Abstract
Context:
Elevated levels of TSH markedly enhance the effectiveness of radioiodine (RAI) therapy in metastatic thyroid cancer.
Objective:
The objective of the study was to compare short-term overall survival in thyroid cancer patients with RAI-avid distant metastases prepared for RAI therapy with either traditional thyroid hormone withdrawal (THW) or recombinant human TSH (rhTSH) stimulation.
Design:
This was a retrospective chart review.
Setting:
The study was conducted at a tertiary care comprehensive cancer center.
Patients:
Patients included 175 patients with RAI avid metastatic disease to lung and/or bone.
Interventions:
In 58 patients, all RAI treatments (remnant ablation and therapy of metastatic disease) were done with rhTSH stimulation. In 35 patients, all RAI treatments were done after THW. In 82 patients, THW was used for initial RAI treatment(s) with subsequent administered activities given after rhTSH stimulation.
Main Outcome Measure:
Overall survival was measured.
Results:
After a median follow-up of 5.5 yr, there were no significant differences in overall survival between patients prepared for RAI therapy with rhTSH alone, THW alone, or THW followed by rhTSH (Kaplan-Meier analysis,P = 0.80). In a multivariate analysis that included clinicopathological features and method of preparation (rhTSH or TWH), only age at diagnosis was an independent predictor of overall survival.
Conclusions:
Preparation for RAI therapy using either THW or rhTSH stimulation was associated with similar 5-yr overall survival rates in patients with RAI avid thyroid cancer metastases to lung or bone.
For more than 50 yr, radioiodine (RAI) has remained the mainstay of therapy for distant metastatic lesions arising from differentiated thyroid cancer. Previous studies have demonstrated that RAI therapy after traditional thyroid hormone withdrawal (THW) is associated with both an improvement in overall survival in patients with RAI avid distant metastases and even complete remissions in patients with small-volume RAI avid metastatic lesions (1,2).
Although recombinant human TSH (rhTSH) was initially approved in the United States in 1998 for diagnostic uses [an adjunct to diagnostic whole body RAI scanning and to stimulate thyroglobulin (Tg) production], numerous clinical studies over the last 10 yr led to its approval for use as preparation for RAI remnant ablation. However, the use of rhTSH in remnant ablation is restricted to those patients without known distant metastases (DM) because the effectiveness of rhTSH in the therapy of metastatic lesions has not been definitively demonstrated. The current American Thyroid Association guidelines note that rhTSH preparation for RAI therapy of DM can be considered in selected patients with underlying comorbidities that make iatrogenic hypothyroidism potentially risky, with pituitary disease that renders them unable to produce adequate TSH and in whom a delay in therapy might be deleterious (3).
It does appear that small-volume disease identified incidentally at the time of remnant ablation can be effectively treated using either traditional THW or rhTSH preparation (4,5). Furthermore, the similar rates of clinical recurrence seen with either method of preparation would also argue that rhTSH provides effective preparation for RAI therapy of small-volume metastatic disease present at the time of ablation (14).
Although no randomized prospective trials have been done, there is a growing body of case reports and retrospective studies suggesting that rhTSH-stimulated RAI therapy may be effective in the treatment of RAI avid DM. However, the observations that rhTSH preparation is associated with more rapid clearance of RAI from the body (6) and possibly lower radiation doses to metastatic lesions (7,8) indicate that additional studies are needed before rhTSH can be routinely recommended as an alternative method of preparation for RAI therapy of known metastatic disease.
Based on positive results seen when rhTSH was used as an adjunct to RAI therapy in the compassionate use program at our center (9), we have offered whole-body, dosimetry-guided RAI therapy after rhTSH stimulation as an alternative to traditional THW for the last 12 yr. In this study, we compared the overall survival rates seen in the first 12 yr of follow-up in patients with RAI avid distant metastases prepared with either rhTSH or THW.
