Abstract
Background and Objective:
Parathormone (PTH) and serum Vitamin D3 (VD3) share a complex interplay where increased VD3 leads to a negative response on parathormone level. Our objective was to determine the correlation of parathormone (PTH) and Vitamin D3 (VD3) levels in nursing mothers and infants 1-6 months’ age from South Punjab, Pakistan.
Methods:
This study is a secondary data analysis of previously conducted cross sectional study which was conducted at the Department of Pediatric Medicine, Nishtar Medical University, Multan, during August 2010 to June 2011. Study included 67 infants 1-6 months of age and 60 nursing mothers. A venous blood sample was drawn for estimation of VD3, calcium, phosphate, alkaline phosphatase, parathormone and albumin. Spearman correlation coefficient was calculated to determine the inverse correlation between PTH and VD3 levels.
Results:
Mean age (in days) of the infants was 83±53.7 days whereas maternal mean age was 25.53 ± 4.12 years. Median VD3 level in infants was 20.90 ng/ml (IQR – 49.5). Median serum PTH levels were 20.90 pg/ml (IQR – 26.17). Median VD3 level in nursing mothers was 21.0 ng/ml (IQR 7.2– 43.8). Median maternal serum PTH levels were 20.89 pg/ml (IQR 2.9 – 232.4). Substantial negative relation between VD3 and parathormone in infants and mothers was not evident (r = - 0.027, p-value 0.83) and (r = 0.156, p-value 0.23) respectively. A significant positive association between infant and maternal VD3 was observed (rs –0.55, p-value < 0.001).
Conclusion:
Our study affirms that the customary negative correlation between VD3 and parathormone levels does not exist.
Keywords: Calcium, Exclusive breastfeeding, Lactating Mother, Parathormone, VD3, Vitamin D
INTRODUCTION
The Parathormone (PTH) and serum Vitamin D3 (VD3) share a complex interplay where increased calcium and VD3 exert a negative feedback on PTH level that has been shown by multiple studies.1 Lower level of VD3 in the body not only decreases intestinal calcium and phosphorus absorption but also triggers the parathormone secretion through calcium-sensing receptors. This increased concentration of PTH in serum leads to increased bony problems like bone mineralization defects leading to loss of bone & higher chances of bone fractures.2 Although this negative relationship of VD3 & PTH is recognized but the optimal serum levels of VD3 that will keep the PTH levels suppressed is still not clear.3
Various studies have differently defined VD3 insufficiency in adults using different cut offs of serum VD3 as 37.5 nmol/L4, 50 nmol/L5, or 75 nmol/L.6 These cutoffs were determined in relation to fracture risk, intestinal calcium absorption or bone mineral density. Based on increased serum PTH in the wake of low VD3 status, the 2008 guidelines by the American Academy of Pediatrics have recommended optimal serum VD3 ≥ 50 nmol/L for children.7
The optimal level to stop increase in PTH level with decreasing VD3 and its inverse relationship does not always hold true. A laboratory database study from Israel included 19, 172 people with complete VD3 and PTH tests. A significant negative correlation (r = - 0. 176, p-value < 0.001) was observed. After excluding the patients with hypercalcemia or renal failure; a VD3 plateau of 46.2 nmol/L was found that stabilized the serum PTH levels.8 However, in a study on 1370 children age 1–6 years, mean reported levels for VD3 and PTH were 86 nmol/L & 2.67 pmol/L, respectively. VD3 plateau level of 107 nmol/L (P < .001) was identified above which PTH levels were minimized.3 However, the studies do not consistently prove this negative relationship between PTH & VD3. Choi YJ et al studied 57 VD3 deficient infants 1 – 6 months age, 20 of these were evaluated for correlation with serum PTH levels and no significant (Pearson p-value – 0.051) correlation was reported.9
Lactating mother-infant pair has been particularly susceptible to VD3 deficiency. Maternal VD3 status determines the VD3 status of exclusively breast-fed infants. In order to evaluate this negative correlation of VD3 and PTH we conducted the study on infant – mother in South Punjab, Pakistan.
