Abstract
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040. 1
Keywords: pathology competencies, organ system pathology, gastrointestinal tract, immune-related disorders of the bowel, Crohn’s disease, ulcerative colitis, idiopathic inflammatory bowel disease
Primary Objectives
GT5.3 Crohn’s Disease and Ulcerative Colitis. Describe the distribution of Crohn’s disease, pathogenesis, and how transmural involvement is related to complications and compare and contrast Crohn’s disease with ulcerative colitis.
Competency 2: Organ System Pathology; Topic GT: Gastrointestinal Tract; Learning Goal 5: Immune-Related Disorders of the Bowel.
GT5.1 Inflammatory Bowel Disease. Compare and contrast the pathophysiology and clinicopathologic features of inflammatory bowel disease.
Competency 2: Organ System Pathology; Topic GT: Gastrointestinal Tract; Learning Goal 5: Immune-Related Disorders of the Bowel
Patient Presentation, 1
A 22-year-old man presents to the emergency department with severe right lower quadrant abdominal pain and abdominal bloating and distension. The patient notes intermittent mild diarrhea for the past 8 to 10 months with abdominal pain and low-grade fevers to 101 °F. The intervening asymptomatic periods ranged from a few weeks to a couple of months. These symptoms seem to have started around the same time the patient began smoking. A 10-pound unintentional weight loss is noted over this period. His parents are immigrants to the United States from Eastern Europe.
Diagnostic Findings, Part 1, Physical Examination
On physical examination, the heart rate is 65, respiratory rate is 18, blood pressure is 118/68, body mass index is 19.5, and his temperature is 98.6 °F. The abdominal examination reveals tenderness to palpation, most prominent in the right lower quadrant. Deep palpation in this area suggests the presence of fullness or ill-defined mass when compared with the left lower quadrant. There is mild associated guarding to deep palpation, but no rebound tenderness.
Questions/Discussion Points, Part 1
What Is the Clinical Differential Diagnosis for This Patient?
The clinical differential diagnosis includes entities such as celiac disease, infectious enteritis, colitis of various types (acute self-limited infectious, pseudomembranous Clostridium difficile, collagenous, lymphocytic), appendicitis, and bowel obstruction. The chronic and progressive course is atypical for the common causes of acute diarrhea such as viral or other infectious enteritis but would be somewhat more in keeping with entities causing chronic diarrhea such as celiac disease or inflammatory bowel disease (IBD). The association of the diarrhea with fevers suggests an inflammatory diarrhea rather than a fatty (malabsorption or maldigestion) or watery (secretory) diarrhea.2,3 Additional laboratory testing can help to narrow this differential.
Diagnostic Findings, Part 2, Initial Laboratory Workup
Laboratory tests were performed as an initial workup of the patient. Initial laboratory test results include a white blood cell count of 14 × 109/L (3.4-10.7 × 109/L) and a neutrophil count of 8 × 109/L (1.56-6.45 × 109/L). The hemoglobin is 14.2 g/dL (12.9-16.9 g/dL) and a tissue transglutaminase antibody is 1 U/mL (<3 U/mL). The stool for C difficile toxin is negative, as are the stool exams for ova and parasites.
Questions/Discussion Points, Part 2
What Laboratory Findings Are Significant and What Remains in the Differential Diagnosis Following These Tests?
The negative test for tissue transglutaminase makes celiac disease unlikely, and the negative testing for C difficle toxin and the negative exam for ova and parasites reduce the likelihood of an infectious etiology. Because of concern for acute bowel obstruction superimposed on the more chronic history, and with an ill-defined potential mass on palpation, a computed tomography (CT) scan is obtained.
Diagnostic Findings, Part 3, Imaging and Pathologic Features
The CT scan shows dilatation of much of the small bowel suggesting a small bowel obstruction. In the region of the distal ileum, there is marked luminal narrowing and thickening of the bowel wall associated with a radiographic “string sign.”4 The appendix is visualized and felt to be within normal limits. The string sign differential diagnosis includes predominantly idiopathic IBD and neoplasm. Due to the obstruction, general surgery is consulted. The patient undergoes surgical resection of a 25-cm length of terminal ileum. The gross and microscopic pathology is shown Figures 1 to 4.