Patients and Methods
Patients
After obtaining appropriate Human Use Committee approval, we reviewed the clinical charts of all follicular cell-derived thyroid cancer patients referred to the Nuclear Medicine Department at Memorial Sloan Kettering Cancer Center between 1993 and 2010 for remnant ablation, therapy of distant metastases or for diagnostic RAI whole-body scanning (DxWBS). All patients receiving RAI therapy after either THW or rhTSH preparation with definite evidence of RAI avid DM to lungs and/or bones found on either the diagnostic (DxWBS) or posttherapy WBS (RxWBS) were included in this study. The RxWBS and DxWBS reports of all patients were reviewed; the actual whole-body scanning (WBS) imaging was available and reviewed in 89% of the patients. Nearly all patients (98%) had cross-sectional imaging of RAI avid metastatic lesions identified on WBS. Lung metastases were classified as micrometastases if all pulmonary lesions were 1 cm or less or macrometastases if any single lesion was greater than 1 cm.
Patients were excluded from analysis for the following reasons: 1) no visible (or only very faint) uptake of RAI in metastatic lesions on either a prior diagnostic or posttherapy scan; 2) uptake of RAI in only a small fraction (<10%) of multiple metastatic lesions; 3) patients with inadequate follow-up data; 4) age less than 20 yr old at diagnosis (because rhTSH preparation was rarely performed in younger patients with distant metastases); 5) patients with another concomitant primary cancer in which the status of the other cancer was unknown or active; 6) patients with DM to organs other than lungs and bones; and 7) patients with anaplastic thyroid carcinoma.
From 245 potential study subjects, 175 patients met the inclusion criteria. We excluded 15 patients with either no definite evidence (or <10% of metastatic lesions) being RAI avid, 13 patients with metastasis in other organs beyond lungs and bones, 15 patients younger than 20 yr old, five patients with a focus of anaplastic thyroid carcinoma within the primary tumor or the metastasis, 18 patients with inadequate follow-up information, and four patients with unknown status of another cancer.
Preparation for RAI treatment
All patients treated with RAI for distant metastases at our center were instructed in a low-iodine diet and underwent whole-body and blood RAI clearance dosimetry studies that were used to determine the maximal therapeutic activity that could be safely administered without expectant damage to the bone marrow (less than 2 Gy) or lungs (less than 80 mCi whole body retention at 48 h) as described previously (10). In 38 patients, one or more of their RAI therapies were done before referral to our center using THW preparation without dosimetry studies. Patients treated before 1998 underwent traditional THW in preparation for RAI scanning and therapy. After rhTSH became commercially available in 1998, the choice of preparation (rhTSHvs. THW) was an individualized decision between each treating physician and the patient with no preset criteria favoring either rhTSH or TWH preparation.
rhTSH preparation
Whole-body and blood dosimetry studies were done as previously published (10). It is important to note that the rhTSH-stimulated RAI therapy described in this report incorporates two injections of rhTSH during wk 1 as preparation for dosimetry and an additional set of two injections during wk 2 before therapy. Briefly, while remaining on suppressive doses of levothyroxine, im injections of rhTSH (0.9 mg) were administered on d 1 and 2. On d 3, 1–5 mCi of 131-I was administered orally, and then WBS, whole body, and blood counts were obtained on d 4, 5, and 6. Two additional injections of rhTSH (0.9 mg, im) were administered on d 8 and 9 and the therapeutic RAI activity was given on d 10. A RxWBS was obtained 5–7 d later.
THW preparation
After levothyroxine withdrawal, most patients received T3 for 2–4 wk before complete THW as previously described (10). In patients treated at our center whole-body and blood RAI clearance studies were done after a 1- to 5-mCi dose of 131-I. In 38 patients receiving RAI therapy outside our center, standard empiric dosing approaches were used. In either setting, a TSH level above 30 mIU/liter was required before therapy. The posttherapy scan was performed approximately 3–10 d after RAI administration.
