. of repeated doses | ||||||
| 1 dose | 55.2% | 57.1% | 0.171 | 78.6% | 66.7% | 0.694 |
| 2 doses | 41.4% | 28.6% | 14.3% | 23.3% | ||
| 3 doses | 3.4% | 0% | 0% | 6.7% | ||
| 4 doses | 0% | 14.3% | 7.1% | 3.3% | ||
| Total cumulative dose (mCi) | 393.4 ± 197.8 | 347.7 ± 297 | 0.055 | 362.9 ± 167.3 | 226.8 ± 128.2 | 0.005a |
| Median % of MTA administered as cumulative dose | 91.1% | n/a | 73.3% | n/a | ||
| Mean urine iodine (μg/liter) | 94.4 ± 66.3 | 125.3 ± 81.7 | 0.475 | 88.8 ± 70.1 | 55.9 ± 70.0 | 0.381 |
| Additional surgical treatment of metastatic lesions | 31.0% | 21.4% | 0.720 | 21.4% | 50% | 0.069 |
| Additional EBRT | 41.4% | 14.3% | 0.073 | 14.3% | 10.0% | 0.515 |
| Treatment with zoledronic acid | 31.0% | 14.3% | 0.291 | n/a | n/a | n/a |
| Total duration of follow-up (months) | 38.5 ± 27.0 | 67.6 ± 41.6 | 0.008a | 40.1 ± 23.3 | 62.5 ± 38.7 | 0.628 |
| Duration of follow-up after the last dose of 131I (months) | 23.5 ± 19.3 | 38.2 ± 32.1 | 0.067 | 29.0 ± 20.5 | 48.9 ± 27.5 | 0.020a |
Patients with distant metastases: First dose activity was significantly higher for the D-Rx group than for the E-Rx group (251 vs. 164 mCi, P = 0.002,), the total cumulative dose was also higher for the D-Rx group, but the difference with marginal statistical significance (393.4 vs. 347.7, P = 0.055, respectively). The cumulative dose administered to the patients from D-Rx group constituted median 91.1% of calculated MTA. Additional surgical excision of metastatic lesions, radiotherapy, or treatment with zoledronic acid for the bone metastases was similar in both the D-Rx and E-Rx groups.
Patients with locally advanced disease: First dose activity and total cumulative dose were significantly higher for the D-Rx group compared to E-Rx (303.5 vs. 148.9, P = 0.000 < 0.05; and 362.9 vs. 226.8, P = 0.005, respectively). The cumulative dose administered to the patients from the D-Rx group constituted median 73.3% of calculated MTA. Application of the other treatment modalities such as additional surgical excision of the metastatic lesions and EBRT was similar in both the D-Rx and E-Rx groups. rhTSH, Recombinant human TSH; n/a, not applicable.
Statistically significant difference.
The assessment of ΔTg after therapy was not possible in two of 29 patients treated with D-Rx and four of 14 patients treated with E-Rx due to the presence of interfering anti-Tg antibodies. In 78% (21 of 27) of patients treated with D-Rx and 60% (six of 10) of patients treated with E-Rx, Tg decreased after treatment (D-Rx vs. E-Rx, P = 0.248). The degree of decrease in Tg levels is presented in Supplemental Table 6.
Patients with locally advanced (LA) disease
Treatment modalities applied to the LA group are described in Table 2. The prescribed activity of the first treatment and the total cumulative prescribed activity were significantly higher for the D-Rx group compared with E-Rx [303.5 mCi (11.2 GBq) vs. 148.9 (5.5 GBq), P < 0.05; and 362.9 mCi (13.4 GBq) vs. 226.8 mCi (8.4 GBq), P < 0.05, respectively]. In this subgroup, 35.7% of patients treated with D-Rx and 43.3% of patients treated with E-Rx had known persistent disease based on positive surgical margins (D-Rx vs. E-Rx, P = 0.444) at the time of RAI therapy. In the remainder of patients with suspected persistent disease, lateral RAI neck uptake highly suggestive for residual disease was similar between the D-Rx and E-Rx groups (D-Rx vs. E-Rx, 44.4 vs. 35.3%; P = 0.81) (Table 3). The outcome as a function of the primary goal of RAI administration (RAI therapy for known persistent disease vs. RAI adjuvant treatment for suspected disease) is presented in Table 3.
Table 3.
