Abstract
6,6′-Aryl trehalose derivatives have been synthesized with a view towards identifying novel Th-17-inducing vaccine adjuvants based on the high affinity Mincle ligand Brartemicin.The initial structure-activity relationships of these novel trehalose-based compounds were investigated. All compounds have been evaluated for their ability to engage the Mincle receptor and induce a potential pro-Th17 cytokine profile from human peripheral blood mononuclear cells based on IL-6 production in human peripheral blood mononuclear cells. The preliminary biological characterization of the designed analogs presented in this paper should aid in the future design and testing of more affine ligands that may foster the discovery of novel adjuvants with improved pharmacological properties.
Keywords: Adjuvant, Brartemicin, Mincle, Tuberculosis, Vaccine, TH-17, trehalose dimycolate, trehalose diester
Graphical Abstract
To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered.
1. Introduction
Tuberculosis (TB) remains a leading cause of death worldwide contributing to 1.5 million deaths and 10 million new cases of Mycobacterium tuberculosis (Mtb) infection reported in 2018.1,2 Development of vaccine adjuvants capable of driving strong, antigen specific immune responses will be essential in preventing Mtb infection.3 The most commonly used and century-old vaccine, Bacille Calmette-Guérin (BCG), does not prevent primary infection or reactivation of latent Mtb infection in adults.4 To address the limitations of the BCG vaccine, subunit vaccines have been developed but have had limited success due to their lack of immunogenicity and inability to mobilize a sufficient immune response for protective immunity.5 Adjuvants are therefore needed to enhance the immunogenicity of subunit vaccines targeting TB. A limited number of adjuvants adjuvants have been approved for human use in combination with a subunit vaccines. These include aluminum salts, monophosphoryl lipid A, QS-21 CpG synthetic oligodeoxynucleotides and oil emulsions. Vaccine adjuvants such as monophosphoryl lipid A (MPL) in combination with QS-21 (AS01) or aluminum hydroxide (Alum; AS04) have proven partially effective against Mtb when combined with subunit vaccines.6 Recent evidence has suggested a Th17 or Th1/Th17 combination response may be beneficial in driving protective immune response against Mtb, but no Th17-inducing adjuvant has been approved for human use.7 There is accumulating evidence that stimulation of the C-type lectin receptors (CLRs) by their cognate ligands induces differentiation of naïve CD4+T cells into Th17 cells.8
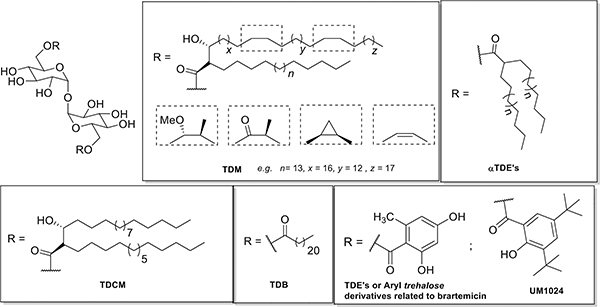
The CLR Pattern Recognition Receptor (PRR) Mincle is essential for the recognition and adjuvanticity of mycobacterial cord factor (trehalose 6,6′-dimycolate (TDM)), the key immunogenic component of the Mtb cell wall,9 and a synthetic analog trehalose-dibehenate (TDB)10 (Figure 1). TDB and TDM have proven useful as adjuvants for mycobacterial subunit vaccines, inducing a strong Th17 and Th1 immune responses when co-administered with vaccine antigen11,12 TDM has proven too reactogenic for human vaccine applications in part due to its highly potent immune-stimulating properties.13 The development of TDB14 and other trehalose diesters (TDEs) (Figure 1),15 have partially met the need for Th17 inducing adjuvants although the physiochemical properties of these adjuvants, being very lipophilic with limited aqueous solubility, limits their application to complex liposomal formulations.16 Therefore, there remains an unmet need to identify novel, safe and efficacious Th17 inducing adjuvants with improved formulation and physiochemical properties. The natural product Brartemicin has been identified as a high-affinity ligand of the carbohydrate-recognition domain (CRD) of the C-type lectin mincle.17,18 In recent years several lipidated diaryl derivatives have been described to elicit Th17 cytokines in a Mincle-dependent fashion with improved adjuvant activity overTDB.15,18a,19 We recently reported the first relatively small 6,6′-aryl trehalose derivatives with demonstrated ability to promote a Th1/Th17 immune response in vitro and in vivo.20b UM1024 was identified one of the most potent compounds and we were interested in further exploring the structural basis for Mincle activation in this class of ligand. We were very interested to determine if the steric bulk of the t-butyl groups was playing a key role in the observed activity and we wanted to determine in the electronic properties of the aryl substituents could be modified using trifluoromethyl aryl derivatives, because of the unique size, electronic properties and excellent metabolic stability, in comparison to small alkyl, nitro and pyridine functionality...
Figure 1.
Representative Mincle ligands: Trehalose Dimycolates (TDM), α-Trehalose Diesters (Vizantin), Trehalose Dicorynomycolates (TDCM), Trehalose dibehenate (TDB), Brartemicin analogs and UM1024.
2. Results and discussions
2.1. Synthesis of brartemicin analogs
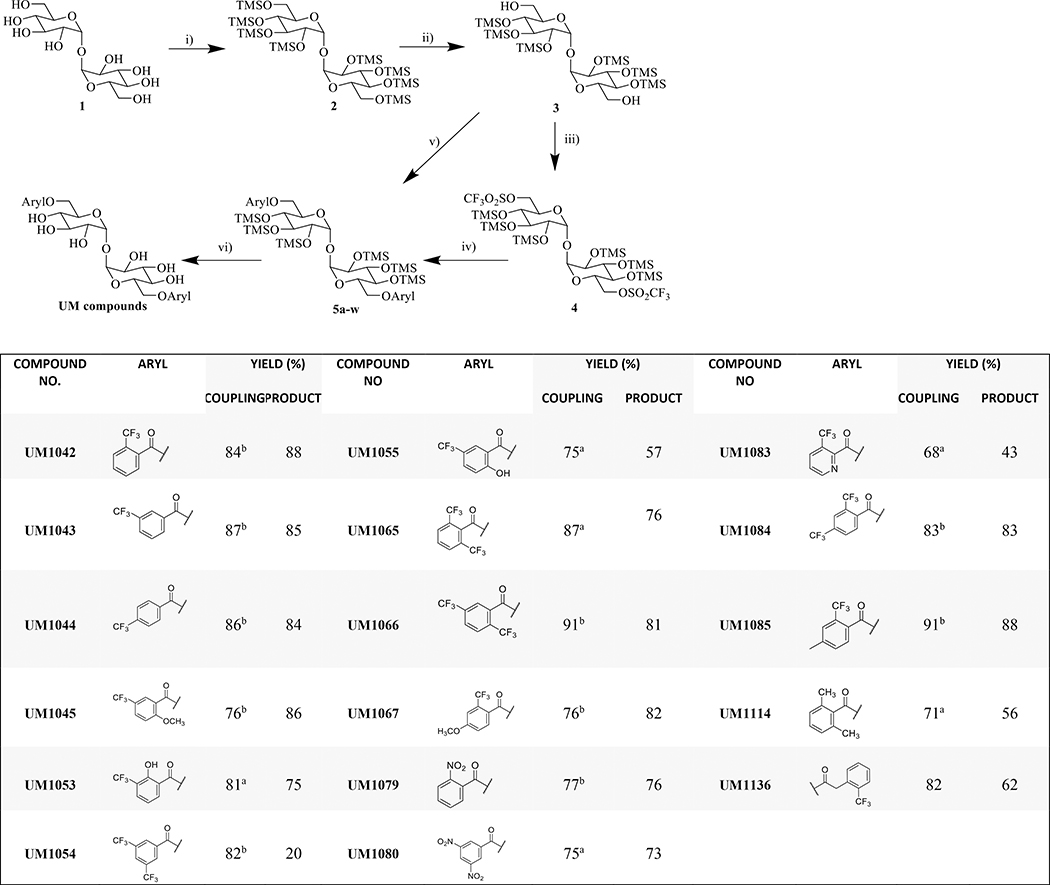
As our continuous interest in exploring the potential effect of the substituents on the aryl ring, we have prepared an additional series of Brartmicin analogs that feature structural modification for better understanding of the electronic factors involved for biological activation and the effect of the position of the substituents on the aryl ring. 6,6′-Di-O-aryl-chemical modification of trehalose is one of the most effective approaches to understand the biological potential of these compounds.22 In the course of our structure-activity studies of 6,6′ aryl trehalose based Mincle ligands,20b we prepared a series of trifluoromethylated substituted brartemicin analogs using trehalose (Scheme 1). These brartemicin analogs were prepared via two synthetic routes based on the reactivity of the substrate (Scheme 1; Table 1). The useful TMS-protected 6,6′-trehalose diol 3 was synthesized using commercially available α,α′-D-trehalose which was per-silylated using N,O-bistrimethylsilylacetamide (BSA) and catalytic TBAF, and the more labile primary TMS ethers were selectively removed via treatment with K2CO3 in methanol in 82% yeild.21,22 This versatile intermediate was then utilized for the efficient double ester coupling with various aryl carboxylic acids (2.5 equiv. per 1 equiv. of TMS-protected trehalose) using an excess of EDCI-MeI or DCC and DMAP. Under these optimized reaction conditions, coupled products were synthesized in a range of isolated yields (30–90%) after purification. We also optimized reaction conditions for coupling aryl acids containing an unprotected hydroxyl moiety (UM1053 and UM1055), electron withdrawing CF3 group on 2- and 6- position of aromatic ring (UM1065) and methyl group on 2- and 6- position (UM1114) that are inaccessible via carbodiimide strategies. These were synthesized in three steps from diol in 80–90% overall yield by employing the highly reactive 6,6′-bis-O-trifluoromethanesulfonate (triflate) 4 in conjunction with potassium salts of aryl carboxylic acids. These salts of the aryl carboxylic acids were generated in quantitative yield in THF using potassium trimethylsilanoate. A solution of the acid (1 equiv.) and KOSiMe3 (1.2 equiv.) were stirred for 30 min at room temperature and then solvent was removed in vacuo to obtain the precipitated salt which was used without further purification. The crude ditriflate (1 equiv.) 4 was condensed with the potassium salts (2.2 equiv.) of aryl carboxylic acid and 18- crown ether in toluene at 80 °C to give symmetrical hexa-O-trimethylsilyl 6,6′-diesters in good to excellent yield (40–85%).23. Having accomplished the synthesis of the protected diesters, global deprotection of TMS groups using Dowex-H+ resin was used to prepare the targeted compounds.19 These reactions do not require the use of strictly anaerobic or scrupulously anhydrous reaction conditions. All these novel compounds were fully characterized by spectroscopic techniques.