Figure 1.
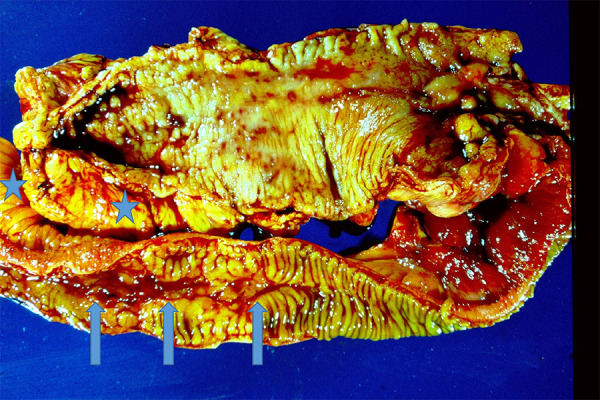
Crohn’s disease gross morphology. There is typical ileal involvement which is discontinuous with areas of relatively normal mucosa separating areas with deep ulceration and long linear fissures (arrows). The mucosa ranges from flattened to a coarse irregular pattern of folds. The mesenteric fat nearly encircles the bowel, so-called the “creeping fat” (stars) which is a response to transmural inflammation.
Figure 2.
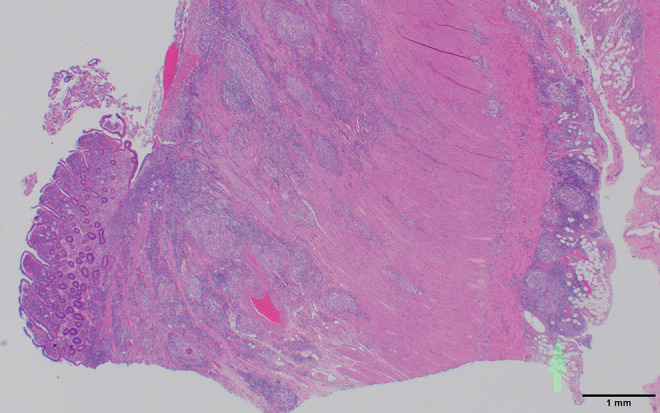
Crohn’s disease histology. The low-power view demonstrates nicely a portion of the deep mucosal linear fissure/ulceration with absent mucosa, as well as the transmural inflammatory process extending to the serosa and involving subserosal adipose tissue (arrow). Scale bar: 1 mm.
Figure 3.
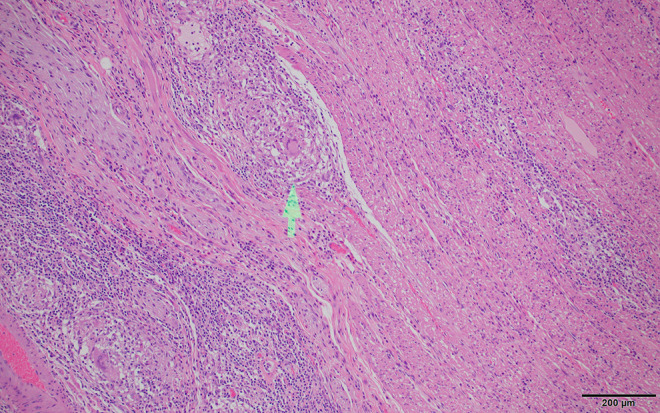
Crohn’s disease histology. Higher magnification demonstrates well-formed non-necrotizing granulomas with multinucleated histiocyte giant cells (arrow). Granulomas are not necessary for the diagnosis of Crohn’s disease and are found in about 35% of cases. Scale bar: 200 µm.
Figure 4.
