Abstract
Malaria caused by Plasmodium falciparum remains the leading single-agent killer of children 1, yet the promise of an effective vaccine remains unfulfilled. Using our differential, blood-stage proteome screening method 2, we identified Pf Glutamic Acid Rich Protein (PfGARP, PF3D7_0113000) as a parasite antigen recognized by antibodies in plasma of children who are relatively resistant, but not by children who are susceptible, to P. falciparum malaria parasitemia. PfGARP is an 80-kDa parasite antigen expressed on the exofacial surface of early to late trophozoite-infected erythrocytes. We demonstrate that antibodies to PfGARP kill trophozoite-infected erythrocytes in culture by inducing parasite programed cell death and vaccination with PfGARP partially protects against P. falciparum challenge in non-human primates. Our longitudinal studies revealed that Tanzanian children without anti-PfGARP antibodies experienced 2.5-fold higher risk of severe malaria and Kenyan adolescents and adults without anti-PfGARP antibodies had 2.0-fold higher parasite densities compared to individuals with these antibodies. By killing trophozoite-infected erythrocytes, PfGARP may synergize with other vaccines targeting hepatocyte invasion and erythrocyte invasion or egress.
To identify novel vaccine candidates for P. falciparum malaria, we pooled plasma collected at age two years from the most resistant individuals and the most susceptible individuals participating in our Tanzanian birth cohort 3 (Supplementary Table 1) and performed differential biopanning experiments on a P. falciparum 3D7 strain blood-stage cDNA library constructed in T7 phage. We differentially biopanned 1.0 x 108 recombinant phage, sequenced n=100 differentially recognized clones, and identified 11 parasite genes whose protein products were uniquely recognized by antibodies in plasma from resistant, but not susceptible individuals (Supplementary Table 2).
Based on its in silico properties, its high degree of enrichment (44 out of 100 differentially biopanned clones), and its representation by clones derived from three overlapping but distinct cDNAs, we focused our attention on PfGARP, encoded by PF3D7_0113000.
In silico analysis (www.PlasmoDB.org and www.OrthoMCL.org) 4,5 predicts that PF3D7_0113000 contains a 2,236 bp gene (PfGARP) that encodes an 80- kDa acidic protein, with one intron near its 5’ end. PfGARP has syntenic orthologs in P. praefalciparum, P. gaboni and P. reichenowi, but not in any other malaria species, or other organism evaluated to date. PfGARP has no significant homology to proteins of known function and contains multiple complex repeat regions and extensive regions of low amino acid complexity with 50% of the protein composed of three amino acids (Lys, Glu, and Asp).
PfGARP expression is highly restricted to the early trophozoite stage 6 and the gene displays minimal sequence variation in the immuno-relevant region, the region encoded by the largest clone identified by resistant sera in our differential screens (nt 1,222-2,022). A recently reported deep-sequencing effort on 227 field samples identified only one non-synonymous SNP in the immuno-relevant region 7, while an expanded, as yet unpublished, analysis of 3,248 field samples has provisionally identified 15 non-synonymous SNPs in the immuno-relevant region (https://www.malariagen.net/apps/pf/4.0/#doc=Doc*AboutData.htm).
Anti-PfGARP-A kills parasites in vitro
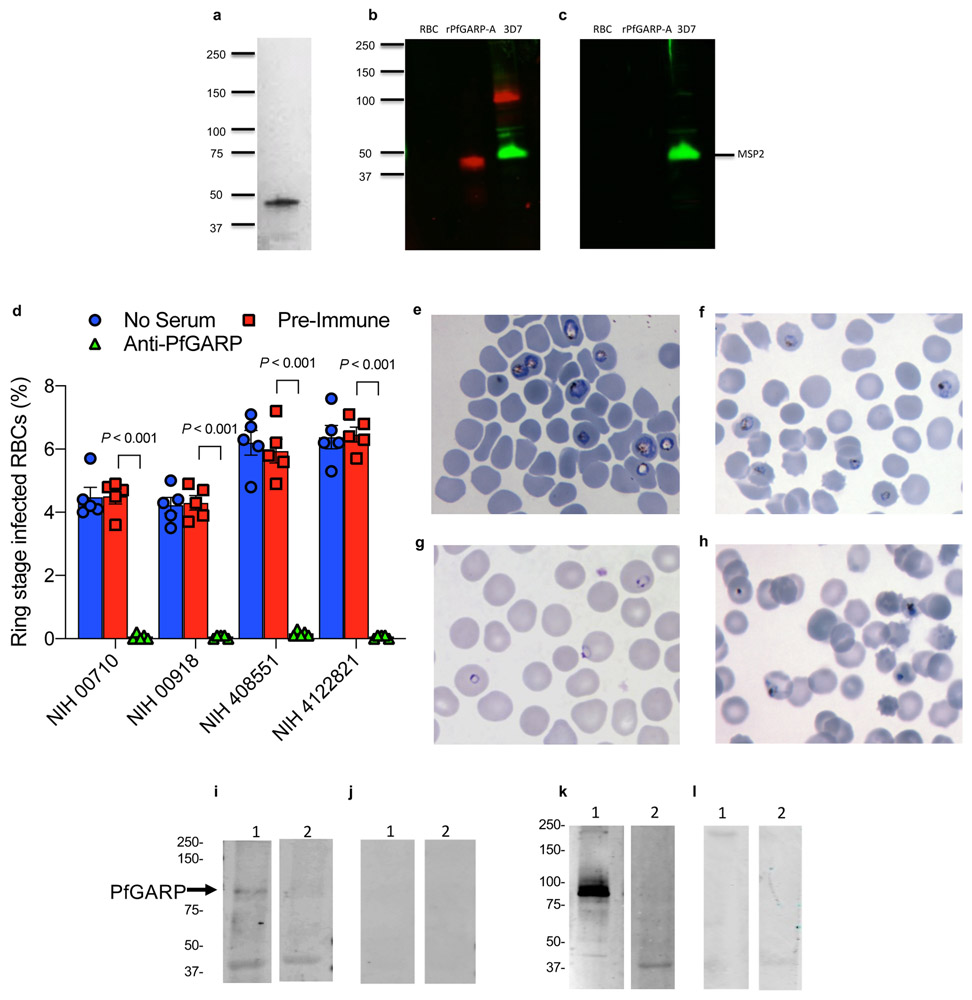
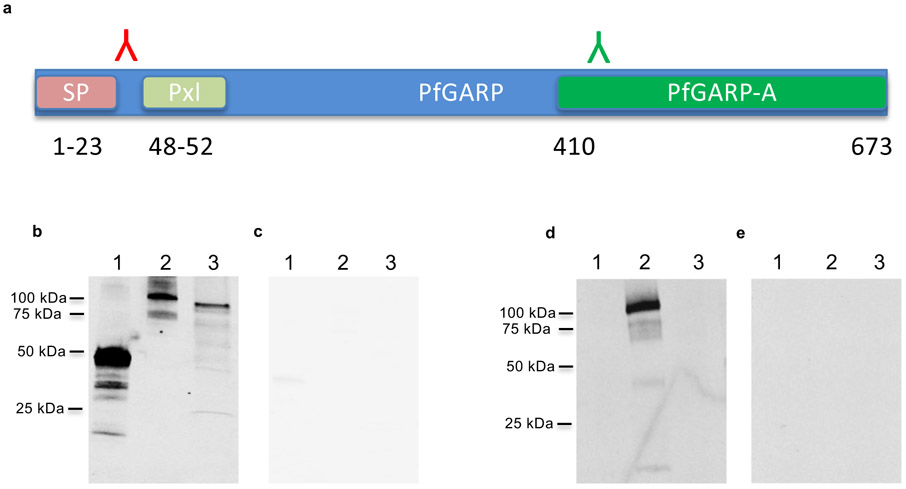
We expressed and purified the polypeptide encoded by the differentially recognized, immuno-relevant region of PfGARP from the referent strain 3D7 (nt 1,228-2,022, aa 410-673) in Escherichia coli and designated this recombinant protein rPfGARP-A (Extended Data Fig. 1a). In addition, we cloned this immuno-relevant region into a eukaryotic expression plasmid (VR2001). To generate anti-PfGARP-A antisera, we immunized mice with either the recombinant protein (rPfGARP-A) in TiterMax adjuvant or the eukaryotic expression plasmid. In western blot analysis, both protein and DNA immunized anti-PfGARP-A antisera recognized a ~100 kDa protein in trophozoite-infected RBCs (Extended Data Fig. 1b and k)- this higher apparent molecular weight is consistent with PfGARP’s acidic composition 8.
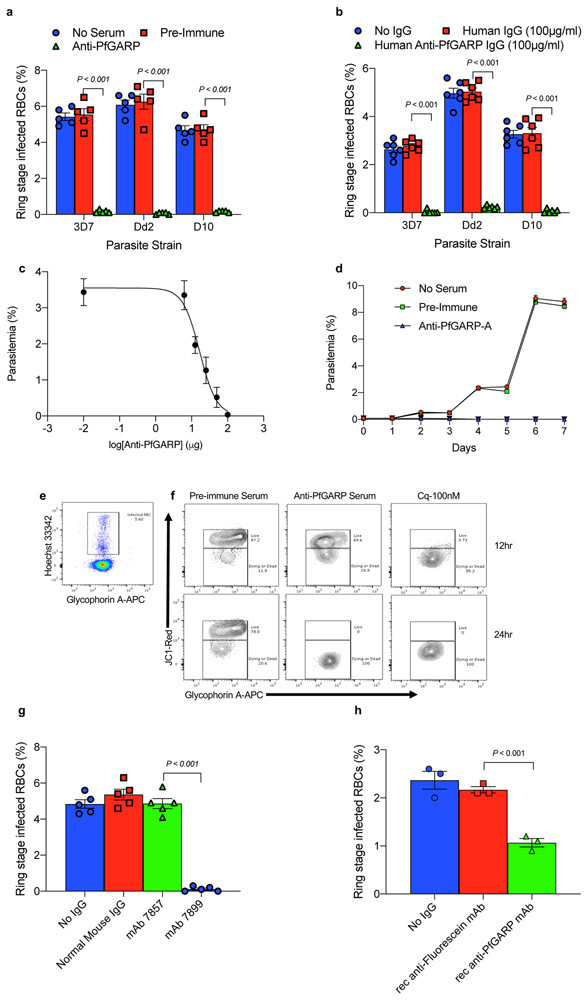
We performed growth inhibition assays (GIA) using anti-PfGARP-A antisera prepared by either DNA or recombinant protein immunization. Anti-PfGARP-A inhibited parasite growth by 94-99% compared to controls in three parasite strains and four freshly isolated parasite lines (all P < 0.001, Fig 1a and Extended Data Fig. 1d). Anti-PfGARP-A treated parasites displayed a dysmorphic, pyknotic appearance consistent with crisis forms associated with dying/dead parasites on Giemsa-stained blood smears 9,10 (Extended Data Fig. 1e-h).
Fig 1. Antibodies to PfGARP inhibit parasite growth.
Murine anti-PfGARP (1:10 dilution) (a) or anti-rPfGARP IgG (100 ug/ml) purified from pooled sera collected from malaria exposed individuals (b) inhibit parasite growth by 94-99%. Negative controls included no anti-sera and pre-immune mouse sera (a), or control media and human IgG purified from human sera obtained from malaria naive individuals (b). The IC50 of anti-PfGARP purified from vaccinated mouse sera is approximately 16.8 ug/ml (c). In (a) and (b), bars represent means of 5 biologically independent replicates, error bars represent SEMs. In (c), points represent the mean of 3 biologically independent replicates. Error bars represent SDs. d) Anti-PfGARP kills P. falciparum parasites in long term cultures. Points represent the mean of 3 biologically independent replicates. Error bars represent SEMs. e and f) Anti-PfGARP disrupts mitochondrial membrane potential. e) Gating strategy for panel f. f) Ring stage 3D7 iRBCs were incubated with pre-immune or anti-PfGARP sera (1:10 dilution) or chloroquine (100 nM). Contour plots demonstrating iRBCs with live parasites in the upper gate and dying/dead parasites in the lower gate defined by JC1 staining. g and h) Monoclonal anti-PfGARP inhibits parasite growth. g) Ring stage 3D7 iRBCs were cultured with media alone, normal mouse IgG (1 mg/ml) or anti-PfGARP monoclonal antibodies (mAb 7857 or mAb 7899, at 1 mg/ml). h) recombinant monoclonal anti-PfGARP produced from VH and VL sequence of mAb 7899 inhibits parasite growth. Ring stage 3D7 iRBCs were cultured with media alone, recombinant anti-fluorescein (250 μg/ml) or recombinant anti-PfGARP monoclonal antibody (250 μg/ml). In (g) and (h), bars represent means of 3 biologically independent replicates, error bars represent SEMs. Panel A is representative of 5 independent experiments. Panels B-H are representative of 3 independent experiments. All P value were calculated by two sided non-parametric Mann-Whitney U test.
We purified human polyclonal anti-PfGARP-A antibodies from plasma pooled from adults living in our Tanzanian field site (Extended Data Fig. 1i and j) and demonstrated that these human anti-PfGARP-A antibodies significantly inhibit parasite growth by 94-99% in three parasite strains compared with controls (all P < 0.001, Fig. 1b). In addition, we purified mouse polyclonal anti-PfGARP-A antibodies from plasma pooled from rPfGARP-A immunized mice (Extended Data Fig. 1k and l) and we demonstrate that the IC50 for parasite growth occurs at 16.8 μg of anti-PfGARP-A per ml of culture media (Fig 1c).
In long term cultures, anti-PfGARP treated parasites never expanded in number (Fig 1d), and by day two, had shrunken in size and appeared pyknotic. By day 4, the parasites were difficult to visualize as they had become small, pyknotic dots. By day 6, they were no longer identifiable as parasite infected RBCs. These data confirmed that anti-PfGARP antibodies, in the absence of complement or cellular effector functions, killed parasite infected RBCs.
We performed GIA assays and quantified parasite viability by flow cytometry using the mitochondrial membrane potential probe, JC-1. Ring stage parasites treated with anti-PfGARP antibodies showed marked loss of mitochondrial membrane potential (a characteristic of programmed cell death) within 12 hours with essentially all parasites losing their mitochondrial function within 24 hours (Fig 1e and f).
