Abstract
Endotoxins and cytokines play an important role in multiple organ failure pathogenesis in patients with severe Gram-negative bacterial infection. We present a clinical case where an oXiris hemofilter was used for continuous renal replacement therapy (CRRT) treatment in a patient with septic shock after liver transplantation. A 35-year-old man with a 20-year history of hepatitis B presented with jaundice, loss of appetite, and decreased urine output. He was diagnosed with decompensated cirrhosis with acute-on-chronic liver failure, and liver transplantation was indicated. The day after surgery, he developed hyperthermia, hypotension, anuria, and a progressive increase in blood inflammatory markers and creatinine. Combined with the donor source and blood culture results, septic shock after transplantation was considered. The patient was immediately treated with endotoxin and cytokine adsorption CRRT (oXiris hemofilter) with tigecycline, caspofungin, and ganciclovir as anti-infectives. After 48 hours on CRRT, his blood pressure gradually stabilized, the CLIF Consortium Acute-on-Chronic Liver Failure score decreased from 63 to 43. Procalcitonin, endotoxin, and the inflammatory factors interleukin (IL)-6 and IL-10 also decreased gradually. The patient’s liver and kidney functions were completely restored. Our experience suggests that oXiris CRRT combined with antibacterial therapy is an effective treatment for septic shock after liver transplantation.
Keywords: Cytokine, endotoxin, liver transplantation, sepsis, continuous renal replacement therapy, oXiris hemofilter
Introduction
Sepsis is the leading cause of death in critically ill patients,1 and infections are the leading cause of mortality immediately after organ transplant.2 Studies have shown that the occurrence rate for septic complications after liver transplantation was 13% to 41%, which was dependent on the patient’s intensive care unit (ICU) stay time.3 The pathophysiology of sepsis is complex, and immune system dysfunction plays an important role in the development and progression of severe infection. After a pathogen invades the body, pro-inflammatory and anti-inflammatory factors are released in large quantities, which leads to an imbalance of the systemic inflammatory response, an uncontrolled, complex, and excessive systemic inflammation network effect, and immune dysfunction. The consequent cascade effect causes abnormal hemodynamics, metabolic disorders, bacterial translocation from the gut, and an imbalance in the internal environment.4,5 Ultimately, these processes lead to immune function deficiency and multiple organ dysfunction, with a high morbidity and mortality rate.
Clinical studies have confirmed that blood purification can remove a wide range of inflammatory mediators.6–8 There are many new techniques, such as high-volume hemofiltration, cascade hemofiltration, plasmapheresis, coupled plasma filtration adsorption, and high-cutoff hemodialysis/filtration,9 but their efficacy in treating sepsis is uncertain, so they are not frequently used clinical treatments. With the progress of membrane materials in continuous renal replacement therapy (CRRT), the use of adsorbents in blood purification techniques has been shown to significantly increase the removal of plasma inflammatory mediators.10 Therefore, application of adsorption technology is an alternative strategy for reducing soluble immune mediators and regulating cellular immunity.
The oXiris hemofilter is a new membrane material that is composed of an AN69-based membrane, which is surface-treated with polyethylenimine and pregrafted with heparin. The AN69 core membrane has an efficient cytokine removal capacity,11,12 the polyethylenimine surface treatment enhances the endotoxin-binding capacity of the AN69 membrane,13 and the heparin coating reduces the membrane thrombogenicity, thereby extending the filter’s life and efficiency.
In this study, a positive outcome was achieved when the oXiris hemofilter for continuous venovenous hemofiltration (CVVH) was used for a patient with multiple organ failure and septic shock following liver transplantation.
