Abstract
Freezing of gait (FoG) in Parkinson’s disease involves deficient anticipatory postural adjustments (APAs) resulting in cessation of step initiation due to supraspinal dysfunction. Individuals with FoG (freezers) may require functional reorganization of spinal mechanisms to perform APAs. As presynaptic inhibition (PSI) is centrally modulated to allow execution of supraspinal motor commands, here we hypothesized a loss of PSI in freezers during APA for step initiation, which would be associated with FoG severity. Seventy individuals (27 freezers, 22 non-freezers, and 21 age-matched healthy controls [HC]) performed a GO-commanded step initiation task on a force platform under 3 conditions: 1) without electrical stimulation; 2) test Hoffman reflex (H-reflex); and 3) conditioned H-reflex. They also performed a control task (quiet stance). In the step initiation task, the H-reflexes were evoked on the soleus muscle when the amplitude of the APA exceeded 10–20% of the mean of the baseline mediolateral force. PSI was quantified by the ratio of the conditioned H-reflex relative to the test H-reflex in both the tasks. Objective assessment of FoG severity (FoG-ratio) was performed. Freezers presented lower PSI levels during quiet stance than non-freezers and HC (P<0.05). During step initiation, freezers presented loss of PSI and lower APA amplitudes than non-freezers and HC (P<0.05). Significant correlations were only found for freezers between loss of PSI and FoG-ratio (r=0.59, P=0.0005) and loss of PSI and APA amplitude (r=−0.35, P<0.036). Our findings suggest that loss of PSI for step initiation in freezers may be due to FoG.
Keywords: freezers, H-reflex, spinal inhibitory mechanism, step initiation, sensorimotor integration, inhibition
Introduction
Freezing of gait (FoG) is one of the most debilitating features of Parkinson’s disease (PD) and one of the major reasons for falls and reduced quality of life (Moore et al., 2007; Giladi & Nieuwboer, 2008; Kerr et al., 2010; Nonnekes et al., 2015). This disabling clinical phenomenon is characterized by brief episodes of inability to step or by extremely short steps that typically occur when initiating gait or when turning (Nutt et al., 2011). FoG has been associated with small and prolonged anticipatory postural adjustments (APAs) during step initiation (Tard et al., 2014; Cohen et al., 2017; Schlenstedt et al., 2018; Heilbronn et al., 2019).
Taking a step is a challenging task given the abrupt transition from bipedal stance posture to an unstable, unipedal gait in which the center of body mass is moved beyond the base of foot support, both forward and toward the initial stance leg (Burleigh et al., 1994; Horak & Macpherson, 1996; Horak, 2006). The sagittal component of the APA pushes the body forward, while the mediolateral component shifts left-right leg support (Burleigh et al., 1994; Horak & Macpherson, 1996). Previous findings have shown that individuals with FoG (freezers) delayed step initiation associated with prolonged APAs (Jacobs & Horak, 2007; Jacobs et al., 2009b; Tard et al., 2014; Cohen et al., 2017).
Deficient APAs in step initiation (Jacobs et al., 2009a; de Lima-Pardini et al., 2017) has been related to disorders of frontocortical areas, which may be more affected in freezers than non-freezers because freezers have smaller activity of the supplementary motor area than non-freezers (Butler et al., 2017). Dysfunction of the supplementary motor area may require functional reorganization of spinal circuitry, given the abnormal projections from this area through reticulospinal tract, which is involved in APA regulation (Schepens & Drew, 2004; Takakusaki, 2013; Takakusaki, 2017). As the reticulospinal tract connects to spinal interneurons that mediate one of the most powerful spinal inhibitory mechanisms, presynaptic inhibition (PSI) (Sirois et al., 2013), it is possible that PSI during APAs may be deficient in freezers compared to non-freezers and age-matched healthy controls (HC).
PSI is required in the execution of supraspinal motor commands, as it is modulated centrally during and at onset of voluntary movements (Hultborn et al., 1987a; Katz et al., 1988; Rudomin & Schmidt, 1999). Hoffman reflex (H-reflex) has been used to measure PSI levels, which is quantified by the ratio of the conditioned H-reflex relative to the test H-reflex (Baudry & Duchateau, 2012; Magalhaes et al., 2015; Silva-Batista et al., 2017). Several studies have demonstrated increased PSI in young healthy individuals during 1) acquisition of a novel visuomotor skill (Perez et al., 2005), 2) co-contraction of antagonist muscles (Nielsen & Kagamihara, 1993), 3) automatic activation of soleus muscles during gait (Dietz et al., 1984; Morin et al., 1984; Faist et al., 1996), and 4) at onset of voluntary movements (Hultborn et al., 1987b). PSI may be involved during APAs, as a recent study has observed larger H-reflex amplitudes in older than in younger and middle-aged individuals during a self-perturbing arm swing (indirectly indicating APAs) (Hortobagyi et al., 2018).
PSI has been assessed only at rest in individuals with PD, with evidence that PSI is smaller in non-freezers than HC (Roberts et al., 1994; Morita et al., 2000; Silva-Batista et al., 2017). PSI has been also negatively correlated with gait speed in non-freezers (Morita et al., 2000). However, PSI remains unexplored in APAs for step initiation in freezers, non-freezers and HC. As FoG may be due to an inability to inhibit postural preparation and initiate voluntary stepping (Nutt et al., 2011; Cohen et al., 2014), we hypothesized that lack of central inhibition of postural preparation to allow stepping to commence would be reflected in loss of PSI in freezers (i.e., higher ratio of the conditioned H-reflex relative to the test H-reflex), which would be associated with FoG severity. We also hypothesized that loss of PSI would be related to lower amplitude and longer duration of APAs in freezers. Thus, the aims of this study were threefold: 1) to compare PSI during APA among freezers, non-freezers and HC; 2) to determine whether PSI correlated with the amplitude and duration of APAs; 3) to determine if PSI and APA amplitudes were correlated with FoG severity. We also measured PSI during quiet stance as a control task.
Methods
Ethical approval
The study was approved by University’s Ethical Committee (School of Physical Education and Sport - ref. 2011/12), registered at the National Clinical Trial (RBR-83VB6B), and performed in accordance with the Declaration of Helsinki. All individuals provided written informed consent.
Participants
The individuals with PD (freezers and non-freezers) were recruited from the Movement Disorders Clinic in the School of Medicine at University of São Paulo. HC were also recruited from the surrounding University area. Individuals with PD were diagnosed by a movement disorders specialist in accordance with the UK Parkinson’s Disease Society Brain Bank diagnostic criteria (Hughes et al., 1992). The eligibility criteria for individuals with PD were: 1) Hoehn and Yahr stage range 2–4; 2) to be on stable dopaminergic therapy; 3) age range 49–80 years; 4) to be able to walk safely for 20 m without walking aids; 5) absence of neurological disorders (other than PD); 6) absence of significant arthritis, musculoskeletal or vestibular disorders, and severe tremor; 7) Mini-Mental State Examination (MMSE) score >23 (Folstein et al., 1975); and 8) lack of regular physical training in the period of 3 months preceding our data collection. Eligibility criteria for the HC included items 3 to 8. A movement disorders specialist assessed probable FoG by using videos of objective tests (e.g., step-over obstacles, turning clockwise and counter clockwise, and walking through a doorway) and the New Freezing of Gait Questionnaire (NFoGQ) (Nieuwboer et al., 2009). Thus, individuals with PD who were defined as having FoG in the videos and scoring >3 on the NFoGQ (Fling et al., 2013) were classified as freezers. The presentation of a short (30 s) video to illustrate FoG was performed at the beginning of the questionnaire application.