METHODS
This study is secondary data analysis of our previous studies that aimed to determine the VD3 status and determinants of low VD3 in nursing mothers and infants 1 – 6 months of age, which was conducted at Department of Pediatric Medicine (both inpatient and outpatient) and approved by ethical review committee of Nishtar Medical University (ERC#15864-77 dated July 14, 2016). This was a cross sectional study conducted from August 2010 to June 2011 at Multan city of South Punjab, Pakistan. A sample of 67 infants and 60 nursing mothers was enrolled through convenience sampling. The eligibility criteria for Infants included 1-6 months of age who visited for routine immunization, those admitted with acute respiratory illness in the Pediatric Medicine Department and whose parents were willing to participate in the study. The infants with any metabolic illness or congenital anomaly and those who were already on VD3 supplementation were excluded from the study. The nursing mothers of these infants were also approached to participate in the study and consenting mothers were enrolled.
Data collection and laboratory samples
We used two structured questionnaires for data collection from the mothers and infants. The questionnaire for mothers included the demographic data about the family, mother, delivery, diet, daily sun exposure and maternal intake of calcium/ VD3 containing vitamins during and after pregnancy. The questionnaire for the infants included the demographic parameters and characteristics including feeding habits that can affect the VD3 status. Three milliliters of venous blood were obtained by the standard procedure from both mother & baby in special vials. All the samples were stocked at -2 to -8°C till the analysis were made. VD3 estimation was done by FDA approved Abbott Laboratories’ chemiluminescent microparticle immunoassay (CMIA – Abbott Park, IL). We also obtained levels of Ca+2, PO4-3, alkaline phosphatase, parathormone and albumin. Taking reference the current recommendations10 the cut-off points for VD3 deficiency and VD3 insufficiency used were <30 nmol/L and <50 nmol/L, respectively. The optimal level of VD3 for infants was taken as >80 nmol/L and >50 nmol/L for nursing mothers.
Data analysis
The data was analyzed using STATA 12.0. The numerical variables are described as the mean ± standard deviation (SD) & qualitative variables as frequency and percentages. The Spearman correlation coefficient was applied to examine relationship between the PTH & VD3 levels.
RESULTS
Over a period of one year 67 infants 1 – 6 months’ age and 60 lactating mothers (data from 7 mothers was not available) were included in the study. Median VD3 level in infants was 20.90 ng/ml (IQR – 49.5). Median serum parathyroid (PTH) levels were 20.90 pg/ml (IQR – 26.17). Mean serum concentration of other biochemical markers included corrected serum calcium (mg/dl) 8.88 ± 0.91, serum phosphate (mg/dl) 5.8 ± 1.03 and serum Alkaline phosphatase (IU/L) 282.4 ± 67. Median VD3 level in nursing mothers was 21.0 ng/ml (IQR 7.2– 43.8). Median serum parathyroid (PTH) levels were 20.89 pg/ml (IQR 2.9 – 232.4). Mean serum concentration of corrected serum calcium (mg/dl) was 8.98 ± 0.73, serum phosphate (mg/dl) 4.19 ± 0.97 and serum alkaline phosphatase (IU/L) was 174.68 ± 53.97 (Table-I).
Table-I.
Biochemical Characteristics of participating infants 1-6 months’ age (n=67) and nursing mothers (n=60).
| Biochemistry | Infants | Mothers | ||
|---|---|---|---|---|
| Serum calcium*, mg/dl (mean ± SD) | 8.88 ± 0.91 | 8.98 ± 0.73 | ||
| Serum phosphate, mg/dl (mean ± SD) | 5.8 ± 1.03 | 4.19 ± 0.97 | ||
| Serum Alkaline phosphatase, IU/L (mean ± SD) | 282.4 ± 72.67 | 174.68 ± 53.97 | ||
| Serum Albumin, g/dl (mean ± SD) | 3.84 ± 0.36 | 3.82 ± 0.26 | ||
| Serum Vit. D, ng/ml (median, (IQR) | 20.90 (49.5) | 21.0 (7.2 – 43.8) | ||
| VD3 status | n | (%) | n | (%) |
| Optimal (> 50 ng/ml) | 21 | (31.3) | 00 | (00) |
| Insufficient (30 - < 50 ng/ml) | 05 | (7.5) | 02 | (3.3) |
| Deficient (<30 ng/ml) | 41 | (61.2) | 58 | (96.7) |
| Serum PTH, pg/ml (median, range) | 20.90 | (26.17) | 20.89 | (2.9-232.4) |
Corrected serum Calcium proportional to Albumin status.