Case report
Review of medical history and patient characteristics
A 35-year-old man was admitted to the hospital with “recurrent jaundice of the skin and sclera for over 2 months and poor appetite for 3 days.” The patient had been diagnosed with hepatitis B during a routine examination 20 years ago, and since then, he had not been treated regularly. Over 2 months before presentation at our hospital, the patient’s jaundice of the skin and sclera increased, and he gradually developed abdominal distension, loss of appetite, and urine volume reduction. Liver transplantation was recommended. The patient was depressed (since the onset of disease) and showed a urine volume of 400 to 500 mL/day, a slight cough, white, sticky phlegm, and no fever. Examination on admission showed the following results: temperature, 37.0°C; pulse, 120 beats/minute; respiration, 16 breaths/minute; blood pressure, 110/70 mmHg; and body weight, 65 kg. The patient was noted to be conscious, with severe jaundice of the skin and sclera. He had clear breathing sounds in both lungs, and no dry or wet rales. There were no abnormalities on cardiac examination. Abdominal distension was noted, which showed a positive shifting dullness sign. Laboratory examinations provided the following results: hemoglobin, 96 g/L (normal, 120–160 g/L); platelet count, 80 × 109/L (normal, 100–300 × 109/L); aspartate aminotransferase, 87.1 U/L (normal, 8–40 U/L); total bilirubin, 547.4 µmol/L (normal, 1.71–17.1 µmol/L); direct bilirubin, 359.7 µmol/L (normal, 1.71–7.0 µmol/L); albumin, 24.2 g/L (normal, 40–55 g/L); prothrombin time, 36.4 s (normal, 11–14 s); activated partial thromboplastin time, 65.2 s (normal, 26–36 s); international normalized ratio, 3.63 (normal, 0.8–1.2); and procalcitonin, 1.34 ng/mL (normal, 0–0.5 ng/mL). Serum creatinine and blood ammonia levels were normal. A computed tomography (CT) scan indicated cirrhosis, liver volume reduction, and ascites. Chest X-ray indicated small amounts of pleural effusion.
On day 3 after admission, the patient underwent piggyback orthotopic liver transplantation. The donated liver was from an ICU patient who had a fever, and his sputum culture presented Acinetobacter baumannii. The operation lasted 8 hours and was completed smoothly, but the patient’s urine volume was only 100 mL during the operation. The patient developed a fever of 39.1°C at 30 minutes after surgery, and in rapid succession, his blood pressure decreased to 70/40 mmHg and he fell into a coma, requiring ventilator assistance for breathing. Re-examination showed a white blood cell count of 7.19 × 109/L (normal, 4.0–10 × 109/L), neutrophil-to-lymphocyte ratio of 83.6% (normal,: 50% to 70%), and serum creatinine of 240.3 µmol/L (normal, 54–133 µmol/L). Blood culture indicated the presence of Acinetobacter baumannii, and the antibiotic sensitivity pattern indicated that it was pan resistant and only sensitive to tegacyclin. The patient’s blood lactate and procalcitonin levels were 3.26 mmol/L (normal, 0.5–1.7 mmol/L) and 61.02 ng/mL (normal, 0–0.5 ng/mL). Based on the Sepsis-3 criteria,14 sepsis shock and grade 3 acute kidney injury (AKI) was diagnosed.
Treatment
The oXiris hemofilter (Gambro Industries, Meyzieu, France) was used for CRRT treatment after surgery. Written informed consent was obtained from the patient and his wife, and all procedures followed hospital CARE guidelines. A vascular access port was established using a dual-chamber 11.5 F hemodialysis catheter that was placed into the femoral vein. CVVH treatment was performed using a PRISMAFLEX dialysis machine (Gambro-Hospal, Stockholm, Sweden) that was equipped with an oXiris hemofilter. The blood flow rate was maintained at 180 to 200 mL/minute. Predilution was used, and the amount of replacement fluid was 4000 mL/hour; the ultrafiltration rate was 100 to 200 mL/hour, and it was adjusted based on the infusion speed. Anticoagulants were not used, and the hemofiltration prescription was adjusted based on the electrolyte and acid–base results. The patient was also concurrently administered tigecycline combined with caspofungin and ganciclovir as anti-infectives, and fluid replacement and norepinephrine were administered as antihypotensives.