Experimental procedures
After explaining the study and obtaining consent, a physical therapist assessed the motor disability with the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) (Fahn & Elton, 1987) and clinical severity of FoG from the individual’s experience with NFoGQ (Nieuwboer et al., 2009) in individuals with PD. Afterwards, FoG severity was also objectively assessed by a 360 degree turning task (FoG-ratio) (Mancini et al., 2017) only in freezers. On the second day, before all of the participants (freezers, non-freezers, and HC) performed the step initiation task, they performed the quiet stance task. H-reflexes were measured during quiet stance on the force platform (AMTI ORG-7) as a control task. We also adjusted the stimulation parameters in quiet stance (Figure 1A) so that the size of the test H-reflex was similar to the H-reflex measured during APA. We matched the size of the H-reflex because it is more susceptible to inhibition/facilitation depending on the size of the test H-reflex relative to maximal motor response (Mmax) (Crone et al., 1990). We evoked the soleus H-reflex in the left leg (the same leg required during initiation of APA), given that the individuals started the step with the right leg and applied the mediolateral force with the left support leg (de Lima-Pardini et al., 2017). The soleus H-reflex was evoked at an intensity corresponding to 20–25% of Mmax, resulting in a soleus H-reflex on the ascending portion of its recruitment curve, and in most individuals a small motor response. Afterwards, all individuals performed the step initiation task on the force platform in 3 conditions, as it follows: 1) without stimulus; 2) test H-reflex; and 3) conditioned H-reflex. Order of conditions was counterbalanced across individuals. Fifteen trials were performed in each condition, with a 10-s interval between trials. Intervals between conditions and tasks (quiet stance and step initiation) lasted 5 minutes. For each condition, a warning luminous stimulus was presented, and 2 s later a verbal imperative signal “GO!” was given. This imperative signal prompted individuals to initiate the step (Figure 1A). Individuals with PD performed both the tasks in the clinically “on” state (fully medicated) within 1.5 hours from taking their morning dose of anti-parkinsonian medication. As levodopa treatment improves APAs (Burleigh-Jacobs et al., 1997; Smulders et al., 2016) and the excitability of the H-reflex in PD (McLeod & Walsh, 1972), individuals with PD remained sat for 20 min in the laboratory after taking their normal dose of levodopa medication.This time window has been considered as appropriate for improvement of their motor state following medication consumption (Colosimo et al., 1996).
Figure 1.
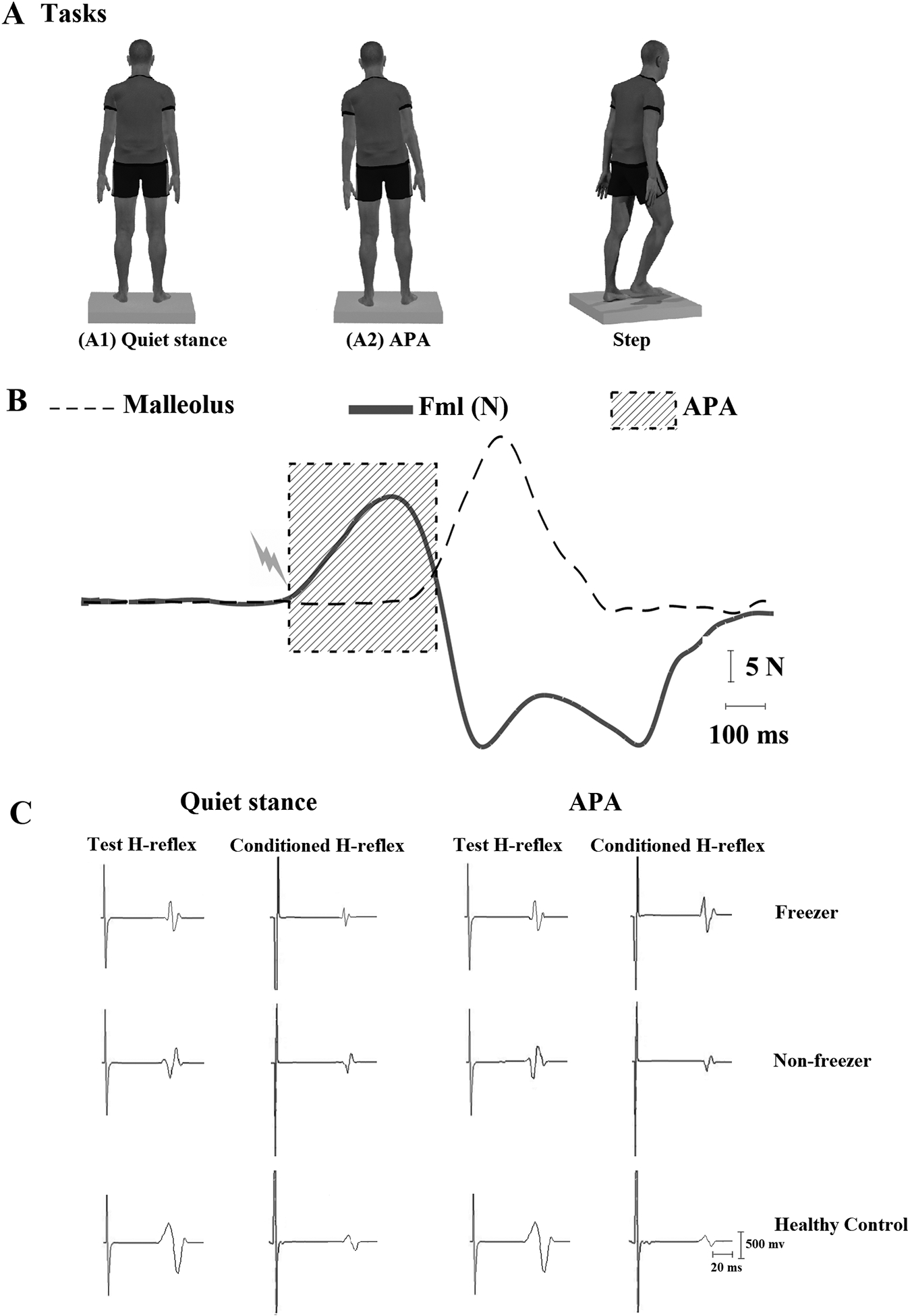
Experimental setup and respective signals. (A) Task examples: (A1) quiet stance; (A2) performing step initiation task with lateral weight shift associated with the anticipatory postural adjustment (APA) after a verbal imperative “GO” signal and stepping. H-reflexes (test and conditioned) were triggered during quiet stance (A1) and during APAs for step initiation (A2). (B) Example of an H-reflex triggered at the onset of APA (duration shaded). Solid line shows mediolateral force (Fml) amplitude during the APA and dashed line shows forward displacement of the reflective marker attached to the ankle during the step. (C) Representative traces of test and conditioned H-reflexes (average over 15 responses) for a freezer, non-freezer, and age-matched healthy individual during quiet stance and in APA for step initiation.
Assessments
Presynaptic inhibition (PSI)
Test H-reflex. The soleus H-reflex was induced by stimulating the posterior tibial nerve in the left leg via a monopolar stimulating electrode (1 ms rectangular pulse) over the popliteal fossa using a constant-current stimulator (Nicolet® Viking Quest portable electromyography [EMG] apparatus, CareFusion, Wisconsin, USA). The anode was placed proximally to the patella. H-reflexes were recorded using two self-adhesive surface disc surface EMG electrodes (1-cm diameter) placed on the soleus muscle, below the insertion of the gastrocnemius muscles, with inter-electrode distance of 3–4 cm. Reflex responses were measured as the peak-to-peak amplitude of the H-reflex. H-reflexes were evoked with 10-s intervals. Stimulus intensities were increased in steps of 0.05 mA, starting below H-reflex threshold and increasing up to supramaximal intensity to measure the Mmax. Sensitivity of the H-reflex to inhibitory and facilitatory effects depends critically on its size (Crone et al., 1990). H-reflex was adjusted to 20–25% of Mmax in all conditions (Crone et al., 1987). The value of Mmax was obtained when no further increase in the peak-to-peak amplitude of the M response with increasing stimulation intensity was observed.
Conditioned H-reflex.
PSI of the soleus H-reflex was evoked by the conditioning stimulation (Capaday et al., 1995; Patikas et al., 2004; Magalhaes et al., 2015; Silva-Batista et al., 2017) (1 ms rectangular pulses) of the common peroneal nerve through bipolar surface electrodes (0.5 cm diameter) placed 1–3 cm distal to the neck of the fibula in the left leg. Motor responses were recorded using two self-adhesive surface disk electrodes (1-cm diameter) placed on the tibialis anterior muscle. Care was taken to ensure that the conditioning stimulus was applied at a position where the threshold for an motor response (motor threshold) in the tibialis muscle was lower than the motor threshold in the peroneal muscle (1.1 × motor threshold), preventing vigorous contractions of the tibialis anterior muscle, which could be monitored throughout the experiments. The specificity of this stimulation was checked several times during experiment. A stimulation intensity of 1.1 × motor threshold is submaximal for activation of all inhibitory interneurons, allowing that both facilitatory and inhibitory effects be observed (Hultborn et al., 1987a; Crone et al., 1990; Patikas et al., 2004; Geertsen et al., 2008). Thus, to measure PSI during quiet stance and step initiation, a interval of 100ms was used between the conditioned H-reflex and the test H-reflex. At a conditioning-test interval of 100 ms, stimulation of the common peroneal nerve evokes an inhibition that is attributed to PSI on the terminals of Ia afferents to soleus motor neurons (Capaday et al., 1995; Iles, 1996; Earles et al., 2001; Patikas et al., 2004). It has been shown that long latencies of 100–120 ms between activation of tibialis muscle and its antagonist (soleus muscle) strongly inhibit soleus H-reflexes during quiet stance and in the early part of the stance phase of walking (Capaday et al., 1995). This effects indicates that a significant portion of the inhibition occurs at a premotoneuronal level, likely via PSI. Several different subsets of interneurons transmit PSI to Ia terminals projecting to several motoneuron pools (see more details in Knikou, 2008). Thus, the average values of 15 test H-reflexes and 15 conditioned H-reflexes for each task (quiet stance and step initiation) were considered for further analysis. Changes in PSI in both the tasks were quantified by the ratio of the conditioned H-reflex relative to the test H-reflex, with lower ratio indicating higher PSI levels (Baudry & Duchateau, 2012; Magalhaes et al., 2015; Silva-Batista et al., 2017). In addition, rectified and averaged EMG (raEMG) recordings during the tasks were measured over a 100 ms epoch that preceded tibial nerve stimulation (test H-reflex) or the common peroneal nerve stimulation (conditioned H-reflex). An epoch of 100 ms was selected to obtain raEMG values that represented the muscle activation at the time of stimulation (Baudry & Duchateau, 2012). A co-contraction ratio was calculated to express the raEMG amplitude for tibial anterior muscle relative to the raEMG for soleus muscle.