Scheme 1: Reagents and reaction conditions:
i) BSA, TBAF, DMF; ii) K2CO3, MeOH (90%); iii) (CF3SO2)2O, pyridine, DCM (95%); iv) K salt of acid, 18-crown ether, toluene, 80 °C (40–85%); v) aryl acid, EDCI-MeI, DMAP, DCM or aryl acid, DCC, DMAP, DCM (30–90%); vi) Dowex-H+, DCM:MeOH (1:1) (70–90%). a) conditions iii, iv were used; b) conditions v were used
2.2. Structure-activity relationships of brartemicin analogs
Previous studies from our group demonstrated that the bulky group at 3 and 5 positions on benzene ring were essential for human Mincle activity20b and herein we anticipated that incorporation of triflouromethylated group on benzene ring may improve the interactions and effectively reduce the requirement for bulky group on benzene ring. For preliminary screening, the compounds were assayed for IL-6 production in human PBMC’s as this is one of the key cytokines for Th17 differentiation,.20c,24 Briefly, compounds were serially diluted in ethanol, applied to tissue culture plates and the solvent was evaporated. Freshly isolated human PBMCs were added and incubated with the compound for 18–24 hours. Supernatants were collected and evaluated for IL-6 levels by ELISA. TDB and TDM, which are Mincle ligands,25, were used as positive controls. Recently, Lang and co-workers26 demonstrated IL6 production by stimulation of isolated APCs with TDM and TDB. The experiments reported here use PBMCs and thus the IL-6 levels are lower than reported in the literature for these Mincle ligands. In addition, the solvent used for plate coating greatly influences the responsiveness of PBMCs to these compounds (data not shown). Here, we used ethanol as the solvent whereas other reports in the literature have used IPA or other solvents. Ethanol was selected to maximize the in vitro activity of our synthetic ligands and may not be optimal for the activity of TDM or TDB. We presume this difference is due to conformation obtained in the drying process of the compound which is critical for the activity of these PRR ligands. Studies are ongoing to further explore the tertiary structural requirements of Mincle receptor binding and activation.
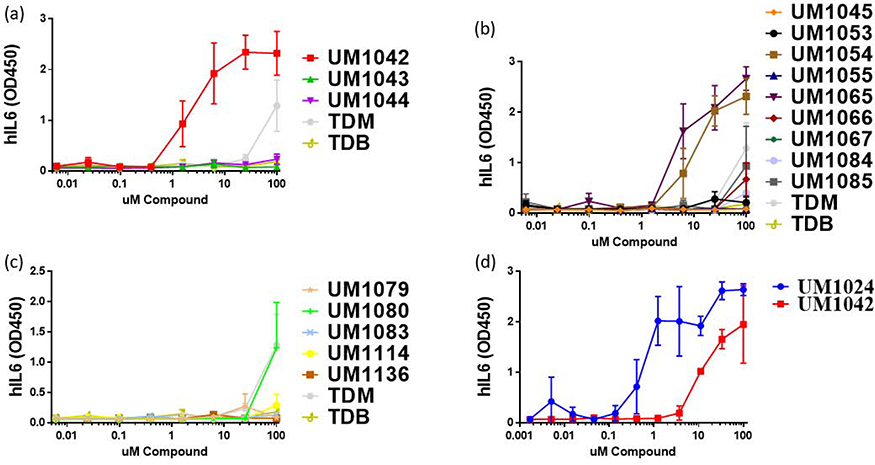
Among the mono-substituted compounds with CF3 substitution at 2-, 3- and 4- position of the aromatic ring, the CF3 group at ortho position (UM1042) resulted in dose-responsive IL-6 production while the meta (UM1043) and para (UM1044) substituted analogs generated little to no IL-6. To determine if the observed activity of ortho substituted compound (UM 1042) is due to the steric or hydrogen bonding properties of CF3 groups or the electronic effects of the electron withdrawing properties of the CF3 on the aromatic ring, we introduced an ortho-nitro group, one of the powerful electron withdrawing groups in structural organic chemistry (UM 1079). This addition proved deleterious and resulted in a complete loss of IL-6 induction. Similarly, replacement of the benzoyl moiety with ortho-CF3 substituted heterocyclic ring (UM 1083) led to the loss of activity (Figure 2c). This stressed the importance of the benzoyl moiety and substitution at 2-position was preferred for the induction of IL-6 response in hPBMCs.
Figure 2:
Cytokine production from primary human mononuclear cells in response to stimulation with synthetic compounds; The indicated compound was dissolved in ethanol and dried to the bottom of a tissue culture plate. hPBMCs were added and incubated at 37 °C for 18–24 hours. IL-6 secretion was measured by ELISA; n=3 independent donors for all experiments; error bars indicate SEM.
For the di-substituted trifluoromethylated substituted trehalose diesters (TDE’s), the CF3 group at 2- and 6- position (UM1065) or 3- and 5- disubstituted (UM1054) were more potent for IL-6 induction compared to 2- and 4- (UM1084) or 2- and 5- (UM1066) disubstituted analogs. The substitution with CF3 group at the 2- and 6- position gave the best results and appeared crucial for activity, therefore its methylated counterpart (UM1114) was synthesized. This compound is inactive as compared to 2,6-disubstituted trifluoromethylated trehalose diester UM1065 (Figure 2). From this, it becomes apparent that electronic factors are important for the activity as compared to the steric factors of this class of molecules. The interesting reduced activity of many of the compounds at higher concentrations was not associated with toxicity or cell deaths (data not shown) and will be a subject of future investigations.
To further elucidate the effect of electron density of the aromatic ring, we designed and synthesized a panel of compounds bearing a variety of substituents such as methoxy and hydroxy in combination with trifluoromethyl groups and replacement of the trifluoromethyl with nitro functionality on the phenyl ring. These compounds (UM1045, UM1053, UM1055, UM1067, UM1085, UM1136) showed no significant response compared to ortho-substituted trifluoromethylated diester (UM1042) and ortho-ortho-disubstituted trifluoromethylated diester (UM1065). Interestingly, the incorporation of two nitro groups into the aromatic residue at meta-position (UM1080) increased the IL-6 response from human PBMCs at the highest dose tested but exhibited lower potency in comparison to ortho- substituted trifluoromethylated diester trehalose (UM1042) (Figure 2). In addition, introducing of methylene spacer between the phenyl ring and carbonyl group (UM1136) resulted in loss of activity. As a working hypothesis, we therefore, suggest that the introduction of CF3 group either at 2- position or at 2- and 6- position or at 3 and 5- position facilitated the active IL-6 response in hPBMCs.. Interestingly, the relative activity of this series of derivatives was lower than that our initial lead UM1024 (Figure 2d).
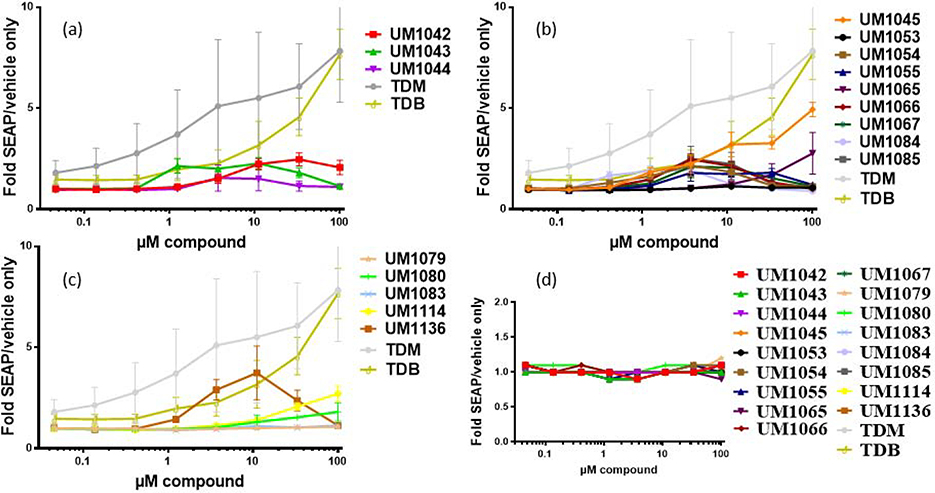
Next, this library of compounds was assessed for their ability to signal specifically through Mincle using human embryonic kidney (HEK) cells along with an NF-κB-driven secreted embryonic alkaline phosphatase (SEAP) reporter. Several of these analogs were able to induce the production of SEAP in a dose dependent manner. HEK null cells (HEK cells containing the NF-κB reporter without Mincle receptor) were used as negative controls to confirm the receptor specificity of the compounds (Figure 3d). ortho- substituted trifluoromethylated diester (UM1042) demonstrated a modest dose response in the hMincle HEK reporter cells (Figure 3a), albeit at much lower levels than TDM and TDB. Similarly, 2- and 6- disubstituted trifluoromethylated diester (UM1065) and 2- and 5- disubstituted aryl TDE’s (UM1045) showed moderate dose-dependent increase in SEAP production from hMincle HEK cells (Figure 3b). Interestingly, introducing a methylene spacer between the phenyl ring and carbonyl group (UM1136) resulted in complete loss of IL-6 production in human PMBCs (Figure 2c) demonstrated activity in the HEK cell line but (figure 3c). In general, compounds with different substitution on aromatic ring other than the trifluoro-substituted heterocyclics were unable to induce measurable SEAP production from hMincle (Figure 3). Further investigations into immunomodulatory properties, adjuvant activity and mode of action of this class of TDEs are currently underway.