Laboratory and pathology studies
Between 1994 and 1997, a variety of thyroglobulin assays were used with functional sensitivities of approximately 1 ng/ml. Starting in 1998, all Tg values were measured using the Dynotest-TgS immunoradiometric assay (Brahms Inc., Berlin, Germany; functional sensitivity 0.6 ng/ml normalized to CRM 457) (10). TSH was measured using Advia Centaur two-site sandwich immunoassay (Bayer Corp., Tarrytown, NY).
Statistical methods
The primary end point of the study was a comparison of overall survival between patients prepared for all their RAI treatments (remnant ablation and RAI therapies) with either rhTSH (n = 58) or THW (n = 35). As an additional comparison group, we describe the overall survival in a cohort of patients (n = 82) that had initial remnant ablation and or RAI therapies with TWH who then had additional follow-up treatments with rhTSH.
Continuous data are presented as median and range values. Comparison between medians was performed using nonparametric tests (Mann-Whitney test). Categorical comparisons were performed with the Fisher's exact test and χ2 test. Kaplan-Meier analysis was performed for overall survival, comparing survival with the log rank test and using the Cox model for multivariate analysis. All the analyses were performed using SPSS software (version 16.0.1; SPSS Inc., Chicago, IL).
Results
Clinicopathological characteristics of the entire cohort
The clinical characteristics at the time of diagnosis of the 175 patients with RAI avid DM are summarized inTable 1. The median age was 56 yr (range 20–86 yr) and 53% of the patients were female. DM were diagnosed at the initial presentation of thyroid cancer in 60% of the patients (M1 patients). The locations of the DM were as follows: 53% only in lung, 28% only in bone, and 19% in lung and bone. Of the entire cohort, 35 patients had all RAI therapies (including radioactive iodine remnant ablation) done following THW (THW only), and 58 had all RAI therapies done after rhTSH stimulation (rhTSH only). The other 82 patients had initial RAI treatments done with TWH with subsequent treatments done with rhTSH. The median follow-up time for the entire cohort was 5.5 yr (range 0.8–21 yr).
Table 1.
Clinical characteristics of the patients
| n = 175 | |
|---|---|
| Age (yr) | |
| Median (range) | 56 (20–86) |
| Gender [n (%)] | |
| Female | 93 (53%) |
| Discovery of distant metastases [n (%)] | |
| At initial presentation (M1) | 105 (60%) |
| During follow-up (M0/Mx) | 70 (40%) |
| AJCC stage [n (%)] | |
| I | 22 (13%) |
| II | 42 (24%) |
| III | 2 (1%) |
| IV | 85 (48%) |
| Unknown | 24 (14%) |
| Distribution of DM [n (%)] | |
| Lung only | 92 (53%) |
| Bone only | 49 (28%) |
| Lung and bone | 34 (19%) |
| Follow-up (yr) | |
| Median (range) | 5.5 (0.8–21.0) |
| Histotype [n (%)] | |
| PTC | 40 (23%) |
| Follicular variant PTC | 32 (18%) |
| Tall cell variant PTC | 12 (7%) |
| Follicular thyroid carcinoma | 20 (12%) |
| Hürthle cell carcinoma | 6 (3%) |
| Poorly differentiated thyroid carcinoma | 54 (31%) |
| Others | 11 (6%) |
| Preparation for RAI treatment(s) [n (%)] | |
| All treatments after THW | 35 (20%) |
| All treatments after rhTSH stimulation | 58 (33%) |
| Initial THW after by subsequent rhTSH stimulation | 82 (47%) |
PTC, Papillary thyroid carcinoma.
Clinicopathological features based on method of preparation
The clinical characteristics of patients based on the method of preparation for RAI treatment are given inTable 2. There are no statistically significant differences between the three groups in age, gender, M stage at the time of diagnosis of thyroid cancer, distribution of DM, size of lung metastases, and presence of multiple bone metastases. Because rhTSH became commercially available in 1998, the median follow-up was significantly longer in the THW-only group (6.9 yr) and in patients who received initial doses prepared with THW followed by rhTSH (6.9 yr) compared with the rhTSH-only group (3.4 yr,P < 0.05). There was no significant difference in the histotype between the two groups.