The locally advanced group outcome as a function of the primary goal of RAI administration (therapeutic vs. adjuvant)
| No. of patients | D-Rx |
E-Rx |
||||||
|---|---|---|---|---|---|---|---|---|
| RAI therapy for known persistent disease [5/14 (35.7%)] |
Adjuvant therapy for suspected disease [9/14 (64.3%)] |
RAI therapy for known persistent disease [13/30 (43.3%)] |
Adjuvant therapy for suspected disease [17/30 (56.6%)] |
|||||
| Central neck uptake | Lateral neck uptake | Central neck uptake | Lateral neck uptake | Central neck uptake | Lateral neck uptake | Central neck uptake | Lateral neck uptake | |
| Total | 4 | 1 | 5 | 4 | 10 | 3 | 11 | 6 |
| CR | 2 | 0 | 3 | 0 | 0 | 0 | 1 | 0 |
| SD | 2 | 1 | 2 | 3 | 9 | 2 | 6 | 6 |
| PD | 0 | 0 | 0 | 1 | 1 | 1 | 4 | 0 |
Application of other treatment modalities such as additional surgical excision of the metastatic lesions and external beam radiation therapy (EBRT) was similar in both the D-Rx and E-Rx groups. There was a significantly higher rate of CR in D-Rx-treated patients compared with the E-Rx group [five of 14 (35.7%) vs. one of 30 (3.3%); P = 0.009] (Fig. 1D and Supplemental Tables 4 and 5). The rate of PR, SD, and PD was similar in patients having D-Rx or E-Rx (Fig. 1D). Once obtained, CR was maintained during the follow-up period (Supplemental Tables 4 and 5), whereas SD was transient in 21.4% (three of 14) of D-Rx patients and 36.7% (11 of 30) of E-Rx patients (P = 0.257). These patients developed PD documented by imaging studies performed in a median frequency every 8.3 months for 29.0 months after the last 131I treatment (95% CI, 17.2–40.9) in the D-Rx group and every 9 months for 48.9 months (95% CI, 38.7–59.2) in the E-Rx group (Fig. 1E).
Although PFS was not significantly different between the D-Rx and E-Rx groups (P = 0.422), there was a trend favoring longer PFS in patients treated with dosimetry (Fig. 1E).
Assessment of ΔTg after treatment was not possible in six of 14 D-Rx patients and 12 of 30 E-Rx patients due to the presence of interfering anti-Tg antibodies. In the six of eight (75%) D-Rx patients and 16 of 18 (89%) E-Rx patients who could be evaluated, baseline Tg decreased after treatment, but no significant difference in the ΔTg was demonstrated (P = 0.359) (Supplemental Table 6).
Frequency of side effects
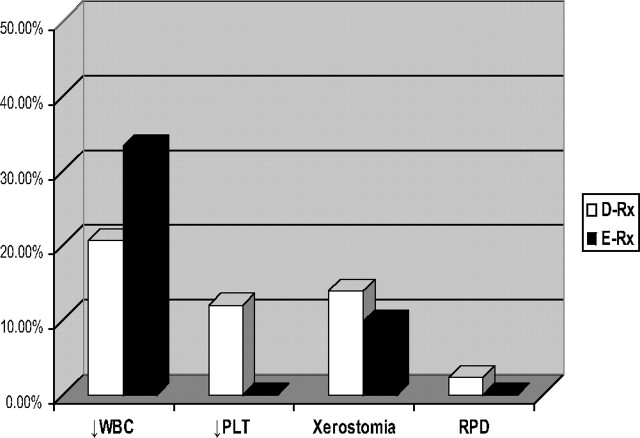
The frequency of side effects was not different between patients treated with D-Rx compared with E-Rx (Fig. 2). Total [white blood cell (WBC)] counts before and 4–6 wk after the 131I therapy were available for 29 of 43 patients from the D-Rx group and for 12 of 44 patients from the E-Rx group, whereas platelet counts were available for 25 of 43 patients from D-Rx and 10 of 44 patients from the E-Rx group. Leukopenia was observed in six of 29 (20.7%) patients treated with D-Rx (grade I according to World Health Organization criteria in four patients and grade II in two patients) and four of 12 (33.3%) patients treated with E-Rx (only grade I) (D-Rx vs. E-Rx, P = 0.894). Thrombocytopenia was observed in three of 25 (12%) patients treated with D-Rx (grade I in one patient, grade II in one patient, and grade IV in one patient) and none of the patients treated with E-Rx (D-Rx vs. E-Rx, P = 0.351). However, transient decreases in blood counts, but not reaching the threshold value for diagnosis of leukopenia or thrombocytopenia, were very common. The total WBC count decreased by more than 10% from the baseline level in 19 of 29 (65%) patients, reaching a nadir approximately 4–6 wk after the treatment in D-Rx patients and in six of 12 (50%) patients treated with E-Rx (D-Rx vs. E-Rx, P = 0.179). Platelet counts decreased by more than 10% in 22 of 25 (88%) patients treated with D-Rx and in six of 10 (60%) patients treated with E-Rx (D-Rx vs. E-Rx, P = 0.084). Xerostomia was observed in 13.9% of patients from the D-Rx group and 9.8% from the E-Rx group. One patient from the D-Rx group developed restrictive pulmonary disease despite the fact that the 48-h whole body 131I retention was lower than 80 mCi (3.0 GBq), the level considered to be consistent with little risk of pulmonary fibrosis in patients with high-volume pulmonary disease (16). Restrictive pulmonary disease in this case could have been due to underlying pulmonary disease compounded by radiation-induced injury. The precise clinical data regarding this patient appear in Supplemental Table 7.