Identification of PSI during APAs.
To trigger H-reflexes (test and conditioned) during APAs for step initiation, we used the force platform (AMTI ORG-7) to detect the abrupt increase of mediolateral force amplitude. When the APA amplitude exceeded 10–20% of the mean of the mediolateral force (corresponding to 2 standard deviations above the mean of the baseline force) an electrical stimulus was automatically triggered (Figure 1B). The baseline force threshold was calculated through LabVIEW software.
Anticipatory postural adjustments (APAs)
APAs were quantified during step initiation as described previously (de Lima-Pardini et al., 2017). Onset of the APA was defined as the time between the abrupt increase of the mediolateral force amplitude (i.e., 2 standard deviations above the mean of the baseline force) and the onset of step. The duration of APA was calculated as the time between the onset of APA and the onset of the step. Step onset was identified by the marker on the right malleolus (2 standard deviations above the mean of the baseline foot displacement in the anteroposterior direction). Mediolateral force amplitude during the step task was normalized by the distance between the malleoli of the individual (unit in N/cm). To quantify step length, a kinematic analysis system (four cameras - Vicon brand, model T10) provided three dimensional coordinates of the foot by tracking the spherical reflective (passive) marker attached to the ankle (14 mm diameter). Amplitude and duration of the APA from condition 1 (without electrical stimulation) were used to compare APAs among the freezers, non-freezers, and HC groups.
Severity of freezing of gait (FoG)
Objective FoG measure (FoG-ratio) was calculated during a 2-minute turning task, in which individuals made 360° turns on the spot, alternating between clockwise and anti-clockwise turns as fast as they could do safely. Inertial sensors (Physilog, Gait Up, Lausanne, Switzerland) were placed on the shins and at the lumbar level. Data were sampled at 128Hz and stored for offline analysis using Matlab 2016b (Mathworks Inc.). Analysis was based on power spectral density from the anteroposterior acceleration data. The FoG-ratio was then calculated as the ratio between the square of the total power in the frequency band corresponding with freezing episodes (3–8 Hz) and the total power in the frequency band corresponding to locomotion (0.5–3 Hz). Higher FoG-ratio scores indicate greater FoG severity (see more details in Mancini et al., 2017).
Subjective FoG measure was performed by NFoGQ that is a useful and comprehensive tool to subjectively assess the severity of FoG and its impact on activities of daily living and quality of life (Nieuwboer et al., 2009). The NFoGQ consists of three parts. Part I detected the presence of FoG using a dichotomous item in which patients were classified as freezers or non-freezers, based on the occurrence of freezing episodes in the past month. Parts II and III were designed for freezers only, providing a score between 0 and 28. Part II (items 2–6, scoring range 0–19) rated the severity of FoG based on its duration and frequency (during turning and initiation of gait). Part III rated the impact of FoG on daily life (items 7–9, scoring range 0–9). Higher NFoGQ scores indicate greater FoG severity (Nieuwboer et al., 2009).
Statistical analyses
Data normality was checked by the Shapiro–Wilk test and non-normal data (conditioned H-reflex relative to the test H-reflex and co-contraction ratio during quiet stance and step initiation) were log transformed. After checking data normality to test for the effects of both tasks on dependent variables (conditioned H-reflex relative to the test H-reflex, test H-reflex amplitude normalized to its corresponding Mmax, raEMG of the soleus muscle, raEMG of the tibial anterior muscle, and co-contraction ratio), a linear mixed model for repeated measures was employed using groups (freezers, non-freezers, and HC) and tasks (quiet stance and step initiation) as fixed factors and subjects as a random factor. Whenever a significant F-value was obtained, a post hoc test with a Tukey’s adjustment was performed.
To compare amplitude and duration of the APAs (condition without electrical stimulation) among the three groups (freezers, non-freezers, and HC), a one-way analysis of variance (ANOVA) was used. In addition, we used disease severity (UPDRS-III) as a covariate (ANCOVA) for this analysis.
To compare the MMSE score, demographic and anthropometric characteristics among the three groups (freezers, non-freezers, and HC), we used an one-way ANOVA.
To compare clinical characteristics between freezers and non-freezers, we used independent t-tests.
Two-tailed Spearman’s rank correlation coefficients were calculated between the PSI levels with amplitude and duration of APA in all individuals, between the PSI levels with the FoG-ratio in freezers, and between APA amplitude with the NFoGQ scores in freezers.
The level of statistical significance was set at P<0.05 for all comparisons. Results are expressed as means ± SD. SAS 9.2 software (SAS Institute, Cary, NC) was used to perform the statistical analyses.
Results
Participants
Seventy individuals (27 freezers, 22 non-freezers, and 21 HC) participated in the study. The demographic, anthropometrical, and clinical characteristics of the individuals in each group are presented in Table 1.
Table 1.
Characteristics of the freezers, non-freezers, and age-matched healthy controls (HC) (mean±SD).
| Characteristics | Freezers | Non-freezers | HC | P-values |
|---|---|---|---|---|
| Demographic | ||||
| Men/women (n) | 18/9 | 18/4 | 18/3 | |
| Age (yr) | 66.7±9.7 | 65.6±9.8 | 67.3±8.5 | 0.778 |
| Anthropometrical | ||||
| Body mass (kg) | 69.5±12.3 | 68.7±9.1 | 70.1±10.8 | 0.362 |
| Heigth (m) | 1.6±0.2 | 1.7±0.1 | 1.6±0.1 | 0.515 |
| Clinical | ||||
| MMSE (score) | 26.0±1.9 | 26.9±2.0 | 28.0±1.7 | 0.851 |
| Years since diagnosis | 8.7±4.5 | 8.1±5.1 | - | 0.648 |
| Hoehn & Yahr Scale (a.u.) | 3.1±0.6 | 2.7±0.5 | - | 0.023 |
| L-Dopa equivalent units (mg/day) | 834.2±226.1 | 747.8±241.4 | - | 0.206 |
| UPDRS-III (score) | 49.7±12.5 | 35.1±14.5 | - | <0.001 |
| PIGD (score) | 8.7±2.0 | 4.2±1.8 | - | <0.001 |
| NFoGQ (score) | 23.9±4.1 | - | - | - |
| FoG-ratio (a.u.) | 37.6±29.6 | - | - | - |
MMSE = Mini-Mental State Examination; UPDRS-III = Unified Parkinson’s Disease Rating Scale part III; PIGD = Postural Instability and Gait Disturbance; NFoGQ = New Freezing of Gait Questionnaire; FoG-ratio = Freezing of gait ratio.
Loss of PSI in the step initiation in freezers
Figure 1C shows examples of test and conditioned soleus H-reflexes for the freezer, non-freezer, and healthy control during quiet stance (A1) and during APA for step initiation (A2).