Table 2.
Comparison between the methods of preparation
| THW only (n = 35) | rhTSH only (n = 58) | Initial THW, followed by rhTSH (n = 82) | |
|---|---|---|---|
| Age (yr) | |||
| Median | 56 | 60 | 55 |
| Range | 24–80 | 20–89 | 20–79 |
| Gender [n (%)] | |||
| Males | 15 (43%) | 28 (48%) | 39 (48%) |
| Females | 20 (57%) | 30 (52%) | 43 (52%) |
| TNM [n (%)] | |||
| M0/Mx | 14 (40%) | 26 (45%) | 30 (37%) |
| M1 | 21 (60%) | 32 (55%) | 52 (63%) |
| AJCC Stage [n (%)] | |||
| I | 6 (17%) | 6 (10%) | 10 (12%) |
| II | 7 (20%) | 19 (33%) | 16 (20%) |
| III | 0 | 1 (2%) | 1 (1%) |
| IV | 17 (49%) | 24 (41%) | 44 (54%) |
| Unknown | 5 (14%) | 8 (14%) | 11 (13%) |
| Distribution of DM [n (%)] | |||
| Lung only | 20 (57%) | 30 (52%) | 42 (51%) |
| Bone only | 9 (26%) | 13 (22%) | 27 (33%) |
| Lung and bone | 6 (17%) | 15 (26%) | 13 (16%) |
| Lung Mets size [n (%)] | |||
| Micro | 18 (75%) | 36 (80%) | 43 (80%) |
| Bone Mets [n (%)] | |||
| Single | 6 (40%) | 9 (32%) | 9 (23%) |
| RAI avid without radiological correlate [n (%)] | 4 (12%) | 16 (28%) | 6 (8%) |
| RAI cumulative administered activity [mCi] | |||
| Median | 522 | 408 | 967a |
| Range | 73–2024 | 75–1167 | 274–3027 |
| Number of RAI treatments (including ablation) | |||
| Median | 2 | 2 | 4a |
| Range | 1–8 | 1–5 | 2–10 |
| Follow-up (median) | 6.9 | 3.4a | 6.9 |
| Range | 1.4–17.1 | 1.3–10.3 | 0.8–20.9 |
| Year of diagnosis of DM [n (%)] | |||
| 1983–1999 | 22 (63%) | 6 (10%)a | 49 (60%) |
| 2000–2010 | 13 (27%) | 52 (90%)a | 33 (40%) |
| Histotype [n (%)] | |||
| PTC | 12 (34%) | 9 (15%) | 19 (23%) |
| Follicular variant PTC | 6 (17%) | 12 (21%) | 14 (17%) |
| Tall cell variant PTC | 2 (6%) | 8 (14%) | 2 (2.5%) |
| Follicular thyroid carcinoma | 6 (17%) | 5 (8%) | 9 (11%) |
| Hürthle cell carcinoma | 0 | 4 (7%) | 2 (2.5%) |
| Poorly differentiated thyroid carcinoma | 8 (23%) | 16 (28%) | 30 (37%) |
| Others | 1 (3%) | 4 (7%) | 6 (7%) |
Mets, Metastases; PTC, papillary thyroid carcinoma.
P < 0.05 compared with the other two groups.
Patients prepared with TWH followed by rhTSH treatments received a median of four treatments compared with only two treatments in both the rhTSH alone and the TWH alone. As a consequence, the cumulative administered activity was higher in this group than in either the TWH only or rhTSH-only groups (median 967vs. 522vs. 408 mCi, respectively,P < 0.05). However, the median administered activity per treatment was higher in patients prepared with rhTSH only (median 263 mCi, range 30–514) than in patients prepared with TWH only (median 200 mCi, range 30–480;P = 0.038). Patients prepared with TWH followed by rhTSH preparation received a median of 207 mCi after TWH (range 30–510 mCi) and a median of 315 mCi following rhTSH (range 10–521 mCi).
