Abstract
Objective:
To determine the association of supraspinatus fat fraction and Goutallier grade to the American Shoulder and Elbow Surgeons (ASES) score in cohorts of older adults with painful full-thickness supraspinatus tendon (SST) tear and controls.
Materials and Methods:
Seventeen controls and 15 study participants with painful full-thickness SST tear (tear cohort) were prospectively recruited with mean ages of 63.0 ± 10.1 and 62.6 ± 9.0 years, respectively. Study participants received shoulder MRI and completed ASES questionnaires at one timepoint. Goutallier grade was assessed on T1-weighted MRI. Fat fraction was measured on 6-point Dixon MRI. Body mass index (BMI) was determined. Descriptive, correlation, reliability, and regression analyses were performed.
Results:
The control and tear cohorts differed in mean supraspinatus fat fraction (3.3% ± 1.4% versus 7.3% ± 5.9%, p = 0.024) and Goutallier grade (0.4 ± 0.5 versus 0.9 ± 0.7, p = 0.022). Fat fraction (p = 0.014) and Goutallier grade (p = 0.017) demonstrated a significant inverse relationship with ASES score only in the tear cohort. The relationship of BMI to ASES score was significant only in controls (p = 0.036). The cohorts demonstrated different correlation between BMI and fat fraction: control cohort (r = 0.676; p = 0.003), tear cohort (r = 0.124; p = 0.687). Fat fraction showed strong inter-observer reliability (intraclass correlation coefficient, 0.903); Goutallier grade showed poor inter-observer reliability (kappa, 0.178).
Conclusion:
The association of ASES score and supraspinatus fat fraction or Goutallier grade differs between patients with painful full-thickness SST tear and asymptomatic controls. While fat fraction shows strong reliability, Goutallier grade should be approached cautiously due to suboptimal reproducibility. Our results also suggest that painful full-thickness SST tear alters the correlation between supraspinatus fat fraction and BMI as compared to controls.
Keywords: shoulder, rotator cuff, MRI, intramuscular fatty infiltration, quantitative
Introduction
Rotator cuff (RC) tear is a leading cause of shoulder-related morbidity, with a prevalence of 25% for adults ≥ 60 years in the general population [1]. Supraspinatus tendon (SST) tear is the most common subtype [2]. As the percentage of older adults continues to rise, the currently estimated $12 billion dollars spent annually on RC tear in the United States also likely will increase [3].
RC tear negatively impacts activities of daily living (ADLs) and hastens age-related functional declines, especially in geriatric populations [4]. Clinical decision-making for symptomatic RC tear is based on a myriad of patient-specific factors, with a lack of consensus for best treatment practices [5–8]. Patients’ perception of shoulder-related pain and disability is a major pillar in the decision-making process, and surveys that measure patient-self-reported outcomes are helpful [4, 7, 8]. The American Shoulder and Elbow Surgeons (ASES) questionnaire is a validated and reliable patient-self-reported survey of shoulder disability based on subjective perception of ADLs, instability and pain. The ASES score ranges from 0 to 100 points; lower scores indicate greater pain and disability [4, 9, 10].
Rotator cuff muscle quality is another key factor for clinical decision-making. Magnetic resonance imaging (MRI) is the leading modality for evaluation of RC tear and rotator cuff intramuscular fatty infiltration (FI) in the United States. Animal and human studies support FI as an imaging biomarker predictive of postsurgical outcomes following rotator cuff repair (RCR) [8, 11–15]. Studies also suggest that significant increases in FI predominantly occur following full-thickness RC tears, but not necessarily partial-thickness RC tears, as compared to RC tendons without tears [16, 17]. The Goutallier classification system, a semi-quantitative 5-point ordinal scale, has been the predominant measure of FI in clinical practice and research [8, 15, 18–20]. Quantitative chemical shift imaging techniques, such as multi-echo Dixon, are touted as reliable and accurate means of stratifying FI in the setting of RC tear [17, 21–24]. Whether the relationship of quantitative and semi-quantitative measures of supraspinatus FI to patient-self-reported outcome measures are comparable in populations of older adults with and without painful full-thickness SST tear remains an open question.
The purpose and uniqueness of our study was to determine the association of quantitative supraspinatus fat fraction by 6-point Dixon MRI, and semi-quantitative supraspinatus Goutallier grade by T1-weighted MRI, to the ASES score in prospective cohorts of older adults with painful full-thickness SST tear and controls. We hypothesized that estimation of FI by quantitative fat fraction would show a stronger association to ASES score and demonstrate superior reliability as compared to semi-quantitative Goutallier grade.
Methods and Materials
Study population
The prospective cross-sectional study was approved by the authors’ institutional review board and complied with Health Insurance Portability and Accountability Act. Informed written consent was obtained from each study participant. From January 2017 to December 2017, 38 study participants were enrolled, following referral to the study from one of three orthopaedic surgeons or self-referral in response to local advertisements.
The study examines two cohorts: (1) participants with painful full-thickness SST tear and (2) asymptomatic controls. Inclusion criteria for the painful full-thickness SST tear cohort: full-thickness SST tear and ipsilateral pain ≥ 2 on an 11-point visual analog scale (VAS), ranging from 0 to 10 (Fig 1a) [5]. Inclusion criteria for the control cohort: no full-thickness SST tear, inclusive of supraspinatus tendons that were intact, with tendinopathy or partial tear; participants who reported ≤ 1 on VAS were defined as asymptomatic (Fig 1b) [5]. Exclusion criteria for both cohorts: contraindication to MRI; age < 40 years or > 85 years; chronic upper extremity paralysis; and history of prior RCR surgery or joint replacement for ipsilateral shoulder of concern. A total of 41 shoulders in 38 study participants were enrolled. Nine shoulders were excluded from analysis: 7 shoulders had no full-thickness SST tear and ipsilateral pain ≥ 2 on a visual analog scale (VAS); 2 shoulders presented with full-thickness SST tear and ipsilateral pain of 0 on VAS. A total of 32 shoulders were included in the analysis: painful full-thickness SST tear cohort (n = 15) and control cohort (n =17). The sub-populations of the two cohorts were mutually exclusive, with no overlap of study participants.