Figure 3:
(a-c) Activation of human Mincle in response to synthesized library. The indicated compounds were plate coated in EtOH and HEK cells transfected with human Mincle and an NF-κB-driven SEAP reporter were added and incubated with the compounds for 18–24 hour followed by assessment of the supernatants for SEAP levels. Data are represented as fold change in OD650 over vehicle treated cells. n=3 for all experiments; error bars indicate SEM. (d) results in HEK null cells.
3. Conclusion
We recently reported on the synthesis of several brartemicin derivatives as a new class of relatively small and potent Mincle receptor selective agonists (e.g UM1024; figure 1) with significantly improved pharmacology over existing Mincle ligands and with improved in vitro and in vivo adjuvant activity.20c In this work, we have prepared an additional series of novel aryl trehalose derivatives that feature various structural modifications on the aromatic ring to demonstrate that electronic factors on the aromatic ring are important for the activity as compared to the steric factors. The targeted analogs were efficiently prepared from commercially available materials (4–5-steps) and in very acceptable overall yield 70–90%. While the activity of this class of compounds is not as high,as UM1024, the structure activity relationships elucidated by this study will aid in the future development of new pharmaceutically acceptable and useful Mincle selective Th17 inducing adjuvants. Our observations confirm the a very strong hydrophobic and steric interaction of the t-butyl groups of UM1024, in addition to the position of the substituents on the ring, are required for the remarkable activity of these molecules, especially when compared to TDM and TDB. Results for this family of CLR compounds indicate low responses in the human Mincle HEK system but strong IL6 production from PBMCs. This disparity may be due to the absence of co-receptors in the transgenic HEK system preventing full activation of the signaling pathways present in the primary human PBMCs. Compound presentation and tertiary structure (plate coating from ethanol) also impacts compound activity across different in vitro systems based on Mincle and co-receptor expression levels.. Expansion of SAR and biological activity of this class of molecules is ongoing in our laboratory.
4. Experimental
General Experimental. All reagents and solvents were used as received. Reactions were monitored by TLC-analysis on Merck Silica gel 60 F254 plates and visualized by UV at 254 nm and dipping in vanillin (vanillin/water/ethanol/sulfuric acid, 0.2 g:5 mL:5 mL:1 mL) or phosphomolybdic acid in ethanol (PMA) and developed with heat. All compounds were confirmed to be >95% pure by NMR and HPLC-CAD analysis. 1H and 13C NMR spectra were recorded on an Agilent or Bruker 400 MHz instrument and were referenced to TMS or a solvent peak. High resolution HPLC-MS analysis was obtained on an Agilent 6520 Q-TOF mass spectrometer utilizing electrospray ionization source in positive or negative mode. Chromatography was performed on Grace or Biotage automated medium pressure chromatography instruments with preloaded Buchi silica gel cartridges. Human Mincle expressing HEK cells were obtained from Invivogen (San Diego, CA). Cells were cultured according to the manufacturer’s instructions in DMEM with 10% FBS, 50 U/ml penicillin, 50 mg/ml streptomycin, 100 mg/ml Normocin, 2 mM L-glutamine, 30 μg/ml blasticidin, 1 μg/ml puromycin and 1x HEK-Blue™ CLR Selection. Intermediate compounds 5j, 5p, and 5q were not isolated and partially characterized by HPLCMS.
2,2′,3,3′,4,4′-Hexa-trimethylsilyl-α,α-D-trehalose (3)
To a solution of trehalose dihydate (25 g, 66 mmol) in dry DMF (100 mL) was added BSA (140 mL) followed by TBAF (4 mL, 0.04 mmol) dropwise. The reaction mixture was stirred for 1 hour and then quenched with 2-propanol (25 mL). Freeze the reaction mixture to −20 °C for 1 hour and then treated it with a cooled solution of K2CO3 (9.1 g, 66 mmol) in 2000 mL of MeOH. After stirring for 10 min, the solution was neutralized with acetic acid and then methanol was removed in vacuo. The resulting residue was extracted with heptane and then washed the organic layer with brine and concentrated in vacuo to afford crude product. The purification of crude product is carried out with column chromatography. White powder (42 g; 82%); 1H NMR (400 MHz, DMSO-d6) δ 4.82 (d, J = 2.93 Hz, 1H), 4.58 (s, 1H), 3.81–3.89 (m, 1H), 3.69 (d, J = 10.51 Hz, 1H), 3.50 (d, J = 8.93 Hz, 2H), 3.40 (dd, J = 3.06, 9.17 Hz, 1H), 3.33 (s, 1H), 0.03–0.16 (m, 27H); 13C NMR (125 MHz, DMSO-d6) δ 93.5, 73.5, 73.3, 72.4, 71.0, 59.8, 1.1, 0.15. HRMS: C30H70O11Si6Calc. [M+ H]+ 775.3606, Found 775.3602.
General procedure for esterification via carbodiimide mediated coupling
To a stirred mixture of 2,2′,3,3′,4,4′-Hexa-trimethylsilyl-α,α-D-trehalose (1 equiv.; 1 mmol), aryl carboxylic acid (2.2 equiv.; 2.2 mmol) and DMAP (3 equiv.; 3 mmol) in anhydrous DCM (10 mL) was added DCC (3 equiv.; 3 mmol) or EDCI-MeI (5 equiv.; 5 mmol) at 0 °C for 30 min and then at room temperature overnight. The reaction mixture was diluted with water and then extracted it with DCM. The combined organic layer was dried over MgSO4 and reduced in vacuo. The crude mixture was subjected to chromatography using the Biotage system with a 12 g silica column and a zero to 20% ethyl acetate in heptane gradient. This yielded the silyl intermediate in good to excellent yields.
General procedure for sulphonylation via SN2 coupling
To a stirred solution of protected trehalose in anhydrous DCM (10 mL/g) and pyridine (6 equiv. for each OH) were added triflic anhydride (2.5 equiv. for each OH) at - 5 °C dropwise. The reaction mixture was allowed to warm gradually at room temperature stirred for 30 min. After this the mixture was diluted with DCM and washed with cold 1 M HCl, aq. NaHCO3 and then with water. The organic layer was evaporated under reduced pressure and used it for next step without any purification for intermediate 4. 1H NMR (400 MHz, CDCl3) δ 4.94 (d, J = 3.06 Hz, 1H), 4.61 (d, J = 1.96 Hz, 1H), 4.55 (d, J = 4.52 Hz, 1H), 4.07–4.14 (m, 1H), 3.87 (t, J = 9.05 Hz, 1H), 3.37–3.47 (m, 2H), 0.12–0.20 (m, 27H); 13C NMR (125 MHz, CDCl3) δ 123.7, 120.5. 117.3, 114.2, 95.6, 75.4, 73.4, 72.7, 71.6, 70.8, 1.3, 1.1, 0.38; HRMS: C32H68F6O15S2Si6 Calc. [M+NH4]+ 1056.2858, Found 1056.2854
The mixture of potassium salt of an aryl acid (2.2 equiv.) [synthesis (2.5 mmol of acid and 1.5 mL of KOTMS were dissolved in THF and stirred for 10 min and then solvent was removed under reduced pressure and used without purification)] triflate (1 equiv. ) and 18-crown-6 (1 equiv. ) were heated in toluene at 70 °C for 12 hrs. After this reaction mixture was diluted with DCM and washed the organic layer with water. The organic layer was dried over MgSO4 and reduced in vacuo. The crude mixture was subjected to chromatography using the Biotage system with a 12 g silica column and a zero to 20% ethyl acetate in heptane gradient.
Synthesis of intermediates for Diaryl ester analogs
Synthesis of 6,6′-Bis(O-2-trifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5a)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (0.23 g, 84%); 1H NMR (400 MHz, CDCl3) δ 7.56–7.86 (m, 4 H), 4.77 (d, J=3.06 Hz, 1H), 4.37 (d, J=2.45 Hz, 1H), 4.20–4.29 (m, 1H), 3.87–3.97 (m, 1H), 3.69–3.82 (m, 1 H), 3.27–3.38 (m, 2 H), 0.03–0.04 (m, 18H), 0.10–0.04 (m, 9H); 13C NMR (125 MHz, CDCl3) δ 165.1, 132.8, 130.9, 129.6, 129.1, 126.5, 94.5, 85.0, 73.5, 72.7, 72.0, 70.9, 64.2, 1.11, 0.89, 0.23; HRMS: C46H76F6O13Si6 Calc. [M+NH4]+ 1136.4144 Found 1136.4140.