Survival analysis and structural response to therapy based on method of preparation
There was no significant difference in overall survival between patients receiving all RAI treatments with TWH-only, rhTSH-only, or initial treatments with TWH followed by subsequent treatments with rhTSH stimulation (P = 0.8). A multivariate analysis that included age at diagnosis, gender, histotype of the primary tumor, presence of bone metastases and method of preparation demonstrated that the only variable significantly associated with a survival difference was age of the patients at diagnosis (seeTable 3). Because18F-fluorodeoxyglucose-positron emission tomography scans were rarely obtained early in the course of these select patients with RAI avid metastatic disease,18F-fluorodeoxyglucose positron emission tomography results were not included as a variable in the multivariate analysis.
Table 3.
Multivariate analysis for overall survival
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Age | |||
| > 45 yrvs. ≤ 45 yr | 4.15 | (1.7–9.9) | 0.001 |
| Gender | |||
| Femalevs. male | 0.65 | (0.4–1.05) | 0.08 |
| Histotype | |||
| c-PTCvs. FV-PTC | 1.09 | (0.5–2.5) | 0.84 |
| TCV-PTCvs.-FV-PTC | 1.63 | (0.6–4.5) | 0.3 |
| FTCvs. FV-PTC | 0.82 | (0.3–2.1) | 0.68 |
| HCCvs. FV-PTC | 1.9 | (0.5–7.3) | 0.3 |
| PDTCvs. FV-PTC | 1.3 | (0.6–2.5) | 1.3 |
| Othersvs. FV-PTC | 1.9 | (0.6–6.3) | 0.3 |
| Presence of bone metastasis | |||
| Not presentvs. present | 0.8 | (0.5–1.3) | 0.3 |
| Preparation | |||
| THW onlyvs. rhTSH only | 0.7 | (0.3–1.5) | 0.4 |
| THS onlyvs. Initial THW followed by rhTSH | 0.8 | (0.5–1.5) | 0.5 |
CI, Confidence interval; PTC, papillary thyroid carcinoma; c-PTC, classical PTC; FV, follicular variant; TCV, tall cell variant; FTC, follicular thyroid carcinoma; HCC, Hürthle cell carcinoma; PDTC, poorly differentiated thyroid carcinoma.
The type of preparation (rhTSH only, TWH only, or THW followed by rhTSH) was not associated with a difference in the overall survival.
Of the 175 patients included in the study, 73 had micrometastases to the lung. Of these patients, 24 were prepared with rhTSH only, 16 were prepared with THW only, and 33 were prepared with THW early in the course of their disease with subsequent treatments after rhTSH stimulation. There was no difference in the survival curves among the three groups (P = 0.79). As expected, the overall survival was superior in patients with micrometastases (median survival 12.5 yr) than in patients with macrometastases (median survival 4.4 yr), regardless of the method of preparation (P < 0.001).
Adequate serial cross-sectional imaging was available to retrospectively evaluate a structural response to therapy in 140 of 175 patients. At the time of final follow-up, no structurally identifiable disease was present at sites of previous RAI avid metastatic lesions in 17% (four of 24) of THW only patients, and 19% (eight of 43) of rhTSH-only patients (P = 0.7, Fisher's exact). Structural disease progression was seen in 54% (13 of 24) of the THW-only and 46% (20 of 43) of the rhTSH-only patients (P = 0.6, Fisher's exact). The remaining 29% (seven of 24) in the THW-only, and 35% (13 of 24) in the rhTSH-only cohort did not demonstrate a clinically significant change in the size of the RAI avid structural lesions (P = 0.14, Fisher's exact).