Figure 2 shows the values of ratio of the conditioned H-reflex relative to the test H-reflex (i.e., PSI) in both the tasks for each group. The linear mixed model showed a significant group × task interaction for PSI levels (F [2, 67] = 203.39, P<0.0001). The non-freezer and HC groups presented higher PSI levels (i.e., lower ratio of the conditioned H-reflex relative to the test H-reflex) in the step initiation than in the quiet stance (mean difference [MD]: −7.1%; 95% confidence interval [CI]: −12.1 to −2.0; P=0.001; and MD: −22.8%; CI: −23.9 to 17.7 to; P<0.0001, respectively), whereas the freezer group presented loss of PSI in the step initiation (i.e., higher ratio of the conditioned H-reflex relative to the test H-reflex), but presented PSI levels in the quiet stance (MD: 23.1%; CI: 18.5 to 27.6; P<0.0001). Post hoc comparisons revealed that the non-freezer and HC groups presented higher PSI levels in the quiet stance than the freezer group (MD: −5.8%; CI: −10.8 to −0.7; P=0.014; and MD: −40.6%; CI: −45.7 to −35.5; P<0.0001, respectively), whereas in the step initiation, the freezer group presented higher ratio of the conditioned H-reflex relative to the test H-reflex (i.e., loss of PSI) than the non-freezer and HC groups (MD: 35.9%; CI: 30.9 to 41.0; P<0.0001; and MD: 63.7%; CI: 58.5 to 68.8; P<0.0001, respectively). The HC group presented higher PSI levels than the non-freezer group in the quiet stance (MD: −11.9%; CI: −17.2 to −6.5; P<0.0001) and in the step initiation (MD: −27.7%; CI: −33.1 to −22.3; P<0.0001) (Figure 2).
Figure 2.
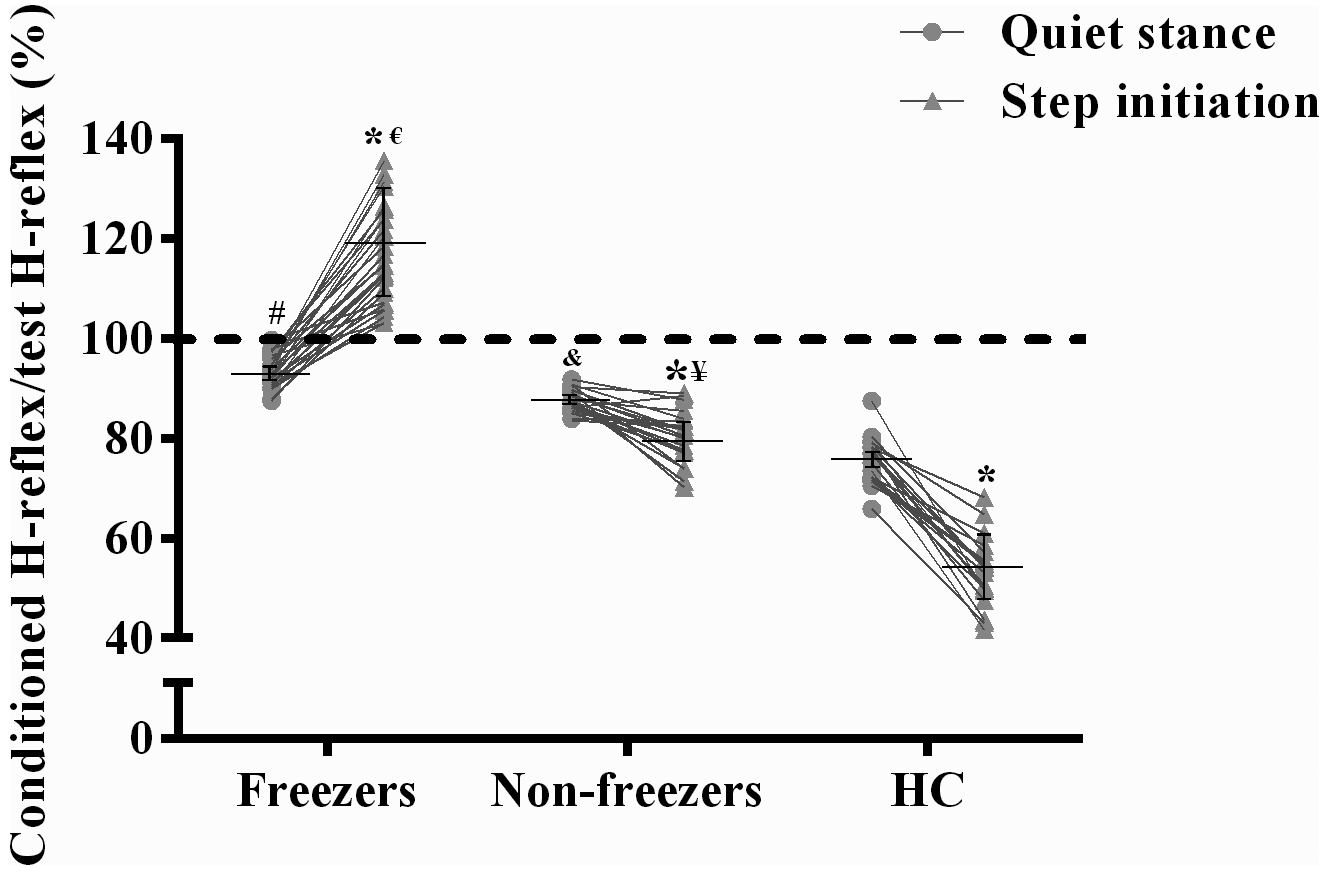
Means ± SD for the ratio of the conditioned H-reflex relative to the test H-reflex (i.e., presynaptic inhibition [PSI]) during quiet stance and step initiation. As can be seen, every freezer showed loss of PSI (i.e., higher ratio of the conditioned H-reflex relative to the test H-reflex) during APA in the step initiation (above the dashed line), but presented PSI levels during quiet stance, whereas every non-freezers and age-matched healthy individuals (HC) showed PSI in both the tasks.
*Different from quiet stance values (P<0.05).
#Different from quiet stance values of the non-freezer and HC groups (P<0.05). &Different from quiet stance values of the HC group (P<0.05).
€Different from step initiation values of the non-freezer and HC groups (P<0.05). ¥Different from step initiation values of the HC group (P<0.05).
Test H-reflex amplitude (%Mmax)
There were no significant differences for the test H-reflex amplitude normalized to its corresponding Mmax (P=0.963) neither among groups nor tasks (Table 2.
Table 2.
Test H-reflex amplitude normalized to its corresponding maximal motor response (Mmax), rectified and averaged electromyography (raEMG) recordings, and co-contraction ratio during quiet stance and step initiation for each group (mean±SD).
| Variables | Tasks | Freezers | Non-freezers | HC |
|---|---|---|---|---|
| Test H-reflex amplitude (%Mmax) | Quiet stance | 32.26 ± 5.76 | 33.24 ± 8.86 | 35.85 ± 6.54 |
| Step Initiation | 32.47 ± 7.97 | 33.96 ± 7.16 | 35.79 ± 5.71 | |
| raEMG of the soleus (mV)& | Quiet stance | 0.07 ± 0.04 | 0.08 ± 0.05 | 0.07 ± 0.03 |
| Step Initiation | 0.08 ± 0.02 | 0.07 ± 0.04 | 0.06 ± 0.04 | |
| raEMG of the tibial (mV)& | Quiet stance | 0.04 ± 0.03 | 0.06 ± 0.03 | 0.04 ± 0.04 |
| Step Initiation | 0.05 ± 0.04 | 0.05 ± 0.03 | 0.07 ± 0.05 | |
| Co-contraction (%)& | Quiet stance | 0.76 ± 0.58 | 0.90 ± 0.59 | 0.73 ± 0.57 |
| Step Initiation | 0.63 ± 0.51 | 0.79 ± 0.44 | 0.96 ± 0.54 |
measured over a 100 ms epoch that preceded stimulation of each nerve.
raEMG recordings
There were no significant differences for the raEMG of the soleus muscle (P=0.810), raEMG of the tibial muscle (P=0.154), and co-contraction ratio (P=0.218) neither among groups nor tasks (Table 2).
APA amplitude and duration
The freezers did not present with any episodes of FoG associated with “trembling of the knees” and multiple APAs (Jacobs et al., 2009b) during the step initiation tasks in this study. In addition, single APAs were observed in all trials of freezers, non-freezers, and HC individuals. The ANOVA showed that the freezer group presented smaller APA amplitudes than the non-freezer group (MD = −0.46N/cm; IC = −0.80 to −0.12; P=0.005; Figure 3A) and the HC group (MD = −0.87N/cm; IC = −1.21 to −0.52; P<0.0001). The non-freezer group showed lower APA amplitudes than the HC group (MD = −0.40N/cm; IC = −0.76 to −0.04; P=0.02; Figure 3A). In addition, the freezer group showed longer APA durations than the HC group (MD = 75.9ms; IC = −30.03 to 121; P=0.0005), and the non-freezer group showed longer APA durations than the HC group (MD = 84.0ms; IC = 36.02 to 132; P=0.0002; Figure 3B). There was no significant difference in APA duration between the freezer and non-freezer groups (MD = −8.15ms; IC = −53.2 to −36.9; P=0.90; Figure 3B).