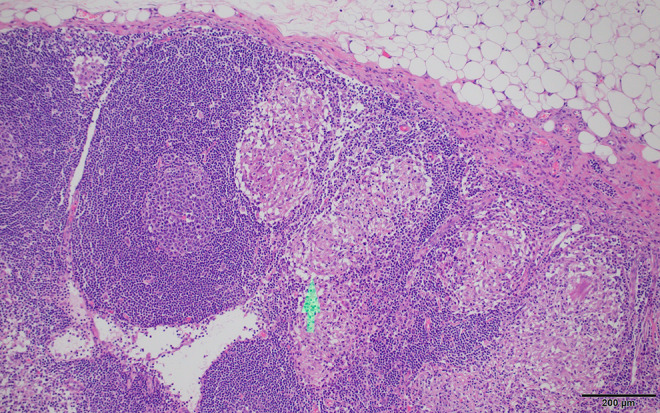
Crohn’s disease histology. Adjacent lymph nodes sometimes also contain non-necrotizing granulomas (arrow). Scale bar: 200 µm.
Questions/Discussion Points, Part 3
What Are the Gross and Microscopic Abnormalities Present and Do They Support the Radiologic Impression?
The gross and histologic findings in the surgical resection are typical of Crohn’s disease (CD) including typical findings of deep mucosal ulceration and active inflammation with transmural inflammation extending into mesenteric fat and the serosa (Figures 2 and 3). Granulomas without necrosis are identified (Figure 3), and granulomas are also found in the mesenteric lymph node (Figure 4). The granulomas are often difficult to find but are unusually well formed and prominent in this case. Neural hypertrophy indicative of a chronic process is also apparent in the submucosa (Figure 5). Because this was the initial diagnostic tissue, and the granulomas were unusually prominent, acid fast and fungal stains were performed to exclude infection, and these were negative for microorganisms.
Figure 5.
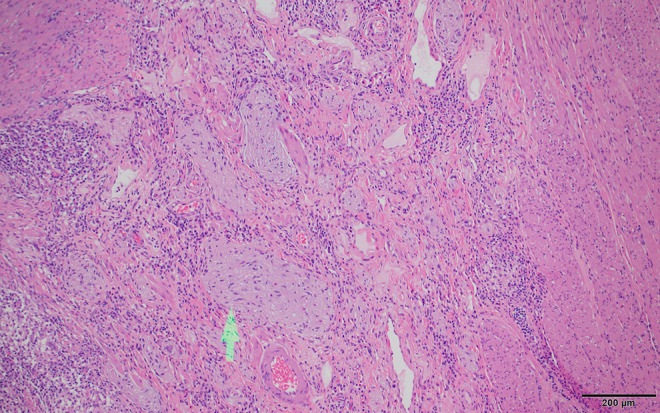
Crohn’s disease histology. Prominent neural hypertrophy (arrow) present in the submucosa in areas of active disease is a fairly common finding. Scale bar: 200 µm.
What Are the Typical Features of CD Including Pattern of Bowel Involvement, Gross Findings, and Histologic Features?
Crohn’s disease is referred to as regional enteritis due to the typical pattern of multifocal involvement with intervening normal tissue, “skip lesions,” which can involve any area of the gastrointestinal tract. Involvement of ileum, ileocecal valve, and cecum is the most common sites at presentation. As in this patient, presentation can occur with the onset of smoking, which is a significant risk factor for CD development, but disease regression does not occur following smoking cessation.
Many of the gross and histologic features that distinguish CD from ulcerative colitis (UC) are from the depth of the inflammatory involvement and the resulting fibrosis in CD.5 Because the inflammation can be transmural and extend to the serosa and into mesentery, complications from CD are often different from those from UC, which is usually more mucosa centered but may involve the superficial muscularis mucosa. Deep linear ulcers and fissures along the bowel length are typical in CD and may result in perforation and fistula formation. Perianal fistulae can be seen with rectal involvement. Bowel wall thickening can cause luminal narrowing and bowel obstruction. Fibrosis involving the mesentery can cause what is called “creeping fat.”