Fig. 1 –
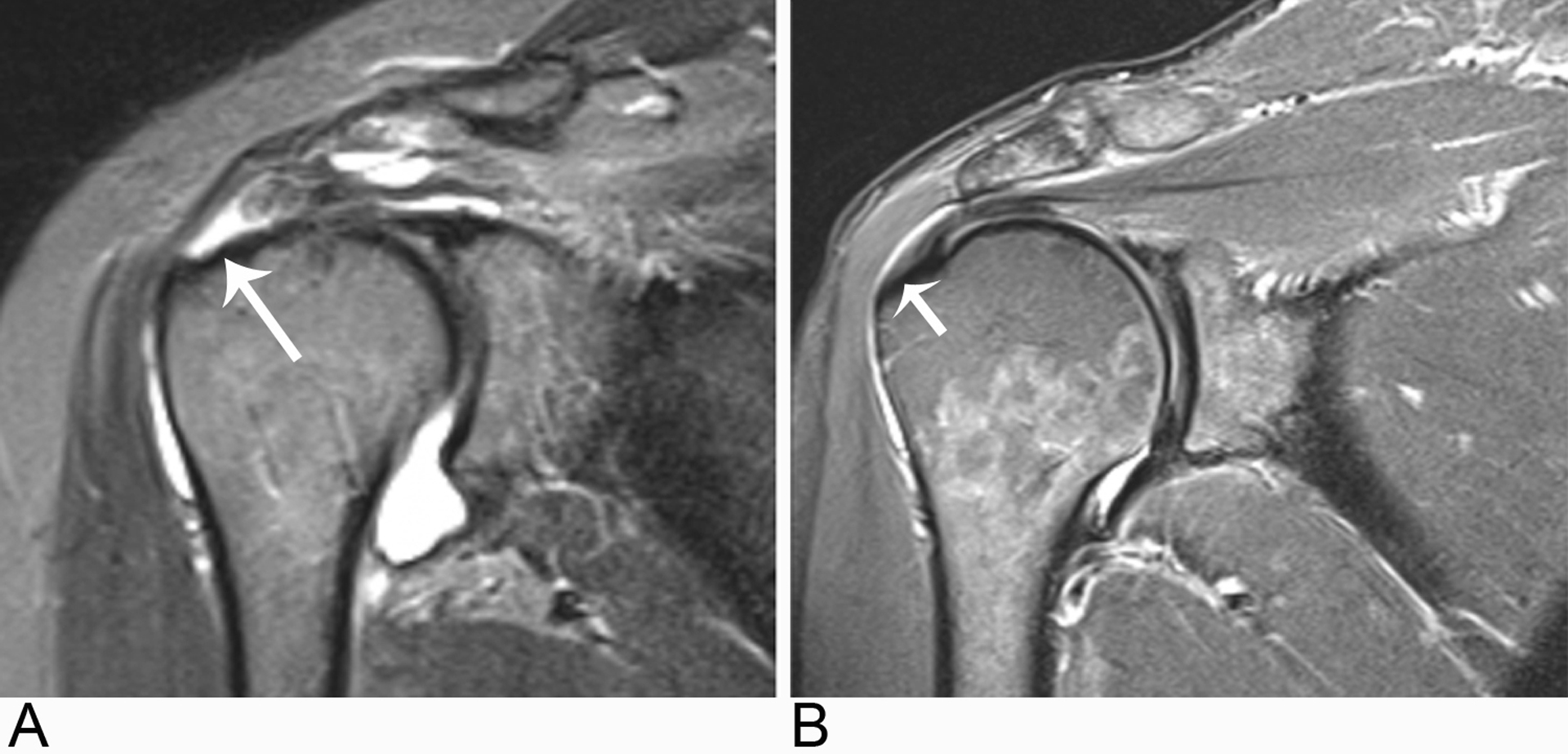
Shoulder MR image examples of study participants in each cohort.
A, Oblique coronal STIR MR image from a study participant in the painful full-thickness supraspinatus tendon (SST) tear (long arrow) cohort.
B, Oblique coronal STIR MR image demonstrating no full-thickness SST tear (short arrow) in a study participant from the control cohort.
MR imaging
Shoulder MR imaging was performed at 3.0T (Magnetom Trio or Magnetom Prismafit; Siemens Healthcare, Erlangen, Germany) using a four-channel flexible coil (see Table 1 for imaging protocol). The 6-point Dixon volumetric sequence was obtained in the sagittal orientation, and fat fraction image maps were reformatted in the oblique sagittal plane.
Table 1.
Shoulder MR imaging sequences and parameters
| Parameter | Oblique sagittal T1-weighted imaging | Oblique sagittal STIR-weighted imaging | Oblique coronal STIR-weighted imaging | Axial STIR-weighted imaging | Sagittal 6-point Dixon imaging |
|---|---|---|---|---|---|
| Imaging technique | 2D | 2D | 2D | 2D | 3D |
| Repetition time/echo time (ms) | 600/24 | 4420/51 | 4420/51 | 4440/51 | 9.31/1.35,2.65, 3.95,5.25, 6.55,7.85 |
| Flip angle (degree) | 9 | ||||
| Field of view (mm2) | 160 × 160 | 160 × 160 | 160 × 160 | 160 × 160 | 400 × 324 |
| Matrix (frequency × phase) | 448 × 202 | 256 × 202 | 256 × 202 | 256 × 202 | 320 × 250 |
| Section thickness (mm) | 4 | 4 | 4 | 4 | 3.5 |
| Inversion time (ms) | 180 | 180 | 180 | ||
| Number of averages | 1 | 1 | 1 | 1 | 1 |
| Total imaging time (min:sec) | 4:06 | 4:00 | 4:53 | 2:41 | 0:38 |
Clinical evaluation
An independent evaluation was performed for each shoulder; study participants completed medical history questionnaires for demographics, history of the present illness and past medical history of the ipsilateral shoulder, ASES score and Charlson Co-Morbidity Index (CCI) [6]. Height and weight were recorded to determine body mass index (BMI).
MR image analysis
De-identified DICOM image modules were created containing a single MR image corresponding to the Y-shaped view, representing the most lateral image where the scapular spine contacts the scapular body, for each individual shoulder on a 3D visualization system viewer (Aquarius iNtuition Edition, version 4.4; TeraRecon Inc., Foster City, California, USA) [19, 25–27]. A blinded musculoskeletal radiologist with > 25 years of experience independently assigned a Goutallier grade for each supraspinatus muscle based on the 5-point Goutallier classification scale: grade 0, no fat; grade 1, streaks of fat; grade 2, muscle> fat; grade 3, muscle=fat; grade 4, muscle<fat (Fig 2a,c) [18, 19]. Four weeks later, the same blinded senior musculoskeletal radiologist independently assigned a Goutallier grade for each supraspinatus muscle to determine intra-observer reliability. A second blinded radiologist with > 25 years of experience independently assigned a Goutallier grade for each supraspinatus muscle in order to determine inter-observer reliability. Blinded musculoskeletal radiologists independently reviewed identical training modules for scoring of Goutallier grade, prior to the assessment of study participant MR images.