Synthesis of 6,6′-Bis(O-3-trifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5b)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (0.32 g, 87%); 1H NMR (400 MHz, CDCl3) δ 8.22 (d, J = 7.81 Hz, 1H), 8.15 (s, 1H), 8.03 (d, J = 7.91 Hz, 1H), 7.71 (t, J = 7.80 Hz, 1H), 4.92 (d, J = 2.93 Hz, 1H), 4.57 (d, J = 2.32 Hz, 1H), 4.35–4.43 (m, 1H), 4.05–4.13 (m, 1H), 3.84–4.00 (m, 1H), 3.51 (d, J = 9.05 Hz, 1H), 3.41 (dd, J = 3.00, 9.35 Hz, 1H), 0.12–0.17 (m, 18H), 0.04–0.07 (m, 9H); 13C NMR (125 MHz, CDCl3) δ 165.1, 132.8, 139.8, 129.5, 126.6, 94.5, 85.0, 73.5, 72.7, 72.0, 70.9, 64.2, 1.11, 0.89, 0.23; HRMS: C46H76F6O13Si6 Calc.[M+NH4]+ 1136.4144, Found 1136.4142.
Synthesis of 6,6′-Bis(O-4-trifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5c)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (0.28 g, 86%); 1H NMR (400 MHz, CDCl3) δ 8.17 (d, J = 8.07 Hz, 2H), 7.92 (d, J = 8.31 Hz, 2H)4.92 (d, J = 2.93 Hz, 1H), 4.57 (d, J = 2.32 Hz, 1H), 4.35–4.43 (m, 1H), 4.05–4.13 (m, 1H), 3.84–4.00 (m, 1H), 3.51 (d, J = 9.05 Hz, 1H), 3.41 (dd, J = 3.00, 9.35 Hz, 1H), 0.12–0.17 (m, 18H), 0.04–0.07 (m, 9H); 13C NMR (125 MHz, CDCl3) δ 166.0, 141.8, 131.8, 130.4, 127.2, 94.1, 73.1, 72.3, 71.6, 70.5, 64.3, 21.1, 0.74, 0.53, 0.28; HRMS: C46H76F6O13Si6 Calc. [M+NH4]+ 1136.4144, Found 1136.4141.
Synthesis of 6,6′-Bis(O-2-methoxy-5-trifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5d)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (0.29 g, 76%); 1H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 2.08 Hz, 1H), 7.74 (dd, J = 2.14, 8.86 Hz, 1H), 7.18 (d, J = 8.80 Hz, 1H), 4.95 (d, J = 2.93 Hz, 1H), 4.51 (d, J = 2.32 Hz, 1H), 4.26–4.38 (m, 1H), 4.05–4.15 (m, 1H), 3.88–4.01 (m, 1H), 3.73 (s, 3H), 3.53–3.61 (m, 1H), 3.44 (dd, J = 3.06, 9.29 Hz, 1H), 0.08–0.22 (m, 27H); HRMS: C48H80F6O15Si6Calc. [M+NH4]+ 1164.4457, Found 1164.4460.
Synthesis of 6,6′-Bis(O-2-hydroxy-3-trifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5e)
The diaryl ester was prepared according to the general procedure for esterification via triflate SN2 coupling. White Powder (0.84 g, 81%); 1H NMR (400 MHz, CDCl3) δ 11.45 (s, 2H), 8.04 (d, J= 7.0 Hz, 2H), 7.78 (d, J= 7.0 Hz, 2H), 7.00 (t, J= 7.5 Hz, 2H), 4.95 (d, J= 2.0 Hz, 2H), 4.65 (d, J= 11.2 Hz, 2H), 4.32 (dd, J= 11.4, 2.6 Hz, 2H), 4.13 (d, J= 8.8 Hz, 2H), 3.97 (t, J= 8.8 Hz, 2H), 3.60 (t, J= 8.9 Hz, 2H), 3.49 (dd, J= 8.9, 2.6 Hz, 2H), 0.18 (s, 18H), 0.15 (s, 18H), 0.14 (s, 18H) 13C NMR (125 MHz, CDCl3) δ 169.39, 159.86, 133.63, 133.21, 118.40, 113.50, 94.73, 73.53, 72.72, 71.91, 70.68, 64.41, 1.10, 0.94, 0.29; LRMS C46H76F6O15Si6 Calc: 1168.4048 [M+NH4]+ Found: 1168.4054 m/z.
Synthesis of 6,6′-Bis(O-3,5-ditrifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis (O-trimethylsilyl)-α,α-trehalose (5f)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (0.53 g, 82%). 1H NMR (400 MHz, CDCl3) δ 8.47 (s, 4H), 8.07 (s, 2H), 4.96 (br, 2H), 4.69 (d, J= 11.7 Hz, 2H), 4.36 (dd, J= 11.7, 3.3 Hz, 2H), 4.10 (d, J= 9.3 Hz, H2), 3.97 (t, J= 8.8 Hz, 2H), 3.57 (t, J= 9.1 Hz, 2H), 3.47 (d, J= 9.3 Hz, 2H), 0.18 (s, 18H), 0.16 (s, 18H), 0.13 (s, 18H) 13C NMR (125 MHz, CDCl3) δ 163.76, 132.46, 132.20, 132.12, 129.77, 129.74, 126.53, 124.18, 121.46, 94.39, 73.40, 72.75, 72.14, 70.91, 64.73, 31.90, 22.71, 14.13, 1.09, 0.88, 0.17; LRMS C48H74F12O13Si6 Calc: 1272.4 [M+NH4]+ Found: 1272.4.
Synthesis of 6,6′-Bis(O-2-hydroxy-5-trifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5g)
The diaryl ester was prepared according to the general procedure for esterification via triflate SN2 coupling. White Powder (0.53 g, 75%); 1H NMR (400 MHz, CDCl3) δ 10.98 (s, 2H), 8.11 (s, 2H), 7.69 (d, J= 9.2 Hz, 2H), 7.08 (d, J= 8.3, 2H), 4.96 (br, 2H), 4.70 (d, J= 12.0 Hz, 2H), 4.33 (d, J= 11.4 Hz, 2H), 4.11 (d, J= 9.7 Hz, 2H), 3.96 (t, J= 9.7 Hz, 2H), 3.57 (t, J= 8.7 Hz, 2H), 3.47 (d, J= 9.9 Hz, 2H), 0.18 (s, 18H), 0.16 (s, 18H), 0.14 (s, 18H); 13C NMR (125 MHz, CDCl3) δ 169.92, 165.2, 133.45, 133.42, 128.73, 122.90, 119.83, 113.50, 95.63, 73.53, 72.72, 71.91, 70.68, 64.41, 1.55, 1.02, 0.30 LRMS: C46H76F6O15Si6 Calc: 1168.4 [M+NH4]+ Found: 1168.4 m/z.
Synthesis of 6,6′-Bis(O-2,6-ditrifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis (O-trimethylsilyl)-α,α-trehalose (5h)
The diaryl ester was prepared according to the general procedure for esterification via triflate SN2 coupling. White Powder (0.36 g, 87%); 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 7.95 Hz, 2H), 7.69 – 7.78 (m, 1H)4.95 (d, J = 2.93 Hz, 1H), 4.51 (d, J = 2.32 Hz, 1H), 4.27 – 4.37 (m, 1H), 4.06 – 4.14 (m, 1H), 3.89 – 4.00 (m, 1H), 3.37 – 3.61 (m, 2H), 0.04 – 0.20 (m, 27H); HRMS: C48H74F12O13NH4+ Calc. [M+NH4]+ 1272.3892 Found 1272.3895.
Synthesis of 6,6′-Bis(O-2,5-ditrifluoromethylbenzoyl)-2,3,4,2′,3′,4′-hexakis (O-trimethylsilyl)-α,α-trehalose (5i)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (0.41 g, 91%); 1H NMR (400 MHz, CDCl3) δ 9.24 (t, J=2.08 Hz, 1 H), 9.15 (d, J=2.08 Hz, 2 H) 5.00 (d, J=2.93 Hz, 1 H), 4.73 (d, J=2.32 Hz, 1 H), 4.42 (br. s., 1 H), 4.09–4.19 (m, 1 H), 4.03–3.94 (m, 1 H), 3.50–3.63 (m, 2 H), 0.02 – 0.31 (m, 27 H); 13C NMR (125 MHz, CDCl3) δ 162.3, 148.7, 133.6, 129.5, 122.5, 94.2, 73.5, 72.0, 70.8, 65.3, 1.06, 0.21; HRMS: C48H74F12O13Si6 Calc.[M+NH4]+ 1272.3892, Found 1272.3898.
Synthesis of 6,6′-Bis(O-2,nitrobenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5k)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. Yellow Powder (0.30 g, 77%); 1H NMR (400 MHz, CDCl3) δ 7.73 (dd, J=7.76, 1.28 Hz, 1 H), 7.66 (dd, J=7.52, 1.53 Hz, 1 H), 7.43 – 7.59 (m, 2 H), 4.77 (d, J=3.06 Hz, 1 H), 4.37 (d, J=2.45 Hz, 1 H), 4.20–4.29 (m, 1 H), 3.88–3.97 (m, 1 H), 3.72–3.82 (m, 1 H), 3.26–3.37 (m, 2 H), 0.30–0.03 (m, 18 H) - 0.11–0.05 (m, 9 H); 13C NMR (125 MHz, CDCl3) δ 165.0, 148.5, 132.6, 131.9, 130.2, 127.1, 123.8, 94.4, 73.3, 72.4, 72.0, 70.8, 65.3, 1.04, 1.04, 0.88, 0.01; HRMS: C44H76N2O17Si6 Calc. [M+Na]+ 1095.3652 Found 1095.3650.