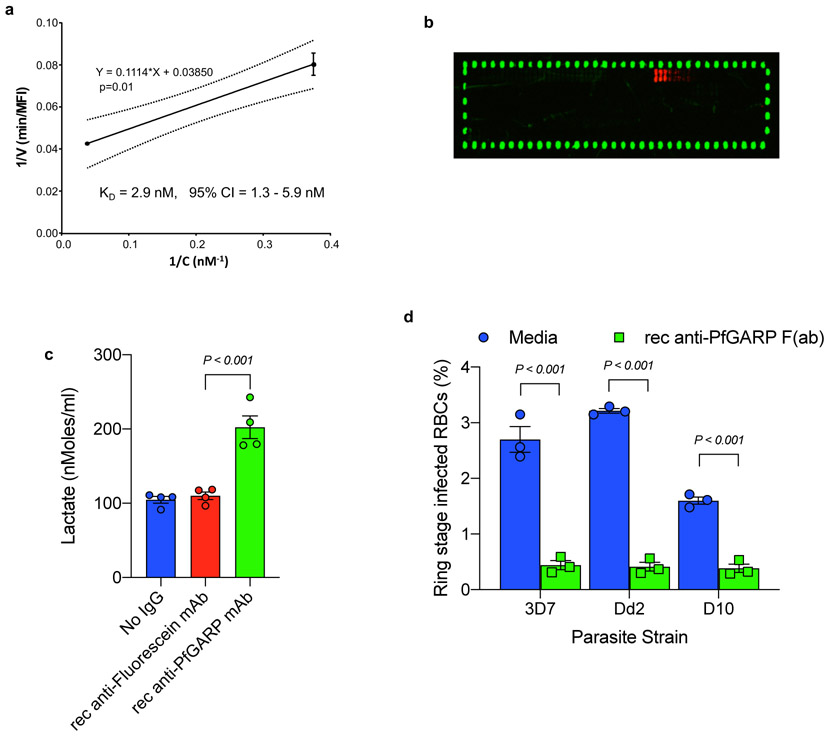
We produced a series of monoclonal antibodies in mice immunized with recombinant PfGARP-A. Of the sixteen mAbs that reacted with PfGARP-A in ELISA based assays, only mAb7899 killed parasites in culture (Fig 1g). We sequenced and expressed the VH and VL of mAb7899 and the recombinant mAb had a KD of 2.9 nM (95% CI = 1.3 – 5.9 nM, Extended Data Fig. 2a), inhibited parasite growth by 51% at a concentration of 250μg/ml in GIA assays (Fig 1h), and recognized aa 443-459 (VKNVIEDEDKDGVEIIN) of full length PfGARP (Extended Data Fig. 2b). Consistent with the loss of mitochondrial function, lactate levels in media from recombinant mAb7899-treated cultures were significantly higher than in controls (Extended Data Fig. 2c). Monovalent Fab mAb7899 inhibited parasite growth by 76-87% across 3 parasite strains (Extended Data Fig. 2d) indicating that anti-PfGARP mediated killing occurred in the absence of complement, cellular effector function, or antigen cross-linking.
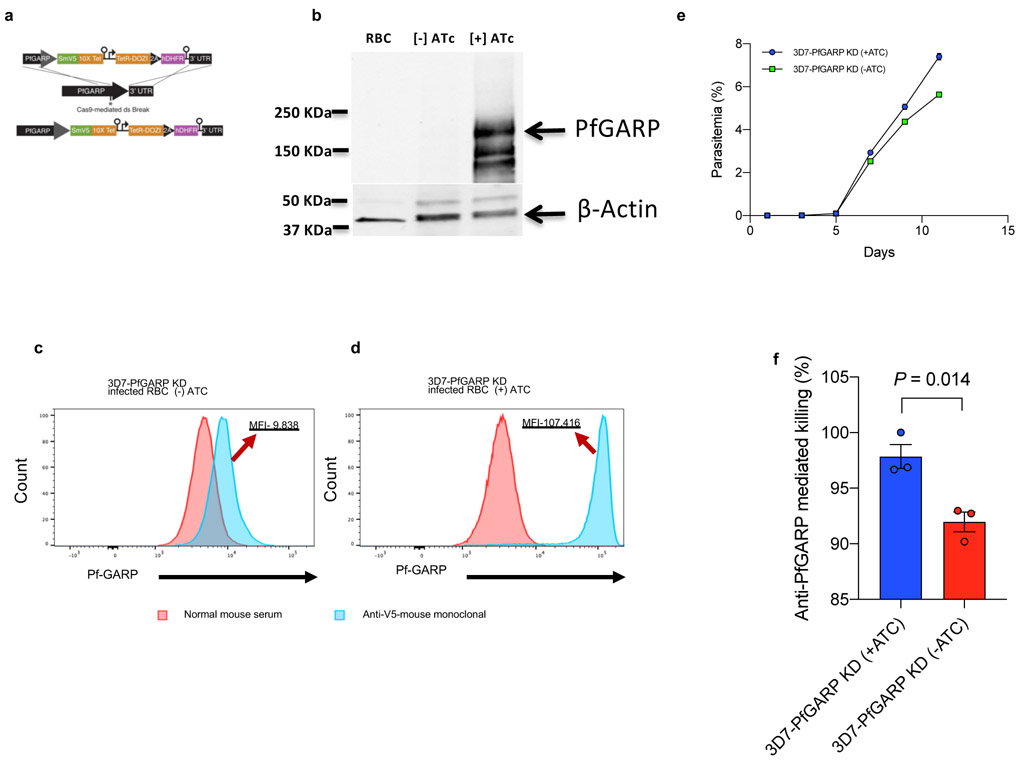
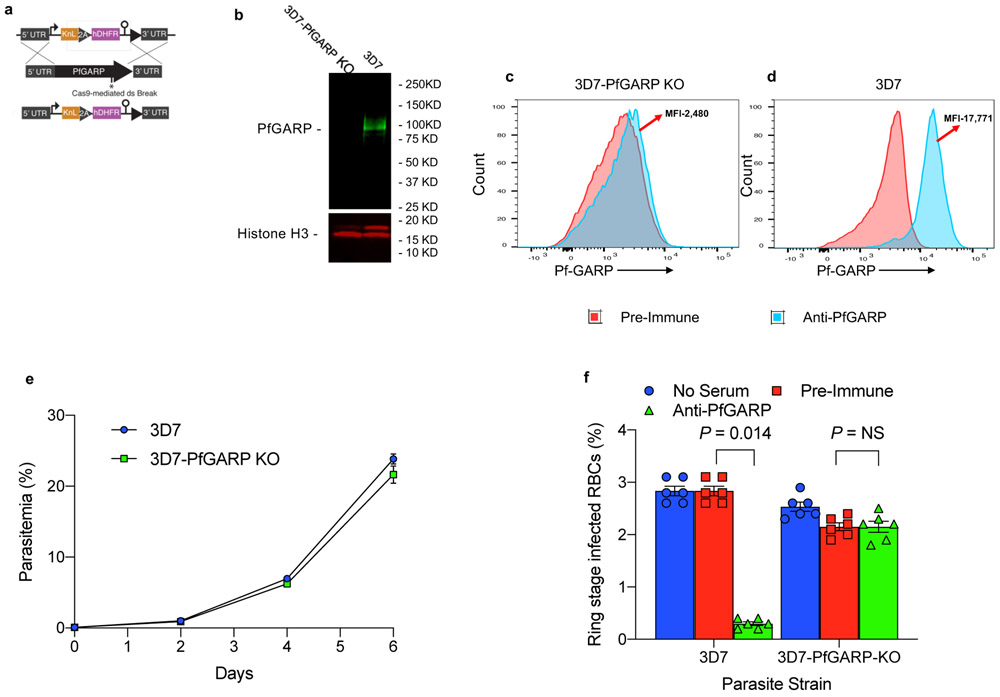
We constructed parasites with a conditional knock-down of PfGARP using the TetR system 11 (Extended Data Fig. 3a and Supplementary Table 3). 3D7-PfGARP KD parasites grown in the absence of the inducer anhydrotetracycline (ATc), did not display an overt growth phenotype in vitro despite a reduction in PfGARP protein levels of up to 90% (Extended Data Fig. 3b-e). In GIA assays, the killing efficacy of anti-PfGARP antibodies was only mildly reduced in 3D7-PfGARP KD parasites cultured without ATc compared to 3D7-PfGARP KD parasites cultured with ATc (P = 0.014, Extended Data Fig. 3f). Anti-PfGARP mediated killing of 3D7-PfGARP KD parasites cultured without ATc may be due to the residual expression of PfGARP even in the absence of the inducer. To test this hypothesis, we constructed PfGARP deleted parasites (3D7-PfGARP KO) (Extended Data Fig. 4a-d) and evaluated them in growth and GIA assays. 3D7-PfGARP KO did not display an overt growth phenotype in vitro (Extended Data Fig. 4e). As expected, the killing efficacy of anti-PfGARP antibodies was completely abrogated in the 3D7-PfGARP KO parasites compared to wild-type 3D7 parasites (Extended Data Fig. 4f).
Immunolocalization of PfGARP
We immunolocalized PfGARP by immunofluorescence confocal microscopy and immunogold transmission electron microscopy (TEM) in 3D7 parasites probed with anti-PfGARP. We also evaluated 3D7-PfGARP KD parasites, in which PfGARP is linked to a carboxy terminal V5 tag, probed with anti-V5 antibodies . In all experiments, PfGARP localized to the exofacial surface of the RBC membrane in early to late trophozoite-infected RBCs (Extended Data Fig. 5,6,7).
Anti-PfGARP disrupts food vacuole integrity
In TEM studies, the food vacuole in anti-PfGARP treated parasites was markedly diminished in size and appeared absent or condensed tightly around hemozoin crystals- a previously unreported effect of antimalarial antibodies (Extended Data Fig. 8a and b). We explored the possibility that PfGARP also localized to the food vacuole membrane by colocalization studies with PfCRT. In the majority of infected RBCs, PfCRT and PfGARP did not co-localize (Extended Data Fig. 7d). We evaluated the impact of anti-PfGARP by confocal microscopy using the calcium binding dye Fluo-4-AM, which preferentially labels the food vacuole 12. Anti-PfGARP treated parasites demonstrated marked loss of food vacuole integrity as evidenced by the redistribution of Fluo-4-AM (and therefore calcium) from the food vacuole 12 to the parasite cytosol (Extended Data Fig. 8c). We further evaluated the impact of anti-PfGARP antibodies on food vacuole integrity by serial block face scanning electron microscopy (SBF-SEM) which demonstrated that treatment with anti-PfGARP resulted in the complete loss of food vacuole integrity with hemozoin dispersed broadly throughout the parasitophorous vacuole (Supplementary Video 1).
Anti-PfGARP activates parasite programmed cell death
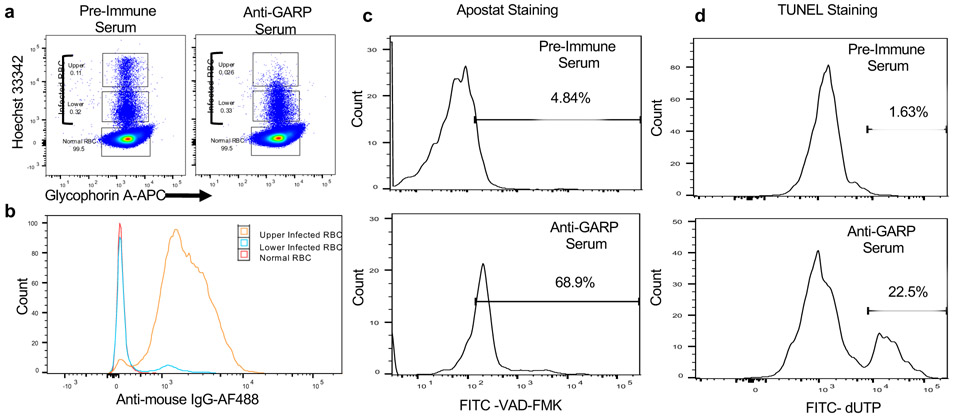
The impact of anti-PfGARP antibodies on parasite morphology, mitochondrial membrane potential, and food vacuole integrity (with elevated intracellular calcium) suggests activation of programmed cell death as a mechanism of parasite killing. Therefore, we evaluated anti-PfGARP treated parasites for the activation of caspase-like enzymes. We performed GIA assays and probed the parasites with Apostat, a fluorescent dye that labels activated caspase-like cysteine proteases. Parasites treated with anti-PfGARP showed marked activation of caspase-like protease as assessed by flow cytometry (Fig. 2a-c). We also performed TUNEL assays to determine if treatment with anti-PfGARP resulted in parasite DNA fragmentation. Parasites treated with anti-PfGARP showed marked fragmentation of their DNA as assessed by flow cytometry (Fig. 2d).
Fig 2. Antibodies to PfGARP bind to P. falciparum infected RBCs leading to activation of caspase-like proteases and DNA fragmentation.
a) Ring stage 3D7 parasites were cultured for 24-30 hours in the presence of 10% pre-immune mouse sera or anti-PfGARP mouse sera generated by DNA vaccination. Representative dot plots demonstrating iRBCs with higher DNA content (mature parasites) in the upper gate, lower DNA content in the middle gate (immature and dying parasites), and uninfected RBCs in the lowest gate. b) Binding of polyclonal anti-PfGARP antibodies to the iRBCs in upper two gates and uninfected RBCs in the lowest gate. Incubation with anti-PfGARP results in activation of caspase-like proteases (assessed by Apostat staining, panel c), and DNA fragmentation (TUNEL staining, d) in parasites present in the upper gate. All data are representative of 3 independent experiments.
Humans cohort studies
Tanzanian Birth Cohort
To evaluate the impact of naturally acquired anti-PfGARP antibodies on clinical malaria, we measured anti-PfGARP IgG antibody levels using a fluorescent, bead-based assay in our birth cohort and related these levels to subsequent malaria outcomes.
We measured anti-PfGARP IgG antibody levels in available plasma obtained at 48 weeks of life in 246 children. The average duration of follow-up was 64 weeks per child. Anti-PfGARP antibodies were detectable in 48.8% of these samples and children were followed for a total of 15,737 child-weeks of observation.