Crohn’s disease can be quite variable in its clinical features.6 Intermittent diarrhea may be a presenting feature, and due to the typical terminal ileal location, right lower quadrant pain mimicking acute appendicitis may occur. Due to the fibrosis and stricture formation, obstruction can be a presenting feature as in this patient. The disease commonly waxes and wanes with flares of activity separated by periods of inactivity. Recurrence following surgical intervention for stenosis/obstruction or fistulae is fairly common, and recurrences are often seen at anastomotic sites.
Extensive small bowel disease can result in malabsorption and hypoalbuminemia. Crohn’s disease is known for more frequent extraintestinal manifestations including uveitis, migratory polyarthritis, and erythema nodosum, all of which are also increased in frequency in patients with UC. Primary sclerosing cholangitis is seen more often than the general population in CD but less frequently than in patients with UC.
Patient Presentation, 2
A 28-year-old woman presents with complaints of mild abdominal discomfort and cramps over the past 6 weeks associated with diarrhea and passage of bloody mucus. The diarrhea was typically nocturnal or postprandial.
Diagnostic Findings, Part 1, Physical Examination
On physical examination, the heart rate is 68, respiratory rate is 17, blood pressure is 125/70, body mass index is 17.5, and her temperature is 98.6 °F. The abdominal examination reveals a nontender abdomen with no rebound tenderness and no guarding. No masses are palpated. No hemorrhoids are seen or palpated on examination and are not seen with anoscopy; however, bloody mucus is present on the glove following digital rectal examination.
Questions/Discussion Points, Part 1
What Is the Clinical Differential Diagnosis for This Patient?
The differential diagnosis for recent onset of bloody diarrhea includes infectious colitis such as Salmonella or Shigella, pseudomembranous colitis of C difficile, ischemic colitis, and idiopathic IBD. Additional laboratory testing can help to narrow this differential.
Diagnostic Findings, Part 2, Initial Laboratory Workup
The complete blood count shows a white blood cell count of 9.5 × 109/L (3.4-10.7 × 109/L), a hemoglobin of 13.5 g/dL (13.0-17.0 g/dL), and a hematocrit of 38% (37.5%-51.0%).
Stool test for C difficile toxin and stool culture for Shigella and Salmonella are all negative. Stool examination is also negative for ova and parasites. The initial lab workup reveals a C-reactive protein (CRP) of 11 mg/L (<3.0 mg/L), an erythrocyte sedimentation rate (ESR) of 45 mm/h (0-20 mm/h), and fecal lactoferrin of 15 µg/g (<7.25 µg/g).
Questions/Discussion Points, Part 2
What Laboratory Findings Are Significant and What Disease Etiologies Remains in the Differential Diagnosis Following These Tests?
The elevated fecal lactoferrin can be seen in infection or IBD, and in patients with IBD, it is associated with disease activity. The negative tests for acute infection decrease the likelihood of Shigella, Salmonella, C difficile, and parasitic infection. The elevated markers of inflammation (CRP, ESR) along with the chronic duration of inflammatory diarrhea greater than 4 weeks raises the possibility of UC and CD, and the patient is referred to gastroenterology for further workup.
Diagnostic Findings, Part 3, Pathologic Features
Colonoscopy reveals markedly inflamed mucosa beginning at the rectum and extending proximally and continuously through the sigmoid colon and ending in the distal descending colon. The remaining colon is without lesion, and biopsies of the abnormal mucosa and normal mucosa are performed. Representative histology from the biopsy is shown (Figure 6).
Figure 6.
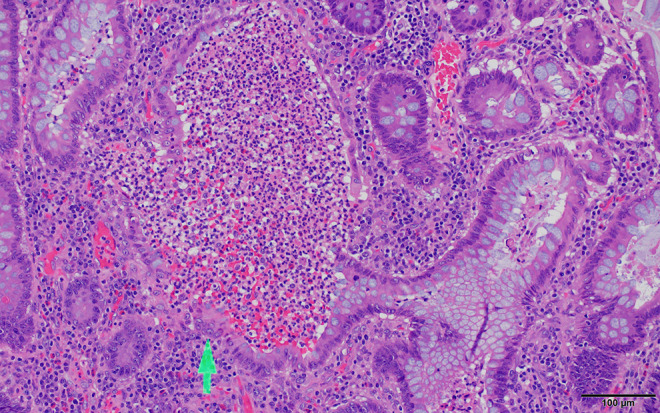
Ulcerative colitis biopsy histology. Dilated glands containing inflammatory cells which at high magnification are recognized as neutrophils, forming crypt abscesses (arrow) typical of ulcerative colitis (UC) but which can also be seen in Crohn’s disease (CD). Scale bar: 100 µm.
