Abstract
The ATP-binding cassette (ABC) transporter genes are ubiquitous in the genomes of all vertebrates. Some of these transporters play a key role in xenobiotic defense and are endowed with the capacity to effl ux harmful toxic substances. A major role in the evolution of the vertebrate ABC genes is played by gene duplication. Multiple gene duplication and deletion events have been identifi ed in ABC genes, resulting in either gene birth or gene death indicating that the process of gene evolution is still ongoing in this group of transporters. Additionally, polymorphisms in these genes are linked to variations in expression, function, drug disposition and drug response. Single nucleotide polymorphisms in the ABC genes may be considered as markers of individual risk for adverse drug reactions or susceptibility to complex diseases as they can uniquely influence the quality and quantity of gene product. As the ABC genes continue to evolve, globalization will yield additional migration and racial admixtures that will have far reaching implications for the pharmacogenetics of this unique family of transporters in the context of human health.
Keywords: ABCtransporters, evolution, pharmacogenetics
Introduction
Human beings have settled and evolved in diverse habitats and conditions. Toxins present in various environments can threaten health and existence; therefore humans have developed and inherited mechanisms to fend off the effect of harmful xenobiotics. Although cell membranes prevent the entry of some detrimental compounds, others may diffuse into the cell. ATP-binding cassette (ABC) transporters are an alternative defense system, using energy from ATP-hydrolysis to pump xenobiotics out of cells (1). ABC transporters comprise a protein superfamily which is ubiquitous in biology. They can be recognized by a consensus ATP-binding region of approximately 90–110 amino acids, including the Walker A and B motifs, in between which lies the dodecapeptide linker region (Walker C region), and some additional areas of homology upstream and downstream from the Walker A and B motifs. The transporters also usually contain transmembrane (TM) domains, which generally consist of six transmembrane helices that confer substrate specificity. While the ABCs are conserved across all organisms, the TMs can present in various combinations of fused subunits (2). There are 48 known ABC transporters which are expressed in humans.
Though the development of ABC transporters is a pillar in the evolutionary history of man, it could also at times act as a stumbling block: a hindrance to treatment or a causative factor for disease. These efflux pumps are often quite indiscriminate, and therefore have a wide range of substrates including compounds used to treat diseases. They therefore can thwart the pharmacokinetics of drugs in the body and limit the accumulation of drugs within target cells (1). In addition, there are at least 13 genetic diseases, including adrenoleukodystrophy, Tangier disease and Dubin-Johnson syndrome, which have been associated with defects in ABC transporters (2). Thus, it is clear that the proper function of ABC transporters is essential to human health and treatment.
As the global scientific community moves closer to the concept of personalized medicine, it is becoming ever more necessary to research and understand differences in human genotypes. The Human Genome Project revolutionized medical research by documenting individual variations in gene sequences and by identifying single nucleotide polymorphisms (SNPs) and their functional effects (3). These ventures, coupled with the knowledge of the pertinence of ABC transporters to drug delivery and response, brought about considerable interest in the implications of the genotypic variations, polymorphisms and overall evolution of the ABC transporter family. It has been found that polymorphisms in ABC genes are associated with differences in protein expression, function, drug disposition and response (4). In fact, single nucleotide polymorphisms can be markers of individual risk for adverse drug reactions or susceptibility to complex diseases (5). Pharmacogenomics of membrane transporters has emerged as a field to determine the role of such transporter polymorphisms in variation of drug response (6).