Outcome
The patient underwent CVVH for 48 hours (with two oXiris hemofilter replacements during this time), and his blood pressure gradually stabilized. The norepinephrine dose was gradually reduced (Figure 1), and it was discontinued after 48 hours. The patient regained consciousness, and he was taken off the ventilator after 3 days. The Chronic Liver Failure (CLIF) Consortium Acute-on-Chronic Liver Failure score (commonly known as CLIF-C ACLFs) decreased from 63 to 43 (Figure 2). After 48 hours of CVVH using the oXiris hemofilters, the procalcitonin and endotoxin levels and the inflammatory factor interleukin (IL)-6 and IL-10 levels also gradually decreased (Table 1). After hemodynamic stabilization, the patient’s treatment was changed, and traditional filters were used with intermittent CVVH. The patient underwent 11 rounds of blood purification. Subsequently, his liver and kidney functions were completely restored. The patient was hospitalized for 51 days, and was discharged after recovery. He was doing well at 1 year after the transplantation.
Figure 1.
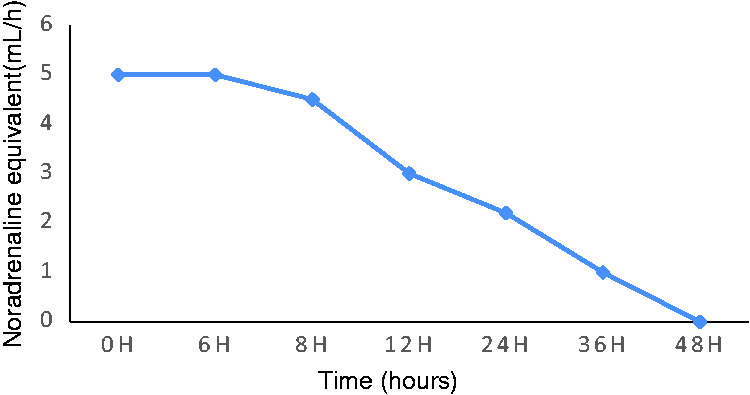
Noradrenaline equivalent use changes over time.
(The concentration of norepinephrine was 18 mg/50 mL).
Figure 2.
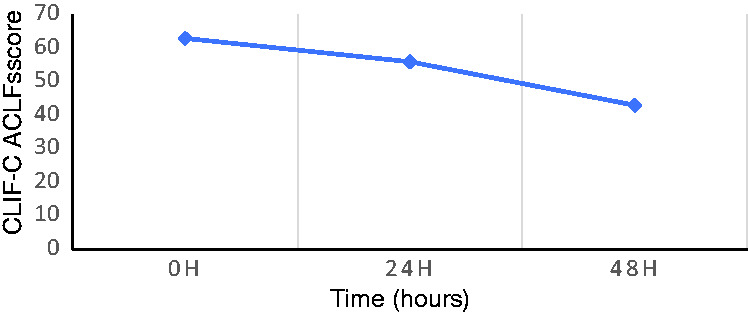
CLIF-C ACLFs (Chronic Liver Failure [CLIF] Consortium Acute on Chronic Liver Failure) score changes over time.
Table 1.
Changes in the inflammatory factor and endotoxin levels before and after 48 hours of CVVH with oXiris and antibiotics.
| 0 hours | 24 hours | 48 hours | |
|---|---|---|---|
| PCT, ng/mL | 61.02 | 27.14 | 25.27 |
| Endotoxin, EU/mL | 0.17 | 0.02 | 0.01 |
| IL-6, pg/mL | 2738 | 1542 | 57.33 |
| IL-10, pg/mL | 1838 | 732.9 | 21.01 |
CVVH, continuous venovenous hemofiltration; EU, endotoxin units; PCT, procalcitonin; IL, interleukin.