Synthesis of 6,6′-Bis(O-3,5-dinitrobenzoyl)-2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5l)
The diaryl ester was prepared according to the general procedure for esterification via triflate SN2 coupling. Yellow Powder (0.30 g, 75%). 1H NMR (400 MHz, CDCl3) δ 9.24 (t, J=2.08 Hz, 1 H), 9.15 (d, J=2.08 Hz, 2 H), 5.00 (d, J=2.93 Hz, 1 H), 4.73 (d, J=2.32 Hz, 1 H), 4.42 (dd, J=16.02, 5.62 Hz, 1 H), 4.08–4.16 (m, 1 H), 3.94–4.05 (m, 1 H), 3.48–3.64 (m, 2 H), 0.11–0.25 (m, 27 H); 13C NMR (125 MHz, CDCl3) δ 162.4, 148.7, 133.4, 129.4, 122.5, 94.2, 73.4, 72.7, 72.0, 70.8, 65.3, 1.06, 0.93, 0.21; HRMS: C44H76N2O17Si6 Calc. [M+Na]+ 1185.3353, Found 1185.3351.
Synthesis of 6,6′-Bis(O-3-trifluoromethylpyridne-2-carbonyl) −2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5m)
The diaryl ester was prepared according to the general procedure for esterification via triflate SN2 coupling. White Powder (0.26 g, 68 %); 1H NMR (400 MHz, CDCl3) δ 8.83 (d, J=4.77 Hz, 1H), 8.07 (d, J=8.07 Hz, 1H), 7.56 (m, J=4.80 Hz, 1H), 4.94 (d, J=2.81 Hz, 1H), 4.56 (d, J=2.08 Hz, 1H), 4.46 (d, J=5.14 Hz, 1H), 4.08–4.15 (m, 1H), 3.82–3.98 (m, 1H), 3.35–3.57 (m, 2H), 0.10–0.19 (m, 27H); 13C NMR (125 MHz, CDCl3) δ 165.3, 152.5, 149.3, 135.1, 125.1, 124.3, 121.6, 94.5, 73.7, 72.9, 72.3, 70.9, 65.9, 1.36, 0.2; HRMS: C44H74F6N2O13Si6 Calc. [M+NH4]+ 1143.3603, Found 1143.3600.
Synthesis of 6,6′-Bis(O-2,4-ditrifluoromethylbenzoyl) −2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5n)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (345 mg, 83 %); 1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H), 7.91 (d, J=0.98 Hz, 2H), 4.92 (d, J=2.93 Hz, 1H), 4.57 (d, J=2.32 Hz, 1H), 4.39 (s, 1H), 4.05–4.13 (m, 1 H), 3.88–3.98 (m, 1H), 3.51 (d, J=9.05 Hz, 1H), 3.41 (dd, J=9.35, 3.00 Hz, 1H), 0.11–0.17 (m, 18 H) 0.03–0.08 (m, 9H); 13C NMR (125 MHz, CDCl3) δ 165.3, 134.5, 133.6, 130.8, 129.9, 129.6, 128.7, 124.2, 124.1, 123.9, 123.8, 94.3, 73.2, 72.5, 71.9, 70.7, 65.3, 1.03. 0.02; HRMS: C48H74F12O13Si6 Calc. [M+NH4]+ 1272.3892, Found 1272.3890.
Synthesis of 6,6′-Bis(O-2-trifluoromethyl-4-methylbenzoyl)- 2,3,4,2′,3′,4′-hexakis(O-trimethylsilyl)-α,α-trehalose (5o)
The diaryl ester was prepared according to the general procedure for esterification via carbodiimide mediated coupling. White Powder (0.46 g, 91%); 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J=7.95 Hz, 1 H), 7.57 (s, 1H), 7.42 (d, J=7.70 Hz, 1H), 4.95 (d, J=2.93 Hz, 1 H), 4.51 (d, J=2.32 Hz, 1H), 4.34 (dd, J=16.63, 4.03 Hz, 1H), 4.06 – 4.13 (m, 1H),, 3.90 – 3.98 (m, 1 H), 3.56 (d, J=9.05 Hz, 1H), 3.44 (dd, J=9.29, 3.06 Hz, 1H), 2.47 (s, 3H), 0.06–0.18 (m, 27H); 13C NMR (125 MHz, CDCl3) δ 166.3, 142.1, 132.1, 130.7, 127.5, 94.4, 73.4, 72.6, 71.9, 70.7, 64.6, 21.4, 1.06, 0.84, 0.04; HRMS: C48H80F6O13Si6 Calc. [M+NH4]+ 1164.4457, Found 1164.4455.
General procedure for Deprotection of silyl Ether
The silyl intermediate (0.333 mmol) was dissolved in equal amount of methylene chloride and methanol (8 mL) treated with Dowex 50WX8 resin (668.4 mg) with magnetic stirring. Upon consumption of the starting material as determined by TLC (20% methanol in methylene chloride and charring with vanillin stain) the reaction was filtered, concentrated and chromatographed on a silica column eluting with a 40% to 80% methylene chloride to methanol gradient on silica gel to provide the desired product.
Synthesis of 6,6′-Bis(O-2-trifluoromethylbenzoyl)-α,α-trehalose (UM1042)
White Powder (55 mg, 88%); 1H NMR (400 MHz, DMSO-d6) δ 7.74–7.90 (m, 4H), 5.20 (d, J = 5.38 Hz, 1H), 4.95 (d, J = 4.89 Hz, 1H), 4.90 (d, J = 5.87 Hz, 1H), 4.86 (d, J = 3.42 Hz, 1H), 4.49 (d, J = 10.27 Hz, 1H), 4.32 (dd, J = 5.32, 11.68 Hz, 1H), 4.07 (dd, J = 3.85, 9.72 Hz, 1H), 3.59 (dt, J = 4.95, 9.02 Hz, 1H), 3.26 – 3.32 (m, 1H), 3.22 (dt, J = 5.50, 9.29 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ 165.8, 132.8, 131.9, 130.1, 127.0, 126.7, 124.6, 121.9, 93.8, 72.7, 71.4, 70.0, 69.7, 64.9; HRMS: C28H28F6O13Calc. [M+NH4]+ 704.1772, Found 704.1770
Synthesis of 6,6′-Bis(O-3-trifluoromethylbenzoyl)-α,α-trehalose (UM1043)
White Powder (52 mg, 85%); 1H NMR (400 MHz, DMSO-d6) δ 8.24 (d, J = 7.83 Hz, 1H), 8.17 (s, 1H), 8.04 (d, J = 7.95 Hz, 1H), 7.79 (t, J = 7.83 Hz, 1H), 5.25 (d, J = 5.38 Hz, 1H), 5.01 (d, J = 4.77 Hz, 1H), 4.90 – 4.96 (m, 2H), 4.46–4.54 (m, 1H), 4.35 (dd, J = 5.93, 11.68 Hz, 1H), 4.12 (ddd, J = 1.71, 5.96, 9.93 Hz, 1H), 3.61 (td, J = 4.60, 9.02 Hz, 1H), 3.25 (dt, J = 5.38, 9.48 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ 164.3, 133.1, 130.8, 130.3, 129.8, 129.7, 129.4, 125.4, 125.0, 122.3, 93.7, 72.8, 71.5, 70.1, 69.7, 64.6; HRMS: C28H28F6O13Calc. [M+NH4]+ 704.1772, Found 704.1775
Synthesis of 6,6′-Bis(O-4-trifluoromethylbenzoyl)-α,α-trehalose (UM1044)
White Powder (51 mg, 84%); 1H NMR (400 MHz, DMSO-d6) δ 8.16 (d, J = 8.07 Hz, 2H), 7.92 (d, J = 8.31 Hz, 2H), 5.25 (d, J = 5.38 Hz, 1H), 5.00 – 5.04 (m, 2H), 4.89 (d, J = 3.67 Hz, 1H), 4.46–4.52 (m, 1H), 4.38 (dd, J = 5.81, 11.68 Hz, 1H), 4.16 (ddd, J = 1.53, 5.78, 9.93 Hz, 1H), 3.57–3.65 (m, 1H), 3.35–3.40 (m, 1H), 3.22–3.31 (m, 1H); 13C NMR (125 MHz, DMSO-d6) δ 164.6, 133.5, 132.7, 130.0, 125.8, 125.1, 122.4, 94.2, 72.8, 71.4, 70.1, 69.8, 64.6; HRMS: C28H28F6O13 Calc.[M+NH4]+ 704.1772, Found 704.1773
Synthesis of 6,6′-Bis(O-2-methoxy-5-trifluoromethylbenzoyl)-α,α-trehalose (UM1045)
White Powder (61 mg, 86%); 1H NMR (400 MHz, DMSO-d6) δ 7.95 (s, 1H), 7.91 (dd, J = 2.14, 8.86 Hz, 1H), 7.35 (d, J = 8.80 Hz, 1H), 5.19 (d, J = 5.38 Hz, 1H), 4.97 (d, J = 4.89 Hz, 1H), 4.91 (d, J = 3.55 Hz, 1H), 4.83 (d, J = 5.99 Hz, 1H), 4.46 (d, J = 10.15 Hz, 1H), 4.26 (dd, J = 5.62, 11.74 Hz, 1H), 4.03 (td, J = 4.00, 5.81 Hz, 1H), 3.90 (s, 3H), 3.61 (dt, J = 4.89, 9.11 Hz, 1H), 3.26–3.32 (m, 1H), 3.20 – 3.26 (m, 1H); 13C NMR (125 MHz, DMSO-d6) δ 164.2, 161.2, 130.8, 128.0, 125.5, 122.8, 121.0, 120.6, 120.5, 113.7, 93.5, 72.9, 71.7, 70.4, 70.0, 64.4, 56.5; HRMS: C30H32F6O15 Calc. [M+Na]+ 769.1538, Found 769.1534
Synthesis of 6,6′-Bis(O-2-hydroxy-3-trifluoromethylbenzoyl)-α,α-trehalose (UM1053)
White Powder (0.37 g, 75%); 1H NMR (400 MHz, DMSO-d6) δ 11.31 (br, 2H), 8.10 (d, J=7.8 Hz, 2H), 7.91 (d, J= 7.7 Hz, 2H), 7.15 (t, J= 7.9 Hz, 2H), 5.27 (d, J= 4.8Hz, 2H), 5.02 (d, J= 5.9 Hz, 2H), 4.99 (d, J= 4.9 Hz, 2H), 4.88(d, J= 3.3 Hz, 2H), 4.52 (d, J= 10.5 Hz, 2H), 4.45 (dd, J= 11.5, 5.4 Hz, 2H), 4.17 (m, 2H), 3.60 (dt, J= 9.0, 4.9 Hz, 2H), 3.27 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ 168.39, 158.28, 134.49, 132.92, 121.95, 119.33, 114.08, 94.29, 72.72, 71.39, 70.07, 69.57, 65.11; HRMS: C28H28F6O15 Calc. [M+NH4]+ 736.1676, Found 736.1662.