No significant negative relation between VD3 and parathormone in infants and mothers was evident by Spearman correlation coefficient (r = -0.027, p-value 0.83) and (r = 0.156, p-value 0.23) respectively. Although Pearson correlation coefficient was not significant between serum calcium and PTH levels in infants (r = 0.088, p-value 0.48) but it was positively correlated in nursing mothers (r = 0.422, p-value 0.001). Similar to infant’s results no significant correlation was observed in mothers between serum phosphate (r = -0.066, p-value 0.60 and r = 0.19, p-value 0.15), alkaline phosphatase (r = 0.037, 0.77 and r= -0.14, p-value 0.29) and PTH levels respectively (Table-II). A moderate positive relationship between mother and child VD3 status was observed (rs – 0.55, p-value < 0.001) (Fig.1).
Table-II.
Correlation of Serum Parathyroid (PTH) with Calcium*, Phosphorus, Alkaline phosphatase and VD3 levels in participating infants 1-6 months age (n=67) and nursing mothers (n=60).
| Infants | Mothers | |||
|---|---|---|---|---|
| PTH, pg/ml | ||||
| Correlationα coefficient | p-value | Correlation coefficient | p-value | |
| Serum total calcium*, mg/dl | 0.088 | 0.48 | 0.422 | 0.001 |
| Serum phosphate, mg/dl | -0.066 | 0.60 | 0.188 | 0.15 |
| Alkaline phosphatase, IU/L | 0.037 | 0.77 | -0.14 | 0.29 |
| VD3β, ng/ml | -0.027 | 0.83 | 0.156 | 0.23 |
Corrected serum Calcium proportional to Albumin status,
Pearson correlation coefficient,
Spearman’s correlation coefficient.
Fig.1.
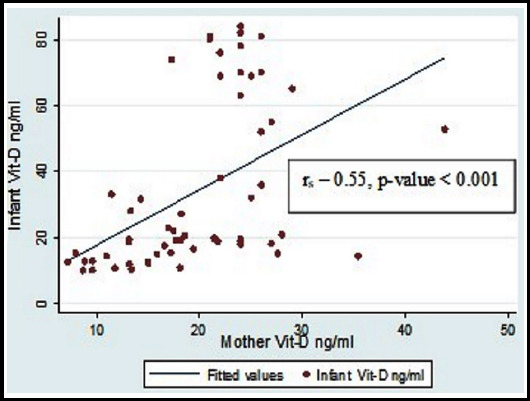
Correlation between Maternal and Infants Vitamin D levels.
DISCUSSION
It has been postulated that increasing level of intact parathyroid hormone (iPTH) in response to low VD3 level is a better biochemical indicator of deficient or insufficient vitamin D levels. Studies from Pakistan have reported VD3 deficiency in healthy adults11,12, pregnant women13, children14, adolescents15 and have also examined the correlation of VD3 with PTH levels16. But none of the studies from Pakistan have documented the correlation of VD3 and PTH in dyad of nursing mothers and their infants.