Fig. 2.
No difference in the frequency of adverse side effects in patients treated with D-Rx vs. E-Rx. PLT, Platelets; RPD, restrictive pulmonary disease.
Discussion
Advantages to approaches by dosimetrically determined prescribed activity include individualized determination of the MTA of 131I and identification of patients for whom empiric fixed prescribed activity would exceed the maximal safe radiation-absorbed dose. This study is the first to document the clinical benefit of therapy with D-Rx compared with E-Rx in high-risk patients with thyroid cancer. Multivariate analysis, including considerations of age, sex, and the status of metastases, revealed that the odds of progressing decreased by a factor of 0.29 for the D-Rx group and showed significant benefit in increasing the likelihood of CR with D-Rx compared with E-Rx. However, the latter result must be interpreted with caution due to the overall small number of patients who obtained CR. Importantly, this advantage can be achieved without increasing adverse side effects.
It is important to underscore that the multivariate model controlled for the status of metastases because of the unequal distribution of patients with DM and LA disease between the D-Rx and E-Rx groups. However, to further elucidate in which subgroup of patients dosimetry-based treatment was specifically beneficial, we provided the separate subgroup analysis of patients with DM and patients with LA disease.
The clinical benefit of dosimetric treatment was specifically apparent in the subgroup of patients with LA DTC, as documented by the significantly higher relative number of patients who obtained CR after D-Rx-based therapy than after E-Rx, despite the more prevalent risk factors for a worse prognosis in this subgroup, including older age and larger tumors.
An advantage of D-Rx was not so clearly apparent for the patients with DM. Although the rate of nonresponders to the treatment presenting with PD was lower in patients given D-Rx, the difference was not statistically significant. Large volume 131I-avid disease is associated with high retention of 131I, thus the administration of dosimetric activity was not significantly higher than the dosage given by E-Rx (393.4 vs. 347.7 mCi, P = 0.055, respectively). However, given that high empiric activity may not be safe (9, 10), individualized D-Rx potentially reduces risk to bone marrow. The absence of patients obtaining PR after treatment was deemed as being due to the high prevalence of patients with exclusively nontarget disease, for whom PR could not be assessed.
Results of the current study cannot be compared directly with previous reports of high-risk DTC patients treated either with a dosimetry-based approach or an empiric fixed one because of different definitions of response. For example, among 70 patients with metastatic DTC given D-Rx by Benua and Leeper (17), 58% were described as “cured” if they had negative findings on x-ray, clinical examination, and radioiodine scan. In more recent studies, treatment efficacy has been assessed predominantly by the observed responses of serum Tg to 131I therapy. The latter parameter has several limitations, especially in highly aggressive cancers that might dedifferentiate and not produce a significant amount of Tg despite a large tumor burden. In a recent small retrospective study, Verburg et al. (18) documented satisfactory biochemical response to treatment with a standard blood (bone marrow) dosimetry-based approach for 10 patients with DTC followed for a median of 24 months (18, 19). However, this study had no control group, and only four patients were treated with an exclusively dosimetry-based approach, whereas six were pretreated with one or multiple empiric fixed treatments of 131I, thus precluding the clear distinction between the cumulative efficacy of previous treatments and the studied dosimetry-based approach. Lee et al. (20) documented that the MTA constitutes effective therapy for patients who fail to respond adequately to conventional fixed therapy. In their protocol, the MTA, once calculated, was administered to 47 patients at 6-month intervals and led to CR in 14.9% of patients, PR in 31.9%, SD in 40.4%, and PD in 12.8%. However, in this study chest CT was performed in only 13 of 21 (62%) patients with lung metastases. For patients who achieved CR, the mean 131I activity administered was higher than that observed in our study [1486 mCi (55.0 GBq) ± 757.1 mCi (28.0 GBq)]. This could relate to achievement of a total activity more closely approximating the MTA in concordance with our observation that CR rate is dependent on percentage of MTA administered to the patient.