Synthesis of 6,6′-Bis(O-3,5-ditrifluoromethylbenzoyl)-α,α-trehalose (UM1054)
White Powder (65.1 mg, 20%); 1H NMR (400 MHz, DMSO-d6) δ 8.45 (s, 2H), 8.43 (s, 4H), 5.28 (d, J= 5.2 Hz, 2H), 5.02 (d, J= 4.7 Hz, 2H), 4.94 (d, J= 3.4 Hz, 2H), 4.90 (d, J=5.7 Hz, 2H), 4.60 (d, J= 10.4 Hz, 2H), 4.36 (dd, J= 11.6, 6.4 Hz, 2H), 4.13 (br, 1H), 3.62 (dt, J= 8.9, 4.5 Hz, 2H), 3.24 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ 163.05, 132.33, 131.16, 130.83, 129.42, 126.89, 124.17, 121.46, 93.57, 72.77, 71.56, 70.17, 69.74, 65.10; HRMS: C30H26F12O13 NH4+ Calc. 840.1526, Found 840.1530.
Synthesis of 6,6′-Bis(O-2-hydroxy-5-trifluoromethylbenzoyl)-α,α-trehalose (UM1055)
White Powder (0.18 g, 57%); 1H NMR (400 MHz, DMSO-d6) 10.93 (br, 2H), 8.02 (s, 2H), 7.83 (d, J= 8.6 Hz, 2H), 7.17 (d, J= 8.6 Hz, 2H), 5.25 (br, 2H), 4.99 (d, J= 3.5 Hz, 2H), 4.95 (br, 2H), 4.87 (br, 2H), 4.52 (d, J= 11.3 Hz, 2H), 4.35 (dd, J= 11.1, 5.5 Hz, 2H), 4.10 (br, 2H), 3.61 (br, 2H), 3.24 (t, J= 9.2 Hz, 2H); 13C NMR (125 MHz, DMSO-d6) δ 166.65, 162.37, 131.75, 127.48, 125.26, 122.56, 118.73, 114.20, 93.49, 72.73, 71.54, 70.15, 69.56, 64.68; HRMS: C28H28F6O15 Calc. [M+NH4]+ 736.1676, Found 736.1670.
Synthesis of 6,6′-Bis(O-2,6-di-trifluoromethylbenzoyl)-α,α-trehalose (UM1065)
White Powder (60 mg, 76%); 1H NMR (400 MHz, DMSO-d6) δ 8.21 (d, J = 7.95 Hz, 2H), 7.97 (t, J = 8.44 Hz, 1H), 5.09 (br. s., 1H), 4.81 (d, J = 3.55 Hz, 1H), 4.70 (br. s., 1H), 4.45–4.52 (m, 1H), 4.32–4.41 (m, 1H), 4.00 (ddd, J = 1.71, 5.23, 9.93 Hz, 1H), 3.57 (t, J = 9.11 Hz, 1H), 3.34 (br. s., 1H), 3.09–3.23 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ 164.9, 132.0, 131.1, 129.7, 127.9, 127.6, 127.3, 126.9, 124.3, 121.6, 93.6, 72.8, 71.4, 70.1, 69.4, 66.3; HRMS: C30H26F12O13 Calc. [M+NH4]+ 840.1519 Found 840.1517.
Synthesis of 6,6′-Bis(O-2,5-di-trifluoromethylbenzoyl)-α,α-trehalose (UM1066)
White Powder, (71 mg, 81%); 1H NMR (400 MHz, DMSO-d6) δ 8.10–8.24 (m, 3H), 5.22 (d, J = 5.38 Hz, 1H), 4.95 (d, J = 4.89 Hz, 1H), 4.89 (d, J = 3.55 Hz, 1H), 4.84 (d, J = 5.87 Hz, 1H), 4.57–4.65 (m, 1H), 4.33 (dd, J = 5.69, 11.68 Hz, 1H), 4.04–4.13 (m, 1H), 3.60 (dt, J = 4.89, 9.11 Hz, 1H), 3.27 (ddd, J = 3.79, 5.81, 9.48 Hz, 1H), 3.19 (dt, J = 5.44, 9.32 Hz, 1H); 13CNMR (125 MHz, DMSO-d6) δ 164.8, 132.6, 131.9, 130.4, 128.9, 128.4, 126.9, 123.9, 93.8, 72.6, 71.4, 70.0, 69.6, 65.4; HRMS: C30H26F12O13 Calc. [M+NH4]+ 840.152, Found 840.1521.
Synthesis of 6,6′-Bis(O-2-trifluoromethyl-4-methoxybenzoyl)-α,α-trehalose (UM1067)
White Powder (61 mg, 82%); 1H NMR (400 MHz, DMSO-d6) δ 7.85–7.98 (m, 2H), 7.35 (d, J = 8.61 Hz, 1H), 5.18 (d, J = 5.48 Hz, 1H), 4.93 (dd, J = 4.11, 14.28 Hz, 2H), 4.81 (d, J = 6.26 Hz, 1H), 4.42–4.49 (m, 1H), 4.19 – 4.31 (m, 1H), 3.98–4.08 (m, 1H), 3.90 (s, 3H), 3.55–3.66 (m, 1H), 3.21 – 3.26 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ 170.0, 133.2, 132.3, 127.6, 125.6, 93.5, 72.6, 71.3, 69.6, 63.8; HRMS: C30H32F6O15 Calc. [M+Na]+ 769.1538, Found 769.1537.
Synthesis of 6,6′-Bis(O-2-nitrobenzoyl)-α,α-trehalose (UM1079)
Brown Powder (43 mg, 76%); 1H NMR (400 MHz, DMSO-d6) δ 8.00–8.05 (m, 1H), 7.78–7.91 (m, 3H), 5.18 (br. s, 1H), 4.90 (br. s., 1H), 4.84 (d, J = 3.55 Hz, 1H), 4.44–4.50 (m, 1H), 4.32 (dd, J = 5.32, 11.68 Hz, 1H), 4.00–4.09 (m, 1H), 3.52–3.60 (m, 1H), 3.28 (d, J = 3.55 Hz, 1H), 3.10–3.22 (m, 1H); 13C NMR (125 MHz, DMSO-d6) δ 164.5, 148.4, 133.6, 133.3, 130.3, 125.9, 124.2, 94.2, 72.9, 71.5, 70.1, 69.9, 65.4; HRMS: C26H28N2O17 Calc. [M+NH4]+ 658.1726, Found 658.1720.
Synthesis of 6,6′-Bis(O-3,5-dinitrobenzoyl)-α,α-trehalose (UM 1080)
Brown Powder (49 mg, 73%); 1H NMR (400 MHz, DMSO-d6) δ 9.03 (t, J = 2.14 Hz, 1H), 8.92 (d, J = 2.08 Hz, 2H), 4.99 (d, J = 3.55 Hz, 2H), 4.69 (d, J = 9.90 Hz, 1H), 4.35 (dd, J = 6.66, 11.55 Hz, 1H), 4.18–4.27 (m, 1H), 3.56–3.64 (m, 1H), 3.39 (dd, J = 3.61, 9.60 Hz, 2H), 3.21 – 3.28 (m, 2H); 13C NMR (125MHz, DMSO-d6) δ 162.4, 148.4, 132.6, 128.9, 122.4, 94.5, 72.8, 71.4, 70.2, 69.8, 65.9; HRMS: C26H26N4O21 Calc. [M+NH4]+ 748.1428, Found 748.1430.
Synthesis of 6,6′-Bis(O-3-trifluoromethylpyridne-2-carbonyl)-α,α-trehalose (UM1083)
Yellow Powder (21 mg, 43%); 1H NMR (400 MHz, DMSO-d6) δ 8.91 (d, J = 4.65 Hz, 1H), 8.38 (d, J = 8.07 Hz, 1H), 7.82 (dd, J = 4.89, 8.07 Hz, 1H), 5.14 (br. s., 1H), 4.86 (d, J = 3.55 Hz, 1H), 4.80 (br. s., 1H), 4.48 – 4.54 (m, 1H), 4.36–4.45 (m, 1H), 4.00–4.08 (m, 1H), 3.57 (t, J = 9.17 Hz, 1H), 3.34 (br. s., 1H), 3.15–3.27 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ 165.1, 153.1, 148.4, 135.9, 126.1, 123.2, 122.8, 121.6, 93.9, 72.9, 71.5, 69.6, 65.8: HRMS: C26H26N2O13F6 Calc. [M+H]+ 689.1412, Found 689.1416.