Discussion
Cytokines play an important role in the pathogenesis of sepsis, septic shock, and multiple organ failure.15,16 Endotoxin blood adsorption can effectively neutralize the pathogenic activity of endotoxins and improve organ dysfunction.17 Cytokine clearance by hemofiltration or blood adsorption can attenuate the effects of cytokine overproduction or overexpression, and restore immune system homeostasis. This also presents clinically as an improvement in the morbidity and mortality rates. Compared with conventional CVVH, CVVH using a hemofilter with enhanced endotoxin/cytokine clearance accelerated the improvement in organ function (within 48 hours).18 A randomized crossover double-blind study19 showed that CRRT with the oXiris filter allowed effective removal of endotoxin and tumor necrosis factor (TNF)-α, IL-6, IL-8, and interferon (IFN)γ in patients with septic shock-associated acute renal failure in the first filter treatment period, and it may be associated with beneficial hemodynamic effects.
For our patient, cirrhosis secondary to hepatitis led to acute-on-chronic liver failure, and liver transplantation was performed. Hepatorenal syndrome was present before the operation, and the patient’s condition was generally poor. Because the donor liver was from an ICU patient who had a fever, the possibility of infection was considered. Our patient had a high fever and showed symptoms of shock on the day after the surgery, and blood culture results yielded a diagnosis of septic shock from a Gram-negative bacterial infection. The patient’s condition was critical. We used the oXiris hemofilter continuously for the 48 hours of CVVH treatment, which resulted in the rapid correction of shock, a marked decrease in inflammatory factor and endotoxin levels, and improved CLIF-C ACLFs. This allowed us time to perform subsequent treatments.
In the field of extracorporeal blood purification therapies for sepsis, most of the available blood purification devices focus on a single target, such as the endotoxin that triggers the immune cascade or cytokine storm that causes organ damage.20 However, the oXiris hemofilter is a unique 4-in-1 device, that combines cytokine and endotoxin removal properties, renal replacement function, and antithrombogenic properties. A study by Rimmelé et al.10 showed that the enhanced adsorption capacity of AN69 oXiris is associated with the charge characteristics of the membrane surface, and that the positively charged surface can adsorb negatively charged endotoxin. In animal models of septic shock, use of the AN69 oXiris for 6 hours can mitigate the clinical symptoms and severity of shock compared with conventional AN69 membranes. It can also cause better stabilization of mean arterial pressure and pulmonary capillary wedge pressure.21 A clinical study by Shum et al.18 also showed that the use of oXiris hemofilters for CVVH can accelerate the improvement of organ dysfunction in patients with acute kidney injury in sepsis. In this patient’s treatment plan, the combination of oXiris CRRT and antibacterial therapy in the early stages of sepsis was effective in blocking the “inflammatory storm” and improving hemodynamics. Recently, Zhang et al.22 presented four cases in which the patients underwent oXiris CRRT in ICUs in China. They suggested that oXiris should be used early in the treatment of septic AKI patients as an adjuvant therapy with good infection source control. However, there are still no randomized trials on the clinical use of oXiris in China.
In the field of organ transplantation, some donor organs come from the ICU setting and may carry pathogenic bacteria, which may represent a potential risk of infection in the recipient. Thus, we believe that CRRT with oXiris can be a beneficial choice for such recipient patients after transplantation. However, this is a case study, and a rigorous, large-sample study is required for confirmation.
Author contributions
Li YN and Yuan F designed the research. Li YN and Zhou LS collected and analyzed the data and drafted the manuscript. Yang LZ performed the endotoxin and cytokine adsorption CRRT. Yuan F wrote and revised the manuscript. All authors have read and approved the final version to be published.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval and informed consent
The Institutional Review Board of the Second Xiangya Hospital of Central South University approved this study (IRB No. 2019-014). Informed consent was obtained from the patient.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81770730) and the Hunan Provincial Natural Science Fund (Grant No. 2017JJ2352).