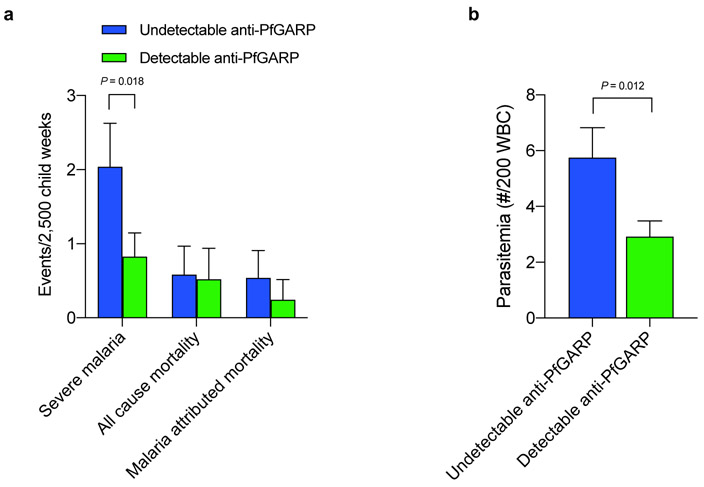
We used generalized estimating equation (GEE) repeated measures models to evaluate the relationship between anti-PfGARP IgG levels and risk of malaria outcomes. When analyzed as a continuous variable, anti-PfGARP IgG levels predicted significantly decreased risk of severe malaria over the follow-up period (P = 0.008). When analyzed dichotomously, individuals with undetectable IgG anti-PfGARP antibodies (n=126 individuals who contributed 7,327 weeks of follow-up) had 2.5-fold higher risk of severe malaria than individuals with detectable IgG anti-PfGARP antibodies (n = 120 individuals who contributed 8,410 weeks of follow-up, 95% CI [1.2, 5.5], P = 0.018, Fig 3a). These results remained significant (OR=2.5, 95% CI [1.1-5.5], P = 0.026) even after adjusting for potential confounders (see Supplemental Methods for modeling approach).
Fig 3. Antibodies to PfGARP predict reduced risk of severe malaria and parasitemia.
a) Tanzanian children with undetectable anti-PfGARP IgG antibodies measured at 48 weeks of age (n=126 individuals who contributed 7,327 weeks of follow-up) had a 2.5-fold higher risk of developing severe malaria compared to children who had detectable IgG anti-PfGARP antibodies (n = 120 individuals who contributed 8,410 weeks of follow-up, 95% CI [1.2, 5.5], P = 0.018. GEE model with two-sided fixed effect test). Bars represent least-square mean number of events/2,500 child weeks, error bars represent SEM adjusted for repeated measures. b) Kenyan males with undetectable IgG anti-rPfGARP-A antibodies (n=61 individuals who contributed 1,018 weeks of follow-up blood smears), had 1.97-fold higher parasite densities over 18 weeks of follow-up compared to individuals with detectable IgG anti-rPfGARP-A antibodies (n=74 individuals who contributed 1,237 weeks of follow-up blood smears) 95% CI [0.94, 4.23], P =0.012. GEE model with two-sided fixed effect test). Bars depict least-square mean parasitemia; error bars depict SEM.
Kenyan Cohort
To generalize these results to a completely independent cohort, we measured anti-rPfGARP-A IgG responses in a cohort of Kenyan males participating in a treatment-reinfection study 13-15. Volunteers (n=135) aged 12-35 years were entered into the study at the beginning of the high transmission season in April, 1997. Volunteers were treated for malaria and followed with weekly blood smears for 18 weeks. Serum was collected 2 weeks post-treatment and stored at −800C. In this age group, clinical or severe malaria is very uncommon.
In GEE based repeated measures models, IgG anti-rPfGARP-A antibodies, when analyzed as a continuous variable, predicted significantly decreased parasite density over 18 weeks of follow-up (P < 0.004). When analyzed dichotomously, individuals with undetectable IgG anti-rPfGARP-A antibodies (n=61 individuals who contributed 1,018 weeks of follow-up blood smears), had 1.97-fold higher parasite densities over 18 weeks of follow-up compared to individuals with detectable IgG anti-rPfGARP-A antibodies (n=74 individuals who contributed 1,237 weeks of follow-up blood smears, 95% CI [0.94, 4.23], P =0.012, see Fig 3b). These results remained significant (OR=1.82, 95% CI [0.9-3.7], P = 0.019) even after adjusting for potential confounders (see Supplemental Methods for modeling approach).
Monkey vaccine trials
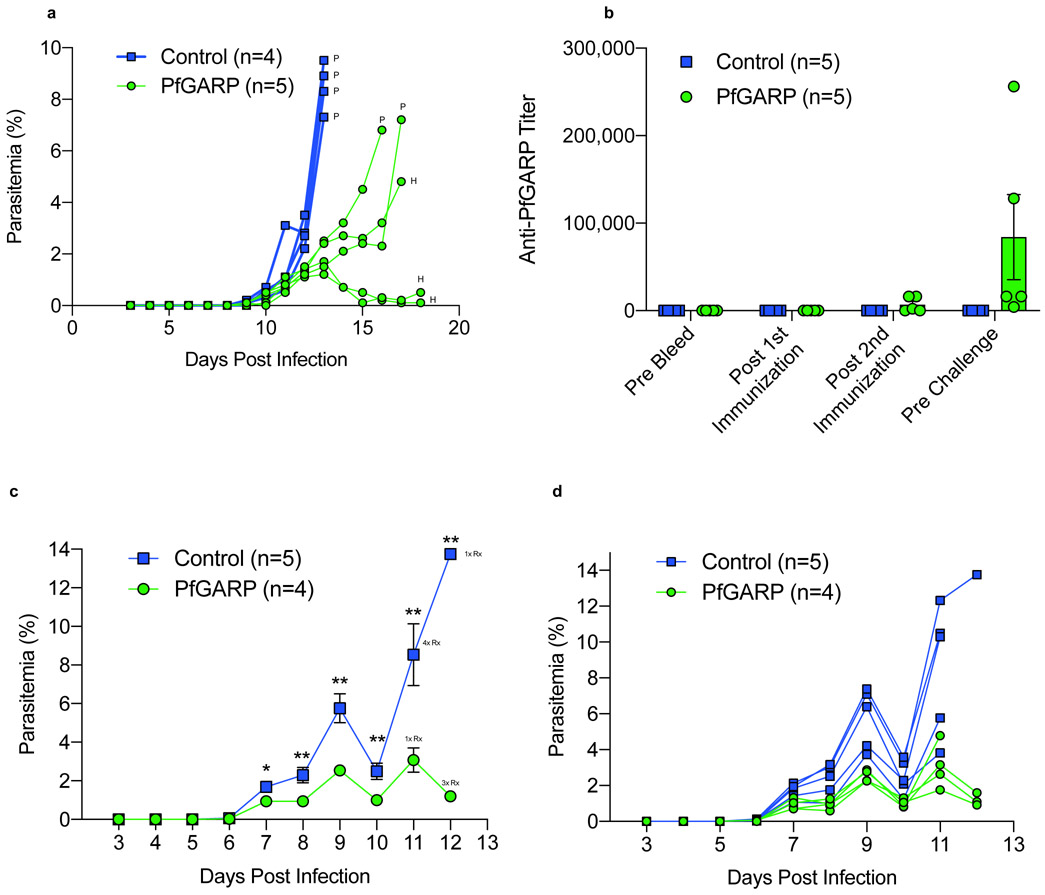
Because PfGARP does not have orthologs in any non-primate malaria species, we conducted a vaccine trial in the P. falciparum/Aotus model. Nucleoside-modified, FPLC-purified mRNA vaccines against Zika and influenza have demonstrated potency in small and large animals 16-18, thus we immunized n=5 monkeys intradermally with 50 μg of lipid nanoparticle (LNP) encapsulated nucleoside-modified mRNA encoding PfGARP-A. Control monkeys (n=4) were intradermally immunized with 50 μg of poly (C) RNA-LNPs. Monkeys received 3 doses at 3-week intervals. Prior to each dose, serum was obtained for antibody assays. On day 63, monkeys were challenged with 104 P. falciparum FVO strain blood stage parasites by IV injection followed by daily blood films. This represents a heterologous challenge with a highly virulent parasite as the sequence for the mRNA-LNP vaccine was derived from the 3D7 strain.
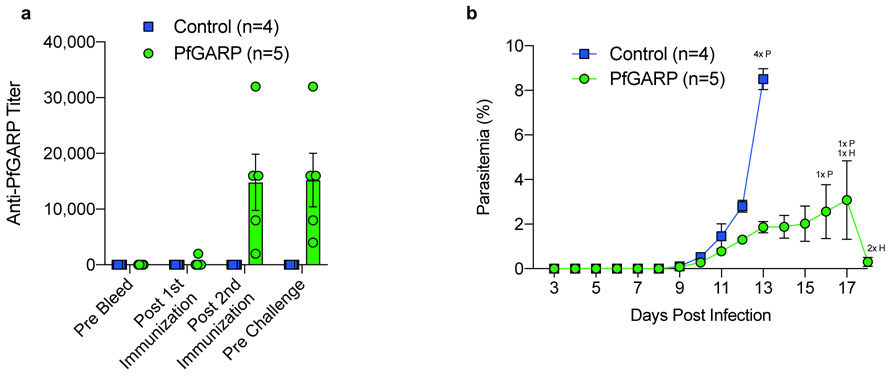
Immunized monkeys generated antibody responses that plateaued after the second injection (Fig 4a). Control monkeys had significantly higher parasitemia than monkeys immunized with PfGARP-A on day 12 (P < 0.009) with a 4.6-fold higher parasitemia on day 13, the final day with complete follow-up of all monkeys (P < 0.001, Fig 4b). All control monkeys met pre-specified criteria for anti-malaria treatment on day 13 due to hyperparasitemia (parasitemia > 7.5%). One PfGARP-A vaccinated monkey was treated for hyperparasitemia on day 16 and one was treated on day 17. One monkey was treated for anemia, a common complication in the Aotus/P. falciparum model 19, on day 17 and the remaining 2 monkeys were treated for anemia on day 18, despite controlling their parasitemias (Extended Data Fig. 9a). Notably, the three monkeys that controlled their parasitemia and required treatment for anemia had higher anti-PfGARP titers (16,000, 16,000 and 32,000) compared to the monkeys that required treatment for their parasitemias (8,000 and 4,000).
Fig 4. Vaccination with PfGARP-A encoding nucleoside-modified mRNA-LNPs protects monkeys from challenge with P. falciparum.
a) Animals were intradermally injected with 50 μg of PfGARP-A mRNA-LNP (n=5 monkeys) or 50 μg of poly(C) RNA-LNP (negative control, n=4 monkeys) at weeks 0, 3 and 6 and PfGARP-specific IgG titers were determined. Bars represent mean titer, error bars represent SEMs. b) Vaccinated Aotus monkeys were challenged IV with 104 P. falciparum FVO strain infected RBC on day 63 and parasitemia was followed daily. Points represent means, error bars represent SEM. Control monkeys had significantly higher parasitemia than monkeys immunized with PfGARP-A on day 12 (two-sided t-Test, P < 0.009) with a 4.6-fold higher parasitemia on day 13, the final day with complete follow-up of all monkeys (two-sided t-Test, P < 0.001). On day 13, all control monkeys required anti-malarial treatment for high parasitemia (indicated by 4x P). On day 16, one vaccinated monkey required anti-malarial treatment for high parasitemia, (1x P). On day 17, one vaccinated monkey required anti-malarial treatment for high parasitemia and one vaccinated monkey required anti-malarial treatment for low hemoglobin (1x H). On day 18, two vaccinated monkeys required anti-malarial treatment for low hemoglobin (2x H).
We also conducted a trial using E. coli expressed recombinant PfGARP-A (aa 410-673) emulsified in Ribi adjuvant as the immunogen in the P. falciparum/Aotus model. We immunized n=4 monkeys with 50 ug of PfGARP-A in Ribi adjuvant or Ribi alone (n=5) sub-cutaneously. Monkeys received 3 doses at 3-week intervals. Prior to each dose, sera were obtained for antibody assays. On day 63, monkeys were challenged with 104 P. falciparum FVO strain blood stage parasites by IV injection followed by daily blood films. Immunized monkeys generated antibody responses that rose after the third injection (Extended Data Fig. 9b). Importantly, control monkeys had significantly higher parasitemia on days 7-12 compared to PfGARP-A vaccinated animals (all P < 0.05, Extended Data Fig. 9c and d). On day 11, the final day with complete follow-up of all monkeys, controls had 3.5 fold higher parasitemia compared to PfGARP-A vaccinated monkeys (P < 0.01). Four control monkeys met pre-specified criteria (parasitemia >7.5%, hematocrit < 25%, or evidence of clinical illness) for drug treatment on day 11 and the final control monkey met criteria on day 12. On Day 11, one PfGARP vaccinated monkey was drug treated despite not meeting pre-specified criteria.
Summary and Conclusions
Malaria remains a leading cause of childhood mortality and vaccines are urgently needed to attenuate this public health threat. Using our vaccine discovery platform, we identified PfGARP, a novel blood stage vaccine candidate that localizes to the exofacial surface of the RBC membrane in trophozoite infected RBCs. Anti-PfGARP significantly attenuates parasite growth by arresting and killing trophozoite infected RBCs in the absence of immune effector cells or complement- a novel activity for an anti-malarial antibody.
Parasites treated with anti-PfGARP displayed several canonical features of programed cell death (PCD) including: 1) shrunken, pyknotic nuclei, 2) loss of mitochondrial membrane integrity, 3) activation of caspase-like proteases, 4) activation of DNA fragmentation, and 5) release of calcium from intracellular stores. P. falciparum lacks classical caspases, but does encode three meta-caspases 20 and activation of PfMCA1 functions as an upstream activator of a caspase-like enzyme leading to parasite PCD 21. Anti-PfGARP is the first example of an antimalarial antibody that activates parasite PCD. Because PfGARP is located on the exofacial surface of the RBC and antibody engagement of PfGARP leads to activation of parasite PCD, we speculate that PfGARP may function in the density dependent regulation of parasitemia by sensing either parasite or host factors 22-24.