Synthesis of 6,6′-Bis(O-2,4-di-trifluoromethylbenzoyl)-α,α-trehalose (UM1084)
White Powder (68 mg, 83%); 1H NMR (400 MHz, DMSO-d6) δ 8.24 (d, J = 8.19 Hz, 1H), 8.19 (s, 1H), 8.09 (d, J = 8.07 Hz, 1H), 5.21 (d, J = 5.38 Hz, 1H), 4.90–4.99 (m, 2H), 4.85 (d, J = 3.55 Hz, 1H), 4.52–4.58 (m, 1H), 4.36 (dd, J = 5.56, 11.68 Hz, 1H), 4.09 (ddd, J = 1.77, 5.50, 9.96 Hz, 1H), 3.54–3.62 (m, 1H), 3.25–3.30 (m, 1H), 3.19 (dt, J = 5.26, 9.35 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ 164.8, 134.5, 131.9, 131.6, 131.4, 130.2, 128.0, 127.7, 124.3, 123.8, 123.7, 121.5, 121.1, 94.1, 72.6, 71.3, 69.7, 65.5; HRMS: C30H26F12O13 NH4+ Calc. 840.152, Found 840.1518.
Synthesis of 6,6′-Bis(O-2-trifluoromethyl-4-methylbenzoyl)-α,α-trehalose (UM1085)
White Powder (54 mg, 88%); 1H NMR (400 MHz, DMSO-d6) δ 7.78 (d, J = 7.95 Hz, 1H), 7.69 (s, 1H), 7.60 (d, J = 7.95 Hz, 1H), 5.19 (d, J = 5.38 Hz, 1H), 4.95 (d, J = 4.89 Hz, 1H), 4.89 (d, J = 5.87 Hz, 1H), 4.86 (d, J = 3.67 Hz, 1H), 4.47 (dd, J = 1.65, 11.55 Hz, 1H), 4.30 (dd, J = 5.50, 11.74 Hz, 1H), 4.06 (ddd, J = 1.77, 5.35, 10.00 Hz, 1H), 3.59 (dt, J = 4.95, 9.14 Hz, 1H), 3.29 (ddd, J = 3.73, 5.90, 9.57 Hz, 1H), 3.21 (dt, J = 5.44, 9.38 Hz, 1H), 2.43 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 166.1, 142.9, 133.4, 130.8, 128.1, 127.6, 127.6, 127.5, 127.2, 125.1, 122.4, 94.2, 73.1, 71.8, 70.4, 70.1, 65.2, 21.2; HRMS: C30H32F6O13 Calc. [M+NH4]+ 732.2087, Found 732.2090.
Synthesis of 6,6′-Bis(O-2,6-di-methylbenzoyl)-α,α-trehalose (UM1114)
White Powder (38 mg, 56%); 1H NMR (400 MHz, DMSO-d6) δ 7.24 (t, J = 7.95 Hz, 1H), 7.09 (d, J = 7.46 Hz, 2H), 5.16 (d, J = 5.38 Hz, 1H), 4.89 (d, J = 5.14 Hz, 1H), 4.85 (d, J = 3.55 Hz, 1H), 4.83 (d, J = 5.99 Hz, 1H), 4.52 (dd, J = 1.77, 11.68 Hz, 1H), 4.33 (dd, J = 5.07, 11.80 Hz, 1H), 3.97–4.07 (m, 1H), 3.57 (dd, J = 5.07, 9.11 Hz, 1H), 3.13–3.28 (m, 2H), 2.24 (s, 6H); 13C NMR (125 MHz, DMSO-d6) δ 169.4, 134.8, 134.3, 129.7, 127.9, 94.2, 73.0, 71.8, 70.5, 70.2, 64.2, 19.6; HRMS: (C30H38O13 + NH4)+ Calc. 624.2651, Found 624.2650.
Synthesis of 6,6′-Bis(O-2-(trifluoromethyl)phenylacetyl)-α,α-trehalose (UM1136)
White Powder (45 mg, 62%); 1H NMR (400 MHz, DMSO-d6) δ 7.71 (d, J = 8.22 Hz, 1H), 7.60–7.67 (m, 1H), 7.45 – 7.56 (m, 2H), 5.03 (d, J = 5.48 Hz, 1H), 4.86 (d, J = 4.70 Hz, 1H), 4.77 (d, J = 3.52 Hz, 1H), 4.72 (d, J = 6.26 Hz, 1H), 4.30 (dd, J = 1.76, 11.54 Hz, 1H), 4.07 (dd, J = 5.48, 11.74 Hz, 1H), 3.84–3.94 (m, 3H), 3.53 (dd, J = 4.89, 9.19 Hz, 1H), 3.25 (ddd, J = 3.91, 6.06, 9.59 Hz, 1H), 3.12 (dd, J = 5.48, 9.39 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ 170.2, 133.4, 132.5, 127.8, 125.8, 93.7, 72.9, 71.5, 70.2, 69.9, 64.1, 37.7; HRMS: C30H32F6O15 Calc. [M+Na]+ 737.1639, Found 737.1645.
Biological Activity
Transgenic HEK cell SEAP assays
Human Mincle expressing HEK cells were obtained from Invivogen (San Deigo, CA). Cells were cultured according to the manufacturer’s instructions in DMEM with 10% FBS, 50 U/ml penicillin, 50 mg/ml streptomycin, 100 mg/ml Normocin, 2 mM L-glutamine, 30 μg/ml blasticidin, 1 μg/ml puromycin and 1x HEK-Blue™ CLR Selection. For assay, indicated compounds were serially diluted in diluent (EtOH), 20 μl of a 10x final concentration were applied to the bottom of a 96-well tissue culture plate and the solvent was evaporated for > 1 hr in a biosafety hood. HEK cells were applied to the plates at a density of 3×105 cells/well and incubated for 18–24 hour at 37 ˚C. Cell supernatants were harvested and analyzed via the manufacturer’s instructions using Hek-Blue™ Detection. SEAP activity was assessed by reading the optical density (OD) at 620–655 nm with a microplate reader; data are expressed as the fold change in OD over vehicle treated cells.
Isolation of PBMC
Peripheral blood samples were collected from healthy adult donors after approval by the University of Montana Institutional Review Board (43–16) and signed written informed consent was obtained from each donor. Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood using Ficoll-Paque. Briefly, heparin-anticoagulated blood was diluted with an equal volume of Dulbeccos phosphate-buffered saline, pH 7.4 (DPBS), 35 mL of diluted blood was layered over 15 ml of the Ficoll-Paque (Sigma). Gradients were centrifuged at 400 × g for 30 min at room temperature in a swinging-bucket rotor without the brake applied. The PBMC interface was carefully removed by pipetting and washed with PBS-5%FBS by centrifugation at 250 × g for 10 min. This was followed by a second wash with PBS-5%FBS. Cells were resuspended in RPMI media with 5% autologous plasma. Cell number and viability were determined using a hemocytometer. Non-viable cells were identified by staining with trypan blue and cell counts calculated on viable cells only.
Cytokine analysis
Compounds were serially diluted in 100% Ethanol to the bottom of a tissue culture plate and solvent was allowed to fully evaporate (i.e. plate coating). Freshly prepared PBMCs purified via Ficoll gradient in RPMI/5% autologous plasma were added to the indicated compound concentrations by addition to plates containing plate coated compounds. Cells were incubated at 37 °C/5% CO2.
Supernatants were harvested from treated cells 18–24 hour post cell application. Supernatants were analyzed using either a DuoSet IL6 ELISA (R&D Systems, Minneapolis, MN) ELISAs were read on a plate reader at 450 nm and raw OD values potted using Prism software.