A high prevalence of VD3 deficiency both in infants and mothers was evident in our previously published studies. Briefly, 67 % (n/N - 45/67) percent of the infants belonged to rural area, 83.6 % (56/67) were having open house, 51 % (n/N - 34/67) of our infants were on exclusive breast feeding and 61 % of the infants (41/67) were VD3 deficient (< 30 ng/ml). Mean age of the mothers (in years) was 25.53 ± 4.12. Seventy-three percent (44/60) belonged to rural areas and 86.7 % (52/60) were having open house. Ninety-six percent (58/60) of the mothers were VD3 deficient (< 30 ng/ml).17,18
Maternal serum calcium in our study was comparable to the levels in a study by Wagner et al (8.98 ± 0.7 vs. 9.4 ± 0.4).19 Similarly, maternal phosphorus in our study was comparable to the mothers recruited from Charleston, SC (4.19 ± 0.97 vs. 4.14 ± 0.62) but it was higher compared to mothers recruited from Rochester, NY (3.8 ± 0.60). A moderate positive correlation (rs – 0.55, p-value < 0.001) between the maternal and infant VD3 was observed. This was concordant with the study by Wagner CL et al showing moderate positive correlation (r – 0.42 – 0.65, p-value < 0.0001) between lactating mother-infant VD3 levels.19 It was also concordant with the study results by Husain et al who showed a moderate positive relation between VD3 levels of mother and infant pair (Pearson coefficient = 0.516, P < 0.001).20 This positive relation between Infant & maternal VD3 concentrations (rs=0.41, P=0.001) was also highlighted by Salameh et al.21
Statistically significant negative relation between VD3 and parathormone levels in infants (Pearson correlation r - 0.156, p-value 0.23) as well as mothers (Pearson correlation r – 0.16, p-value 0.23) was not demonstrated in our study. This is in line with a number of studies not showing negative correlation of serum PTH and serum VD3 levels in infants and mothers. Elsammak et al studied fifty-two women and eighty-seven men blood donors (N = 139) from Saudi Arabia. A statistically significant inverse relationship between PTH & VD3 levels was not observed in either of the groups (men, r = 0.35, p=0.75 and women, r = 0.11, p=0.44 respectively).22 However, a local study on 50 healthy female nursing staff verified a significantly strong negative relationship between VD3 and PTH levels (r-value: -0.781, p-value: <0.001).23
However, Husain et al have demonstrated a strong inverse relationship between PTH & VD3 (r = -0.66, p-value < 0.001).20 Kramer CK et al also described the negative correlation (r = −0.37; P < .0001) in mothers at three months postpartum.24 Study by Wagner et al. also highlighted the inverse relationship between VD3 & PTH levels in lactating mothers (r = - 0.32) as well as in infants (r = -0.35).19 So, the evidence for inverse relationship between VD3 & PTH is not conclusive in either way.
Limitations of the study
One of the study limitations leading to lack of inverse correlation between VD3 and PTH in infants and mothers include smaller sample size. This is due to the fact that this was a secondary analysis of data and study was not powered for this objective. Other limitations include variation in measurement method of PTH levels and non-availability of data on serum magnesium levels (hypomagnesemia) that may influence serum PTH levels.
CONCLUSION
The negative relation between VD3 and parathormone levels, as claimed, is not evident in our study. We need to have well-controlled and high-powered studies to elucidate this dilemma.
Author`s Contribution
GM conceived, designed the study and & editing and final approval of the manuscript. Also responsible for the integrity and accuracy of the work
MK performed data analysis and interpretation & first draft of manuscript.
IA did data collection, manuscript writing and review of final draft.
MAT did data collection and manuscript writing and review of final draft review.
Footnotes
Grant Support & Financial Disclosures: None.
REFERENCES
- 1.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D:Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2015;96(1):365–408. doi: 10.1152/physrev.00014.2015. doi:10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr Physiol. 2011;6(2):561–601. doi: 10.1002/cphy.c140071. doi:10.1002/cphy.c140071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire JL, Birken C, Thorpe KE, Sochett EB, Parkin PC. Parathyroid hormone as a functional indicator of vitamin D sufficiency in children. JAMA Pediatrics. 2014;168(4):383–385. doi: 10.1001/jamapediatrics.2013.5379. doi:10.1001/jamapediatrics.2013.5379. [DOI] [PubMed] [Google Scholar]