Figure 3.
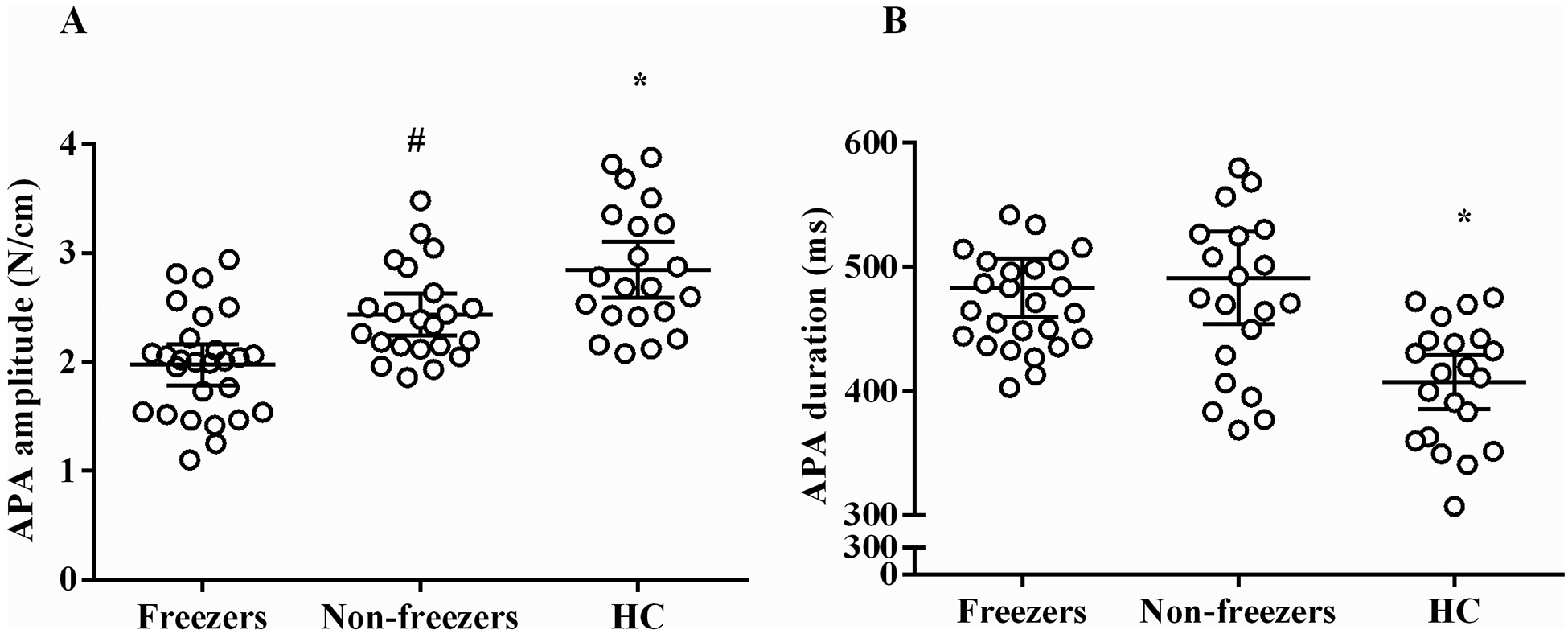
Means ± SD for the amplitude (A) and duration (B) of the anticipatory postural adjustments (APA) in the step initiation for the freezer, non-freezer, and age-matched healthy controls (HC) groups.
*HC presented higher APA amplitudes and shorter APA durations than the individuals with PD (P<0.05).
#Non-freezer presented higher APA amplitudes than the freezers (P<0.05).
Correlational analysis
Significant correlations were observed only for freezers between the loss of PSI in the step initiation and APA amplitude (P<0.05), between the loss of PSI in the step initiation and the FoG-ratio (P<0.05), and between the NFoGQ scores and the APA amplitude (P<0.05) as shown in Figure 4. For non-freezers and HC, no significant correlations were found between PSI level and any APA related variable as shown in Table 3.
Figure 4.
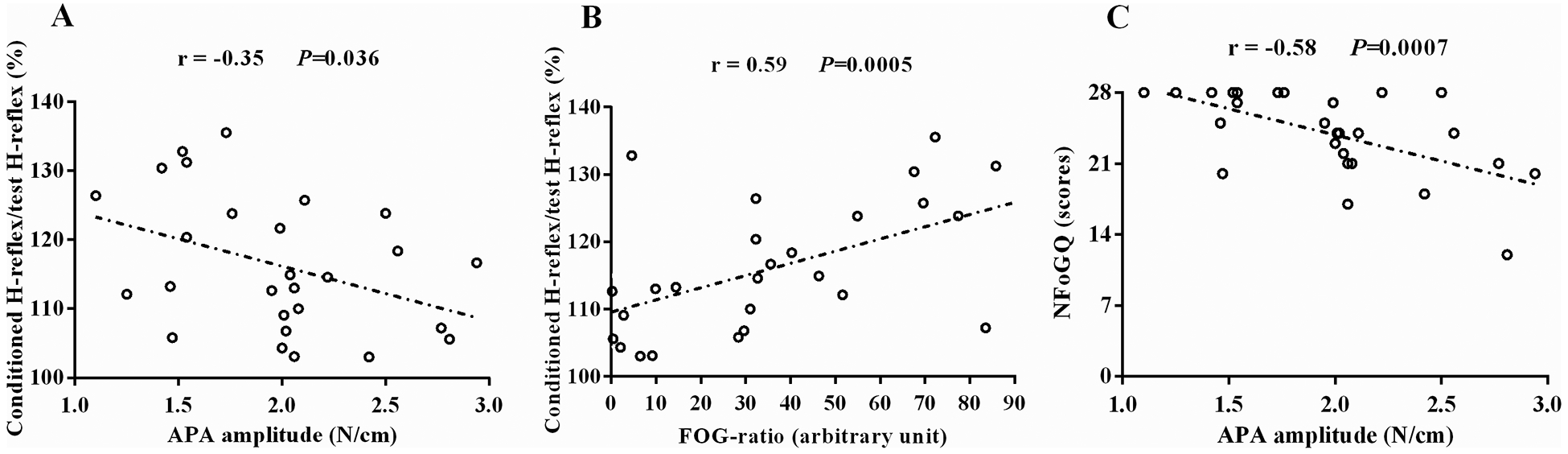
Correlation of loss of presynaptic inhibition (i.e., higher ratio of the conditioned H-reflex relative to the test H-reflex) with anticipatory postural adjustment (APA) amplitude (A) and with freezing severity (FoG-ratio) (B), and correlation of the subjective severity of freezing (New Freezing of Gait Questionnaire [NFoGQ] scores) with APA amplitude (C). Spearman’s r-values and P-values are shown.
Table 3.
Spearman’s rank correlation coefficients between conditioned H-reflex relative to the test H-reflex (cond-H/test-H) and amplitude and duration of the anticipatory postural adjustment (APA) for each group, and between cond-H/test-H and severity of freezing of gait (FoG) for freezers.
| Freezers | Non-freezers | HC | ||||
|---|---|---|---|---|---|---|
| Cond-H/test-H (%) | Cond-H/test-H (%) | Cond-H/test-H (%) | ||||
| Variables | r | P value | r | P value | r | P value |
| APA amplitude (N/cm) | −0.35 | 0.036 | −0.16 | 0.226 | −0.05 | 0.401 |
| APA duration (ms) | 0.15 | 0.225 | 0.19 | 0.188 | 0.21 | 0.170 |
| FoG-ratio (a.u.) | 0.59 | 0.0005 | ||||
HC = age-matched healthy controls.
Discussion
The current study is the first to identify spinal reflex involvement during an important functional task for freezers (step initiation), as well as the importance of PSI to prevent freezing. Our main findings were the following: 1) Freezers presented loss of PSI (i.e., higher ratio of the conditioned H-reflex relative to the test H-reflex) whereas non-freezers and HC presented PSI during APAs, and 2) Significant correlations were found between loss of PSI and FoG-ratio and small APA amplitude for freezers. Our data provide novel insights about the important role of central and spinal inhibitory mechanisms to manage the transition from standing to walking.