In non-human primates, immunization with PfGARP as an mRNA- or recombinant protein-based immunogen conferred marked protection against parasitemia following heterologous P. falciparum challenge compared with controls. This is the first report of protection against P. falciparum following vaccination with an mRNA-based vaccine.
In longitudinal cohort studies, naturally occurring anti-PfGARP predicted decreased risk of severe malaria in children and parasitemia in adolescents and adults. Together, our data define PfGARP as a rationally identified vaccine candidate for P. falciparum malaria and suggest that our recombinant monoclonal anti-PfGARP antibody could serve as a platform for developing therapeutic and prophylactic antibody-based interventions and function in high-throughput drug screens targeting PfGARP-induced PCD. By killing trophozoite infected RBCs, immunization with PfGARP may synergize with other vaccines targeting hepatocyte invasion 25 and red cell invasion 26 or egress 2.
Methods
No statistical methods were used to predetermine sample size. The experiments were not randomized. The investigators were blinded to allocation during the non-human primate experiments and outcome assessments. Investigators were blinded to clinical status during blood smear reading for the human cohort studies. The studies complied with all relevant ethical regulations for both animal and human studies.
Tanzanian Birth Cohort
Study population
Subjects participated in the Mother-Offspring Malaria Studies (MOMS) project, which is based at Muheza Designated District Hospital (DDH), in north eastern Tanzania. Mothers presenting at Muheza DDH for delivery were enrolled and provided signed, informed consent prior to participation of themselves and their newborns in the study. The entomologic inoculation rate in our study site exceeds 400 infectious mosquito bites per person per year 27. Details of the MOMS study design, enrolment methods, and exclusion criteria have been published elsewhere 3,28.
Inclusion criteria and clinical monitoring
We monitored N=785 children for P. falciparum infection from birth up to 3.5 years of age. Children were evaluated at routine, well-child visits by a clinician every two weeks from birth to one year of age, and monthly thereafter, including blood smear analysis. Routine blood samples were collected once every 6 months from 1.5 to 3.5 years of life. Blood smears and blood samples were also collected any time the child became sick. Sick children were examined by a medical officer upon presentation to the hospital or mobile clinic. Treatment outside the study was minimized by active, weekly surveillance by our mobile clinics.
Clinical malaria was defined as asexual P. falciparum parasitemia by blood smear coupled with symptoms suggestive of malaria such as temperature > 37.5 °C, nausea or vomiting, irritability, and poor feeding. Prompt treatment was provided to sick children according to the guidelines of the Tanzanian Ministry of Health, and study participants were instructed to obtain all medications including antimalarial through the project staff.
Sample collection and processing
Venous blood was collected and stored at 4°C until processing. Following centrifugation, plasma was stored at −80°C. P. falciparum parasitemia was determined by Giemsa-stained thick blood smears prepared from the capillary or venous blood. Parasite density was expressed as the number of asexual stage parasites/200 white blood cells in the thick smear. Sickle cell trait was determined by electrophoresis (Helena Laboratories, Beaumont, TX USA). Hemograms were obtained on an impedance-based analyzer (Abbott Cell Dyne® 1200).
Case definitions
Mild malaria was defined as a positive blood smear and one or more of the following: 1) anemia defined by Hgb <8 g/dL; 2) vomiting; 3) diarrheal disease or gastroenteritis; 4) lower respiratory infection, or 5) oral temperature >= 38 deg C.
Severe malaria was defined as a positive bloodsmear and one or more of the following: 1) respiratory distress defined by respiratory rate of >40/min for children older than two months of age or a respiratory rate of >50/min for children less than two months of age; 2) a history of one or more convulsions in the twenty-four hours prior to or during hospitalization; 3) prostration defined by inability to sit unaided; 4) hypoglycemia defined by glucose < 2.2 mmol/L; 5) severe anemia defined by Hgb <6 g/dL; or 6) oral temperature > 40 deg C.
Malaria-associated mortality was defined as death with a positive blood film obtained during the terminal illness. One child who died of bacterial meningitis, but had a positive blood film was adjudicated as a non-malarial death.
Kenyan Cohort
Study population
To generalize the protective nature of anti-PfGARP antibodies, we measured anti-PfGARP antibody levels in an entirely distinct longitudinal cohort using epidemiologic data and blood samples that were collected in 1997 as part of a treatment-reinfection study 14,29. Volunteers were residents of subsistence farming; falciparum endemic villages in western Kenya north of Lake Victoria. The entomological inoculation rate in this area can exceed 300 infectious bites per year 30. After obtaining informed consent, 144 males aged 12 to 35 years were entered into the study at the beginning of the high transmission season in April 1997. Detectable parasitemia was eradicated in 143 of the 144 participants with quinine sulfate (10 mg/kg twice daily for 3 days) and doxycycline (100 mg twice daily for 7 days). One volunteer remained parasitemic during the week following treatment and was removed from the analysis, and five volunteers did not have available serum samples, thus our analytic sample size of n=138. Immunologic and epidemiologic analyses of this cohort have been reported elsewhere 14,29,31,32.
Malaria assessment:
Thick and thin blood smears were obtained from each volunteer prior to treatment and then weekly for 18 weeks after treatment to quantify reinfection. Each smear was interpreted by two microscopists who were blinded to the clinical status of the subjects and the mean of the two values recorded.
Entomology measurements:
The intra-domiciliary female anopheline abundance was measured weekly for 18 weeks in each volunteer’s domicile using the Daytime Resting Indoors (DRI) method 33 as previously described 29.
Blood collection.
Two weeks after treatment with quinine and doxycycline, volunteers donated 10 ml of blood into heparinized tubes. Within four hours of collection, samples were centrifuged, and plasma was aliquoted and stored at −80°C for subsequent analysis.
Clinical assays:
Sickle cell trait was determined by electrophoresis (Helena Laboratories, Beaumont, TX USA).
Differential Screening and Characterization of PfGARP
Selection of resistant and susceptible individuals for differential screening assays
Strategy:
Our overall purpose is to identify acquired differences in antibody repertoire that mediate resistance to parasitemia. In our cohort, parasitemia does not decline until after the age of 2 years (see Fig S1 from reference 2). Any differences in parasitemia between groups that are detectable in the first 2 yrs of life, are unlikely due to differences in acquired antibody repertoire- the children have made relatively little specific antibody by this age. Therefore, we want to ensure that the susceptibility to parasitemia is similar (and high) between our resistant and susceptible groups in the first two years of life and then diverges after 2 yrs of age. The divergence after age two is unlikely due to a constitutive (i.e. genetic) feature, but rather more likely due to an acquired phenomena (i.e. antibody repertoire). Thus, we selected children for our two groups (resistant vs susceptible) to have similar parasitemias in the first two years of life, and then to have very divergent parasitemias from age 2–4 yrs.
From our Tanzanian Birth Cohort, we excluded individuals with less than 9 of the total n=18 scheduled monthly blood smears collected between the ages of 2-3.5 yrs, individuals with less than 200 ul of plasma available from the plasma sample obtained at age 2 (+/− 2 weeks), and individuals who were parasitemic when the 2 yrs (+/− 2 weeks) plasma sample was obtained. We then rank ordered individuals based on the mean parasite density on all blood films collected between ages 2 and 3.5 yrs. This mean parasite density included the scheduled monthly blood smears as well as positive blood smears obtained during sick visits. Individuals from the low and high extremes of this distribution were chosen to comprise the Resistant (N=12) and Susceptible (N=14) groups. To minimize differential exposure as a possible confounder, Resistant individuals were selected from those children who did not sleep under bed nets, while Susceptible individuals were selected from those children who did sleep under bed nets. Selections were made with matching based on the village of residence and sex. Potential confounders examined included: Hgb phenotype, the presence of placental malaria, maternal age, birth season, and # of previous pregnancies (Supplementary Table 1). By matching and demonstrating that potential non-immunologic variables influencing resistance (i.e., HbS) were not differentially distributed between the resistant and susceptible groups, we dramatically reduced the chance that these covariates were confounding the relationship between antibody specificities discovered and the outcome of resistance or susceptibility.
Whole blood-stage proteome differential screening
We constructed a P. falciparum blood-stage cDNA expression library prepared in T7Select 10-3b vector (Invitrogen) using RNA prepared from freshly isolated parasites collected in our Tanzanian field site. This vector displays 5-15 copies of the cloned gene on the surface of phage capsids as a fusion with phage 10B protein.
We bound Immulon 4HB (Thermo Fisher, USA) ELISA wells with 100 μl of 1:100 dilution of sera pooled from malaria resistant children (n=12, see Supplementary Table 1) for one hour at room temperature. Wells were washed five times with 1x TBST (10mM Tris HCL, 150mM NaCl, 0.05% Tween 20, pH 7.4) and blocked with 2% BSA in 1xTBST for one hour at room temperature. Wells were probed with 108 phage in 100 μl of 1xTBST and incubated for one hour at room temperature. Unbound phage were removed and the wells were washed five times with 1xTBST. Bound phages were then eluted in 100 ul of 5 M NaCl. Eluted phage were amplified and titered using BLT5403 bacteria according to manufacturer’s instructions. Amplified eluted phage were used as input phage for 3 additional rounds of amplification.
After four rounds of positive selection, the titer of eluted phage was determined and adjusted to 105/ml in 1x TBST buffer. For negative selection, we bound Immulon 4HB (Thermo Fisher, USA) ELISA wells with 100 μl of 1:100 dilution of sera pooled from malaria susceptible children (n=14, see Supplementary Table 1) for one hour at room temperature. 100 μl of the diluted phage (10,000 total phage) were added to the well, incubated for one hour at room temperature, and the unbound phage were collected. Unbound phage were transferred to an additional well coated with sera pooled from susceptible children, incubated for one hour at room temperature, and unbound phage collected. This process was repeated a total of five times.
Following negative selection, the titer of eluted phage was determined and 100 individual plaques were isolated and their cDNA inserts were amplified by PCR using the vector specific T7SelectUP (5'- GGAGCTGTCGTATTCCAGTC - 3’) and T7Select Down (5’- AACCCCTCAAGACCCGTTTA-3’) and the PCR products were sequenced.
PfGARP expression and purification
We subcloned the ORF encoding aa 410-673 of PfGARP into pET30 (Novagen) and transformed the resulting plasmid into the expression host E. coli BL21(DE3) (Novagen). The pET30 vector encodes a His tag at both the amino and carboxy ends of the recombinant protein, thus facilitating purification by metal chelate chromatography. Transformants were grown in Terrific broth supplemented with 100 μg/mL kanamycin, at 37 deg C in a 10 L fermenter with oxygen sparging (10L/min) until OD600 = 8.0. Isopropyl-b-D-thiogalactopyranoside was added to a final concentration of 1 mmol/L, and the culture was fed continuously with 0.3 g/ml glucose, 0.09 g/ml yeast extract at 50 ml/hr for 12 h. Cultures were harvested by centrifugation and 750 gr of wet cell paste was resuspended in 10 L of 10 mmol/L potassium phosphate, 150 mmol/L NaCl, 10 mmol/L imidazole, 0.5% Tween 20 , and 0.5% Triton X 100, (pH 8.0) and lysed by high-pressure disruption at 20,000 PSI (Microfluidics, Model 110-T). The lysate was clarified by tangential flow microfiltration (filter area 1m2, pore size 1 μm, Millipore) and 8 L of clarified lysate was recovered. Protein purification was achieved by a 4-step process on BioPilot chromatography equipment (Pharmacia). Briefly, clarified lysate was applied to a FineLine Pilot 35 (GE Healthcare) column containing 90 mL of Ni-NTA Superflow Resin (Novagen). The protein of interest was eluted with a stepped gradient containing increasing concentrations of imidazole. Fractions containing the protein of interest were pooled, adjusted to 400 mmol/L ammonium sulfate, 10 mmol/L DTT and further purified, by hydrophobic-interaction chromatography on a Fine Line Pilot 35 (GE Healthcare) column containing 150 ml of Source 15PHE (GE Healthcare). Recombinant proteins were eluted with a linear gradient of elution buffer (10 mmol/L Tris, 1 mmole/L DTT, 1 mmol/L EDTA [pH 8.0]). Fractions containing the protein of interest were pooled and further purified, by anion exchange chromatography on a Fine Line Pilot 35 (GE Healthcare) column containing 130 ml of MacroPrep High Q (BioRad). Recombinant proteins were eluted with a linear gradient of elution buffer (10 mmol/L Tris, 1 mole/L NaCl, 1 mmole/L DTT, 1 mmol/L EDTA [pH 8.0]). Final purification was achieved by ceramic hydroxyapatite chromatography on a FineLine Pilot 35 (GE Healthcare) column containing 70 ml of CHT type 1 (BioRad). Recombinant proteins were eluted with a linear gradient of elution buffer (500 mmoles/L potassium phosphate, and 1 millimole/L DTT, pH 7.4)
Purified recombinant protein, designated rPfGARP-A, was buffer exchanged into 10 mmol/L sodium phosphate, 0.05% Tween 20, 3% sucrose and concentrated to 500 μg/ml by tangential flow ultrafiltration (filter area 50 cm2, pore size 5 kDa, Pall). rPfGARP was lyophilized at 500 μg/vial and stoppered under nitrogen. Endotoxin levels were less than 2EU/mg protein as determined by an FDA cleared assay (Lonza). Typical yields are > 50 mg rPfGARP per 750 gr of wet cell paste.
Importantly, rPfGARP-A expresses immuno-relevant epitopes, which generate functional polyclonal antibodies that block parasite growth and kill trophozoites (Fig 1) and recognize native PfGARP by western blot (Extended Data Fig 1).
Parasite strains and culture
P. falciparum strains (3D7, Dd2, and D10) were obtained from MR4. Two parasite isolates from adults and two parasite isolates from children were collected from our Tanzanian field site and culture adapted. The parasites were cultured in vitro according to the methods of Trager and Jensen with minor modifications 34. Briefly, parasites were maintained in RPMI 1640 medium containing 25 mm HEPES, 5% human O+ erythrocytes, 5% Albumax II (Invitrogen), 24 mm sodium bicarbonate, and 10 μg/ml gentamycin at 37 °C with 5% CO2, 1% O2, and 94% N2.