ORCID iD
Fang Yuan https://orcid.org/0000-0002-5034-6603
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310. DOI: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gayowski T, Marino IR, Singh N, et al. Orthotopic liver transplantation in high-risk patients: risk factors associated with mortality and infectious morbidity. Transplantation 1998; 65: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mor E, Cohen J, Erez E, et al. Short intensive care unit stay reduces septic complications and improves outcome after liver transplantation. Transplant Proc 2001; 33; 2939–2940. DOI: 10.1016/s0041-1345(01)02260-6. [DOI] [PubMed] [Google Scholar]
- 4.Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ 2019; 9: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui HX, Xu JY, Li MQ. Efficacy of continuous renal replacement therapy in the treatment of severe acute pancreatitis associated acute respiratory distress syndrome. Eur Rev Med Pharmacol Sci 2014; 18: 2523–2526. [PubMed] [Google Scholar]
- 6.Peng ZY, Carter MJ, Kellum JA. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit Care Med 2008; 36: 1573–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudry S, Ricard JD, Leclaire C, et al. Acute kidney injury in critical care: experience of a conservative strategy. J Crit Care 2014; 29: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 8.RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361: 1627–1638. [DOI] [PubMed] [Google Scholar]
- 9.Atan R, Peck L, Visvanathan K, et al. High cut-off hemofiltration versus standard hemofiltration: effect on plasma cytokines. Int J Artif Organs 2016; 39: 479–486. [DOI] [PubMed] [Google Scholar]
- 10.Rimmelé T, Assadi A, Cattenoz M, et al. High-volume haemofiltration with a new haemofiltration membrane having enhanced adsorption properties in septic pigs. Nephrol Dial Transplant 2009; 24: 421–427. [DOI] [PubMed] [Google Scholar]
- 11.Bouman CS, Van Olden RW, Stoutenbeek CP. Cytokine filtration and adsorption during pre- and postdilution hemofiltration in four different membranes. Blood Purif 1998; 16: 261–268. DOI: 10.1159/000014343. [DOI] [PubMed] [Google Scholar]
- 12.De Vriese AS, Colardyn FA, Philippé JJ, et al. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol 1999; 10: 846–853. [DOI] [PubMed] [Google Scholar]
- 13.Mitzner S, Schneidewind J, Falkenhagen D, et al. Extracorporeal endotoxin removal by immobilized polyethylenimine. Artif Organs 1993; 17: 775–781. DOI: 10.1111/j.1525-1594.1993.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 14.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen J. The immunopathogenesis of sepsis. Nature 2002; 420: 885–891. DOI: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 16.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005; 365: 63–78. DOI: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 17.Cruz DN, Perazella MA, Bellomo R, et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care 2007; 11: R47. DOI: 10.1186/cc5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shum HP, Chan KC, Kwan MC, et al. Application of endotoxin and cytokine adsorption haemofilter in septic acute kidney injury due to Gram-negative bacterial infection. Hong Kong Med J 2013; 19: 491–497. DOI: 10.12809/hkmj133910. [DOI] [PubMed] [Google Scholar]
- 19.Broman ME, Hansson F, Vincent JL, et al. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: a randomized crossover double-blind study. PLoS One 2019; 14: e0220444. DOI: 10.1371/journal.pone.0220444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monard C, Rimmelé T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif 2019; 47: 1–14. DOI: 10.1159/000499520. [DOI] [PubMed] [Google Scholar]
- 21.Davies B, Cohen J. . Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect Dis 2011; 11: 65–71. DOI: 10.1016/S1473-3099(10)70220-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Yan Tang GK, Liu S, et al. Hemofilter with adsorptive capacities: case report series. Blood Purif 2019; 47: 1–6. DOI: 10.1159/000499357. [DOI] [PubMed] [Google Scholar]