Loss of PSI during the APA in freezers
Three possible explanations for the loss of PSI during the APA in freezers include abnormal alterations in some mechanisms that underlying FoG in PD, such as abnormal supraspinal motor commands, abnormal proprioceptive inputs, and or a combination of both alterations (i.e., deficits in sensorimotor integration). APAs are thought to be generated by the supplementary motor area, as lesions in supplementary motor area result in absent APAs in humans (Gurfinkel et al., 1988; Viallet et al., 1992). Additionally, inhibition of supplementary motor area via repetitive low frequency transcranial magnetic stimulation of the pre-supplementary motor area prolongs APAs, especially in individuals with PD (Jacobs et al., 2009a). The supplementary motor area and the peduncolopontine nucleus have significant inputs to pontomedullary reticular formation, in addition to the spinal cord (Keizer & Kuypers, 1989; Matsuyama & Drew, 1997; Schepens & Drew, 2004). The pontomedullary reticular formation has neurons related to APAs, different neurons related to stepping, and a third set of neurons related to both APAs and stepping (Schepens & Drew, 2004). In freezers, both the supplementary motor area and peduncolopontine nucleus may send abnormal motor commands to the reticulospinal tract, which may coordinate APAs with step initiation (Schepens & Drew, 2004; Takakusaki, 2013; van Lith et al., 2018). Alternatively, the supplementary motor area may forward programs of precise leg-foot movement to the primary motor cortex, which, in turn, sends motor command via the corticospinal tract (Hoshi & Tanji, 2007; Takakusaki, 2013). Recently, corticospinal involvement during the APA has been observed, as corticospinal excitability of the erector spinae muscle increased 40 ms prior to rapid shoulder flexion in healthy individuals (Chiou et al., 2018). Both the reticulospinal and corticospinal tracts project to the spinal motoneuron pool (Takakusaki, 2013; Takakusaki, 2017), which send drive to excitatory and inhibitory interneurons that mediate PSI during voluntary movements (Lundberg & Voorhoeve, 1962; Iles, 1996; Meunier & Pierrot-Deseilligny, 1998; Sirois et al., 2013). Therefore, an intact drive from frontocortical and subcortical areas to spinal circuits controlling PSI may be important for flexible modulation of APAs.
In fact, the small APA amplitude of freezers was inversely correlated with loss of PSI (i.e., higher ratio of the conditioned H-reflex relative to the test H-reflex), with smaller APAs related to more loss of PSI (Figure 4A). In addition, smaller APAs were negatively correlated with higher NFoGQ scores (Figure 4C). Freezers showed smaller APA amplitudes than the other groups (Figure 3A), corroborating findings from previous studies (Schlenstedt et al., 2018). Thus, our results would suggest that reticulospinal and corticospinal tracts send irregular or no drive to interneurons mediating PSI of the soleus muscles during deficient APA in freezers. In addition, FoG has been hypothesized be due to an inability to inhibit stance postural control and initiate stepping (Jacobs & Horak, 2007; Jacobs et al., 2009b; Nutt et al., 2011; Cohen et al., 2014). Furthermore, freezers show loss of white matter in the inhibition pathway between the right supplementary motor area and right subthalamic nucleus, as well as between the right supplementary motor area and right peduncolopontine nucleus (Fling et al., 2013). This right-sided pathway has been implicated in cognitive inhibition in healthy control individuals (Coxon et al., 2012). Thus, we believe that lack of central inhibition of stance posture to allow stepping to commence is reflected in loss of PSI in freezers. However, other possible explanations for loss of PSI include proprioceptive deficits and/or abnormal sensorimotor integration.
Abnormal proprioception may also contribute to the observed loss of PSI in freezers, given that freezers may present greater proprioceptive deficits than non-freezers and HC (Tan et al., 2011). These proprioceptive deficits might interfere negatively with APAs prior to a step, since presynaptic proprioceptive signals may also project to the sensorimotor cortex and cerebellum to coordinate posture and movement (Morita et al., 1998). However, further studies should determine if greater proprioceptive deficits are related to loss of PSI during APAs in freezers. Evidence has suggested that even non-freezers have deficits in sensorimotor integration (Lewis & Byblow, 2002). It might interfere negatively in the reorganization of PSI during APA, given that the APA motor program can be adjusted during its execution (Delval et al., 2018). This process might involve rapid and direct sensorimotor integration, such as reorganization of the supraspinal and spinal motor systems and proprioceptive afference for the ongoing APA (Takakusaki, 2013; Takakusaki, 2017). We hypothesize that abnormal sensorimotor integration may also contribute to loss of PSI during APA in freezers. Further studies are necessary to confirm this hypothesis.
Correlation between loss of PSI and FoG-ratio
Loss of PSI was associated with higher FoG severity (FoG-ratio values) (Figure 4B). The FoG-ratio is based on the assumption that the high frequency weight shift represent “trembling of the knees” and the lower frequencies represent the gait cadence. So although there was no clinically apparent FoG during the study the freezers demonstrated prefreezing characteristics involving rapid left-right weight shifts during APAs that delayed onset of a step (prolonged APAs). Although APAs were small and prolonged none step initiation trials in freezers resulted in FoG, including multiple APAs (trembling of the knees) (Jacobs et al., 2009b) in this study. The lack of freezing is likely because individuals were tested in the clinically “on” levodopa state and freezing is usually reduced in the “on” state (Nutt et al., 2011). In addition, the step initiation on our study was triggered by an external cue, a verbal “GO” command. It is well known that freezing can be overcome with these types of external cues (Ginis et al., 2018). We calculated the FoG-ratio during 360 degree turns. Evidence has suggested that continuous turning for 2 min with direction reversals every 360 degrees is the best way to induce FoG events, because it imposes temporal and spatial asymmetry between steps (Plotnik et al., 2008; Snijders et al., 2012; Mancini et al., 2017). Temporal and spatial asymmetry between steps are associated with problems in rhythmic movement coordination (Plotnik et al., 2008), which also is controlled by a spinal cord network (Takakusaki, 2013; Takakusaki, 2017). As PSI is important for modulating muscles’ coordination by adjusting both supraspinal motor commands and sensory feedback at the spinal level (Nielsen, 2004), the loss of PSI found in freezers may contribute to FoG severity.
Methodological care
We took care to prevent two methodological possibilities to facilitation, instead of inhibition, following a conditioning stimulus to the tibialis anterior muscle. First, the conditioning stimulation depolarizes Ia afferents from peroneal muscles that have facilitatory effects on the soleus muscle (Meunier et al., 1993). Second, the H-reflex is more susceptible to inhibition/facilitation depending on the size of the test H-reflex (Crone et al., 1990). To minimize these two factors, contraction of the peroneal muscle was highly controlled in the present study to isolate activation of the tibialis anterior muscle with stimulation. We checked that the stimulation evoked a motor response in the tibialis anterior muscle without a motor response in the peroneal muscles. In addition, the size of the test H-reflex was maintained constant throughout the experiments, thus reducing the bias in the amount of inhibition (PSI), as it depends on the size of the test H-reflex. Since the soleus H-reflex is often depressed in the quiet stance (Katz et al., 1988; Capaday et al., 1995; Baudry & Duchateau, 2012), and mainly in the early part of the stance phase of walking (Capaday et al., 1995), the test stimulus intensity during the APA was adjusted so that the reference (unconditioned) H-reflex attained the same size as in the quiet stance. In fact, we also observed that all freezers had PSI during quiet stance in which the size varied by group: freezers < non-freezers < age-matched healthy controls (Figure 2). Thus, loss of PSI during the APA in the freezers may be due to FoG.
Possible influences on loss of PSI during APAs
If the APAs are reduced in step initiation, but tibial muscle activity is not inhibited, one may suggest that cocontraction and/or prolonged activation of tibialis muscles during APAs could also be another possible explanation. PSI is known to increase during cocontraction of ankle muscles (Nielsen & Kagamihara, 1993). Freezers have more cocontraction than non-freezers (Schlenstedt et al., 2018) and non-freezers have higher cocontraction levels than HC (Dimitrova et al., 2004; Horak, 2006), even so, we found that non-freezers presented lower PSI levels than HC, whereas freezers did not present PSI at all. Thus, cocontraction may not have interfered. In addition, using the co-contraction ratio at the time of stimulation, there were no between-group differences (Table 2). Moreover, there was no correlation (P > 0.09) between the co-contraction ratio with loss of PSI in freezers and with the PSI leves in non-freezers and HC (data not shown). On the other hand, evidence has demonstrated that in individuals with PD, during stepping, the freezing events are anticipated by early with prolonged activation of tibialis muscles (Nieuwboer et al., 2004) that may reflect unsuccessful compensatory attempts to facilitate PSI in the soleus muscle. This may be a possible explanation for the loss of PSI during deficient APAs in freezers. However, freezers did not present any freezing events in the present study. Moreover, there were no between-group differences for the arEMG of the tibial muscle at the time of stimulation. Thus, further studies should investigate if prolonged activation of tibialis muscles during APAs in freezing events may be related with loss of PSI.