Anti-PfGARP antisera
Mouse anti-PfGARP antisera were produced by either DNA, recombinant protein, or mRNA based immunization. For DNA immunization, we subcloned the ORF encoding aa 410-673 of PfGARP into VR2001, transformed into the host E. coli NovaBlue (Novagen), and purified endotoxin-free plasmid (Endofree Giga, Qiagen). BALB/cJ mice were immunized with 100 μg of plasmid (25 μg intramuscular injection in each hind leg and 50 μg intradermal injection at the base of tail) followed by 50 μg intradermal injections at the base of tail every two weeks for a total of four doses.
For protein immunization, we emulsified rPfGARP-A in an equal volume of TiterMax adjuvant (CytRx Corporation) and injected 50 μg of rPfGARP-A intraperitoneally at two-week intervals for a total of four doses.
For mRNA-based immunization, BALB/cJ mice were immunized intradermally with 10 μg lipid encapsulated mRNA (see below) encoding aa 410-73 of PfGARP every three weeks for a total of 3 doses.
Affinity purification of anti-PfGARP antisera
To purify polyclonal mouse and human anti-PfGARP IgG, we coupled 6 mg of rPfGARP-A to one ml of NHS-activated Sepharose 4 Fast Flow (GE Health Sciences) according to the manufacturer’s instructions. For mouse anti-PfGARP IgG, we used plasma pooled from rPfGARP-A immunized mice. For human anti-PfGARP IgG, we used plasma pooled from placental blood collected from women delivering in our Tanzanian birth cohort.
Briefly, rPfGARP-A coupled resin was incubated with 600 μl of pooled mouse or human plasma diluted in 6 ml of PBS. After extensive washing in PBS, 0.05% Tween 20, bound antibody was eluted in 0.1M glycine, pH 2.5 and immediately neutralized with 1M Tris HCl, pH8. Eluted antibodies were buffer exchanged into PBS by diafiltration in spin columns (Centricon) and sterilized prior to use in immunoblot and in vitro growth assays.
Western blot
Parasite pellets were prepared by treatment of parasitized RBCs with 0.15% saponin in phosphate buffered saline (PBS), pH 7.4 on ice for 10 min followed by centrifugation (3,000 x g, 5 min), and resuspension in cold PBS, and centrifugation (3,000 x g, 5 min). Alternatively, mature stage infected RBCs were purified on a magnetic column and resuspended in distilled water (1:25 v/v) to lyse the RBCs followed by centrifugation (3,000 x g, 5 min) to collect parasite pellets.
Parasite pellets or rPfGARP-A were dissolved in SDS sample loading buffer with reducing agent (Bio-Rad), heated to 95 deg C for 10 min, and proteins were separated in 4-11% gradient SDS-PAGE gels. Separated proteins were transferred to nitrocellulose membranes which were blocked in 5% milk PBS (pH 7.4) and 0.05% Tween 20 for 1 h. Membranes were probed with polyclonal anti-PfGARP or pre-immune mouse sera, detected by use of anti-mouse IgG antibody conjugated to fluorescent tagged secondary antibodies and imaged on a LI-COR (Odyssey Imaging Systems).
Growth inhibition assays
Growth inhibition assays (GIA) were carried out with anti-PfGARP polyclonal serum or IgG monoclonal and polyclonal antibodies, control anti-fluorescein monoclonal antibodies, control mouse serum or IgG as described 35-37 with minor modifications. Briefly, anti-PfGARP antibodies or control were dialyzed overnight in PBS, pH7.4, heat inactivated at 56°C for 30 min and pre-incubated with human RBC for 1 hour before use in GIA assays. GIA assays were carried out using W2, 3D7, D10 or 4 newly adapted isolates of P. falciparum collected at our Tanzanian field site. Parasites were synchronized to the ring stage by treatment with 5% sorbitol 38 for three successive replication cycles and cultured to the ring stage. Parasites at 0.3-0.4% parasitemia and 2% hematocrit were incubated with anti-sera or IgG, in a final volume of 100 μl in microtiter wells. Cultures were performed in triplicate with five replicates (comprising a total of 15 individual wells) prepared for each treatment condition. After 48 hr, blood films were prepared from each replicate, stained with Giemsa, a microscopist blinded to the treatment conditions enumerated RBCs infected with ring stage parasites, and the results from the three wells were averaged. The relationship between the treatment group and parasitemia outcome of the five replicates was analyzed by Mann-Whitney U test.
In some GIA assays (Fig 1D), parasites were plated at 0.08% parasitemia and were incubated with anti-sera, in a final volume of 100 μl in microtiter wells. Cultures were examined by microscopy daily for 7 days. Media (with appropriate anti-sera) was changed daily. Cultures were performed in triplicate with five replicates (comprising a total of 15 individual wells) prepared for each treatment condition.
Monoclonal antibody production and epitope mapping
We immunized BALB/cJ IP three times at two-week intervals with 25ug of rPfGARP-A emulsified in TiterMax and boosted with 25 ug of rPfGARP-A IV three days prior to fusion of splenocytes with P3X63Ag8.653 myeloma cells (ATCC) according to our published method (33).
Hybridomas were culture and cloned by limited dilutions. Hybridomas producing anti-PfGARP antibodies were screened and used for large-scale production of monoclonal antibodies.
The heavy and light chain variable regions of hybridoma clone 7899 (IgG1 kappa) were sequenced, and recombinant plasmids constructed and used for the production of recombinant mAb in HEK293 cells according to manufacturer’s protocols (Absolute Antibody). Recombinant mAb expressing the variable heavy and light chains from clone 7899 on a murine IgG1 framework were purified by Protein A chromatography. Recombinant mAb expressing the variable heavy and light chains from clone 7899 on a murine IgG1 Fab framework (monovalent) were purified by Ni-NTA chromatography.
For epitope mapping, a custom 15-mer peptide microarray was designed and printed. The array contained 264 different peptides which spanned the PfGARP-A sequence (aa 410-673). The peptides overlapped by a single amino acid and were printed in duplicate, framed by HA control peptides. The array was probed with rec mAb7899 (red) and anti-HA (green) and imaged on a LI-COR Odyssey according to the manufacturer’s protocol (PepperPrint).
Mitochondrial membrane potential
Mitochondrial membrane potential was assessed with the dye JC1, a cationic dye that exhibit potential-dependent accumulation in mitochondria. Ring stage infected RBC from P. falciparum in vitro cultures were harvested at 5-10% parasitemia and incubated with anti-glycophorin A antibodies (Invitrogen) for 30 minutes at room temperature followed by Hoechst 33342 dye at 1μg/ml. JC1 staining was performed at a final concentration of 2μM for 30 minutes at 370 C in the dark with constant agitation and analyzed by flow cytometry.
Caspase activation assay
The activation of caspase-like proteases was quantified using a cell permeable, FITC-conjugated pan-caspase inhibitor (FITC-VAD-FMK, ApoStat, R&D Systems) which irreversibly binds and labels activated cysteine proteases. Antibody treated P. falciparum infected RBCs (treated with anti-PfGARP or control) were incubated with FITC-VAD-FMK, at a final concentration of 1%, 1hr before harvest. Samples were washed with PBS to remove unbound reagent, followed by staining with anti-glycophorin A antibodies (Invitrogen) and Hoechst 33342 (Thermo Scientific) dye as above. Samples were analyzed by flow cytometry.
TUNEL assay
Fragmentation of intracellular DNA in the cultured P. falciparum parasites was evaluated by TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labeling) assay, using the APO-DIRECT™ Kit (BD Biosciences). Antibody treated P. falciparum infected RBCs (treated with anti-PfGARP or control) were harvested, washed and stored in ice cold 70% ethanol for at least 18 hrs before staining with 50μl of DNA labeling solution, prepared as per manufacturer’s instructions. After about 1hr incubation with the DNA labeling solution, samples were stained with anti-glycophorin A antibodies (Invitrogen) and Hoechst 33342 (Thermo Scientific) dye as above and analyzed by flow cytometry.
Lactate assay
The spent media from 3D7 parasites incubated with recombinant anti-PfGARP monoclonal antibody or control recombinant anti-fluorescein in GIA assays was collected and assayed for lactate levels (Lactate assay kit II, Sigma-Aldrich, MAK065) according to the manufacturer's protocol.
Generation of 3D7-PfGARP KD and 3D7-PfGARP KO parasite lines
For 3D7-PfGARP KD parasites, approximately 100 ug of pRR203 was linearized with EcoRV, purified, and co-transfected with 100 ug pRR183 into 3D7 parasites. Parasites were maintained on 500 nM ATc. One day post-transfection, drug pressure was applied with 2.5 nM WR99210 and the PfDHODH-inhibitor N-(3-Chloro-4-methylphenyl)-5-methyl-2-(trifluoromethyl)[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (MMV665874 or AD1) at 150 nM. Five days after transfection, AD1 selection was removed. Parasites were cloned by limiting dilution.
3D7-PfGARP KO parasites were made as above, with the exception that pRR248 was used as the homology-directed repair plasmid. pRR248 was linearized with AvrII, NotI, and SapI and transfected with pRR183 into a strain of 3D7 parasites with episomal expression of SpCas9. Only WR99210 drug pressure was applied after transfection.
For 3D7-PfGARP KD parasites, integration of the targeting construct was confirmed by PCR with oJDD1027/oJDD4507 (control), oJDD1027/oJDD2933 (integration), and oJDD4279/4280 (locus size). Tet aptamer size was confirmed by amplifying the aptamer with oJDD3560/oJDD44 and digesting the PCR fragment with PspOMI and KpnI. For 3D7-PfGARP KO parasites, integration of the targeting construct was similarly confirmed by PCR.
Construction of PfGARP homology-directed repair plasmid
To prevent homology directed repair from occurring between the SpCas9-directed cut site and the 3’ end of PfGARP, we synthesized caPfGARP where the last 438bp of PfGARP are codon altered. We amplified the synthesized gene with oJDD4506/oJDD3566, and the 500bp preceding the codon altered region from 3D7 genomic DNA with oJDD3563/oJDD4507. We amplified 500bp from the 3’ end of PfGARP for the 3’ homology region with oJDD3561/oJDD3562 from 3D7 gDNA. We again used PCR SOE to create a PfGARP 3’HR-EcoRV-PfGARP 5’HR-caPfGARP fusion. We cloned this fusion into pRR69 39 with NotI and NcoI to generate pRR203.
Construction of PfGARP-targeting SpCas9 Vector and generation of knock down parasite line
A PfGARP-targeting guide was cloned into the U6 cassette of pBAM203 39 by PCR SOE. The U6 promoter with guide was amplified by oJDD3058/oJDD4088 and gRNA and U6 terminator with guide amplified with oJDD3059/oJDD4089. The cassette was cloned into pBAM203 with EcoRI and AvrII to generate pRR183. The 3D7 parasites were transfected with pRR183 and genome integrated parasites were selected by serial dilution.
Construction of PfGARP KO homology-directed repair plasmid
To knock out PfGARP we generated a cassette for double crossover that integrated into the 5’ UTR of PfGARP and at the 3’ end of the sequence coding for PfGARP. We amplified the 5’ UTR of PfGARP from gDNA with oJDD4077/4979 and cloned it into a plasmid containing a cassette expressing the KAHRP signal sequence fused to nanoluciferase followed by a 2A skip peptide and the hDHFR selection marker. The 5’ UTR of PfGARP was cloned upstream of the cassette with NotI/AflIII. The 3’ homology region was amplified with oJDD5029/4083 and cloned downstream of the selection cassette with EcoRI/AvrII to generate pRR248.
Immunofluorescence assays
Blood smears of asynchronous 3D7 strain parasite cultures were prepared, fixed in cold methanol for 15 minutes, and probed with anti-PfGARP prepared by DNA vaccination, rabbit anti-PfMSP-4 (MR4) or anti-rabbit glycophorin A were diluted 1:200 in PBS, 5% BSA, pH 7.4. Blood smears were incubated with primary antibodies for 1 hr at 25 deg C, washed three times in PBS, 0.05 % Tween-20 and incubated with goat anti-mouse IgG conjugated with Alexa fluor 488 (Molecular Probes) and goat anti-rabbit IgG conjugated with Alexa Fluor 594 (Molecular Probes). Blood smears were incubated for 10 minutes in 1 μg/ml of 4′,6′-diamino-2-phenylindole (DAPI, Sigma) to label nuclei and cover-slipped with ProLong Gold anti-fade reagent (Invitrogen). Blood smears were imaged using a confocal microscope (Leica SP2, Leica Microsystems, Exton, PA) equipped with a 100× oil immersion objective and sequential Z-sections of the infected RBC were collected. Immuno-florescence assays were also performed using Anti-smV5 monoclonal antibodies and control IgG as described above.
To assess co-localization of PfGARP with the food vacuole membrane, we probed parasites with both anti-PfGARP as well as anti-PfCRT, a protein which localizes to the food vacuolar membrane 40.
In some experiments, parasite infected RBCs were incubated with primary antibodies prior to fixation and slide preparation to ensure that the methanol used for fixation did not permeabilize the RBC membrane.
Transmission electron microscopy
To assess the impact of anti-PfGARP on parasite ultrastructure, we performed TEM on parasites after 24 hr incubation with anti-PfGARP or control antibodies. 3D7 strain parasites were grown to high parasitemia (10%) consisting of predominantly trophozoites. Parasites were incubated with anti-PfGARP prepared by DNA or rPfGARP-A vaccination at 10% serum concentration for 24 hr at 37 °C. Pre-immune mouse sera at 10% serum concentration were used as a negative control.
Parasites were washed three times in 1 x PBS, and were fixed for 30 min at 4°C with 2% glutaraldehyde, 1% paraformaldehyde in 0.1 M sodium cacodylate buffer. Samples were dehydrated, embedded in Epon (EMS), sectioned on an ultra-microtome, counter stained for 10 min in 5% aqueous uranyl acetate and examined on a Philips CM10 electron microscope.
For immunoelectron microscopy, we performed live cell staining followed by fixation. Parasitized RBCs were blocked for 1 hour at 25 °C in 1x PBS containing 2% BSA. Samples were incubated with anti-PfGARP prepared by DNA or rPfGARP-A vaccination (diluted 1:200 in PBS) for 3 hr at 25 °C. Pre-immune mouse sera were used as a negative control. The samples were washed three times in 1 x PBS and probed with gold conjugated anti-mouse antibodies for 1 hour at 25 °C at 1:2 dilution (Invitrogen). Samples were washed three times in 1 x PBS, fixed and processed as described above.