All of the above-mentioned studies were based on the classic Benua and Leeper dosimetry-based approach, focusing on the calculation of the MTA to the blood (bone marrow) (8, 11). This approach does not assess what activity is taken up by the individual metastatic lesions. Thomas et al. (21) demonstrated that the radiation-absorbed dose to metastatic lesion, e.g. lymph nodes, can be individually identified and quantified. They observed that the likelihood of cure could be increased from 20% to approximately 90% by increasing the prescribed activity delivered to a lesion from 40 to 80 rad (cGy) (21). This observation formed the rationale for the application of another dosimetric approach combining blood (bone marrow) dosimetry with lesional dosimetry. Using this approach, Dorn et al. (22) achieved CR with tumor doses ranging from 10,000 to 15,000 rad (100 to 150 Gy). In this study, 12 patients (38%) showed decreased Tg levels of below 1 ng/ml after 131I treatment, and nine of them maintained Tg levels below 1 ng/ml over a mean follow-up of 4.3 yr. During the follow-up period, 19% died of disease, and 13% died of other causes.
It is worthwhile to compare the relative efficacy of dosimetry-based RAI treatment with the reported response to treatment with empiric prescribed activity. Widely cited data on the efficacy of the empiric fixed dosage approach has been reported by Schlumberger et al. (23), who observed a remission rate for lung metastases of 50% with a 10-yr survival rate of 61%, a remission rate of 10% for bone metastases with a 10-yr survival rate of 21%, and a remission rate of 7% for lung and bone metastases with a 10-yr survival rate of 13%.
The strength of the present study is the direct comparison of the therapeutic efficacy between the patients treated exclusively with a dosimetry-based approach with individuals treated solely with the empiric fixed one based on the same RECIST criteria of the response to treatment. To more clearly interpret the data and in contrast to earlier studies, patients who had 131I treatments with both empiric and dosimetry regimens were not included in the study population.
Our study has limitations that include its retrospective design and potential confounders that could affect treatment efficacy. However, age, sex, and status of metastases were taken into consideration in multivariate analysis. Although the study sample did not allow adjustments for additional confounders, the distribution of those was equal between the study groups. Clearly, the results have potential significant clinical implications, providing important evidence of the advantage of dosimetry-based treatment with 131I relative to the standard empiric fixed approach, and should form a basis for larger prospective studies to confirm these findings.
Supplementary Material
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- CI
Confidence interval
- CR
complete response
- DM
distant metastases
- D-Rx
dosimetry-based prescribed activity of 131I
- DTC
differentiated thyroid cancer
- EBRT
external beam radiation therapy
- E-Rx
empiric prescribed activity of 131I
- LA
locally advanced
- MTA
maximum tolerated activity
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- PTC
papillary thyroid cancer
- RAI
radioactive iodine
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- Tg
thyroglobulin
- ΔTg
change in Tg levels
- WBC
white blood cell
- WBS
whole body scan.