Clinical Implications
FoG is a disabling clinical phenomenon that is thought to involve defective APAs resulting in difficulty or cessation of step initiation. Our findings show that loss of PSI is associated with both small APAs and FoG severity. Therefore, this study has implications for informing the design of treatment methods to focus on restoring PSI levels and improving APA amplitude, which could improve FoG severity. As the loss of PSI during APAs observed in freezers may also be explained by deficits in sensorimotor integration, treatment strategies to enhance sensorimotor integration may be helpful. Spinal cord stimulation is a recent, semi-invasive method that may enhance the sensorimotor integration in freezers. It activates multiple structures along the somatosensory pathway and desynchronizes the pathological cortico-striatal oscillations responsible for the manifestation of Parkinson’s disease symptoms (Fuentes et al., 2009; Yadav & Nicolelis, 2017). In addition, proprioceptive signals run primarily through some of the largest myelinated axons that comprise the dorsal columns of the spinal cord during electrical stimulation (Fuentes et al., 2009; Yadav & Nicolelis, 2017). Although spinal cord stimulation has improved APA duration (de Lima-Pardini et al., 2018) and FoG episodes (de Lima-Pardini et al., 2018; Samotus et al., 2018), evidence is still inconclusive as these findings were recorded in a small number of individuals.
Alternatively, exercise strategies that require high levels of sensorimotor integration may be helpful for restoring PSI and improving APAs to reduce FoG severity. In fact, we recently demonstrated that three months of challenging exercise with high levels of sensorimotor integration (i.e., resistance training with instability) improved PSI levels at rest (Silva-Batista et al., 2017) and improved APAs by a clinical scale (Silva-Batista et al., 2018). Future studies should investigate the effects of this type of exercise program in freezers.
Conclusion
The current study demonstrates that freezers consistently show loss of PSI, unlike non-freezers and HC during their APAs. Furthermore, the loss of PSI was associated with small APA size and worse FoG severity. From these observations, we suggest that loss of PSI of stance posture in preparation for a step may be due to FoG.
Key Points.
Individuals with freezing of gait (FoG) due to Parkinson’s disease (PD) have small and long anticipatory postural adjustments (APA) associated with delayed step initiation.
Individuals with FoG (freezers) may require functional reorganization of spinal mechanisms to perform APAs due to supraspinal dysfunction. As presynaptic inhibition (PSI) is centrally modulated to allow execution of supraspinal motor commands, it may be deficient in freezers during APAs.
We show that freezers presented PSI in quiet stance (control task), but they presented loss of PSI (i.e., higher ratio of the conditioned H-reflex relative to the test H-reflex) during APAs prior to step initiation (functional task), whereas non-freezers and healthy control individuals presented PSI in both the tasks.
The loss of PSI in freezers was associated with both small APA amplitudes and FoG severity.
We hypothesize that loss of PSI during APA for step initiation in freezers may be due to FoG.
Acknowledgements
We would like to thank participants from Movement Disorders Clinic from School of Medicine of the University of São Paulo for their commitment to study, Eugenia Casella Tavares Mattos and Éden Marcos Braga de Oliveira who helped in the technical support, Martina Mancini who reviewed the manuscript, FAPESP, CNPq, and CAPES.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo under award numbers 2015/13096-1, 2016/13115-9 and 2018/16909-1, the Conselho Nacional de Desenvolvimento Científico e Tecnológico under award numbers 406609/2015-2 and 03085/2015-0, National Institutes of Health under award number R01AG006457, and Department of Veterans Affairs Merit Award number 5I01RX001075.
Footnotes
Competing interests
Fay B. Horak has a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. Fay B. Horak also consultants with Biogen, Neuropore, Sanofi, Adamus, Abbott, and Takeda. This potential individual conflict has been reviewed and managed by Oregon Health & Science University.
References
- Baudry S & Duchateau J. (2012). Age-related influence of vision and proprioception on Ia presynaptic inhibition in soleus muscle during upright stance. J Physiol 590, 5541–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG & Obeso JA. (1997). Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord 12, 206–215. [DOI] [PubMed] [Google Scholar]
- Burleigh AL, Horak FB & Malouin F. (1994). Modification of postural responses and step initiation: evidence for goal-directed postural interactions. J Neurophysiol 72, 2892–2902. [DOI] [PubMed] [Google Scholar]
- Butler JS, Fearon C, Killane I, Waechter SM, Reilly RB & Lynch T. (2017). Motor preparation rather than decision-making differentiates Parkinson’s disease patients with and without freezing of gait. Clin Neurophysiol 128, 463–471. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA & Comeau F. (1995). Differential effects of a flexor nerve input on the human soleus H-reflex during standing versus walking. Can J Physiol Pharmacol 73, 436–449. [DOI] [PubMed] [Google Scholar]
- Chiou SY, Hurry M, Reed T, Quek JX & Strutton PH. (2018). Cortical contributions to anticipatory postural adjustments in the trunk. J Physiol 596, 1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RG, Klein KA, Nomura M, Fleming M, Mancini M, Giladi N, Nutt JG & Horak FB. (2014). Inhibition, executive function, and freezing of gait. J Parkinsons Dis 4, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RG, Nutt JG & Horak FB. (2017). Recovery from Multiple APAs Delays Gait Initiation in Parkinson’s Disease. Front Hum Neurosci 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo C, Merello M, Hughes AJ, Sieradzan K & Lees AJ. (1996). Motor response to acute dopaminergic challenge with apomorphine and levodopa in Parkinson’s disease: implications for the pathogenesis of the on-off phenomenon. J Neurol Neurosurg Psychiatry 60, 634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon JP, Van Impe A, Wenderoth N & Swinnen SP. (2012). Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. J Neurosci 32, 8401–8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B & Nielsen J. (1987). Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol 389, 163–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J & Pierrot-Deseilligny E. (1990). Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81, 35–45. [DOI] [PubMed] [Google Scholar]
- de Lima-Pardini AC, Coelho DB, Souza CP, Souza CO, Ghilardi M, Garcia T, Voos M, Milosevic M, Hamani C, Teixeira LA & Fonoff ET. (2018). Effects of spinal cord stimulation on postural control in Parkinson’s disease patients with freezing of gait. Elife 7, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima-Pardini AC, de Azevedo Neto RM, Coelho DB, Boffino CC, Shergill SS, de Oliveira Souza C, Brant R, Barbosa ER, Cardoso EF, Teixeira LA, Cohen RG, Horak FB & Amaro E Jr. (2017). An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Sci Rep 7, 43088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delval A, Braquet A, Dirhoussi N, Bayot M, Derambure P, Defebvre L, Tard C & Dujardin K. (2018). Motor Preparation of Step Initiation: Error-related Cortical Oscillations. Neuroscience 393, 12–23. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J & Berger W. (1984). Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neurosci Lett 44, 131–135. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Horak FB & Nutt JG. (2004). Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J Neurophysiol 91, 489–501. [DOI] [PubMed] [Google Scholar]
- Earles D, Vardaxis V & Koceja D. (2001). Regulation of motor output between young and elderly subjects. Clin Neurophysiol 112, 1273–1279. [DOI] [PubMed] [Google Scholar]
- Fahn S & Elton R. (1987). UPDRS Program Members. Unified Parkinson’s disease rating scale In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease, Vol. 2 Florham Park, NJ: Macmillan Healthcare Information, p.153–163, 293–304. [Google Scholar]
- Faist M, Dietz V & Pierrot-Deseilligny E. (1996). Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res 109, 441–449. [DOI] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA & Horak FB. (2013). Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136, 2405–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE & McHugh PR. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Fuentes R, Petersson P, Siesser WB, Caron MG & Nicolelis MA. (2009). Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science 323, 1578–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geertsen SS, Lundbye-Jensen J & Nielsen JB. (2008). Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J Appl Physiol (1985) 105, 915–922. [DOI] [PubMed] [Google Scholar]
- Giladi N & Nieuwboer A. (2008). Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord 23 Suppl 2, S423–425. [DOI] [PubMed] [Google Scholar]
- Ginis P, Nackaerts E, Nieuwboer A & Heremans E. (2018). Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann Phys Rehabil Med 61, 407–413. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Lipshits MI & Lestienne FG. (1988). Anticipatory neck muscle activity associated with rapid arm movements. Neurosci Lett 94, 104–108. [DOI] [PubMed] [Google Scholar]