Questions/Discussion Points, Part 3
What Is the Differential Diagnosis on the Biopsy?
There is chronic active colitis most consistent with active IBD. Granulomas are not found. The biopsy diagnosis of IBD often cannot distinguish CD and UC based on histology alone as the biopsies are superficial mucosal biopsies, and both CD and UC can show cryptitis, crypt abscess, and chronic architectural gland distortion, while granulomas are not always seen in CD. Thus, the endoscopic findings and description of disease distribution are critically important in separating UC and CD. With the pattern of endoscopic involvement seen, the findings in this patient are most consistent with UC. Initial first-line therapy with oral and intracolonic mesalamine was not effective. Systemic corticosteroids also did not provide relief nor did a course of cyclosporin. The patient remains symptomatic despite several months of medical management and elects surgical intervention. A rectosigmoid excision is performed.
Diagnostic Findings, Part 4, Gross and Microscopic Features
The gross pathology is shown in Figure 7. Microscopic images for this second colon specimen are given for your review in Figures 6, 8, and 9.
Figure 7.
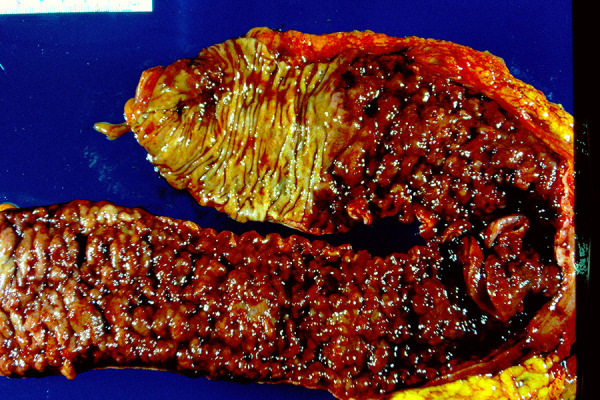
Ulcerative colitis gross morphology. There is the typical involvement beginning at the rectum (almost always involved) and extending proximally in a continuous fashion without skip lesions. Ulcers are diffusely present giving rise to a somewhat uniform cobblestone appearance to the mucosa, but long linear fissures typical of Crohn’s disease are absent. The transition from involved tissue to uninvolved is typically quite abrupt.
Figure 8.
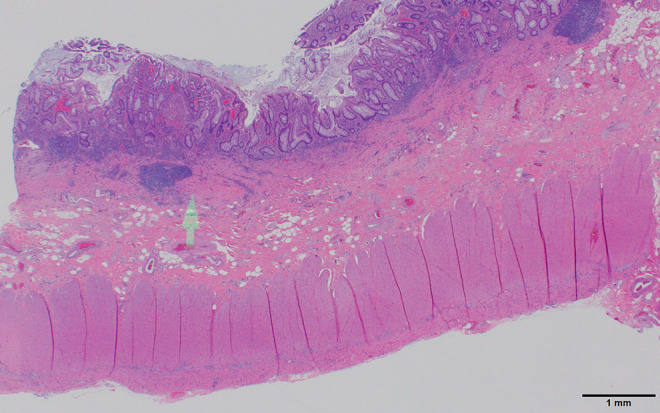
Ulcerative colitis histology. The low-power view shows a markedly expanded lamina propria inflammatory cell component which does not extend into the submucosa (arrow). There is crypt distortion with irregular shaped and branched glands as well as loss of glands which are features of chronicity (also seen in Crohn’s disease) separating inflammatory bowel disease from acute self-limited (infectious) colitis. Scale bar: 1 mm.