Discussion
This is the first report to demonstrate very similar five overall survival rates in thyroid cancer patients with RAI avid distant metastases prepared with either traditional THW or a four-injection, rhTSH-stimulated, dosimetry-guided treatment protocol. Furthermore, the method of preparation (rhTSHvs. TWH) had no impact on overall survival in a multivariate analysis. These data suggest that rhTSH may have a role as an adjunct to RAI therapy of distant metastases in addition to its role in diagnostic testing (whole body RAI scanning and/or stimulated Tg) and remnant ablation.
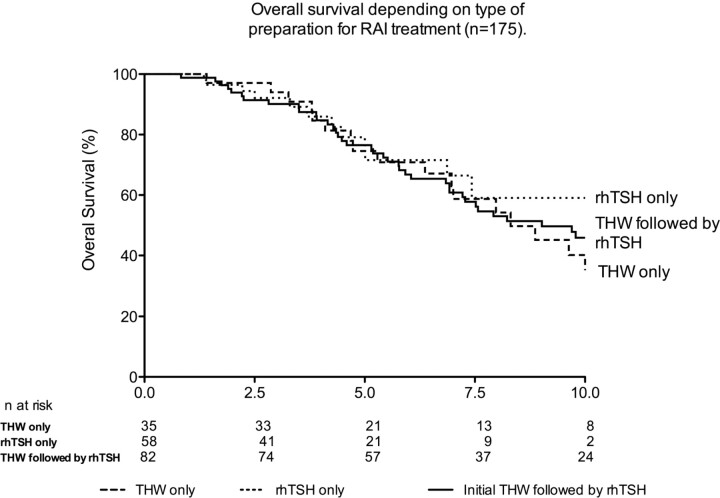
Although much longer studies will be needed to assess the impact of preparation methods on long-term survival, initial observations over the first 5 yr of follow-up are an important starting point. Analysis of both the Surveillance Epidemiology and End Results data (11) and the 53,856 cases in the National Cancer Data Base (12) demonstrates a 50% mortality in American Joint Committee on Cancer (AJCC) stage IV patients over the first 5 yr of follow-up. Because 41% of our rhTSH-only and 49% of our TWH-only patients were stage IV, it is likely that if major differences in short-term overall survival existed between these two groups, we would have detected either a trend or statistical difference over this initial 5-yr follow-up period. Furthermore, analysis of the Kaplan-Meier curve presented in the Durante series (1) shows a 5-yr survival rate of approximately 75–80% in patients with RAI avid distant metastases in which all RAI treatments were given after traditional TWH. This rate is nearly identical to the 5-yr survival rates of 75–80% that we report with TWH only, rhTSH only, or TWH followed by rhTSH RAI treatments (seeFig. 1). However, because metastatic thyroid cancer is a disease that can extend over many years, it is possible that despite very similar rates of short-term overall survival, differences could develop as these cohorts are followed up over the next several decades.
Fig. 1.
Overall survival curves based on method of preparation for RAI therapy. rhTSH-only patients had all RAI treatments (ablation and any additional therapies) done with rhTSH preparation. Similarly, THW-only patients had all treatments done after THW. The final group had at least one initial treatment (either ablation or treatment of DM) with TWH followed by one or more additional treatments using rhTSH stimulation (THW followed by rhTSH). Overall survival was similar in all three groups (P = 0.80 by Kaplan-Meier analysis).
Our findings suggesting that rhTSH preparation is an effective adjunct to RAI in the therapy of distant metastatic lesions is consistent with previous studies demonstrating similar effectiveness in destroying small-volume metastatic disease found incidentally at the time of radioactive iodine remnant ablation (4,5). Furthermore, the similar rates of recurrence seen with either THW or rhTSH preparation for radioactive iodine remnant ablation also suggests a therapeutic benefit that goes beyond simply destroying residual normal thyroid bed tissue.