References
- 1. Jemal A , Siegel R , Xu J , Ward E. 2010. Cancer statistics 2010. CA Cancer J Clin 60:277–300 [DOI] [PubMed] [Google Scholar]
- 2. Davies L , Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1988–2005. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 3. Ries LA , Eisner MP , Kosary CL. 2006. SEER cancer statistics review, 1975–2000/2003. Bethesda, MD: National Cancer Institute; seer.cancer.gov/csr/1975_2001/ [Google Scholar]
- 4. Chen AY , Jemal A , Ward EM. 2009. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 5. Rego-Iraeta A , Pérez-Méndez LF , Mantinan B , Garcia-Mayor RV. 2009. Time trends for thyroid cancer in northwestern Spain: true rise in the incidence of micro and larger forms of papillary thyroid cancer. Thyroid 19:333–340 [DOI] [PubMed] [Google Scholar]
- 6. Enewold L , Zhu K , Ron E , Marrogi AJ , Stojadinovic A , Peoples GE , Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS , Doherty GM , Haugen BR , Kloos RT , Lee SL , Mandel SJ , Mazzaferri EL , McIver B , Pacini F , Schlumberger M , Sherman SI , Steward DL , Tuttle RM. 2009Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 8. Van Nostrand D , Atkins F , Yeganeh F , Acio E , Bursaw R , Wartofsky L. 2002. Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid 12:121–134 [DOI] [PubMed] [Google Scholar]
- 9. Kulkarni K , Van Nostrand D , Atkins F , Aiken M , Burman K , Wartofsky L. 2006. The relative frequency in which empiric dosages of radioiodine would potentially overtreat or undertreat patients who have metastatic well-differentiated thyroid cancer. Thyroid 16:1019–1023 [DOI] [PubMed] [Google Scholar]
- 10. Tuttle RM , Leboeuf R , Robbins RJ , Qualey R , Pentlow K , Larson SM , Chan CY. 2006. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med 47:1587–1591 [PubMed] [Google Scholar]
- 11. Benua RS , Cicale NR , Sonenberg M , Rawson RW. 1962. The relation of radioiodine dosimetry to results and side effects in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Ther Nucl Med 87:171–182 [PubMed] [Google Scholar]
- 12. Samuel AM , Rajashekharrao B , Shah DH. 1998. Pulmonary metastases in children and adolescents with well-differentiated thyroid cancer. J Nucl Med 39:1531–1536 [PubMed] [Google Scholar]
- 13. Lassmann M , Reiners C , Luster M. 2010. Dosimetry and thyroid cancer: the individual dosage of radioiodine. Endocr Relat Cancer 17:R161–R172 [DOI] [PubMed] [Google Scholar]
- 14. Eisenhauer EA , Therasse P , Bogaerts J , Schwartz LH , Sargent D , Ford R , Dancey J , Arbuck S , Gwyther S , Mooney M , Rubinstein L , Shankar L , Dodd L , Kaplan R , Lacombe D , Verweij J. 2009. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247 [DOI] [PubMed] [Google Scholar]
- 15. Van Nostrand D , Freitas J. 2006. Side effects of 131-I for ablation and treatment of well differentiated thyroid carcinoma. In: , Wartofsky L , Van Nostrand D eds. Thyroid cancer: a comprehensive guide to clinical management. 2nd ed Totowa, NJ: Humana Press; 459–485 [Google Scholar]
- 16. Sgouros G , Song H , Ladenson PW , Wahl RL. 2006. Lung toxicity in radioiodine therapy of thyroid carcinoma: development of a dose-rate method and dosimetric implications of the 80-mCi rule. J Nucl Med 47:1977–1984 [PMC free article] [PubMed] [Google Scholar]
- 17. Benua RS , Leeper RD. 1986. A method and rationale for treating metastatic thyroid carcinoma with the largest safe dose of I-131. In: , Medeiros-Neto G , Gaitan E eds. Frontiers in thyroidology. Vol. 2 New York: Plenum Medical Book Co.; 1317–1321 [Google Scholar]
- 18. Verburg FA , Hänscheid H , Biko J , Hategan MC , Lassmann M , Kreissl MC , Reiners C , Luster M. 2010. Dosimetry-guided high-activity (131)I therapy in patients with advanced differentiated thyroid carcinoma: initial experience. Eur J Nucl Med Mol Imaging 37:896–903 [DOI] [PubMed] [Google Scholar]
- 19. Lassmann M , Hänscheid H , Chiesa C , Hindorf C , Flux G , Luster M, EANM Dosimetry Committee 2008EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur J Nucl Med Mol Imaging 35:1405–1412 [DOI] [PubMed] [Google Scholar]
- 20. Lee JJ , Chung JK , Kim SE , Kang WJ , Park do J , Lee DS , Cho BY , Lee MC. 2008. Maximal safe dose of I-131 after failure of standard fixed dose therapy in patients with differentiated thyroid carcinoma. Ann Nucl Med 22:727–734 [DOI] [PubMed] [Google Scholar]
- 21. Thomas SR , Maxon HR , Kereiakes JG. 1976. In vivo quantitation of lesion radioactivity using external counting methods. Med Phys 3:253–255 [DOI] [PubMed] [Google Scholar]
- 22. Dorn R , Kopp J , Vogt H , Heidenreich P , Carroll RG , Gulec SA. 2003. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med 44:451–456 [PubMed] [Google Scholar]
- 23. Schlumberger M , Challeton C , De Vathaire F , Travagli JP , Gardet P , Lumbroso JD , Francese C , Fontaine F , Ricard M , Parmentier C. 1996. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med 37:598–605 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.