Birth and death evolution in ABC transporters
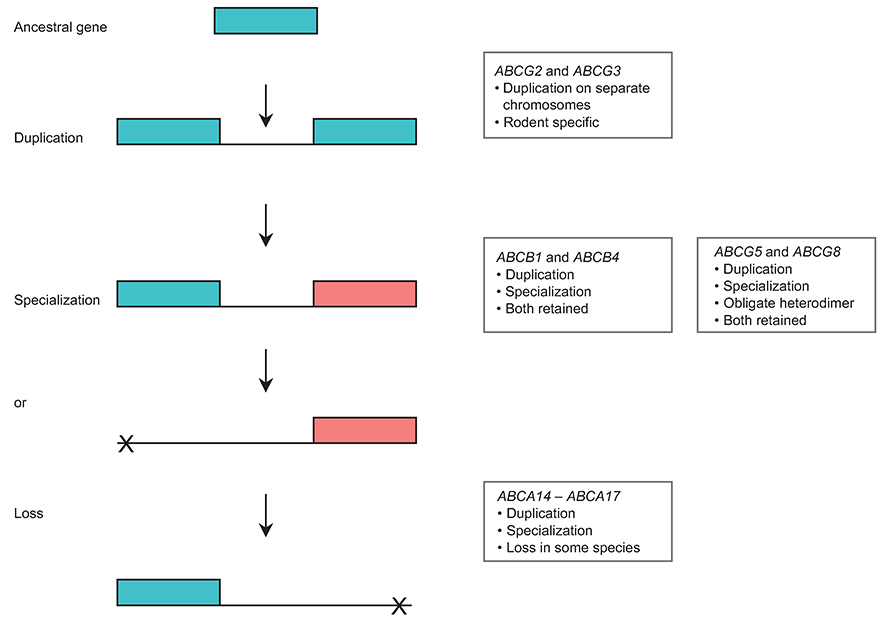
Selective pressure for specific function has epigenetic effects on the continuing evolution of ABC transporters. This continuous evolution is evidenced by multiple duplication and deletion events in ABC genes (7). Studies have revealed three possible scenarios where two identical genes may be generated by duplication (Figure 1). Most frequently, one of the copies is silenced by mutations (pseudogenization) or even completely lost (complete deletion) (Figure 1). In an alternative scenario one copy of the gene may retain its original function, while the other gains or acquires a new or specialized function (Figure 1). This process is called neofunctionalization. Another possibility is subfunctionalization. This occurs when the original function of the gene is separated (either spatially or by timing of expression) between the duplicate copies. ABC transporter genes are highly conserved in evolution and are assumed to encode proteins whose functions have changed very little over time (7). Over 94% of all human ABC genes have an ortholog in each studied mammal, and 85% of the genes are orthologous in chicken, 77% in zebrafish, and 40% in Ciona intestinalis. Additional studies on the functionality of ABC genes in multiple species could shed insight into the process of gene evolution. One of the important processes for supplying raw genetic material for biological evolution is thus gene duplication. Duplication occurs in an individual, and can be fixed or lost in the population, similar to a point mutation. If a new allele comprising duplicate genes is selectively neutral it only has a small probability of being fixed in a diploid population. This fact would suggest that many duplicated genes over time will be lost. If the genes become fixed in the population, the long-term evolutionary fate of duplication will be determined by functions of the duplicate genes. Birth-and-death evolution is a form of independent evolution in which new genes are created. The creation of genes by repeated gene duplication is called ‘gene birth'. Some of these duplicate genes may remain in the genome for a long time, whereas others may be deleted or become non-fuctional. When/if the gene becomes deleted or non-functional it is known as ‘gene death' (7). A number of ABC genes have arisen by the process of duplication leading to gene birth. We have previously documented the active gene duplication process occurring in the ABC transporter family in vertebrates. These events include ancient events, such as the apparent whole-genome duplication in fish as well as more recent events, such as the duplication of the ABCG3 gene which is specific to rodents, and the ABCA10 gene that has been lost in rodents and is a pseudogene in dogs (7). Other duplication events leading to gene birth include duplications of ABCB1 and ABCB4, along with ABCG5 and ABCG8 (Figure 1). On the other hand, gene death in ABC transporters includes the loss of the ABCA14, ABCA15 and ABCA16 genes in primates, the ABCC13 gene in rodents and apes, as well as the loss of the ABCCll gene in rodents.
