Abstract
Background
No validated treatments have been identified for the COVID-19 pandemic virus; several are currently in randomized clinical trials. Diagnostic instruments are rapidly evolving. Symptoms range from those of a common cold to acute respiratory distress syndrome (ARDS), to sepsis arising from the flood of inflammatory bacterial and viral pathogens in the blood. Mortality generally arises from cytokine storms of uncontrolled inflammation, oxidative injury, and damage to the alveolar-capillary barrier, with secondary bacterial infection. To address the indisputably urgent need for therapeutics for COVID-19, a specialized interdisciplinary medical panel convened in Shanghai in March 2020 to consider all relevant clinical and experimental evidence on the possible utility of intravenous (IV) ascorbate in the treatment of COVID-19-related ARDS.
Methods
The panel convened multidisciplinary medical experts and reviewed all relevant in vitro, in vivo, clinical studies and randomized controlled trials on IV ascorbate and issued a consensus report on 23 March 2020 noting that substantial differences in serum concentrations of ascorbate are achieved through IV administration in contrast with the oral route.
Findings
The Shanghai panel, and a parallel medical group in Guangzhou, are advising the use of high-dose IV ascorbate for the treatment of ARDS, along with other supportive therapies, including Vitamin D and zinc. We report preliminary progress in using this treatment for 50 consecutive cases treated in Shanghai hospitals, consistent with earlier reports from a meta-analysis of the use of IV ascorbate to treat sepsis. We provide an instructive clinical anecdote regarding a single family where one elderly member with cardiac and other major comorbidities developed and survived ARDS-related sepsis following daily treatments that included 15 g of IV ascorbate. None of her adult caregivers who had ingested between 2 and 10 g of ascorbate daily developed COVID-19.
Keywords: public health, global health, covid-19, intravenous ascorbate
Background
While randomized controlled trials (RCTs) remain the gold standard for therapies, given the unprecedented scale and scope of the COVID-19 pandemic, medicine is compelled to take a hard look at interventions as they are evolving. We report here that a special expert medical panel convened by the Shanghai government on 23 March 2020 advised treating physicians to expand clinical uses of ingested ascorbate for prophylaxis and higher dose intravenous (IV) therapy for COVID-19.1 Vitamin C, also known as ascorbic acid or ascorbate, clearly has antioxidant properties and appears generally to be depleted in those with underlying infections.2
The Physicians Desk Summary (PDQ) on the U.S. National Cancer Institute website reports that IV ascorbate has been evaluated in RCTs for the treatment of sepsis, for the prevention of viral diseases, and in the treatment of advanced cancer along with a number of chemotherapy agents for cases of advanced gastric, glioblastoma multiforme, and pancreas cancer. IV ascorbate combined with Vitamin D and zinc as supportive therapies has also been shown to be safe and efficacious in some cases involving viral infections that lead to lung capillary endothelial cell activation, neutrophil infiltration, and increased reactive oxygen species (ROS) and reactive nitrogen species (RNS).3
Ascorbate Pharmacokinetics
Critically different results have been reported in duration and levels of ascorbate depending on route of exposure. Experimental and clinical investigations led by Mark Levine at the U.S. National Institutes of Health demonstrate that ascorbate plasma concentrations can be maintained at <100 µM.4 Levels in serum following oral administration are limited by gastrointestinal absorption, renal function, tissue uptake, and metabolic rate.4,5
In contrast, IV ascorbate can achieve up to 25 times higher serum ascorbate levels than when the same amount is ingested.3,6 IV administered ascorbate provides immune-supportive as well as anti-oxidant, anti-carcinogenic, and anti-mutagenic effects, including neutrophil chemotaxis, phagocytosis, and consequent microbial clearance.7 In addition, ascorbate promotes T-cell and natural killer (NK) cell proliferation and modulates their functions against cancers and viral proliferation by enhancing macrophage function.8,9
The inability of earlier studies to show efficacy for ascorbate may lie with a fundamental failure to differentiate between nutritional and pharmacological doses. For therapeutic applications for COVID-19, the Shanghai protocol recommends that dosing regime should allow sustained high plasma levels to be achieved through twice daily doses of 12 to 15 g administered at 12 ml/h. The dosage recommendation will vary with the severity of the illness ranging from 50 to 200 mg/kg/day to as much as 16,000 mg/kg/day administered IV.