Fig. 2–
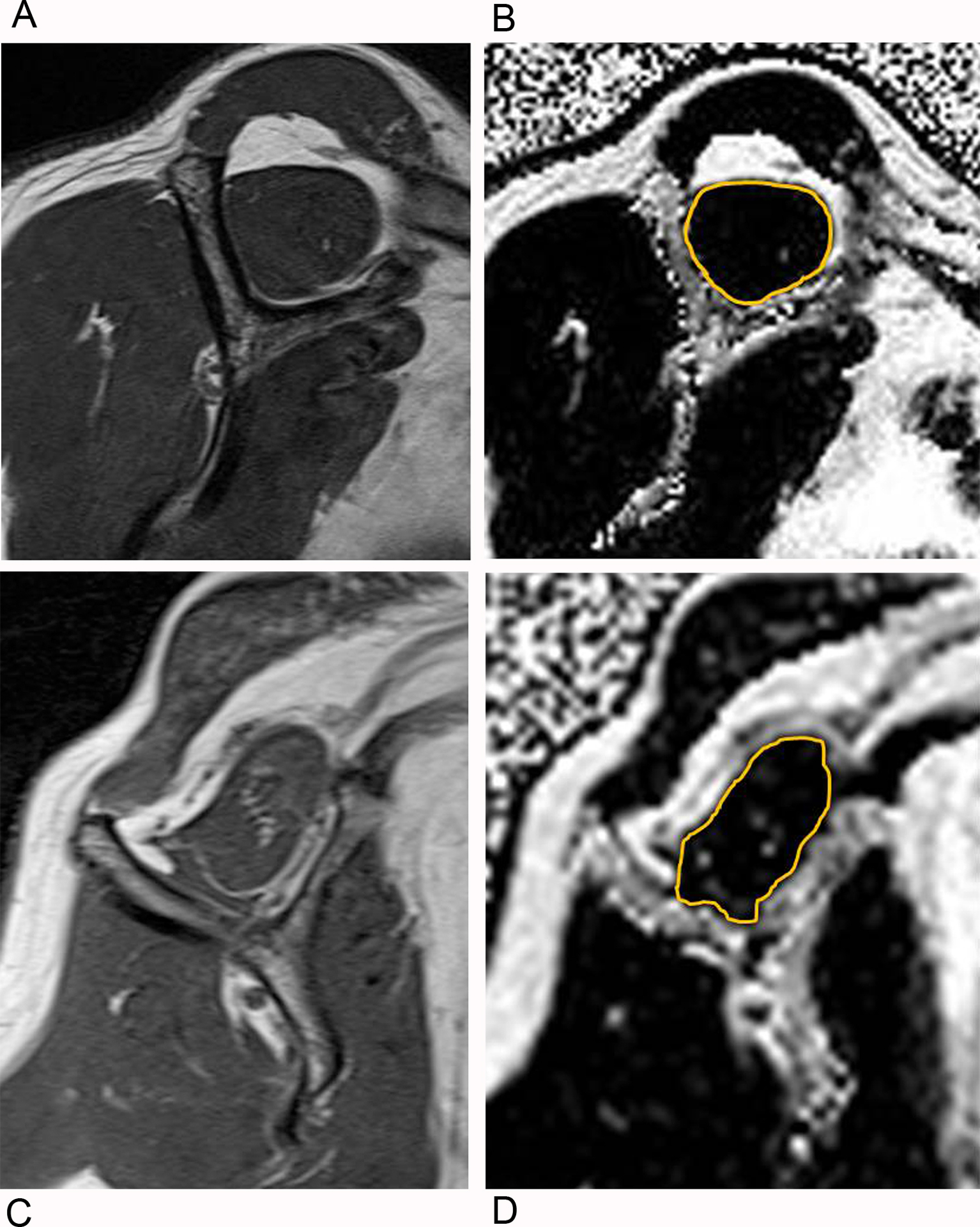
Example shoulder MR images from study participants with differing amounts of supraspinatus intramuscular fatty infiltration.
A and B. Example 1. Oblique sagittal T1-weighted MRI Y-shaped view for Goutallier grade (A) and oblique sagittal Dixon fat fraction map image (B), corresponding to the oblique sagittal T1-weighted MRI Y-shaped view . The gold outline about the supraspinatus muscle denotes the region of interest placed by manual segmentation for measurement of fat fraction.
C and D. Example 2. Oblique sagittal T1-weighted MRI Y-shaped view (C) and corresponding oblique sagittal Dixon fat fraction map image (D).
De-identified DICOM image modules were created containing a single oblique 6-point Dixon MR fat fraction map image corresponding to the same slice location as the MR T1-weighted Y-shaped view. A blinded radiology resident independently calculated fat fraction for each supraspinatus muscle on 6-point Dixon MR fat fraction maps by manual segmentation using Medical Image Processing, Analysis and Visualization software (MIPAV, version 7, National Institutes of Health, Bethesda, Maryland, USA) (Fig 2b,d). Care was taken to place the region of interest at the perimeter of the supraspinatus muscle without including perimuscular fat or bone. Four weeks later, the same blinded radiology resident independently calculated the fat fraction again for each supraspinatus muscle to determine intra-observer reliability. A second blinded radiology resident independently calculated the fat fraction for each supraspinatus muscle on 6-point Dixon MR fat fraction maps by manual segmentation using the MIPAV software, in order to determine inter-observer reliability.
A musculoskeletal radiologist with 8 years of experience independently reviewed each shoulder MRI to determine the presence of tendinopathy, partial tear, and/or full-thickness tear of the supraspinatus, infraspinatus, subscapularis and teres minor tendons. The mediolateral and anteroposterior dimensions of full-thickness RC tears were also recorded.
Statistical analysis
Statistical analysis was performed using Stata statistical software version 14 (StataCorp LP, College Station, Texas). Cohorts were compared with the unpaired t test and χ2 test. Intra-observer and inter-observer reliability for fat fraction were assessed by intraclass correlation coefficient [28]. Intra-observer and inter-observer reliability for Goutallier grade were assessed by kappa. ASES score was the primary outcome for univariate and multivariate linear regression analyses. The primary independent variables were fat fraction (6-point Dixon MRI) and Goutallier grade (T1-weighted MRI). Co-variables were age, BMI, and CCI. Tests for statistical interactions were conducted between supraspinatus tear-status (presence versus absence full-thickness SST tear) and supraspinatus fat fraction, supraspinatus Goutallier grade, age, BMI, and CCI. Univariate and multivariate linear regression analyses were stratified by supraspinatus tear-status. Model 1 for multivariate linear analysis included supraspinatus fat fraction, age, BMI and CCI; supraspinatus Goutallier grade, age, BMI and CCI were included in model 2. Pearson correlation (r) was performed between supraspinatus fat fraction and mean maximum tear size, and also between supraspinatus Dixon fat fraction and BMI. Spearman rank order correlation (rs) was performed between mean maximum tear size and supraspinatus Goutallier grade, and also between supraspinatus Goutallier grade and BMI. A p value < 0.05 was considered to indicate a significant difference.
Results
Baseline characteristics are shown in Table 2. The presence or absence of a full-thickness SST tear produced heterogeneity of effect for fat fraction and Goutallier grade in the study sample. With ASES score as an outcome, there was significant statistical interaction: fat-fraction*tear status (p= 0.004, n=30) and Goutallier-grade*tear-status (p= 0.020, n=32). There was no significant statistical interaction for age*tear-status, BMI*tear-status, or CCI*tear-status (n =32).
Table 2.