- Heilbronn M, Scholten M, Schlenstedt C, Mancini M, Schollmann A, Cebi I, Potter-Nerger M, Gharabaghi A & Weiss D. (2019). Anticipatory postural adjustments are modulated by substantia nigra stimulation in people with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. [DOI] [PubMed] [Google Scholar]
- Horak FB. (2006). Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35 Suppl 2, ii7–ii11. [DOI] [PubMed] [Google Scholar]
- Horak FB & Macpherson JM. (1996). Postural Orientation and Equilibrium In Rowell LB, & Sheperd JT (Eds.), Handbook of Physiology, Section 12. Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 255–292. [Google Scholar]
- Hortobagyi T, van de Waardt LE, Tokuno CD, Taube W & Papegaaij S. (2018). Age-related reversal of spinal excitability during anticipatory postural control. Eur J Appl Physiol 118, 2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E & Tanji J. (2007). Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol 17, 234–242. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L & Lees AJ. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55, 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C & Pierrot-Deseilligny E. (1987a). Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J Physiol 389, 729–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E & Shindo M. (1987b). Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. (1996). Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491 (Pt 1), 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV & Horak FB. (2007). External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp Brain Res 179, 29–42. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Lou JS, Kraakevik JA & Horak FB. (2009a). The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 164, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M & Horak FB. (2009b). Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol 215, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, Meunier S & Pierrot-Deseilligny E. (1988). Changes in presynaptic inhibition of Ia fibres in man while standing. Brain 111 (Pt 2), 417–437. [DOI] [PubMed] [Google Scholar]
- Keizer K & Kuypers HG. (1989). Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res 74, 311–318. [DOI] [PubMed] [Google Scholar]
- Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM & Silburn PA. (2010). Predictors of future falls in Parkinson disease. Neurology 75, 116–124. [DOI] [PubMed] [Google Scholar]
- Knikou M (2008). The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171, 1–12. [DOI] [PubMed] [Google Scholar]
- Lewis GN & Byblow WD. (2002). Altered sensorimotor integration in Parkinson’s disease. Brain 125, 2089–2099. [DOI] [PubMed] [Google Scholar]
- Lundberg A & Voorhoeve P. (1962). Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol Scand 56, 201–219. [DOI] [PubMed] [Google Scholar]
- Magalhaes FH, Elias LA, da Silva CR, de Lima FF, de Toledo DR & Kohn AF. (2015). D1 and D2 Inhibitions of the Soleus H-Reflex Are Differentially Modulated during Plantarflexion Force and Position Tasks. PLoS One 10, e0143862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Smulders K, Cohen RG, Horak FB, Giladi N & Nutt JG. (2017). The clinical significance of freezing while turning in Parkinson’s disease. Neuroscience 343, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K & Drew T. (1997). Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 389, 617–641. [DOI] [PubMed] [Google Scholar]
- McLeod JG & Walsh JC. (1972). H reflex studies in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 35, 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S & Pierrot-Deseilligny E. (1998). Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res 119, 415–426. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E & Simonetta M. (1993). Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Exp Brain Res 96, 534–544. [DOI] [PubMed] [Google Scholar]
- Moore O, Peretz C & Giladi N. (2007). Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord 22, 2192–2195. [DOI] [PubMed] [Google Scholar]
- Morin C, Pierrot-Deseilligny E & Hultborn H. (1984). Evidence for presynaptic inhibition of muscle spindle Ia afferents in man. Neurosci Lett 44, 137–142. [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N & Nielsen J. (1998). Gating of somatosensory evoked potentials during voluntary movement of the lower limb in man. Exp Brain Res 120, 143–152. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Ikeda S & Yanagisawa N. (2000). Decrease in presynaptic inhibition on heteronymous monosynaptic Ia terminals in patients with Parkinson’s disease. Mov Disord 15, 830–834. [DOI] [PubMed] [Google Scholar]
- Nielsen J & Kagamihara Y. (1993). The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol 464, 575–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB. (2004). Sensorimotor integration at spinal level as a basis for muscle coordination during voluntary movement in humans. J Appl Physiol (1985) 96, 1961–1967. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Janssens L & Stijn V. (2004). Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain 127, 1650–1660. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T & Giladi N. (2009). Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait & Posture 30, 459–463. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N & Bloem BR. (2015). Freezing of gait: a practical approach to management. Lancet Neurol 14, 768–778. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB & Nieuwboer A. (2011). Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10, 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patikas DA, Kotzamanidis C, Robertson CT & Koceja DM. (2004). The effect of the ankle joint angle in the level of soleus Ia afferent presynaptic inhibition. Electromyogr Clin Neurophysiol 44, 503–511. [PubMed] [Google Scholar]
- Perez MA, Lungholt BK & Nielsen JB. (2005). Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol 568, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik M, Giladi N & Hausdorff JM. (2008). Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur J Neurosci 27, 1999–2006. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Part NJ, Farquhar R & Butchart P. (1994). Presynaptic inhibition of soleus Ia afferent terminals in Parkinson’s disease. J Neurol Neurosurg Psychiatry 57, 1488–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P & Schmidt RF. (1999). Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129, 1–37. [DOI] [PubMed] [Google Scholar]
- Samotus O, Parrent A & Jog M. (2018). Spinal Cord Stimulation Therapy for Gait Dysfunction in Advanced Parkinson’s Disease Patients. Mov Disord 33, 783–792. [DOI] [PubMed] [Google Scholar]
- Schepens B & Drew T. (2004). Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92, 2217–2238. [DOI] [PubMed] [Google Scholar]
- Schlenstedt C, Mancini M, Nutt J, Hiller AP, Maetzler W, Deuschl G & Horak F. (2018). Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson’s Disease? Front Aging Neurosci 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Batista C, Corcos DM, Kanegusuku H, Piemonte MEP, Gobbi LTB, de Lima-Pardini AC, de Mello MT, Forjaz CLM & Ugrinowitsch C. (2018). Balance and fear of falling in subjects with Parkinson’s disease is improved after exercises with motor complexity. Gait Posture 61, 90–97. [DOI] [PubMed] [Google Scholar]
- Silva-Batista C, Mattos EC, Corcos DM, Wilson JM, Heckman CJ, Kanegusuku H, Piemonte ME, Tulio de Mello M, Forjaz C, Roschel H, Tricoli V & Ugrinowitsch C. (2017). Resistance training with instability is more effective than resistance training in improving spinal inhibitory mechanisms in Parkinson’s disease. J Appl Physiol (1985) 122, 1–10. [DOI] [PubMed] [Google Scholar]
- Sirois J, Frigon A & Gossard JP. (2013). Independent control of presynaptic inhibition by reticulospinal and sensory inputs at rest and during rhythmic activities in the cat. J Neurosci 33, 8055–8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders K, Dale ML, Carlson-Kuhta P, Nutt JG & Horak FB. (2016). Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism Relat Disord 31, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders AH, Haaxma CA, Hagen YJ, Munneke M & Bloem BR. (2012). Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord 18, 149–154. [DOI] [PubMed] [Google Scholar]
- Takakusaki K (2013). Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord 28, 1483–1491. [DOI] [PubMed] [Google Scholar]
- Takakusaki K (2017). Functional Neuroanatomy for Posture and Gait Control. J Mov Disord 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T, Almeida QJ & Rahimi F. (2011). Proprioceptive deficits in Parkinson’s disease patients with freezing of gait. Neuroscience 192, 746–752. [DOI] [PubMed] [Google Scholar]
- Tard C, Dujardin K, Bourriez JL, Destee A, Derambure P, Defebvre L & Delval A. (2014). Attention modulates step initiation postural adjustments in Parkinson freezers. Parkinsonism Relat Disord 20, 284–289. [DOI] [PubMed] [Google Scholar]
- van Lith BJH, Coppens MJM, Nonnekes J, van de Warrenburg BPC, Geurts AC & Weerdesteyn V. (2018). StartReact during gait initiation reveals differential control of muscle activation and inhibition in patients with corticospinal degeneration. J Neurol 265, 2531–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallet F, Massion J, Massarino R & Khalil R. (1992). Coordination between posture and movement in a bimanual load lifting task: putative role of a medial frontal region including the supplementary motor area. Exp Brain Res 88, 674–684. [DOI] [PubMed] [Google Scholar]
- Yadav AP & Nicolelis MAL. (2017). Electrical stimulation of the dorsal columns of the spinal cord for Parkinson’s disease. Mov Disord 32, 820–832. [DOI] [PubMed] [Google Scholar]