Assessment of food vacuole integrity
We further evaluated the impact of anti-PfGARP antibodies on food vacuole integrity by confocal microscopy using the calcium binding dye Fluo-4-AM, which specifically labels the food vacuole. Ring stage 3D7 parasites were incubated with culture media (negative control), 1μM chloroquine (positive control), or anti-PfGARP prepared by rPfGARP-A immunization for 24 hours. Parasites were washed with 1x PBS three times and incubated with Fluo-4-AM (a cell-permeable calcium-sensing dye that fluoresces green when bound with Ca+2) at a final concentration of 2μM and DAPI for 30 minutes, at 370 C. The parasites were washed with 1x PBS and observed under a fluorescent microscope. Under these conditions, Fluo-4-AM localizes to the food vacuole as a punctate structure, while chloroquine disrupts the food vacuole leading to dispersion of the dye into the parasite’s cytosol.
Serial block face scanning electron microscopy
To further assess the impact of anti-PfGARP on parasite ultrastructure, we performed SBF-SEM on parasite infected RBCs that had been treated with anti-PfGARP antisera or preimmune antisera. Ring stage 3D7 strain parasites were grown to 5% parasitemia and incubated with anti-PfGARP antisera (prepared by DNA vaccination) or preimune sera (10% final serum concentration) for 30 hr at 37 °C. Parasites were washed three times in 1 x PBS, and fixed for 1 hour at 4°C with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer containing 1mM CaCl2 and 1mM MgCl2. Samples were washed 5 times with sodium cacodylate buffer. Samples were incubated at 4 °C for 1 hour with 1% osmium tetroxide and 1.5% potassium ferrocyanide in sodium cacodylate buffer followed by 5 washes with deionized water. Samples were incubated in 1% aqueous thiocarbohydrazide for 20min followed by 5 washes with deionized water. Samples were incubated in 1% aqueous osmium tetroxide for 30min, followed by overnight incubation at 4 °C in 1% uranyl acetate in 70% ethanol followed by 5 washes with deionized water. Samples were finally incubated in 0.2% aqueous lead citrate for 30min before being dehydrated, treated with propylene oxide, and embedded in Epon resin (EMS). Once cured, samples were cut and mounted on a 6.6mm specimen mount with conductive silver epoxy (MG Chemicals). Specimens were coated with gold palladium using an Emitech K550 sputter coater before being sectioned and imaged with a Thermo Apreo Volume Scope SEM under high vacuum. Acquired images were processed and the final three-dimensional reconstruction was performed with Amira 2019.2 software.
Processing of PfGARP
PfGARP encodes a predicted amino terminal signal sequence/transmembrane region (aa 1-22) and an appropriately located PEXEL element (aa 48-52). To determine whether parasites process and cleave the PEXEL element, we probed western blots of parasite extracts and recombinant PfGARP-A using peptide specific antisera generated against peptides that flank the PEXEL element (aa 31-48 and aa 504-522).
Anti-PfGARP antibody assays
Initial, confirmatory antibody assays were performed with rPfGARP coated ELISA plates according to our published methods. To measure IgG anti-rPfGARP antibody levels in the Kenyan cohort; we developed a bead-based assay according to our published methods 41. Briefly, 100 μg of rPfGARP-A or 100 μg of BSA was conjugated to 1.25 x 107 microspheres (Luminex), and conjugated rPfGARP and BSA beads were pooled and lyophilized in single-use aliquots. Reconstituted beads were incubated for 30 min at 37 deg C with human plasma samples at 1:80 dilution in Assay Buffer E (ABE, PBS pH 7.4 containing 0.1% BSA, 0.05% Tween-20, and 0.05% sodium azide) in microtiter filter bottom plates (Millipore). Beads were washed three times in ABE by vacuum filtration and incubated for 30 min at 37 deg C with biotinylated anti-human IgG (Pharmingen) diluted 1:1000 in ABE. Beads were washed three times in ABE by vacuum filtration and incubated for 10 min at 37 deg C with phycoerythrin-conjugated streptavidin (Pharmingen) diluted 1:500 in ABE. Beads were washed three times in ABE by vacuum filtration, resuspended in ABE and analyzed on a BioPlex 200 multi-analyte analyzer. Fluorescence values for BSA beads were subtracted from rPfGARP beads. The cut-off for detectable anti-PfGARP antibody levels was defined as fluorescence values greater than the mean + 2SD fluorescence level of of 22 healthy North American adults.
To measure IgG anti-rPfGARP antibody levels in the Tanzanian cohort, we performed bead-based assays using aa 23-673 of PfGARP expressed and purified from COS-7 cells as the target antigen. We expressed and purified aa 23-673 of PfGARP (excluding initial signal sequence/transmembrane domain) in a eukaryotic expression system (COS-7 cells) according to our published methods 42-44. Briefly, the coding sequence for aa 23-673 of PfGARP was PCR amplified with primers which encoded BamH1 and EcoR1 restriction sites, gel purified, digested with BamH1 and EcoR1 and ligated into BamH1 and EcoR1 digested vector pAdEx 42-44 Integrity of the PfGARP construct was verified by sequencing on both strands. Transfection of COS-7 cells, expression, extraction, and immobilization of recombinant PfGARP protein and control AdEx protein on the surface of BioPlex beads have been described in detail in our previous publications 42-44. Immobilization of recombinant PfGARP on beads was verified by reactivity with mouse anti-PfGARP antibodies. PfGARP and AdEx only beads were incubated for 30 min at 37 deg C with human plasma samples at 1:80 dilution in Assay Buffer E (ABE, PBS pH 7.4 containing 0.1% BSA, 0.05% Tween-20, and 0.05% sodium azide) in microtiter filter bottom plates (Millipore). Beads were washed three times in ABE by vacuum filtration and incubated for 30 min at 37 deg C with biotinylated anti-human IgG (Pharmingen) diluted 1:1000 in ABE. Beads were washed three times in ABE by vacuum filtration and incubated for 10 min at 37 deg C with phycoerythrin-conjugated streptavidin (Pharmingen) diluted 1:500 in ABE. Beads were washed three times in ABE by vacuum filtration, resuspended in ABE and analyzed on a BioPlex 200 multi-analyte analyzer. Fluorescence values for AdEx beads were subtracted from rPfGARP beads. The cut-off for detectable anti-PfGARP antibody levels was defined as fluorescence values for rPfGARP beads exceeding the value for AdEx beads.
Role of anti-PfGARP antibodies in resistance to malaria - statistical analyses
Tanzanian Birth Cohort
To assess the relationship between anti-PfGARP antibody responses and resistance to clinical malaria outcomes, we developed GEE based repeated measures models with antibody levels as a continuous variable (SAS version 9.3, Cary, NC). These models were used to evaluate the relationship between anti-PfGARP antibody levels (log transformed) and risk of malaria outcomes.
Potential confounders and effect modifiers, including hemoglobin phenotype, birthweight, and transmission season at birth, were evaluated with the two-sided fixed effect test and retained in the model if their P value was less than 0.1 or their inclusion altered the beta coefficient for the anti-PfGARP term by more than 10%. Only hemoglobin phenotype met the pre-specified criteria for inclusion (P < 0.1). We report both the unadjusted results as well as the model adjusted for hemoglobin phenotype.
Kenyan Cohort
To assess the relationship between anti-PfGARP antibody responses and resistance to P. falciparum parasitemia, we developed GEE based repeated measures models using JMP version 10 (Cary, NC). We evaluated the relationship between detectable anti-PfGARP IgG antibodies and parasite density measured on 18 post-treatment blood films. We assessed several potential confounders and effect modifiers including age, week of follow-up, exposure to Anopheles mosquitoes, and hemoglobin phenotype using the two-sided fixed effect test. Variables, including the week of monitoring, were retained in the model if their P value was less than 0.1 or they changed the parameter estimate for the antibody of interest by >10%. Age, exposure and hemoglobin phenotype were retained for face validity, though they did not meet the pre-specified criteria for inclusion, and PfGARP antibodies remained a significant predictor of parasite density with their exclusion.
PfGARP-A mRNA production
mRNAs were produced as previously described 45 using T7 RNA polymerase (Megascript, Ambion) on a linearized plasmid encoding codon-optimized 46 PfGARP-A. mRNAs were transcribed to contain 101 nucleotide-long poly(A) tails. One-methylpseudouridine (m1Ψ)-5’-triphosphate (TriLink) instead of UTP was used to generate modified nucleoside-containing mRNA. RNAs were capped using the m7G capping kit with 2’-O-methyltransferase (ScriptCap, CellScript) to obtain cap1. mRNA was purified by Fast Protein Liquid Chromatography (FPLC) (Akta Purifier, GE Healthcare), as described 47. All mRNAs were analyzed by denaturing or native agarose gel electrophoresis and were stored frozen at −20°C.
LNP formulation of the mRNA
Poly(C) RNA (Sigma) and FPLC-purified m1Ψ-containing mRNAs were encapsulated in LNPs using a self-assembly process in which an aqueous solution of mRNA at pH=4.0 is rapidly mixed with a solution of lipids dissolved in ethanol 48. LNPs used in this study were similar in composition to those described previously 49, which contain an ionizable cationic lipid (proprietary to Acuitas)/phosphatidylcholine/cholesterol/PEG-lipid (50:10:38.5:1.5 mol/mol) and were encapsulated at an RNA to total lipid ratio of ~0.05 (wt/wt). They had a diameter of ~80 nm as measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, UK) instrument. mRNA-LNP formulations were stored at −80°C at a concentration of mRNA of ~1μg/μl.
LNP-mRNA based Aotus monkey vaccination studies
We immunized n=5 monkeys intradermally with 50 μg of PfGARP-A as mRNA-LNP or 50 μg Poly(C) (n=4) intradermally on four sites of the shaved back. Monkeys received 3 doses at weeks 0, 3, and 6. Prior to each immunization, sera were obtained for antibody assays. At week 9, animals were challenged with 104 P. falciparum FVO strain (a heterologous challenge as the sequence for rPfGARP was derived from the 3D7 strain) blood-stage parasites by IV injection followed by daily blood films. Antibody assays were performed with PfGARP-A coated beads according to our published methods 41 using biotin-conjugated anti-monkey IgG antibody (Invitrogen) for detection of bound anti-PfGARP. Monkeys were monitored daily from day 4 post-challenge with blood samples to quantify parasitemia and hemoglobin concentration. Monkeys with parasitemia greater than 7.5%, hematocrit < 25%, or exhibiting signs of illness (fever, immobility, decreased food intake, etc.) were treated with oral Mefloquine in accordance with our animal protocol.
Recombinant PfGARP-A based Aotus monkey vaccination studies
We immunized n=5 monkeys intradermally with 50 μg of recombinant, E. coli produced PfGARP-A (aa 410-673) emulsified in Ribi adjuvant or Ribi adjuvant alone as controls (n=5). Monkeys received 3 doses at weeks 0, 3, and 6. Prior to each immunization, sera were obtained for antibody assays. At week 9, animals were challenged with 104 P. falciparum FVO strain (a heterologous challenge as the sequence for rPfGARP was derived from the 3D7 strain) blood-stage parasites by IV injection followed by daily blood films. Antibody assays were performed with PfGARP-A coated beads according to our published methods 41 using biotin-conjugated anti-monkey IgG antibody (Invitrogen) for detection of bound anti-PfGARP. Monkeys were monitored daily from day 4 post-challenge with blood samples to quantify parasitemia and hemoglobin concentration. Monkeys with parasitemia greater than 7.5%, hematocrit < 25%, or exhibiting signs of illness (fever, immobility, decreased food intake, etc.) were treated with oral Mefloquine in accordance with our animal protocol.
Data availability
All relevant data are available in the manuscript or as Source Data.
Extended Data
Extended Data Figure 1 ∣. Expression of PfGARP, killing of parasites by anti-PfGARP, and purification of anti-PfGARP antibodies.
a) SDS-PAGE gel of purified recombinant PfGARP-A (250ng). b and c) Extracts prepared from uninfected or 3D7 strain trophozoite-infected RBCs, or recombinant PfGARP-A were analyzed by western blot. b) probed with anti-PfGARP-A murine polyclonal sera generated by plasmid immunization and anti-MSP2 (rabbit polyclonal sera) and detected with anti-mouse IgG (red) and anti-rabbit IgG (green). c) probed with pre-immune mouse sera and anti-MSP2 (rabbit polyclonal sera) and detected with anti-mouse IgG (red) and anti-rabbit IgG (green). Panels a, b, and c are representative of 5 independent experiments. d) Growth inhibition assay (GIA) performed on parasites collected from our Muheza, Tanzania field site following short term culture adaptation. Assays were performed using polyclonal anti-PfGARP-A antibodies generated by recombinant protein immunization in mice. Ring stage malaria parasites adapted from adults (NIH 00710 and NIH 00918), or children (NIH 408551 and NIH4122821) were cultured in the presence of anti-PfGARP mouse sera at 1:10 dilution. Negative controls included no anti-sera and pre-immune mouse sera. Parasites were cultured for 48 hours at 37°C and ring and early trophozoite stage parasites were enumerated by microscopy. Bars represent the mean of 5 biologically independent replicates, error bars represent SEMs. P values calculated by two-sided non-parametric Mann-Whitney U test are indicated. Data are representative of 5 independent experiments. e) 3D7 parasites were synchronized to the ring stage and plated at 5% parasitemia in the presence of pre-immune (e and g) and anti-rPfGARP-A mouse sera (f and h) at 1:10 dilution. Parasites were cultured for 24 hours (e and f) or 48 hours (g and h), stained with giemsa, and photographed by light microscopy. Panel e, f, g, and h are representative of 3 independent experiments. i) Purification of anti-PfGARP antibodies from human (i and j) and mouse (k and l) serum. Sera was affinity purified using PfGARP-A coupled to Sepharose beads. The specificity of the purified anti-PfGARP antibodies was determined by western blot on extracts prepared from unsynchronized 3D7 parasite infected RBCs. In all panels, Lane 1- 3D7 infected RBCs extracted in RIPA buffer, Lane 2- uninfected RBCs extracted in RIPA buffer. i) probed with anti-PfGARP purified from serum pooled from adults living in a holoendemic area of Tanzania (2ug/ml) or j) malaria-naive human immunoglobulin (2ug/ml). k) probed with anti-PfGARP purified from serum prepared from PfGARP-A immunized mice (10ug/ml) or l) malaria-naive mouse immunoglobulin (10ug/ml). Panels i, j, k, and l are representative of 2 biologically independent experiments.