Baseline characteristics of the study participants
| Control (N = 17) |
TearA (N = 15) |
P value | |
|---|---|---|---|
| Age, years | 63.0B ± 10.1C | 62.6 ± 9.0 | 0.907 |
| Male, % | 53% | 53% | 0.982 |
| ASES score (best 100, worst 0) | 97.4 ± 4.0 | 52.0 ± 25.8 | < 0.001 |
| Fat fraction, supraspinatusD | 3.3% ± 1.4%E | 7.3% ± 5.9% | 0.024 |
| Goutallier grade, supraspinatus | 0.4 ± 0.5 | 0.9 ± 0.7 | 0.022 |
| Body mass index, kg/cm2 | 25.4 ± 3.8 | 31.5 ± 4.9 | < 0.001 |
| Charlson co-morbidity index | 2.4 ±1.5 | 2.7 ±2.2 | 0.640 |
= Painful full-thickness supraspinatus tendon tear;
= Mean;
= Standard deviation;
= Two study participants in the tear cohort did not have a fat fraction map available for analysis (n = 13);
= fat fraction is expressed as a percentage
Shoulders in the painful full-thickess SST tear cohort had a mean maximum tear size of 2.2 ± 1.7 cm corresponding to the larger of the mediolateral or anteroposterior dimension for each SST tear and a (2) mean SST tear retraction of 2.0 ± 1.8 cm corresponding to the mediolateral tear dimension. There were 7 isolated full-thickness SST tear, 6 full-thickness SST tear in combination with full-thickness infraspinatus tendon tears, and 2 full-thickness SST tear in combination with full-thickness infraspinatus and subscapularis tendon tears. There was a significant moderate correlation between mean maximum tear size and supraspinatus fat fraction (r = 0.566, p = 0.044) and a strong correlation between mean maximum tear size and supraspinatus Goutallier grade (rs = 0.717, p = 0.003). The study sample failed to show a statistically significant correlation between maximum tear size and supraspinatus fat fraction or Goutallier grade, when considering the isolated full-thickness SST tear subset only or for the subset of study participants with full-thickness supraspinatus and infraspinatus tendon tears.
In the control cohort, there was no significant association between fat fraction, Goutallier grade, age or CCI with ASES score during univariate linear regression (n = 17) (Table 3). BMI (p= 0.036) did have a significant association with ASES score (n = 17). There was no significant association of ASES score with fat fraction, BMI, age or CCI during multivariate linear regression for the controls in model 1 (Table 4); in model 2, only BMI (p= 0.038, n=17) had a statistically significant association with ASES score.
Table 3.
Univariate linear regression parameter estimates for ASES score outcomes by tear status
| β-Estimate (95% CI)A | P value | |
|---|---|---|
| TearB cohort | ||
| Age | 0.67 (−0.62 to 1.96) | 0.280 |
| BMIc | −1.71 (−3.76 to 0.35) | 0.096 |
| CCID | −0.09 (−6.41 to 6.24) | 0.977 |
| Fat fractionE | −2.46 (−4.31 to −0.60) | 0.014 |
| Goutallier gradeF | −18.41 (−32.97 to −3.85) | 0.017 |
| Control cohort | ||
| Age | −0.05 (−0.23 to 0.12) | 0.531 |
| BMI | 0.37 (0.03 to 0.71) | 0.036 |
| CCI | 0.23 (−0.70 to 1.16) | 0.604 |
| Fat fraction | 0.31 (−0.23 to 0.85) | 0.237 |
| Goutallier grade | −0.92 (−5.71 to 3.87) | 0.687 |
= 95% Confidence Interval;
= Painful full-thickness supraspinatus tendon tear;
= Body mass index;
= Charlson co-morbidity index;
= supraspinatus muscle;
= supraspinatus muscle
Table 4.
Multivariate linear regression parameter estimates for ASES score outcomes by tear status
| Model 1 | |||||
|---|---|---|---|---|---|
| β-Estimate (95% CI)A | P value | β-Estimate (95% CI) | P value | ||
| TearB cohort | Control cohort | ||||
| Age | 1.77 (−0.83 to 4.36) | 0.155 | Age | −0.10 (−0.37 to 0.17) | 0.445 |
| BMIC | −1.14 (−3.28 to 1.00) | 0.256 | BMI | 0.41 (−0.13 to 0.96) | 0.121 |
| CCID | −8.02 (−17.41 to 1.37) | 0.084 | CCI | 0.58 (−0.79 to 1.95) | 0.375 |
| Fat fractionE | −2.10 (−4.26 to 0.06) | 0.056 | Fat fraction | −0.28 (−1.56 to 1.00) | 0.639 |
| Model 2 | |||||
| β-Estimate (95% CI)A | P value | β-Estimate (95% CI) | P value | ||
| Tear cohort | Control cohort | ||||
| Age | 0.61 (−1.43 to 2.64) | 0.522 | Age | −0.02 (−0.28 to 0.23) | 0.860 |
| BMI | −2.71 (−4.99 to −0.43) | 0.024 | BMI | 0.40 (0.03 to 0.77) | 0.038 |
| CCI | −1.35 (−7.96 to 5.26) | 0.659 | CCI | 0.46 (−0.56 to 1.48) | 0.342 |
| Goutallier gradeF | −26.79 (−42.65 to −10.93) | 0.004 | Goutallier grade | −2.39 (−8.92 to 4.15) | 0.442 |
= 95% Confidence Interval;
= Painful full-thickness supraspinatus tendon tear;
= Body mass index;
= Charlson co-morbidity index;
= supraspinatus muscle;
= supraspinatus muscle
In the painful full-thickness SST tear cohort, there was a significant inverse association of fat fraction with ASES score (p= 0.014, n=13) (Fig 3) during univariate linear regression; and also significant inverse association between Goutallier grade and ASES score (p = 0.017, n=15). During multivariate linear regression for the painful full-thickness SST tear cohort in model 1, fat fraction (p = 0.056, n=13) and CCI (p= 0.084) had an inverse association with ASES score, with fat fraction at near statistical significance; in model 2, Goutallier grade (p= 0.004, n=15) and BMI (p= 0.024) had a significant inverse association with ASES score.