Figure 9.
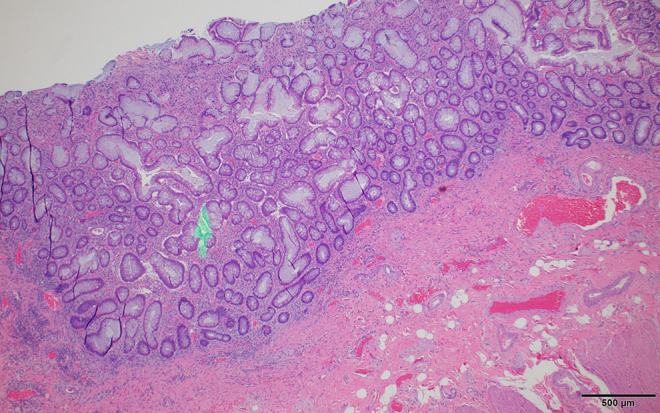
Ulcerative colitis histology. Less inflamed areas of mucosa show the distortion of the normal gland architecture with irregular gland spacing and abnormally branched and forked glands (arrow) consistent with prior inflammation and damage, often called quiescent colitis. Scale bar: 500 µm.
Questions/Discussion Points, Part 4
What Is Different in the Gross Pattern of Colon Involvement Compared to the Previous Patient?
The tissue shows rectal involvement and continuous diseased tissue extending proximally, without linear fissures and with an abrupt transition to normal appearing tissue.
What Are the Microscopic Features of Disease in This Patient and How Do They Differ From Those of CD?
The histologic findings in the surgical excision are typical for UC. There is increased mucosal inflammation apparent at low magnification which is confined to the mucosa and superficial muscularis mucosa (Figure 8) and does not involve the submucosa, muscularis propria, or serosa. Crypt abscesses filled with neutrophils are present (Figure 6), but granulomas are absent. There is no neural hypertrophy in the submucosa, which contrasts with that seen in the first patient. Less inflamed areas demonstrate the architectural mucosal changes in chronicity (also seen in CD), including irregular spacing of glands, and with glandular branching and dilatation (Figure 9), a pattern referred to as quiescent colitis when found without active inflammation.
How Do Clinical and Pathological Features of UC in the Second Patient Differ From Those of CD in the First Patient?
Ulcerative colitis and CD are closely related but in UC disease is usually restricted to the rectum and colon without skip lesions. The rectum is typically involved in UC with disease extending proximally in a continuous fashion with an abrupt end and sharp transition to uninvolved bowel.7 There are 2 exceptions to rectal involvement. Lack of rectal involvement can occasionally be seen in pediatric UC, and in addition, during treatment, the rectum may heal first giving the appearance of rectal sparing mimicking CD. Although the small bowel is uninvolved, if UC extends to the ileal cecal valve, a mild terminal ileitis may be seen described as “backwash ileitis.” The involved UC mucosa can be granular and red or involved with extensive broad ulcers or a more cobblestone appearance as seen in this patient; however, this cobblestone pattern is more common and more typical of CD than UC. When ulceration is extensive, small islands of preserved mucosa are described as pseudopolyps. Progression can lead to mucosal flattening and atrophy. In contrast to CD, patients with UC do not develop strictures as the inflammation is not transmural and fibrosis does not result. The inflammation of UC can alter motility and toxic megacolon can result with risk of perforation. Microscopic changes in UC are similar to CD; however, granulomas are absent and the inflammation is limited to the mucosa with only rare extension to the upper portion of submucosa.
What Epidemiologic Features Are Characteristic for IBD?
Inflammatory bowel disease presents most commonly in a bimodal distribution with an initial peak in the 20- to 40-year range and a second peak in the sixth decade of life. In the United States where 1.4 million individuals are affected, the disease is more common in Caucasians than other races, and there is an increased incidence in Ashkenazi Jews of 3- to 5-fold compared to the general population.