Recent evidence for a prophylactic role for ingested ascorbate against rhinovirus was demonstrated by a randomized double-blind study of 1444 healthy military recruits in basic training from Korea treated for 1 month from February to March of 2018. Those receiving prophylactic ascorbate had 80% fewer upper respiratory infections compared with those receiving the placebo, with the greatest benefit to never-smokers.10
Clinical Trials of Vitamin C and One Instructive Anecdote
In further support of the use of ascorbate both prophylactically and therapeutically, we wish to report what we regard as a highly instructive corroborated anecdote that provides some insight into the possible role of ascorbate for the current pandemic. A family of 6 well-educated adults in Wuhan, China, spent considerable time taking care of their eldest member who had a number of comorbidities and developed COVID-19. Each one of her non-cohabiting caregivers took between 3 and 10 g daily of ascorbate with their food in 2 divided doses. When the ill family member entered intensive care and was placed on a mechanical respirator, treating physicians agreed to administer IV ascorbate in addition to supportive therapy. Unlike more than half of those in her condition in Wuhan in February, she recovered.
Not a single member of the exposed caregivers came down with the virus.
Of course, this anecdote might simply be the result of a coincidence. In effect this story can be construed as a small-scale trial insofar as exposures certainly took place to COVID-19 for the patient and each one of her caregivers who did not use personal protective equipment. Yet none developed the disease after ingesting prophylactic doses of ascorbate throughout her illness.
Interpretations From the Front Lines and Other Instructive Anecdotes
In an impressive series of papers, Prof. Paul Marik previously demonstrated the stunning capacity of ascorbate with steroids, Vitamin D, and other supportive treatment to reverse sepsis among critically ill patients.11 Further support is provided by a meta-analysis of 3 studies, including 146 patients in intensive care units that randomly administered IV ascorbate finding significant benefits in those being treated.11–13 When sepsis occurs, the cytokine surge and neutrophil accumulation in the lungs destroy alveolar capillaries. These clinical studies have shown that vitamin C can effectively prevent this process and can reduce alveolar fluid by inhibiting the activation and accumulation of neutrophils and reducing alveolar epithelial water channel damage that can induce vascular injury.
This work is now effectively corroborated by additional reports from the medical frontlines in China and Korea in the treatment of COVID-19. A medical director in Wuhan Tongji Hospital reports the following:
On the afternoon of 20 February 2020, another 4 patients with severe new coronaviral pneumonia recovered from the C10 West Ward of Tongji Hospital [Wuhan]. In the past 8 patients have been discharged from hospital … [H]igh-dose vitamin C achieved good results in clinical applications … [H]gh-dose vitamin C can not only improve antiviral levels, but more importantly, can prevent and treat acute lung injury (ALI) and acute respiratory distress (ARDS).
From South Korea, a treating physician, Hyoungjoo Shin, provides the following report from March 2020:
At my hospital in Daegu, South Korea, all inpatients and all staff members have been using vitamin C orally … . Some people this week had a mild fever, headaches and coughs, and those who had symptoms got 30,000 mg intravenous vitamin C. Some people got better after about 2 days, and most had symptoms go away after one injection.