Fig. 3—
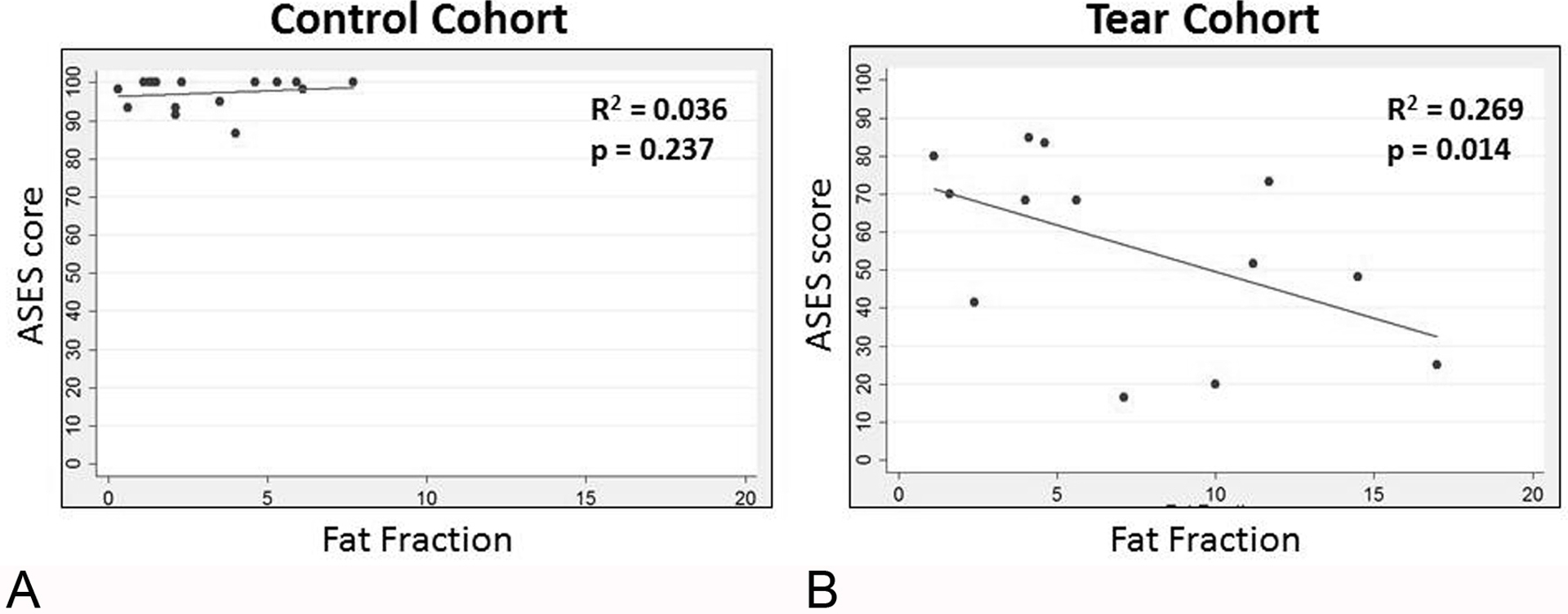
Relationship of the American Shoulder and Elbow Surgeons (ASES) score with supraspinatus fat fraction. Fat fraction is expressed as a percentage.
A, Scatterplot for the control cohort.
B, Scatterplot for the painful full-thickness supraspinatus tendon tear cohort.
There was a strong positive correlation between supraspinatus fat fraction and BMI for the control cohort, while no significant correlation was found for the painful full-thickness SST cohort (Fig 4). There was no statistically significant correlation between supraspinatus Goutallier grade and BMI in either cohort.
Fig. 4—
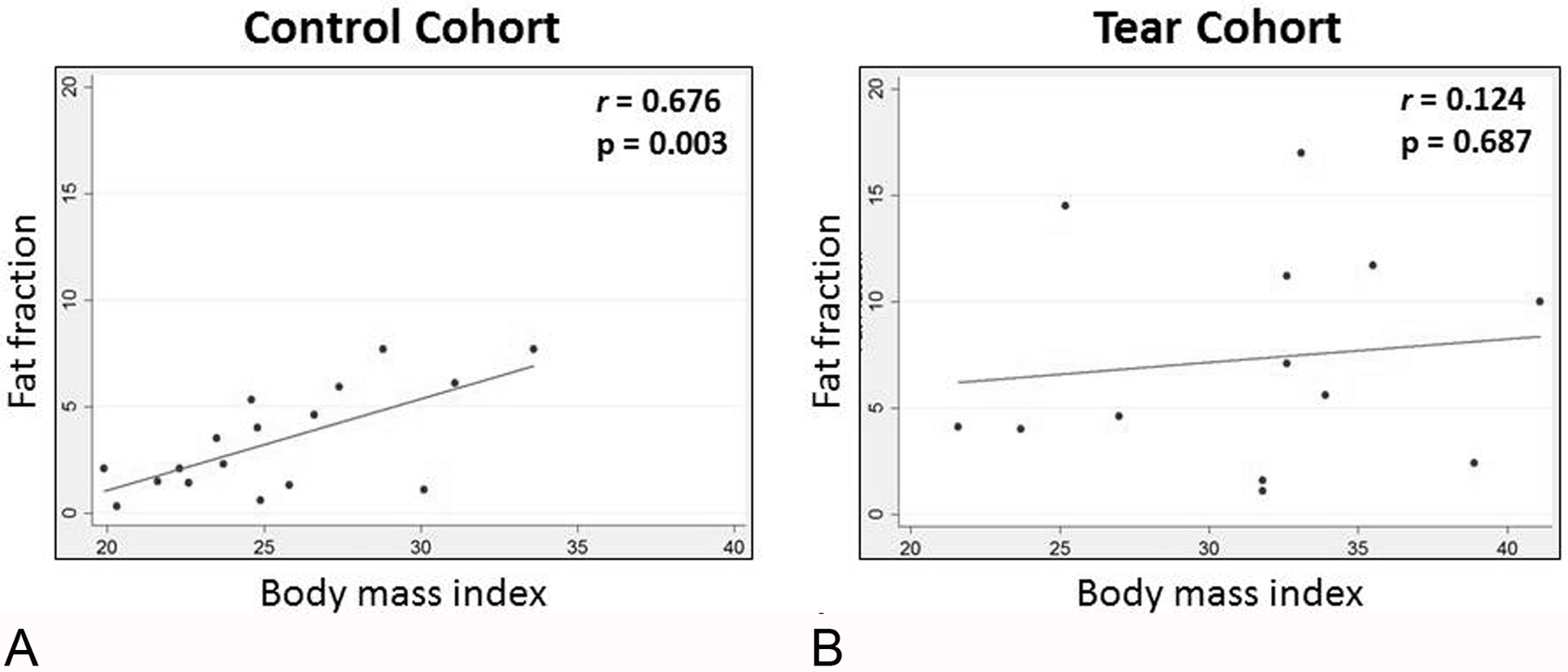
Correlation between supraspinatus fat fraction and body mass index. Fat fraction is expressed as a percentage.
A, Scatterplot for the control cohort.
B, Scatterplot for the painful full-thickness supraspinatus tendon tear cohort.
Inter-observer and intra-observer reliability were strong to near perfect for fat fraction, with intraclass correlation coefficients of 0.903 and 0.975, respectively. Inter-observer reliability was poor for the 5-point ordinal Goutallier grade (kappa, 0.178). When using the modified 3-point scale proposed by Fuchs et al., inter-observer reliability was improved to fair (kappa, 0.362) [19]. Intra-observer reliability was moderate and strong for the 5-point (kappa, 0.593) and modified 3-point scales (kappa, 0.788), respectively.
The mean and standard deviation of supraspinatus muscle Dixon fat fraction stratified by Goutallier grade for study participants: Grade 0, fat fraction 2.5% ± 1.9%; Grade 1, fat fraction 6.1% ± 3.2%; and Grade 2, fat fraction 15.75% ± 1.8%. No study participant had a Goutallier grade of 3 or 4 in the study.
Discussion
Our study suggests that the association of ASES score with MRI measures of supraspinatus FI differs between patients with painful full-thickness SST tear and control participants. Supraspinatus quantitative fat fraction and semi-quantitative Goutallier grade each separately demonstrate a significant inverse association with ASES score in shoulders with painful full-thickness SST tear, but fat fraction is more reliable than Goutallier grade as an imaging measure of FI. The lack of a significant association between ASES score and fat fraction or Goutallier grade for control participants adds to the body of evidence that RC tear is a sentinel event that alters associated muscle architecture and function [17, 29, 30].