Our findings are also consistent with previously reported series examining the clinical effectiveness of rhTSH stimulation before therapy of metastatic disease, usually in the setting of compassionate use in patients unable to increase endogenous TSH or in whom THW was deemed too risky. In a report on European patients, Lusteret al. (13) showed that from115 patients in which clinical outcome could be analyzed, 2% achieved complete remission, 36% partial response, and 27% disease stabilization. Robbinset al. (9) reported the effect of RAI treatment after rhTSH preparation as part of the Thyrogen Compassionate Use Program in 115 patients from the United States and Canada with metastatic disease. Clinical outcome was assessed by the serum Tg level trend, which was lower than baseline in 75% of patients assessed at 12 months. The results in both of these retrospective studies suggest that rhTSH-stimulated RAI therapy does have a therapeutic effect on RAI avid metastatic disease and is therefore consistent with our findings of similar rates of overall survival in both groups.
As with any retrospective study, there are several important limitations that must be considered. First and foremost is the potential for a selection bias that might result in patients with higher-risk disease being offered THW rather than rhTSH. Although we cannot rule out a subtle selection bias, comparison of the standard clinicopathological features between patients prepared with THW and rhTSH failed to identify significant differences. Prospective, randomized studies would be necessary to address this potential bias.
The other important limitation of this study is that our results are dependent on a series of four rhTSH injections (d 1, 2, 8, and 9) before administration of the therapeutic activity of RAI. Although we have previously published that a two-dose rhTSH regimen is associated with similar rates of successful ablation and recurrence as a four-dose regimen, the efficacy of a two-injection approach (d 1 and 2 with RAI therapy on d 3) in the treatment of macroscopic metastatic disease cannot be determined from our data (14). Additionally, because we routinely obtain whole-body and blood RAI clearance studies before therapy, we usually administer rather large activities of RAI as demonstrated by the median administered activity in the rhTSH cohort of 263 mCi. Therefore, our data cannot be used to determine whether a treatment approach using two injections of rhTSH followed by empiric 150- to 200-mCi dosing of RAI would result in similar clinical benefit. Unfortunately, the number of patients treated with rhTSH alone are too small to allow for a meaningful evaluation of the minimum empirical administered activity required to achieve the same results we achieved using our dosimetry guided approach.
In conclusion, in this retrospective study, the 5-yr overall survival is similar in thyroid cancer patients with RAI avid distant metastases prepared with whole-body and blood dosimetry with either thyroid hormone withdrawal or recombinant human TSH before receiving RAI. Therefore, a four-injection, rhTSH-stimulated, dosimetry-guided approach to the therapy of patients with RAI avid metastatic lesions appears to be an effective alternative to traditional THW-stimulated RAI treatments.
Acknowledgments
This work was supported by the Audrey Meyer Mars Clinical Training Grant from the American Cancer Society provided to H.T. (University Desarrollo/Clinica Alemana, Chile), 2009–2010.
Disclosure Summary: R.M.T. and R.R. have been consultants to and received research support from Genzyme. The other authors have nothing to disclose.
Abbreviations:
- AJCC
American Joint Committee on Cancer
- DM
distant metastases
- RAI
radioiodine
- rhTSH
recombinant human TSH
- RxWBS
posttherapy WBS
- Tg
thyroglobulin
- THW
thyroid hormone withdrawal
- WBS
whole-body scanning.