- 4.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among african american and white women of reproductive age:Third national health and nutrition examination survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. doi:10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 5.Mithal A, Wahl DA, Bonjour J-P, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807–1820. doi: 10.1007/s00198-009-0954-6. doi:10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. doi:10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 7.Wagner CL, Greer FR. American academy of pediatrics section on breastfeeding, american academy of pediatrics committee on nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. doi:10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 8.Saliba W, Barnett O, Rennert HS, Lavi I, Rennert G. The relationship between serum 25 (oh) D and parathyroid hormone levels. Am J Med. 2011;124(12):1165–1170. doi: 10.1016/j.amjmed.2011.07.009. doi:10.1016/j.amjmed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Choi YJ, Kim MK, Jeong SJ. Vitamin D deficiency in infants aged 1 to 6 months. Korean J Pediatr. 2013;56(5):205–210. doi: 10.3345/kjp.2013.56.5.205. doi:10.3345/kjp.2013.56.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press (US); 2011. doi:10.17226/13050. [PubMed] [Google Scholar]
- 11.Jadoon SA, Ahmed A, Alam MA. Vitamin D deficiency in Pakistan:tip of iceberg. J Ayub Med Coll Abbottabad. 2017;30(1):78–80. [PubMed] [Google Scholar]
- 12.Roomi MA, Farooq A, Ullah E, Lone KP. Hypovitaminosis D and its association with lifestyle factors. Pak J Med Sci. 2015;31(5):1236–1240. doi: 10.12669/pjms.315.7196. doi:10.12669/pjms.315.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasir JA, Imran M, Zaidi SA. Pattern of vitamin D among Pakistani pregnant women. J Coll Physicians Surg Pak. 2018;28(3):233–237. doi: 10.29271/jcpsp.2018.03.233. [DOI] [PubMed] [Google Scholar]
- 14.Jawa A, Riaz SH, Assir MZ, Afreen B, Riaz A, Akram J. Causes of short stature in Pakistani children found at an Endocrine Center. Pak J Med Sci. 2016;32(6):1321–1325. doi: 10.12669/pjms.326.11077. doi:10.12669/pjms.326.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadeem S, Munim TF, Hussain HF, Hussain DF. Determinants of Vitamin D deficiency in asymptomatic healthy young medical students. Pak J Med Sci. 2018;34(5):1248–1252. doi: 10.12669/pjms.345.15668. doi:10.12669/pjms.345.15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan S, Muzammil SM, Jafri L, Khan AH. An audit of clinical laboratory data of 25 [OH] D at Aga Khan University as reflecting vitamin D deficiency in Pakistan. J Pak Med Assoc. 2015;65(11):1247–1250. [PubMed] [Google Scholar]
- 17.Mustafa G, Asadi MA, Iqbal I, Bashir N. Low vitamin D status in nursing pakistani mothers in an environment of ample sunshine:A cross-sectional study. BMC Pregnancy Childbirth. 2018;18(1):426. doi: 10.1186/s12884-018-2062-0. doi:10.1186/s12884-018-2062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustafa G, Aslam M, Iqbal I, Ahmad I, Talib A, Bashir N. Low vitamin D status in infants aged 1-6 months &risk factors. Pak Pediatr J. 2016;40(2):104–112. [Google Scholar]
- 19.Wagner CL, Howard CR, Hulsey TC, Lawrence RA, Ebeling M, Shary J, et al. Maternal and infant vitamin D status during lactation:Is latitude important? Health. 2013;5(12):2004–2013. doi:10.4236/health.2013.512271. [Google Scholar]
- 20.Husain M, Verma M, Jora R, Soni JP, Sharma P. Correlation of serum vitamin D levels in lactating mothers and their infants. Indian J Endocrinol Metab. 2018;22(6):801–805. doi: 10.4103/ijem.IJEM_186_17. doi:10.4103/ijem.IJEM_186_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salameh K, Al-Janahi NSA, Reedy AM, Dawodu A. Prevalence and risk factors for low vitamin D status among breastfeeding mother–infant dyads in an environment with abundant sunshine. Int J Womens Health. 2016;8:529–535. doi: 10.2147/IJWH.S107707. doi:10.2147/IJWH.S107707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsammak M, Al-Wossaibi A, Al-Howeish A, Alsaeed J. High prevalence of vitamin D deficiency in the sunny eastern region of saudi arabia:A hospital-based study. East Mediterr Health J. 2011;17(4):317–322. [PubMed] [Google Scholar]
- 23.Nasim A, Salim B, Niazi S, Fatima N. Healthy lookig hospital nurses showing vitamin D deficiency:correlation of vitamin D levels with their levels of parathhyroid hormone and bone turnover markers. Pak Armed Forces Med J. 2015;65(1):145–148. [Google Scholar]
- 24.Kramer CK, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. The relationship between parathyroid hormone and 25-hydroxyvitamin D during and after pregnancy. J Clin Endocrinol Metab. 2016;101(4):1729–1736. doi: 10.1210/jc.2015-4060. doi:10.1210/jc.2015-4060. [DOI] [PubMed] [Google Scholar]