Extended Data Figure 2 ∣. Characterization of recombinant monoclonal anti-PfGARP.
a) Kinetics of binding between rec mAb7899 and PfGARP-A was measured at two antibody concentrations. For each concentration, two biologically independent replicates were performed. The experiment was performed twice and the data was pooled. The 95% confidence intervals (CI) is shown by dashed lines. Error bars represent standard. Formula shows linear regression. V, initial velocity of binding; c, concentration of biotinylated rec mAb7899 ; min, minutes, MFI, Median Fluorescence Intensity. b) Epitope mapping of rec mAb7899. We printed a custom 15-mer peptide microarray contained 264 different peptides which spanned the PfGARP-A sequence (aa 410-673). The peptides overlapped by a single amino acid and were printed in duplicate, framed by HA control peptides. The array was probed with rec mAb7899 (red) and anti-HA (green) and imaged on a LI-COR Odyssey. c) Ring stage 3D7 parasites were cultured in the presence of media, recombinant mAb anti-PfGARP, or recombinant mAb anti-fluorescein. Parasites were cultured for 48 hours at 37°C and lactate levels were measured in culture supernatant. Bars represent means of 4 biologically independent replicates, error bars represent SEMs. P value calculated by non-parametric two-sided Mann-Whitney U test is indicated. d) Ring stage 3D7, D10, or Dd2 parasites were cultured in the presence of media alone or recombinant anti-PfGARP Fab antibody (1 mg/ml) for 48 hrs at 37°C and ring or early trophozoite stage parasites were enumerated by microscopy. Bars represent means of 3 biologically independent replicates, error bars represent SEMs. P values calculated by non-parametric two-sided Mann-Whitney U test are indicated. Data representative of two biologically independent experiments.
Extended Data Figure 3 ∣. Construction and characterization of PfGARP knock down parasite line.
a) Targeting strategy for creating 3D7-PfGARP KD parasites, b) Immunoblot analysis of 3D7-PfGARP KD. Parasites were sorbitol-synchronized at the ring stage and incubated with or without anhydrotetracycline (Atc) for 20 hours and probed with anti-V5. The expected molecular weight of the PfGARP-V5 tagged protein is 124 kDa, however its apparent mobility is 165 kDa due to its acidic composition. Lane 1, uninfected RBCs, Lane 2- 3D7-PfGARP KD infected RBCs cultured without ATc, lane 3, 3D7-PfGARP KD infected RBCs cultured with ATc. c and d) Expression of PfGARP on the surface of human RBCs infected with 3D7-PfGARP KD parasites. Ring stage 3D7-PfGARP KD parasites were cultured to the trophozoite stage in the absence (C) or presence (D) of ATc. PfGARP expression on surface of infected human RBCs (fixed but not permeabilized) was determined by flow cytometry, using monoclonal anti-V5 as primary and anti-mouse IgG-FITC as secondary antibody. Infected RBCs were gated and identified as described in Fig. 1e. e) Growth curves for 3D7-PfGARP KD parasites. Ring-stage parasites were cultured with or without ATc. Parasitemia was measured by microscopy. Each data point represents the mean of 3 biologically independent replicates, error bars indicate SEM. f) Growth inhibition assay using 10% anti-rPfGARP-A antisera or pre-immune antisera on 3D7-PfGARP-KD parasites cultured with or without ATC. Bars represent means of 3 biologically independent replicates, error bars represent SEM. P value calculated by non-parametric two-sided Mann-Whitney U test are indicated. Panels b, d, e, and f are representative of 3 independent experiments. Panel c is representative of 5 independent experiments.
Extended Data Figure 4 ∣. Construction and characterization of PfGARP knock out parasite line.
a) Targeting strategy for creating PfGARP KO parasites, b) Immunoblot analysis of 3D7-PfGARP-KO. Trophozoite stage 3D7 or 3D7-PfGARP KO parasites were probed with anti-PfGARP and anti-actin as a loading control. Lane 1, 3D7-PfGARP KO infected RBCs, Lane 2- 3D7 infected RBCs. c and d) Expression of PfGARP on the surface of human RBCs infected with 3D7-PfGARP KO parasites. Ring-stage stage 3D7 or 3D7-PfGARP KO parasites were cultured to the trophozoite. PfGARP expression on the surface of infected human RBCs (fixed but not permeabilized) was determined by flow cytometry, using anti-PfGARP as primary and anti-mouse IgG-FITC as secondary antibody. Infected RBCs were gated and identified as described in Fig. 1e. e) Growth curve for 3D7-PfGARP KO parasites. Ring-stage stage 3D7 or 3D7-PfGARP KO parasites were plated at 0.5% parasitemia and cultured for 6 days. Parasitemia was measured by microscopy. Each data point represents the mean of 3 biologically independent replicates, error bars indicate SEM. f) Ring stage 3D7 or 3D7-PfGARP KO parasites with targeted deletion of PfGARP were cultured at 1:10 dilution in the presence of anti-PfGARP-A mouse sera generated by mRNA LNP immunization. Negative controls included no anti-sera and pre-immune mouse sera. Parasites were cultured for 48 hours at 37°C and ring and early trophozoite stage parasites were enumerated by microscopy. Bars represent the mean of 6 biologically independent replicates, error bars represent SEMs. P values calculated by non-parametric two-tailed Mann-Whitney U test are indicated. Data are representative of two independent experiments.
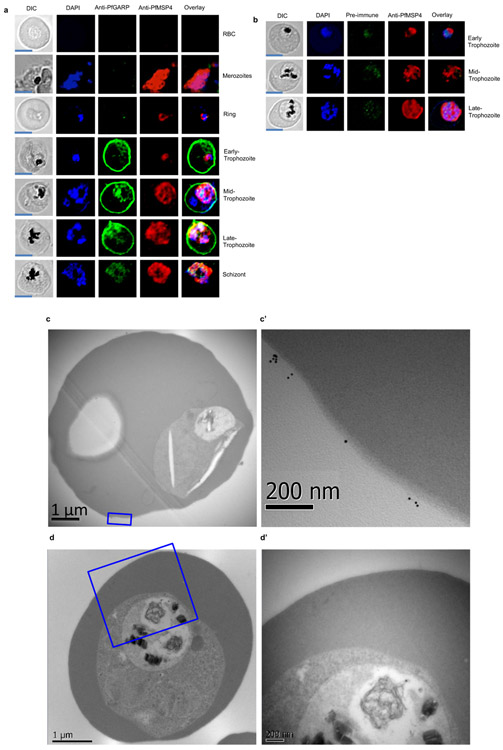
Extended Data Figure 5 ∣. Immunolocalization of PfGARP.
a) Uninfected and infected RBCs were probed with mouse anti-PfGARP prepared by DNA vaccination (green) and rabbit anti–PfMSP-4 (red) and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) to label parasite nuclei. PfGARP is detected on early, mid and late-trophozoite-infected RBC membranes and does not co-localize with PfMSP-4 (which localizes to the parasite membrane, scale bars 5 μm). b) Trophozoite-infected RBCs do not label when probed with pre-immune mouse sera by immunofluorescence microscopy (scale bars 5 μm). Non-permeabilized, un-fixed trophozoite infected RBCs (c and d) were incubated with anti-rPfGARP polyclonal (c) or control mouse serum (d), probed with anti-mouse IgG labeled with 10 nm gold particles, fixed, embedded and visualized by transmission electron microscopy. PfGARP localized to the outer leaflet of trophozoite infected RBCs. Figures labeled with primes represent higher magnifications views of the parent figure. Panel a and b are representative of 5 biologically independent experiments. Panel c and d are representative of 2 biologically independent experiments.
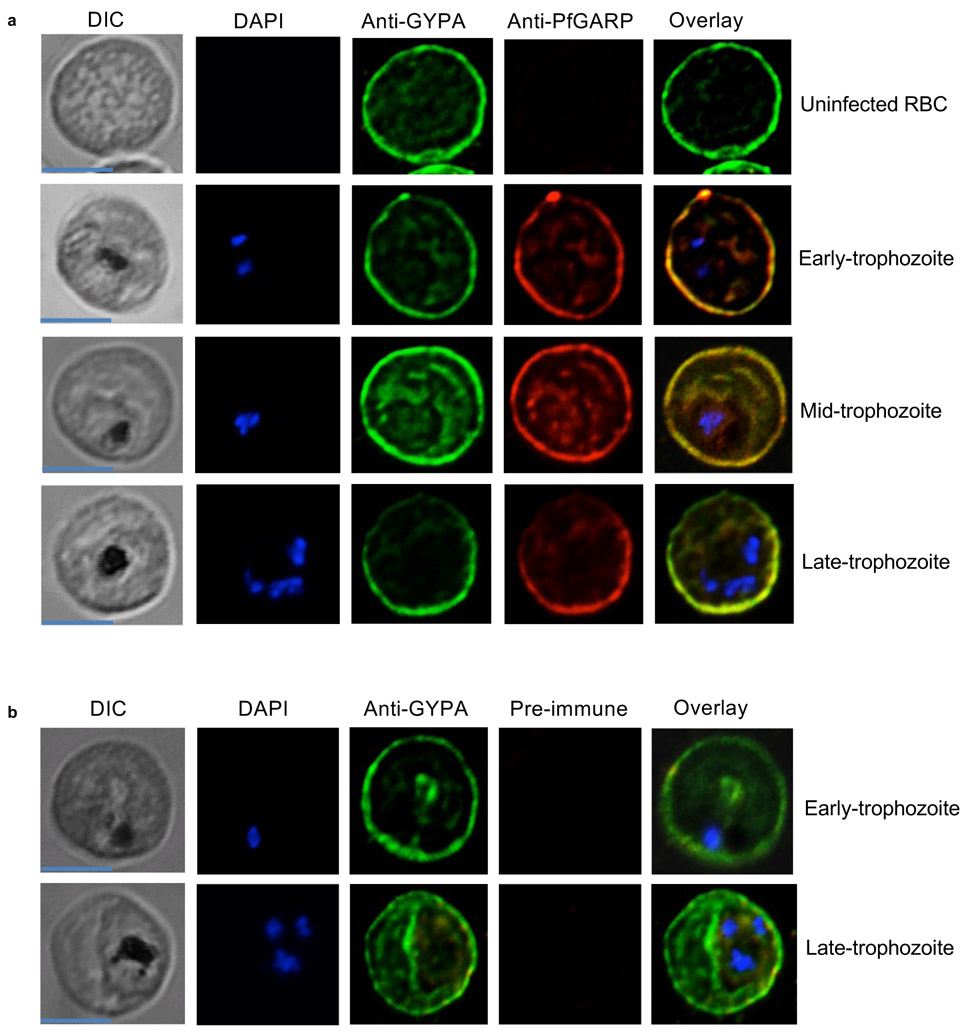
Extended Data Figure 6 ∣. PfGARP co-localizes with glycophorin A to the exofacial surface of trophozoite infected RBC membranes.
a) Uninfected and infected RBCs were probed with rabbit anti-glycophorin A (green) and recombinant DNA vaccine immunized mouse anti-PfGARP (red) and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) to label parasite nuclei. PfGARP is detected only in trophozoite-infected RBCs and co-localizes with human glycophorin A on the RBC membrane. Scale bars for all the images are 5 μm. b) Neither early nor late-trophozoite-infected RBCs label when probed with pre-immune mouse sera. Scale bar for all the images is 5 μm. Panels A and B are representative of 3 independent experiments.
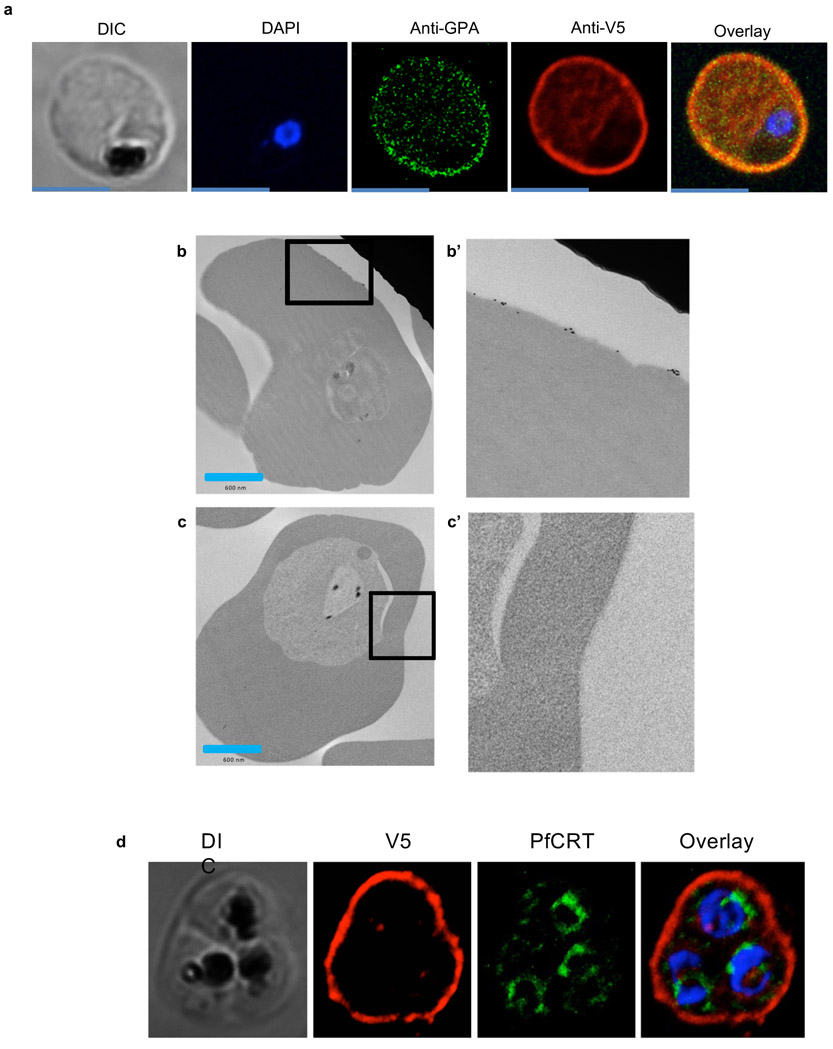
Extended Data Figure 7 ∣. Localization of V5 tagged PfGARP.
a) 3D7-PfGARP KD parasites, in which PfGARP is tagged with the V5 epitope, were grown in the presence of ATc to induce the expression of PfGARP and probed with rabbit anti-glycophorin A (green) and anti-V5 mouse antibodies (red) and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) to label parasite nuclei. PfGARP tagged with the V5 epitope co-localizes with glycophorin A to the exofacial surface of trophozoite-infected, non-permeablized, RBCs. Scale bar is 5 μm. Data are representative of 3 biologically independent experiments. b) 3D7-PfGARP KD parasites were grown in the presence of ATc to induce the expression of PfGARP. Trophozoite-stage 3D7-PfGARP KD were fixed and incubated with anti-V5 mouse IgG (b) or buffer (c) and probed with anti-mouse IgG labeled with 10 nm gold particles. V5 tagged PfGARP localized to the outer leaflet of 3D7-PfGARP KD infected, non-permeabilized RBCs. Figures labeled with primes represent higher magnifications views of the parent figure. Panels b and c are representative of 2 biologically independent experiments. e) 3D7-PfGARP KD parasites were grown in the presence of ATc to induce the expression of PfGARP and probed with anti-PfCRT (green), anti-V5 antibodies (red) and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) to label parasite nuclei. PfGARP does not co-localize with PfCRT to the food vacuole in the majority of trophozoite infected RBCs. Panel d is representative of 5 biologically independent experiments.