References
- 1. Durante C ,Haddy N ,Baudin E ,Leboulleux S ,Hartl D ,Travagli JP ,Caillou B ,Ricard M ,Lumbroso JD ,De Vathaire F ,Schlumberger M. 2006. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 2. Robbins RJ ,Schlumberger MJ. 2005. The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J Nucl Med 46(Suppl 1):28S–37S [PubMed] [Google Scholar]
- 3. Cooper DS ,Doherty GM ,Haugen BR ,Haugen BR ,Kloos RT ,Lee SL ,Mandel SJ ,Mazzaferri EL ,McIver B ,Pacini F ,Schlumberger M ,Sherman SI ,Steward DL ,Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 4. Pilli T ,Brianzoni E ,Capoccetti F ,Castagna MG ,Fattori S ,Poggiu A ,Rossi G ,Ferretti F ,Guarino E ,Burroni L ,Vattimo A ,Cipri C ,Pacini F. 2007. A comparison of 1850 (50 mCi) and 3700 MBq (100 mCi) 131-iodine administered doses for recombinant thyrotropin-stimulated postoperative thyroid remnant ablation in differentiated thyroid cancer. J Clin Endocrinol Metab 92:3542–3546 [DOI] [PubMed] [Google Scholar]
- 5. Tuttle RM ,Lopez N ,Leboeuf R ,Minkowitz SM ,Grewal R ,Brokhin M ,Omry G ,Larson S. 2010. Radioactive iodine administered for thyroid remnant ablation following recombinant human thyroid stimulating hormone preparation also has an important adjuvant therapy function. Thyroid 20:257–263 [DOI] [PubMed] [Google Scholar]
- 6. Hänscheid H ,Lassmann M ,Luster M ,Thomas SR ,Pacini F ,Ceccarelli C ,Ladenson PW ,Wahl RL ,Schlumberger M ,Ricard M ,Driedger A ,Kloos RT ,Sherman SI ,Haugen BR ,Carriere V ,Corone C ,Reiners C. 2006. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med 47:648–654 [PubMed] [Google Scholar]
- 7. Pötzi C ,Moameni A ,Karanikas G ,Preitfellner J ,Becherer A ,Pirich C ,Dudczak R. 2006. Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patients. Clin Endocrinol (Oxf) 65:519–523 [DOI] [PubMed] [Google Scholar]
- 8. Freudenberg LS ,Jentzen W ,Petrich T ,Frömke C ,Marlowe RJ ,Heusner T ,Brandau W ,Knapp WH ,Bockisch A. 2010. Lesion dose in differentiated thyroid carcinoma metastases after rhTSH or thyroid hormone withdrawal: (124)I PET/CT dosimetric comparisons. Eur J Nucl Med Mol Imaging 37:2267–2276 [DOI] [PubMed] [Google Scholar]
- 9. Robbins RJ ,Driedger A ,Magner J. 2006. Recombinant human thyrotropin-assisted radioiodine therapy for patients with metastatic thyroid cancer who could not elevate endogenous thyrotropin or be withdrawn from thyroxine. Thyroid 16:1121–1130 [DOI] [PubMed] [Google Scholar]
- 10. Robbins RJ ,Larson SM ,Sinha N ,Shaha A ,Divgi C ,Pentlow KS ,Ghossein R ,Tuttle RM. 2002. A retrospective review of the effectiveness of recombinant human TSH as a preparation for radioiodine thyroid remnant ablation. J Nucl Med 43:1482–1488 [PubMed] [Google Scholar]
- 11. Altekruse SF ,KC ,Krapcho M ,Neyman N ,Aminou R ,Waldron W ,Ruhl J ,Howlader N ,Tatalovich Z ,Cho H ,Mariotto A ,Eisner MP ,Lewis DR ,Cronin K ,Chen HS ,Feuer EJ ,Stinchcomb DG ,Edwards BK, eds.Surveillance Epidemiology and End Results Cancer Statistics Review, 1975–2007.Bethesda, MD:National Cancer Institute; http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010 [Google Scholar]
- 12. Hundahl SA ,Fleming ID ,Fremgen AM ,Menck HR. 1998. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 13. Luster M ,Lippi F ,Jarzab B ,Perros P ,Lassmann M ,Reiners C ,Pacini F. 2005. rhTSH-aided radioiodine ablation and treatment of differentiated thyroid carcinoma: a comprehensive review. Endocr Relat Cancer 12:49–64 [DOI] [PubMed] [Google Scholar]
- 14. Tuttle RM ,Brokhin M ,Omry G ,Martorella AJ ,Larson SM ,Grewal RK ,Fleisher M ,Robbins RJ. 2008. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med 49:764–770 [DOI] [PubMed] [Google Scholar]