Imaging assessment of FI following RC tear has been a central measure in clinical decision-making due to the observation that preoperative FI is predictive of post-surgical outcomes [8, 15, 18, 19]. The presence of Goutallier grade 3 is a relative contraindication to RCR surgery [30]. Animal and human studies support the hypothesis that FI does not improve after successful RCR surgery and may continue to progress [8, 11–15]. Most studies have relied on the semi-quantitative Goutallier grade to support their conclusions [8, 14, 15, 31]. Our finding of a significant difference in supraspinatus Goutallier grade between painful full-thickness SST tear and control cohorts is in-line with prior literature [30]. However, the exact mechanism for intramuscular adipocyte deposition on the cellular level remains controversial and a matter of considerable debate [17, 29, 30].
Despite its ease of use, the known major drawback of the Goutallier classification is poor to moderate reliability [17, 23, 24, 32–34]. Our results confirm that Goutallier grade has suboptimal reproducibility. Fuchs et al., proposed a 3-point ordinal scale, by combining grades 0 and 1, and grades 3 and 4 to improve reproducibility [19]. Other researchers also have suggested additional variations of 3-point ordinal scales [33, 35]. Use of the 3-point Fuchs modified scale improved Goutallier grade inter-observer reliability from poor to fair in our study, consistent with the literature.
In an attempt to facilitate meaningful conclusions, certain studies have resorted to dichotomizing the Goutallier classification system to a simple 2-point scale [8, 22]. This highlights the difficulties faced by research investigators, and suggests that potential misclassification errors of rotator cuff intramuscular fatty infiltration is a major shortcoming of the Goutallier classification scale, since reliability is improved at the expense of less stratification of study subjects.
Quantitative chemical shift imaging techniques offer the potential for accurate and reliable stratification of FI. Multi-echo Dixon techniques allow high spatial resolution for quantification of intramuscular fat signal [36]. These methods allow for measurement of FI as a continuous variable, on a scale from 0 to 100 [22]. Agten et al., demonstrated that multi-echo Dixon fat fraction was comparable to single voxel spectroscopy for determination of supraspinatus muscle FI [21]. Fat fraction is preferable as a quantitative measure of FI, as compared to MR spectroscopy, due to its superior reproducibility and greater capacity to assess larger areas of skeletal muscles [37].
Dixon MRI can also quantify the small amount of FI that is not visible for Goutallier grade 0 muscles [21]. The 6-point Dixon MRI method, in particular, offers improved accuracy for measurement of FI in comparison to earlier iterations of the Dixon-based strategies by mitigating T2* decay and T1 effects through a multi-peak fat spectral model [21]. Grimm et al., in a study comparing 2-point, 3-point and 6-point Dixon MRI, advocated that Dixon MRI protocols designed to measure fat fraction should be performed with 3 or more echoes, since some 2-point Dixon sequences may not entirely separate fat from water, thereby leading to an overestimation of fat signal [38]. Our study finding of strong to near perfect inter-observer and intra-observer reliability for supraspinatus FI on 6-point Dixon MR imaging is also in agreement with previous human and animal studies [21, 25, 39].
Agten et al., in a study of the supraspinatus muscle in persons with symptomatic shoulders and younger asymptomatic controls suggested that shoulders with full-thickness SST tear had a higher fat fraction as compared to those with no full-thickness tear, although there were only four shoulders with full-thickness SST tear in their study [21]. Our study findings also support the hypothesis that fat fraction by multi-echo Dixon MRI is capable of discriminating differences of FI between populations with and without full-thickness RC tear.
Patient-self-reported surveys are useful in the clinical decision-making process for RC tear by measuring patients’ perception of pain and disability, which are especially important for the maintenance of functional independence in geriatric populations [4, 8]. Several patient-self-reported outcome measures exist for the evaluation of shoulder dysfunction in the setting of RC tear. Vidt et al., found moderate correlation between the ASES score, Western Ontario Rotator Cuff Index and Simple Shoulder Test, but favored prioritizing ASES score for older adults to measure decline in functional ADLs [4]. Gladstone et al., did not find supraspinatus Goutallier grade to have significant correlation to ASES score in subjects with RC tear [8]. In contrast, our study suggests that supraspinatus Goutallier grade and fat fraction each have a significant inverse association with ASES score in persons with painful full-thickness SST tear.
A spectrum of patient-specific factors must be considered during clinical decision-making for symptomatic RC tear, including age, BMI, co-morbidity, and patients’ expected long-term future activity level among others [5–7]. Due to the complexity of these factors, best treatment practices for symptomatic RC tear remain controversial [5]. Prior studies have suggested that older adults with intact rotator cuffs show incremental increases in supraspinatus FI during normal aging, but an incident tendon tear is known to rapidly accelerate intramuscular adipocyte deposition in this population [17, 30]. Kweon et al., in a prospective cohort study of subjects with symptomatic full-thickness RC tear suggested that advancing age and high BMI were factors associated with individuals more likely to receive conservative management rather than surgical repair [7]. BMI has been shown to correlate with skeletal muscle adipocyte deposition in studies of obesity and diabetes mellitus [17]. Kiefer et al., in a study of abdominal myosteatosis demonstrated a positive correlation between abdominal muscle fat fraction and BMI [40]. The impact of BMI on the muscle quality of rotator cuff muscles is not well studied in the orthopedic literature, although Lee et al., suggested that higher BMI leads to higher levels of FI in rotator cuff muscles [17]. By contrast, our study suggests that RC tear is a sentinel event that alters the correlation of BMI and quantitative measures of FI, when stratifying the study population by the presence or absence of full-thickness RC tear. Fat fraction correlated positively with BMI in controls without full-thickness RC tear, but shows no correlation in the cohort with full-thickness RC tear. The impact of co-morbidities on clinical decision-making remains controversial, with conflicting data in the literature. However, persons with many co-morbidities may be considered as less optimal candidates for RCR surgery [6, 7].
The routine evaluation of muscle fat fraction is becoming feasible as MRI vendors make available software packages that facilitate multi-echo Dixon MRI techniques in clinical practice. Commercially available 1.5 and 3.0 Tesla MRI scanners now exist that allow for the automated creation of fat fraction maps to be interpreted by radiologists on clinical picture archive and communication systems. Measurement of rotator cuff muscle fat fraction on fat fraction maps only requires placing an ROI, a function commonly familiar to most radiologists who routine perform tasks such as measuring Hounsfield Units on computed tomography. In general, manual placement of an ROI of supraspinatus muscles took less than one minute on average in our study, although we did not formally record the time of this activity. The fast acquisition time of multi-echo Dixon sequences – less than one minute in our study – should further encourage radiologists that rapid-paced quantitative assessment of FI is possible in clinical practice.