In March 2020, high-dose IV administration successfully treated 50 moderate to severe COVID-19 patients in Shanghai, China.14 The doses ranged from 2 g to 10 g per day, given over a period of 8 to 10 hours, for 5 to 7 days. For this group of recent patients, the oxygenation index improved in real time and all were discharged from intensive care and released from the hospital as of 23 March 2020.1
It is important to note that high-dose ascorbate has been clinically used for several decades with no serious side effects reported, aside from those that are carriers of G6PD who cannot receive more than 15 g a day because this increases their risk of hemolytic anemia and rare instances of oxalate nephropathy after long-term treatments. In fact a recent NIH expert panel document states clearly that a regimen of 1.5 g/kg body weight is safe and without major adverse events.3
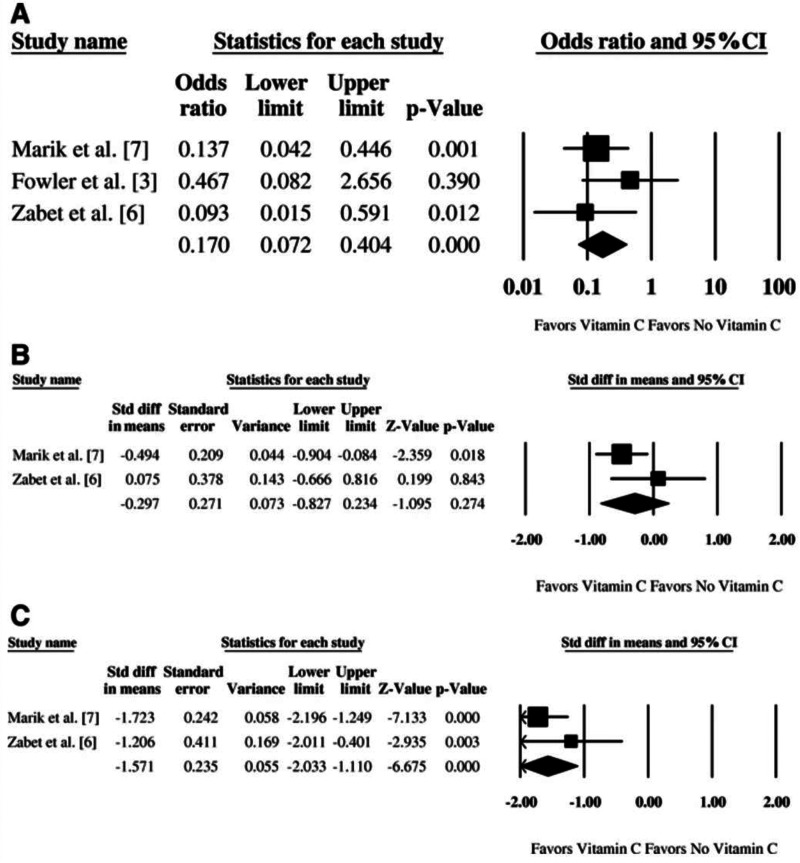
As of April 10, 2020, there were over a dozen RCTs registered on clinicaltrials.gov that are assessing the effects of vitamin C administration in critically ill patients, particularly those with sepsis, and 28 studies listed on ascorbate and cancer.15 Two ongoing trials in Canada and China are assessing ascorbate in patients admitted to the ICU with sepsis, including COVID-19.16,17 (Figure 1)
Clinical Trials and Relative Risk Profiles of Investigational Agents Against COVID-19
We note with interest that Northwell Health facilities on New York’s Long Island has reportedly begun using IV ascorbate with critically ill patients in that hospital system as is the University of Wisconsin Critical Care Unit.16 Other efforts to employ bolus infusions of ascorbate and other nutritional supplementation for COVID-19 patients are underway under the medical direction of Miriam Rahav in New York City. In addition, George Moskowitz, MD, a family physician in Borough Park, Brooklyn, New York, reported recently that COVID-19 positive patient that had developed unresponsive respiratory symptoms was successfully and uneventfully treated with a single course of 40 grams of IV ascorbate. As this report was being prepared, we learned that high doses of IV ascorbate were part of the successful treatment given to a gravely ill 44-year-old emergency room physician in Seattle. As glassy lung developed, he was placed on a mechanical ventilator at Swedish Hospital. A combination of twice daily infusions of up to 25 g of ascorbate and the rheumatoid arthritis medication Actemra are credited with preventing his demise.
Of course, there is no dispute that the gold standard for clinical evidence remains the RCT. There is also no dispute that under these grave circumstances of an exponentially growing pandemic, where more than a quarter of all children who are positive for COVID-19 have few or no symptoms and many transmitters of the disease are asymptomatic it is critically important to devise innovative approaches both to prevent and treat the disease.17 The use of ascorbate, a relatively low-cost and nontoxic option, should be considered an important option in light of the growing impact COVID-19 is having on public health and the global economy.
In conclusion, we note that the history of medicine is replete with instances where well-drawn case reports have guided the creation of life-saving innovations. The catheterization of the human heart by Andre Cournand and Dickinson Richards provides an especially apt instance of such a world-changing clinical case report, depicted in Cournand’s Nobel Prize in Medicine acceptance speech in 1956.18
We present these cases and findings fully aware of their limitations and the unprecedented nature of the challenges posed by this global pandemic. Ultimately RCTs will prove pivotal. Currently, supportive care is the only proven remedy for those with advanced COVID-19. Were reliable, nontoxic treatments such as the use of ascorbate validated for this novel virus, this would greatly facilitate the return to a new normal for medicine and for civil society.