What Is the Pathophysiology of IBD?
Inflammatory bowel disease is an abnormal inflammatory process, often with a genetic predisposition, involving altered mucosal epithelial barrier functions, altered microbiology of the gastrointestinal tract, and contributing to the subsequent abnormal mucosal inflammatory response.5 Evidence of a genetic component includes a concordance CD rate of nearly 50% and UC of 15% in monozygotic twins, as well as increased risk when there is an affected first-degree relative. Three genes which have been implicated include NOD2, ATG16L1, and IRGM. All are involved in recognizing and responding to intracellular pathogens, suggesting an abnormal inflammatory response to luminal bacteria or their products.8,9 A number of cytokines are also implicated, and Interleukin 10 mutations are associated with severe IBD with early onset. Defects in the epithelial barrier tight junctions have been described, and altered microbiota have been found. Thus, a model of pathogenesis is emerging suggesting altered microbiota shed bacterial fragments, which can enter the submucosal space due to epithelial defects, which then incite the abnormal inflammatory response. Clinical trials suggest that probiotic or fecal transplants may help patients with IBD, but anti-inflammatory therapy is still the mainstay of treatment.
Describe Endoscopic, Gross, and Microscopic, and Laboratory Findings That Can Help You Distinguish CD and UC
Standard diagnostic clinical criteria are lacking for idiopathic IBD. A definitive diagnosis requires a thorough endoscopic evaluation and histopathological confirmation and exclusion of other possible causes by clinical and microbiology data. Tables 1 and 2 outline the major differences between CD and UC. However, some patients have features that appear to overlap. When a specific diagnosis cannot be definitively made, these patients are put in a category of IBD, type indeterminate, or indeterminate colitis. Serology may help in these patients as 75% of UC patients will have perinuclear antineutrophil cytoplasmic antibodies, seen in only about 10% of patients with CD.5 Conversely anti-Saccharomyces cerevisiae antibodies are present in about 70% of patients with CD and only 15% of patients with UC. Thus, serology for these markers can distinguish CD and UC with about 50% sensitivity and 90% specificity.5,10 Medical management of UC and CD is similar enough that a diagnosis of indeterminate colitis is not a barrier to effective treatment. Once the diagnosis is confirmed, laboratory tests such as ESR and CRP can be used to help monitor disease activity.
Table 1.
Gross Findings of Crohn’s Disease Versus Ulcerative Colitis.
| Finding | Crohn’s disease | Ulcerative colitis |
|---|---|---|
| Location | Ileum and colon | Colon and rectum |
| Continuity of disease | Skip lesions | Continuous |
| Fistula formation | Common | Rare |
| Formation of strictures | Yes | No (rare) |
| Bowel wall | Thickened | Thin |
| Creeping fat | Present | Absent |
Table 2.
Microscopic Findings of Crohn’s Disease Versus Ulcerative Colitis.
| Finding | Crohn’s disease | Ulcerative colitis |
|---|---|---|
| Depth of inflammation | Transmural | Mucosa only |
| Depth of ulcers | Deep | Superficial |
| Lymphoid response | Marked | Moderate |
| Fibrosis | Common and marked | Uncommon and mild |
| Serositis | Common and marked | Rare and mild |
| Granulomas | Yes (about 35% of cases) | none |
| Neural hyperplasia | Yes | No |
What Other Risk Factors Are There in Patients With Long-Term IBD Colitis?