The fat fraction percentage corresponding to Goutallier grade in our study was consistent with the literature [22, 41]. The Goutallier classification scheme denotes that grade 3 muscles have 50% FI, while grade 4 muscle have > 50% FI. However, prior studies suggest that Goutallier grade over estimates the level of fat present in a muscle. Alizia et al., found that calf muscles categorized as Goutallier grade 3 and grade 4 muscles, had mean fat fractions corresponding to < 20% [41]. Similarly, Nardo et al., described rotator cuff muscles having mean fat fractions < 30% in muscles determined to be Goutallier grade 3 and 4 [22]. Future studies are needed to determine new cut-off points for clinical decision-making and long term outcomes based on fat fraction instead of Goutallier grade.
Limitations include the small sample size and cross-sectional design of this pilot study, but the study sample was still able to identify several statistically significant differences. We also did not stratify the painful full-thickness SST tear cohort by tear severity, although self-reported shoulder pain and dysfunction are known to be poor predictors of tear severity [5, 42]. The study population had an overall low burden of co-morbidity. Our findings may not be applicable to populations with a high burden of co-morbidities. The study population was a convenience sample and results may not be generalizable to the general population. The study also may be limited by selection bias. Motivated study participants who self-referred in response to advertisements may not be representative of the general population. Also, the participants who agreed to be referred to the study from their orthopaedic surgeon may not be representative of the population of symptomatic patients who present to orthopaedic clinics with shoulder complaints. We analyzed supraspinatus muscle Dixon fat fraction and Goutallier grade on the single 2D Y-shaped view oblique sagittal image that is well-described in the literature [25–27]. The single slice 2D image may not be representative of the entire volumetric FI content of the supraspinatus muscle. However, feasible current methods for quantification of fat content for the entire supraspinatus muscle are not available [43]. Our study results are not generalizable to populations with Goutallier grade 3 or 4 supraspinatus muscles, since participants with these classifications were not present in the study. However, prior investigators have commented that study participants that lack advanced stages of FI are the ideal population for study, since these patients are most relevant for possible surgical repair in the setting of symptomatic RC tear [19]. Future studies may be needed to address the reproducibility of the multi-echo Dixon measures of fat fraction for Goutallier grade 4 muscles which may pose a greater challenge for manual placement of a region of interest when attempting to distinguish between the border of a severely fatty infiltrated muscle and the adjacent perimuscular fatty tissue present in the suprascapular fossa on fat fraction maps. The study was designed to examine the association of supraspinatus FI with ASES score, but future studies will be needed to assess the association of infraspinatus FI to patient self-reported outcome measures in patients with isolated full-thickness infraspinatus tears, although such full-thickness tears of the infraspinatus tendon not found in combination with adjacent full-thickness SST tear are rare [44–46].
In conclusion, the association of ASES score and quantitative fat fraction, or semi-quantitative Goutallier grade, differs between control study participants and older adults with painful full-thickness SST tear. Counter to our hypothesis, supraspinatus fat fraction and Goutallier grade performed similarly relative to the ASES score. However, while fat fraction shows strong reliability, the use of Goutallier grade should be considered with caution secondary to poor reproducibility. Our results also suggests that painful full-thickness SST tear alters the correlation between supraspinatus fat fraction and BMI as compared to controls.
Funding information:
This manuscript was supported by a research seed grant in 2016 from the Radiological Society of North America Research & Education Foundation & Hitachi Medical Systems (PI: Dr. Derik L. Davis).
Disclosures:
Dr. Derik L. Davis receives partial salary support from the University of Maryland Claude D. Pepper Older Americans Independence Center (NIA 2P30AG028747).
Contributor Information
Derik L. Davis, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, Maryland 21201.
Jiachen Zhuo, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, Maryland 21201.
Ranyah Almardawi, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, Maryland 21201.
Michael E. Mulligan, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, Maryland 21201.
Charles S. Resnik, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, Maryland 21201.
Selwan B. Abdullah, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland Medical Center, 22 S. Greene Street, Baltimore, Maryland 21201.
Hussain Al Khalifah, Department of Radiology and Radiological Science, The Johns Hopkins Hospital, 600 N. Wolfe Street, Baltimore, Maryland 21287.
R. Frank Henn, 3rd, Department of Orthopaedics, University of Maryland School of Medicine, 110 S. Paca Street, Baltimore, Maryland 21201.
Mohit N. Gilotra, Department of Orthopaedics, University of Maryland School of Medicine, 110 S. Paca Street, Baltimore, Maryland 21201.
S. Ashfaq Hasan, Department of Orthopaedics, University of Maryland School of Medicine, 110 S. Paca Street, Baltimore, Maryland 21201.
Rao P. Gullapalli, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, Maryland 21201.
References
- 1.Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 2010; 19:116–120 [DOI] [PubMed] [Google Scholar]
- 2.Melis B, Nemoz C, Walch G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res 2009; 95:319–324 [DOI] [PubMed] [Google Scholar]
- 3.McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA 3rd. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med 2015; 43:491–500 [DOI] [PubMed] [Google Scholar]
- 4.Vidt ME, Santago AC 2nd, Hegedus EJ, et al. Can self-report instruments of shoulder function capture functional differences in older adults with and without a rotator cuff tear? J Electromyogr Kinesiol 2016; 29:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon PJ, Prasad A, Francis KA. What is the prevalence of senior-athlete rotator cuff injuries and are they associated with pain and dysfunction? Clin Orthop Relat Res 2014; 472:2427–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varkey DT, Patterson BM, Creighton RA, Spang JT, Kamath GV. Initial medical management of rotator cuff tears: a demographic analysis of surgical and nonsurgical treatment in the United States Medicare population. J Shoulder Elbow Surg 2016; 25:e378–e385 [DOI] [PubMed] [Google Scholar]
- 7.Kweon C, Gagnier JJ, Robbins CB, Bedi A, Carpenter JE, Miller BS. Surgical Versus Nonsurgical Management of Rotator Cuff Tears: Predictors of Treatment Allocation. Am J Sports Med 2015; 43:2368–2372 [DOI] [PubMed] [Google Scholar]
- 8.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007; 35:719–728 [DOI] [PubMed] [Google Scholar]
- 9.McClure PW ML. Measures of Adult Shoulder Function. Arthritis & Rheumatism 2003; 49:S50–S58 [Google Scholar]