Supplementary Material
ACKNOWLEDGMENT
Funding Sources
This work was supported by a NIAID Adjuvant Discovery Program Contract (HHSN272201400050C). The authors acknowledge University of Montana Core Services of the Center for Biomolecular Structure and Dynamics (CBSD) and the Department of Chemistry and Biochemistry supported by the National Institutes of General Medical Science (NIH CoBRE grant P20GM103546) for NMR and MS instrumentation.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting Information
The Supporting Information is available free of charge
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1 a).Patil K; Bagade S; Bonde S; Sharma S; Saraogi G; Biomed. & Pharm, 2018, 99, 735–745; [DOI] [PubMed] [Google Scholar]; b) Swarts MB; Holsclaw CM; Jewett JC; Alber M; Fox DM; Siegrist M,S; Leary JA; Kalscheuer R; Bertozzi CR, J. Am. Chem. Soc, 2012, 134, 16123–16126; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Backus K,M; Boshoff H,I; Barry CS; Boutureira O; Patel MK; D’Hooge F; Lee SS; Via LE, Tahlan K, Barry CE III; Davis BG; Nat. Chem. Bio, 2011, 7, 228–235; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Winslow GM; Cooper A; Reiley W; Chatterjee M; Woodland DL; Immunol. Rev, 2008, 225, 284–299; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Daniel TM, Respir. Med, 2006, 100(11), 1862–1870. [DOI] [PubMed] [Google Scholar]
- 2.WHO, Global tuberculosis report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 3.Schrager LK; Harris RC; Vekemans J; F1OOORes, 2018, 7, 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 a).Copland A; Diogo GR; Hart P; Harris S; Tran AC; Paul MJ; Singh M; Cutting SM; Reljic R; Front. Immunol, 2018, 9, 346; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Moliva JI; Turner J; Torrelles JB; 2017, Front. Immunol, 8, 407–424; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Andersen P; Doherty TM, Nat. Rev. Microbiol, 2005, 3 (8), 656–62; [DOI] [PubMed] [Google Scholar]; d) Colditz GA; Brewer TF; Berkey CS; Wilson ME; Burdick E; Fineberg HV; Mosteller F, JAMA, 1994, 271 (9), 698–702. [PubMed] [Google Scholar]
- 5.Foged C, Therapeutic Delivery, 2011, 2(8), 1057–1077. [DOI] [PubMed] [Google Scholar]
- 6 a).Nemes E; Geldenhuys H; Rozot V; Rutkowski KT; Ratangee F; Bilek N; Mabwe S; Makhethe L; Erasmus M; Toefy A; Mulenga H; Hanekom WA; Self SG; Bekker L-G; Ryall R; Gurunathan S; DiazGranados CA; Andersen P; Kromann I; Evans T; Ellis RD; Landry B; Hokey DA; Hopkins R; Ginsberg AM; Scriba TJ; Hatherill M, Engl N. J. Med, 2018, 379 (2), 138–149; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dis EV; Sogi KM; Rae CS; Sivick KE; Surh NH; Leong ML; Kanne DB; Metchette K; Leong JJ; Bruml JR; Chen V; Heydari K; Cadieux N; Evans T; McWhirter SM; Dubensky TW Jr.; Portnoy DA; Stanley SA; Cell Rep, 2018, 23(5), 1435–1447; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Meeren OVD; Hatherill M; Nduba V; Wilkinson M; Muyoyeta M; Brakel EV; Ayles HM Henostroza G; Thienemann F; Scriba TJ; Diacon A; Blatner GL; Demoitie M-A; Tameris M; Malahleha M; Innes JC; Hellstrom E; Martinso N; Singh T; Akite EJ; Khatoon azam A; Bollaerts A; Ginsberg AM; Evans TG; Gillard P; Tait DR, N. Engl. J. Med, 2018, 379, 1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 a).Ahmed M; Jiao H; Domingo-Gonzalez R; Das S; Griffiths KL; Rangel-Moreno J; Nagarajan UM; Khader SA; J. Leukoc. Biol, 2017, 101(6), 1373–1381; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Khader SA; Cooper AM, Cytokine 2008, 41 (2), 79–83; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Khader SA; Bell GK; Pearl JE; Fountain JJ; Rangel-Moreno J; Cilley GE; Shen F; Eaton SM; Gaffen SL; Swain SL; Locksley RM; Haynes L; Randall TD; Cooper AM, Nat. Immunol, 2007, 8 (4), 369–377. [DOI] [PubMed] [Google Scholar]
- 8.Leibund Gut-Landmann S; Gross O; Robinson MJ; Osorio F; Slack EC; Tsoni SV; Schweighoffer E; Tybulewicz V; Brown GD; Ruland J; Reis e Sousa C, Nat. Immunol, 2007, 8(6), 30–638 [DOI] [PubMed] [Google Scholar]
- 9 a).Schoenen H; Bodendorfer B; Hitchens K; Manzanero S; Werninghaus K; Nimmerjahn F; Agger EM; Stenger S; Andersen P; Ruland J; Brown GD; Wells C; Lang R, J. Immunol, 2010, 184 (6), 2756–2760; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ishikawa E; Ishikawa T; Morita YS; Toyonaga K; Yamada H; Takeuchi O; Kinoshita T; Akira S; Yoshikai Y; Yamasaki S, J. Exp. Med, 2009, 206 (13), 2879–2888; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fomsgaard A; Karlsson I; Gram G; Schou C; Tang S; Bang P; Kromann I; Andersen P; Andreasen LV, Vaccine, 2011, 29 (40), 7067–7074; [DOI] [PubMed] [Google Scholar]
- 11 a).Werninghaus K, Babiak A, Gross O, Holscher C, Dietrich H, Agger EM; J. Exp. Med, 2009, 206, 89–97; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hunter RL, Olsen MR; Jagannath C; Actor JK; Ann. Clin. Lab. Sci, 2006; 36, 371–386. [PubMed] [Google Scholar]
- 12.Desel C; Werninghaus K; Ritter M; Jozefowski K; Wenzel J; Russkamp N; Schleicher U; Christensen D; Wirtz S; Kirschning C; Agger E,M; Prazeres da Costa C; Lang R; PLoS One, 2013, 8 (1), e53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen D, Schijns, Virgil EJC In Immunopotentiators in Modern Vaccines (Second Edition), O’ Hagan DT, Ed. Academic Press: New York; 2017; pp 333–345. [Google Scholar]
- 14 a).Fomsgaard A; Karlsson I; Gram G; Schou C; Tang S; Bang P; Kromann I; Andersen P; Andreasen LV, Vaccine 2011, 29 (40), 7067–7074. [DOI] [PubMed] [Google Scholar]; b) Agger EM; Rosenkrands I; Hansen J; Brahimi K; Vandahl BS; Aagaard C; Werninghaus K; Kirschning C; Lang R; Christensen D; Theisen M; Follmann F; Andersen P, PLoS One, 2008, 3 (9), e3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto H; Oda M; Nakano M; Watanabe N; Yabiku K; Shibutani M; Inoue M; Imagawa H; Nagahama M; Himeno S; Setsu K; Sakurai J; Nishizawa M, J. Med. Chem, 2013, 56 (1), 381–385. [DOI] [PubMed] [Google Scholar]
- 16.Davidsen J; Rosenkrands I; Christensen D; Vangala A; Kirby D; Perrie Y; Agger EM; Andersen P, Biochimica et biophysica acta, 2005, 1718 (1–2), 22–31. [DOI] [PubMed] [Google Scholar]
- 17 a).Braganza CD; Teunissen T; Timmer MSM; Stocker BL; Front Immunol, 2017, 8, 1940; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Feinberg H; Rambaruth NDS; Jégouzo SAF; Jacobsen KM; Djurhuus R; Poulsen TB; Weis WI; Taylor ME; Drickamer K, J. Biol. Chem, 2016, 291 (40), 21222–21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18 a).Foster AJ; Nagata M; Lu X; Lynch AT; Omahdi Z; Ishikawa E; Yamasaki S; Timmer SM; Stocker BL; J. Med. Chem, 2018, 61, 3, 1045–1060; [DOI] [PubMed] [Google Scholar]; b) Bird JH; Khan AA; Nishimura N; Yamasaki S; Timmer MSM; Stocker BL; Liu Y-L; Ge L-P, Han X,-Z; Liu Z-P; Chem. Bio & Drug Des, 2015, 86, 5, 1017–1029; [DOI] [PubMed] [Google Scholar]; c) Jiang Y-L; Tang L-Q, Miyanaga S; Igarashi Y; Saiki I; Liu ZP; Bioorg. Med. Chem. Lett, 2011, 21, 1089–1091; [DOI] [PubMed] [Google Scholar]; d) Jacobsen KM; Keiding UB; Clement LL; Schaffert ES; Rambaruth DS; Johannsen M; Drickamer K; Poulsen TB; Med. Chem. Comm, 2015, 6, 647–653; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Foster AJ; Kodar K; Timmer MSM; Stocker BL; Org. Biomol. Chem, 2020, 18, 1095–1103. [DOI] [PubMed] [Google Scholar]
- 19 a).Stocker BL; Kodar K; Wahi K; Foster AJ; Harper JL; Mori D; Yamasaki S; Timmer MSM, Glycoconj. J, 2019, 36, 69–78; [DOI] [PubMed] [Google Scholar]; b) Khan A; Kodar K; Timmer MSM; Stocker BL, Tetrahedron, 2018, 74 (12), 1269–1277. [Google Scholar]
- 20 a).Burkhart D; Ettenger G; Evans J; Ryter KT; Smith A; PCT Int. Appl, 2019, 131 pp., WO2019165114; [Google Scholar]; b) Ryter KT; Ettenger G; Rasheed OK; Buhl C; Child R; Miller SM; Holley D; Smith AJ; Evans JT, J. Med. Chem, 2020, 63, 309–320; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Smith AJ; Miller SM; Buhl C; Child R; Whitacre M; Schoener R; Ettenger G; Burkhart D; Ryter K; Evans JT, Front. Immunol, 2019, 10, 338; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson DA, Carbohydrate Research 1992, 237, 313–318. [Google Scholar]
- 22 a).Khan AA; Stocker BL; Timmer MSM; Carbohydrate Res, 2012, 356, 25–36; [DOI] [PubMed] [Google Scholar]; b) Khan AA; Chee SH; Mclaughlin RJ; Harper JL; Kamena F; Timmer MSM; Stocker BL; ChemBioChem, 2011, 3, 2572–2576. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DA; Livesay MT, In J Carbohydr. Chem, 1998, 17, 969–974. [Google Scholar]
- 24 a).Srenathan U; Steel K; Taams LS, Immunology Lett, 2016, 178, 20–26; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kimura A; Kishimota T; Eur. J. Immunol, 2010, 40, 1830–1835. [DOI] [PubMed] [Google Scholar]
- 25 a).Williams SJ Frontiers in Immunology, 2017, 8(1662); [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bird JH; Khan AA; Nishimura N; Yamasaki S; Timmer MSM; Stocker BL; J. Org. Chem; 2018, 83, 7593–7605. [DOI] [PubMed] [Google Scholar]
- 26.Ostrop J; Jozefowski K; Zimmermann S; Hofmann K; Strasser E; Lepenies B, Lang R; J. Immunol, 2015, 2417–2428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.