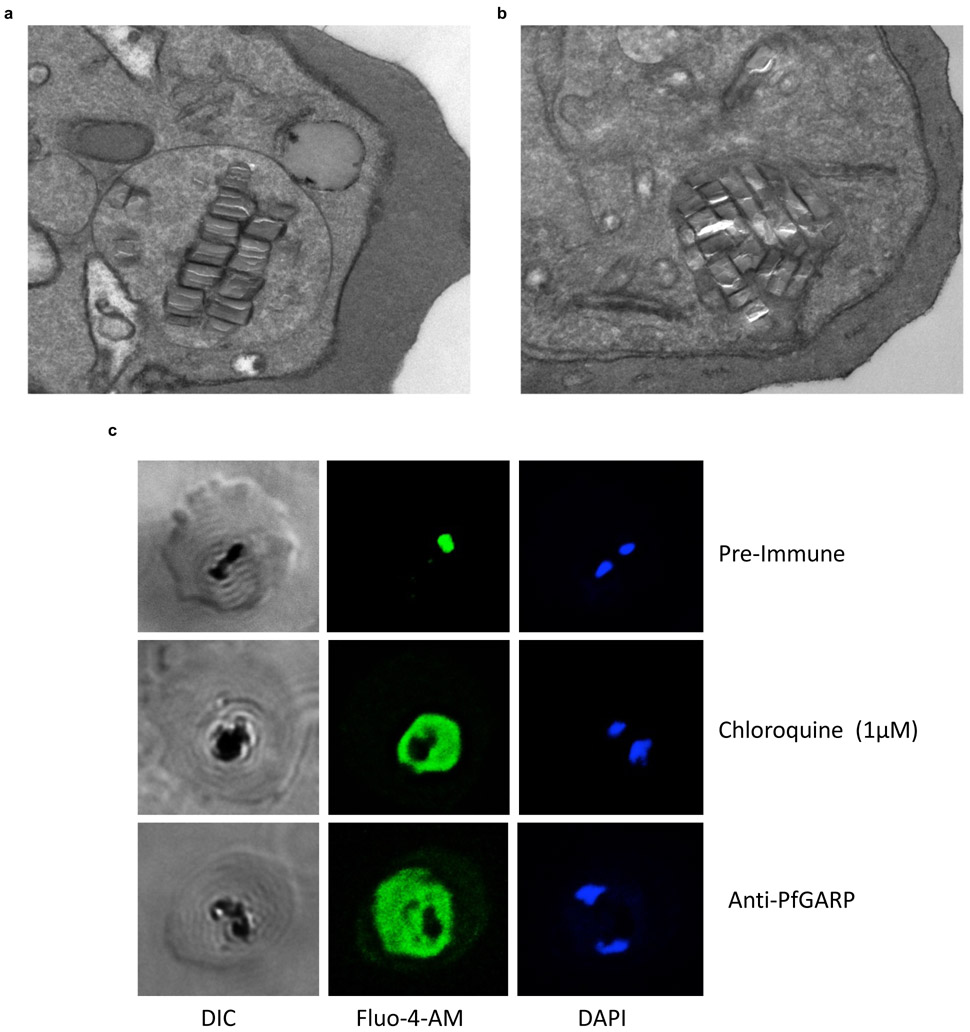
Extended Data Figure 8 ∣. Anti-PfGARP antibodies disrupt food vacuole integrity.
3D7 parasites were synchronized to the ring stage and plated at 5% parasitemia in the presence of pre-immune (a) or anti-rPfGARP-A mouse sera (b) at 1:10 dilution. Parasites were cultured for 24 hours and processed for transmission electron microscopy. Panel a and b are representative of all trophozoites observed in 3 biologically independent experiments. c) Ring stage 3D7 parasites were treated with pre-immune sera (1:10), 1μM chloroquine, or anti-PfGARP antisera (1:10) for 24 hours, followed by staining with DAPI and Fluo-4-AM. Data are representative of 2 biologically independent experiments.
Extended Data Figure 9 ∣. Vaccination with PfGARP protects monkeys from P. falciparum challenge.
a) Individual parasitemia data from monkey trial presented in Fig 5. Aotus monkeys vaccinated with rPfGARP-A formulated as a lipid encapsulated mRNA (n=5) and control monkeys vaccinated with lipid encapsulated poly C (n=4) were challenged IV with 105 P. falciparum infected RBC. H indicates treatment for low hemoglobin, P indicates treatment for high parasitemia. b) Animals were injected subcutaneously with 50 μg of rPfGARP-A emulsified in 100 ul of Ribi (n=5 monkeys) or Ribi alone (negative control, n=4 monkeys) at weeks 0, 3 and 6 and PfGARP-A specific IgG titers were determined. Bars represent mean titer, error bars represent SEMs. c) Vaccinated Aotus monkeys were challenged IV with 104 P. falciparum FVO strain infected RBC on day 63 and parasitemia was followed daily. Points represent means, error bars represent SEM. Control monkeys had significantly higher parasitemia on days 7-12 compared to PfGARP vaccinated animals. On day 11, the final day with complete follow-up of all monkeys, controls had 3.5 fold higher parasitemia compared to PfGARP vaccinated monkeys. Four control monkeys met pre-specified criteria for drug treatment on day 11 and the final control monkey met criteria on day 12. On Day 11, one PfGARP vaccinated monkey was drug treated despite not meeting pre-specified criteria. * indicates P < 0.05. ** indicates P < 0.01 in two-sided t-Tests without adjustment for multiple comparisons. d) Individual parasitemia data from monkey trial presented in panel c.
Extended Data Figure 10 ∣. The PEXEL element is processed and cleaved in mature PfGARP.
a) Schematic depicting the binding sites for the peptide specific antibodies. Immunoblot of recombinant PfGARP-A (lane 1), recombinant full length PfGARP (lane 2), and an extract of trophozoite infected RBCs (lane 3) probed with b), antisera raised against aa 504-522 of PfGARP, c) pre immune sera, d) antisera raised against aa 31-48 of PfGARP, or e) pre immune antisera. Only antibodies raised against aa 504-522 recognized native PfGARP in trophozoite infected RBCs, while the anti-sera raised against aa 31-48 only recognized the full length recombinant PfGARP confirming that the PEXEL element is cleaved during the processing of native PfGARP. Abbreviations: SP-signal peptide, Pxl- pexel element. Data are representative of 3 biologically independent experiments.
Supplementary Material
Acknowledgments
The authors wish to thank MOMS Project staff for their effort collecting clinical data, sample processing and interpreting malaria blood smears as well as the study subjects and their families. Ethical clearance was obtained from the IRBs of SBRI and Rhode Island Hospital, the Medical Research Coordinating Committee of the National Institute for Medical Research, Tanzania, and the Kenyan Medical Research Institute. Ethical clearance for animal studies was obtained from the relevant review boards of Brown University, Rhode Island Hospital, and the NIH.
This work was supported by grants from the US National Institutes of Health (grant R01-AI076353, R01-AI127699, and R01-AI110699) and an internal Rhode Island Hospital Research Pilot Award Grant to JDK, grants from the US National Institutes of Health (grant R01-AI52059) and the Bill & Melinda Gates Foundation (grant 1364) to PED, the Intramural Research Program of the NIAID-NIH, NIH grant R01AI092120 and Florida Atlantic University start-up funds to AVO, NIH R37 AI50234 to DAF, and R01 AI145941 and R01 AI102907 to JDD.
We also acknowledge research core services provided by the RIH imaging core (Ginny Hovanesian), the Leduc bioimaging facility (Geoff Williams) and core services supported by COBRE CCRD (P20GM103421). The Thermo Apreo VS SEM used for the serial block face imaging was purchased with a high-end instrumentation grant from the Office of the Director at the National Institutes of Health (S10 OD023461).
The DNA sequence for PfGARP is available in PlasmoDB (www.Plasmodb.org) as ID# PF3D7_0113000. The work presented in this manuscript has been submitted in partial support of patent application # PCT/US12/67404 filed with the United States Patent Office. Authors Lynn Lambert, Sachy Orr-Gonzalez, Michal Fried and Patrick E. Duffy are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Current address for Nicholas Hilton is Department of Microbiology and Molecular Genetics, University of California, Davis, Davis, CA 95616, USA
Footnotes
Competing Interests None to declare.
References
- 1.WHO. World health statistics 2018: monitoring health for the SDGs, sustainable development goals., Vol. https://www.who.int/data/gho/publications/world-health-statistics (World Health Organization, 2018). [Google Scholar]
- 2.Raj DK et al. Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 344, 871–877, doi: 10.1126/science.1254417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mutabingwa TK et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS medicine 2, e407, doi: 10.1371/journal.pmed.0020407 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurrecoechea C et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic acids research 37, D539–543, doi: 10.1093/nar/gkn814 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, Mackey AJ, Stoeckert CJ Jr. & Roos DS OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic acids research 34, D363–368, doi: 10.1093/nar/gkj123 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Barragan MJ et al. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC genomics 12, 587, doi: 10.1186/1471-2164-12-587 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manske M et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487, 375–379, doi: 10.1038/nature11174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari P, Kaila P & Guptasarma P Understanding anomalous mobility of proteins on SDS-PAGE with special reference to the highly acidic extracellular domains of human E- and N-cadherins. Electrophoresis 40, 1273–1281, doi: 10.1002/elps.201800219 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Ockenhouse CF, Schulman S & Shear HL Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol 133, 1601–1608 (1984). [PubMed] [Google Scholar]
- 10.Jensen JB, Boland MT & Akood M Induction of crisis forms in cultured Plasmodium falciparum with human immune serum from Sudan. Science 216, 1230–1233 (1982). [DOI] [PubMed] [Google Scholar]
- 11.Ganesan SM, Falla A, Goldfless SJ, Nasamu AS & Niles JC Synthetic RNA-protein modules integrated with native translation mechanisms to control gene expression in malaria parasites. Nature communications 7, 10727, doi: 10.1038/ncomms10727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ch'ng JH, Liew K, Goh AS, Sidhartha E & Tan KS Drug-induced permeabilization of parasite's digestive vacuole is a key trigger of programmed cell death in Plasmodium falciparum. Cell Death Dis 2, e216, doi: 10.1038/cddis.2011.97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtis J, Lanar D, Opollo M & Duffy P in Am. J. Trop. Med. Hyg 309. [Google Scholar]
- 14.Kurtis JD, Mtalib R, Onyango FK & Duffy PE Human resistance to Plasmodium falciparum increases during puberty and is predicted by dehydroepiandrosterone sulfate levels. Infect Immun 69, 123–128, doi: 10.1128/IAI.69.1.123-128.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nixon CP et al. Antibodies to rhoptry-associated membrane antigen predict resistance to Plasmodium falciparum. J. Infect. Dis 192, 861–869, doi: 10.1086/432550 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Pardi N et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. The Journal of experimental medicine 215, 1571–1588, doi: 10.1084/jem.20171450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardi N et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251, doi: 10.1038/nature21428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardi N et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nature communications 9, 3361, doi: 10.1038/s41467-018-05482-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan AF, Fabucci ME, Saul A, Kaslow DC & Miller LH Aotus New World monkeys: model for studying malaria-induced anemia. Blood 99, 3863–3866 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Vandana, Dixit R, Tiwari R, Katyal A & Pandey KC Metacaspases: Potential Drug Target Against Protozoan Parasites. Frontiers in pharmacology 10, 790, doi: 10.3389/fphar.2019.00790 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meslin B, Beavogui AH, Fasel N & Picot S Plasmodium falciparum metacaspase PfMCA-1 triggers a z-VAD-fmk inhibitable protease to promote cell death. PloS one 6, e23867, doi: 10.1371/journal.pone.0023867 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutai BK & Waitumbi JN Apoptosis stalks Plasmodium falciparum maintained in continuous culture condition. Malaria journal 9 Suppl 3, S6, doi: 10.1186/1475-2875-9-S3-S6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelbrecht D & Coetzer TL Plasmodium falciparum exhibits markers of regulated cell death at high population density in vitro. Parasitology international 65, 715–727, doi: 10.1016/j.parint.2016.07.007 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Chou ES et al. A high parasite density environment induces transcriptional changes and cell death in Plasmodium falciparum blood stages. The FEBS journal 285, 848–870, doi: 10.1111/febs.14370 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Agnandji ST et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. The New England journal of medicine 365, 1863–1875, doi: 10.1056/NEJMoa1102287 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Crosnier C et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534–537, doi: 10.1038/nature10606 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available in the manuscript or as Source Data.