- 10.Smith MV, Calfee RP, Baumgarten KM, Brophy RH, Wright RW. Upper extremity-specific measures of disability and outcomes in orthopaedic surgery. J Bone Joint Surg Am 2012; 94:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am 2004; 86-A:1973–1982 [DOI] [PubMed] [Google Scholar]
- 12.Uhthoff HK, Matsumoto F, Trudel G, Himori K. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. J Orthop Res 2003; 21:386–392 [DOI] [PubMed] [Google Scholar]
- 13.Uhthoff HK, Coletta E, Trudel G. Effect of timing of surgical SSP tendon repair on muscle alterations. J Orthop Res 2014; 32:1430–1435 [DOI] [PubMed] [Google Scholar]
- 14.Liem D, Lichtenberg S, Magosch P, Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am 2007; 89:1770–1776 [DOI] [PubMed] [Google Scholar]
- 15.Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg 2014; 134:985–990 [DOI] [PubMed] [Google Scholar]
- 16.Kim HM, Dahiya N, Teefey SA, Keener JD, Galatz LM, Yamaguchi K. Relationship of tear size and location to fatty degeneration of the rotator cuff. J Bone Joint Surg Am 2010; 92:829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Lucas RM, Lansdown DA, et al. Magnetic resonance rotator cuff fat fraction and its relationship with tendon tear severity and subject characteristics. J Shoulder Elbow Surg 2015; 24:1442–1451 [DOI] [PubMed] [Google Scholar]
- 18.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994:78–83 [PubMed] [Google Scholar]
- 19.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 1999; 8:599–605 [DOI] [PubMed] [Google Scholar]
- 20.Somerson JS, Hsu JE, Gorbaty JD, Gee AO. Classifications in Brief: Goutallier Classification of Fatty Infiltration of the Rotator Cuff Musculature. Clin Orthop Relat Res 2016; 474:1328–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agten CA, Rosskopf AB, Gerber C, Pfirrmann CW. Quantification of early fatty infiltration of the rotator cuff muscles: comparison of multi-echo Dixon with single-voxel MR spectroscopy. Eur Radiol 2016; 26:3719–3727 [DOI] [PubMed] [Google Scholar]
- 22.Nardo L, Karampinos DC, Lansdown DA, et al. Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. J Magn Reson Imaging 2014; 39:1178–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis DL, Kesler T, Gilotra MN, et al. Quantification of shoulder muscle intramuscular fatty infiltration on T1-weighted MRI: a viable alternative to the Goutallier classification system. Skeletal Radiol 2019; 48:535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horiuchi S, Nozaki T, Tasaki A, et al. Reliability of MR Quantification of Rotator Cuff Muscle Fatty Degeneration Using a 2-point Dixon Technique in Comparison with the Goutallier Classification: Validation Study by Multiple Readers. Acad Radiol 2017; 24:1343–1351 [DOI] [PubMed] [Google Scholar]
- 25.Nozaki T, Tasaki A, Horiuchi S, et al. Predicting Retear after Repair of Full-Thickness Rotator Cuff Tear: Two-Point Dixon MR Imaging Quantification of Fatty Muscle Degeneration-Initial Experience with 1-year Follow-up. Radiology 2016; 280:500–509 [DOI] [PubMed] [Google Scholar]
- 26.Mellado JM, Calmet J, Olona M, et al. Surgically repaired massive rotator cuff tears: MRI of tendon integrity, muscle fatty degeneration, and muscle atrophy correlated with intraoperative and clinical findings.AJR Am J Roentgenol 2005; 184:1456–1463 [DOI] [PubMed] [Google Scholar]
- 27.Yoo JC, Ahn JH, Yang JH, Koh KH, Choi SH, Yoon YC. Correlation of arthroscopic repairability of large to massive rotator cuff tears with preoperative magnetic resonance imaging scans. Arthroscopy 2009; 25:573–582 [DOI] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174 [PubMed] [Google Scholar]
- 29.Valencia AP, Lai JK, Iyer SR, et al. Fatty Infiltration Is a Prognostic Marker of Muscle Function After Rotator Cuff Tear. Am J Sports Med 2018; 46:2161–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashry R, Schweitzer ME, Cunningham P, Cohen J, Babb J, Cantos A. Muscle atrophy as a consequence of rotator cuff tears: should we compare the muscles of the rotator cuff with those of the deltoid? Skeletal Radiol 2007; 36:841–845 [DOI] [PubMed] [Google Scholar]
- 31.Fucentese SF, von Roll AL, Pfirrmann CW, Gerber C, Jost B. Evolution of nonoperatively treated symptomatic isolated full-thickness supraspinatus tears. J Bone Joint Surg Am 2012; 94:801–808 [DOI] [PubMed] [Google Scholar]
- 32.Lippe J, Spang JT, Leger RR, Arciero RA, Mazzocca AD, Shea KP. Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy 2012; 28:154–159 [DOI] [PubMed] [Google Scholar]
- 33.Slabaugh MA, Friel NA, Karas V, Romeo AA, Verma NN, Cole BJ. Interobserver and intraobserver reliability of the Goutallier classification using magnetic resonance imaging: proposal of a simplified classification system to increase reliability. Am J Sports Med 2012; 40:1728–1734 [DOI] [PubMed] [Google Scholar]
- 34.Spencer EE Jr., Dunn WR, Wright RW, et al. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging. Am J Sports Med 2008; 36:99–103 [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y, Yokoya S, Harada Y, Shiraishi K, Adachi N, Ochi M. The prospective evaluation of changes in fatty infiltration and shoulder strength in nonsurgically treated rotator cuff tears. J Orthop Sci 2017; 22:676–681 [DOI] [PubMed] [Google Scholar]
- 36.Kumar D, Karampinos DC, MacLeod TD, et al. Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis Cartilage 2014; 22:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimm A, Meyer H, Nickel MD, et al. Repeatability of Dixon magnetic resonance imaging and magnetic resonance spectroscopy for quantitative muscle fat assessments in the thigh. J Cachexia Sarcopenia Muscle 2018; 9:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm A, Meyer H, Nickel MD, et al. Evaluation of 2-point, 3-point, and 6-point Dixon magnetic resonance imaging with flexible echo timing for muscle fat quantification. Eur J Radiol 2018; 103:57–64 [DOI] [PubMed] [Google Scholar]
- 39.Gerber C, Meyer DC, Fluck M, Benn MC, von Rechenberg B, Wieser K. Anabolic Steroids Reduce Muscle Degeneration Associated With Rotator Cuff Tendon Release in Sheep. Am J Sports Med 2015; 43:2393–2400 [DOI] [PubMed] [Google Scholar]
- 40.Kiefer LS, Fabian J, Rospleszcz S, et al. Assessment of the degree of abdominal myosteatosis by magnetic resonance imaging in subjects with diabetes, prediabetes and healthy controls from the general population. Eur J Radiol 2018; 105:261–268 [DOI] [PubMed] [Google Scholar]
- 41.Alizai H, Nardo L, Karampinos DC, et al. Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol 2012; 22:1592–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am 2014; 96:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santago AC 2nd, Vidt ME, Tuohy CJ, et al. Quantitative Analysis of Three-Dimensional Distribution and Clustering of Intramuscular Fat in Muscles of the Rotator Cuff. Ann Biomed Eng 2016; 44:2158–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Othman AY, Taylor GJ. Traumatic avulsion of the bony insertion of infraspinatus tendon. The Journal of trauma 2001; 50:575–577 [DOI] [PubMed] [Google Scholar]
- 45.Lunn JV, Castellanos-Rosas J, Tavernier T, Barthelemy R, Walch G. A novel lesion of the infraspinatus characterized by musculotendinous disruption, edema, and late fatty infiltration. J Shoulder Elbow Surg 2008; 17:546–553 [DOI] [PubMed] [Google Scholar]
- 46.Kolbe AB, Collins MS, Sperling JW. Severe atrophy and fatty degeneration of the infraspinatus muscle due to isolated infraspinatus tendon tear. Skeletal Radiol 2012; 41:107–110 [DOI] [PubMed] [Google Scholar]