A recent consensus statement from a group of renowned infectious disease clinicians observed that vaccine programs have proven ill-suited to the fast-changing viruses underlying these illnesses, with efficacy ranging from 19% to 54% in the past few years. Accordingly, they advise that:
supplementing above the RDA for certain immune-supporting vitamins, promotes optimal immune function, helps to control the impact of infections, and could help limit the emergence of novel, more virulent strains of pathogenic viruses. We, therefore, strongly encourage public health officials to also include nutritional strategies in their arsenal to improve public health and to limit the impact of seasonal and emerging viral infections.19
Acknowledgments
The authors would like to thank Meg Sears, PhD, and Mark A. Tuckfelt, MD, for their provided instructive comments and review, and Candace Woodbury for her research assistance in completing this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Figure 1.
Meta-analyses show that ascorbate shortens use of vasopressors and reduces mortality from sepsis. Adapted from Li16 (see https://ccforum.biomedcentral.com/articles/10.1186/s13054-018-2191-x#Fig1).
ORCID iD
Devra Davis https://orcid.org/0000-0003-1759-8275
References
- 1.Shanghai 2019 coronary virus disease comprehensive treatment expert consensus. Chin J Infect Dis 2020;38. doi:10.3760/cma.j.issn.1000-6680.2020.0016
- 2.Cantley L, Yun J. Intravenous high-dose vitamin C in cancer therapy. National Cancer Institute. https://www.cancer.gov/research/key-initiatives/ras/ras-central/blog/2020/yun-cantley-vitamin-c. Published 2020. Accessed April 3, 2020.
- 3.PDQ® integrative, alternative and CTEB. PDQ high-dose vitamin C. National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq. Published 2020. Accessed February 8, 2020.
- 4.Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci of USA. 1996; 93(8):3704–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LRJ, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997; 14(9):1133–1139. [DOI] [PubMed] [Google Scholar]
- 6.Ohno S, Ohno Y, Suzuki N, Soma G-I, Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009; 29(3):809–815. [PubMed] [Google Scholar]
- 7.Levy TE. Primal Panacea. Henderson, NV: MedFox Publishing; 2011. [Google Scholar]
- 8.Meng L, Zhao X, Zhang H. HIPK1 interference attenuates inflammation and oxidative stress of acute lung injury via autophagy. Med Sci Monitor Int Med J Exp Clin Res. 2019; 25:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel V, Dial K, Wu J, et al. Dietary antioxidants significantly attenuate hyperoxia-induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway HMGB1. Int J Mol Sci 2020; 21(3). doi:10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TK, Lim HR, Byun JS. Vitamin C supplementation reduces the odds of developing a common cold in Republic of Korea Army recruits: randomised controlled trial. BMJ Military Health Published online March 2020. doi:10.1136/bmjmilitary-2019-001384 [DOI] [PubMed]
- 11.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017; 151(6):1229–1238. [DOI] [PubMed] [Google Scholar]
- 12.Hemilä H, Chalker E. Vitamin C can shorten the length of stay in the ICU: A meta-analysis. Nutrients. 2019; 11(4). doi:10.3390/nu11040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabzdyk CS, Bittner EA. Vitamin C in the critically ill – indications and controversies. World J Crit Care Med. 2018; 7(5):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng RZ. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov Published online March 2020. doi:10.1016/J.MEDIDD.2020.100028 [DOI] [PMC free article] [PubMed]
- 15.Li J. Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis. Crit Care. 2018; 22(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masse MH, Ménard J, Sprague S, et al. Lessening Organ dysfunction with VITamin C (LOVIT): protocol for a randomized controlled trial. Trials. 2020; 21(1). doi:10.1186/s13063-019-3834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitamin C infusion for the treatment of severe 2019-nCoV infected pneumonia – tabular view – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT04264533. Accessed April 12, 2020.
- 18.André F. Cournand – banquet speech – NobelPrize.org. https://www.nobelprize.org/prizes/medicine/1956/cournand/speech/. Accessed April 15, 2020.
- 19.Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020; 12(4):1181. [DOI] [PMC free article] [PubMed] [Google Scholar]