Long-term colitis secondary to UC or CD is a risk factor for the development of colorectal adenocarcinoma. The risk of carcinoma is similar in patients with UC and colonic CD, as long as the duration and extent of disease are similar.6 Polypoid dysplasias can be seen endoscopically and removed endoscopically for histologic classification similar to colorectal carcinoma screening in patients without IBD. However, IBD-associated dysplasia also occurs in flat nonpolypoid mucosa that cannot be seen on routine endoscopy and when found on random biopsies is graded as low or high grade. The increase in cancer risk correlates with duration and severity of disease inflammation and frequency of activity. Risk increases steeply after 8 to 10 years of disease, and patients with colitis involving the entire colon (pancolitis) are at greater risk than patients with colitis only on the left side. If sclerosing cholangitis is also present, the risk of neoplasia is even greater, and surveillance is begun without delay. Endoscopic surveillance with multiple mucosal biopsies are performed at set intervals to look for flat nonvisible dysplastic precursor lesions to carcinoma, and any visible polypoid lesions are also sampled. A diagnosis of high-grade dysplasia on surveillance biopsy may be associated with a carcinoma at the biopsy site or elsewhere and is typically an indication for colectomy. Low-grade dysplasia may be followed or be an indication for colectomy based on additional patient factors.
Teaching Points
Both CD and UC are the major forms of IBD, with the first peak of incidence in the 20- to 30-year age group and a second peak especially for UC in the sixth decade.
Diagnosis of IBD requires endoscopic and histopathologic evaluation.
Crohn’s disease is characterized by ileal and ileocecal disease and “skip lesions,” while UC typically begins in the rectum and extends proximally in a continuous fashion.
Inflammation in UC is limited to the mucosa and superficial muscularis mucosa, while in CD, the inflammation is transmural.
Bowel thickening, fibrosis, stenosis, and obstruction are typical complications of CD but rare in UC and are related to the transmural inflammation of CD.
Crohn’s disease inflammation contains non-necrotizing granulomas in ∼35% of patients, which aid in the distinction from UC but are not necessary for the diagnosis of CD.
Ulceration of mucosa is present in both CD and UC, but linear fissures and or fistulae are typical of CD and rare in UC.
Pathophysiology includes genetic predisposition, abnormal epithelial barrier function, altered microbiota, and an abnormal inflammatory response.
Patients with UC or CD with long-standing colitis are at similar increased risk for developing colonic adenocarcinoma, the risk is dependent on severity and duration of disease, and routine endoscopic screening with biopsies is required.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Publication cost provided by the Department of Biomedical Sciences.
ORCID iD: Carl T. McGary  https://orcid.org/0000-0003-4625-3030
https://orcid.org/0000-0003-4625-3030
References
- 1. Knollmann-Ritschel BEC, Regula DP, Borowitz MJ, Conran R, Prystowsky MB. Pathology competencies for medical education and educational cases. Acad Pathol. 2017:4 doi:10.1177/2374289517715040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sweetser S. Evaluating the patient with diarrhea: a case-based approach. Mayo Clin Proc. 2012;87:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juckett G, Trivedi R. Evaluation of chronic diarrhea. Am Fam Physician. 2011;84:1119–1126. [PubMed] [Google Scholar]
- 4. Masselli G. The gastrointestinal string sign. Radiology. 2007;242:632–633. [DOI] [PubMed] [Google Scholar]
- 5. Turner JR. The gastrointestinal tract In: Kumar V, Abbas AK, Aster JC. eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed Elsevier Inc; 2015:749–819. [Google Scholar]
- 6. Friedman S, Blumberg RS. Inflammatory bowel disease In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. eds. Harrison’s Principles of Internal Medicine. 20th ed McGraw-Hill; 2018:2258–2275. [Google Scholar]
- 7. Robert ME, Skacel M, Ullman T, Bernstein CN, Easly K, Goldblum JR. Patterns of colonic involvement at initial presentation in ulcerative colitis. Am J Clin Pathol. 2004;122:94–99. [DOI] [PubMed] [Google Scholar]
- 8. Cohen LJ, Cho JH, Gevers D, Chu H. Genetic factors and the intestinal microbiome guide development of microbe-based therapies for inflammatory bowel diseases. Gastroenterology. 2019;156:2174–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramos GP, Papadakis KA. Mechanism of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siddiqi HA, Salwen MJ, Shaikh MF, Bowne WB. Laboratory diagnosis of gastrointestinal and pancreatic disorders. In: McPherson RA, Pincus MR, eds. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 23rd ed Elsevier Inc; 2017:306–323.e2. [Google Scholar]