Figure 1. Gene duplication and fate of duplicated genes in ABC transporters.
The transporter genes ABCG2 and ABCG3 duplicated from an ancestral gene to carry out different specialized functions. ABCB1 and ABCB4 underwent duplication (both located adjacent to each other on the same chromosome) but became specialized to perform distinct functions. ABCG5 and ABCG8 duplicated from the ancestral gene and also became specialized as obligate heterodimers to perform a similar function, while the genes ABCA14-ABCA17 underwent duplication and specialization in certain mammals but were lost in primates.
It is believed that ABC genes have evolved in response to the toxicants and other environmental conditions to which each species is exposed (8). This may be evidenced on a smaller scale through cell culture and the development of acquired mutations. For example, genomic sequencing reveals that wild-type ABCG2 has an arginine at position 482. However, two mutations (R482G and R482T) in the gene may be acquired during the course of drug selection leading to a gain of function. The conditions of drug selection foster an environment that facilitates mutations, which yield differential drug efflux and sensitivity patterns among ABCG2-overexpressing cell lines (9). The continuing evolution of ABC transporters is also apparent through varying allelic frequencies and the range of polymorphic haplotypes among different populations. As a consequence, there is a large number of orthologs of ABC genes. It has been found that variants with reduced function are rarer than variants that retain function, thus reinforcing the notion that ABC transporters are integral to health and development (10). It is not surprising, therefore, that mutations in a number of ABC genes have been implicated in human disease and influence variations in drug metabolism (11). Rare polymorphisms may be more likely to be deleterious than common ones as the affected amino acids are functionally important thus preventing mutations from occurring at high frequency. The scarcity of reduced function polymorphisms suggests that such variants have no evolutionary favorable phenotype, and therefore are not under positive selection pressure (8). In this review we evaluate the implications of the variants of ABCB1, ABCC1,ABCG2, ABCG5 and ABCG8.
ABCB1
Among the members of the ABC transporter family, P-glycoprotein (P-gp) is perhaps the most well-studied member. This transporter is encoded by the human ABCB1 gene, which extends over more than 100 kb and maps to 7q21.1. P-glycoprotein can be found throughout the body, at high levels in the apical surfaces of epithelial cells in tissues, such as the small intestine, the liver, kidneys, blood-brain barrier and placenta (12, 13). It is believed that P-gp plays a role in xenobiotic defense, and as an efflux pump its substrates vary greatly ranging from organic Cations, carbohydrates, and antibiotics to polysaccharides, proteins and anti-cancer drugs. The removal of drugs from intracellular compartments allows P-gp to influence drug absorption, distribution and elimination. It also allows for the development of multidrug resistance (MDR); P-gp is also known as the multidrug resistance gene (MDR1).
Because of the influence of P-gp on pharmacokinetics and pharmacodynamics, it is important to understand the implications of polymorphisms in the ABCB1 gene. There are at least 1630 SNPs of ABCB1, with only 56 reported as non-synonymous SNPs (nsSNPs) (Table 1). However, one of the most interesting ABCB1 SNPs is C3435T, which is a synonymous polymorphism (silent mutation) in exon 26 (13). This SNP is a wobble mutation which translates to isoleucine, and is well conserved in different animal species, from humans to mice and pigs (1). Because it is a silent polymorphism, C3435T does not produce an alteration in the coding sequence, and is thus not expected to change the function of P-gp. However, interestingly, considerable evidence indicates that C3435T and perhaps other silent polymorphisms could affect pharmacogenomics (14). In fact, Kimchi-Sarfaty et al. have demonstrated that even with similar mRNA and protein expression levels, wild-type and polymorphic (C3435T) P-gp function differently due to altered conformations causing variations in substrate specificity (15). Unfortunately, data on C3435T and other ABCB1 SNPs and their consequential effects on pharmacology are not only limited, but the current findings are often contradictory.
Table 1.
Number of SNPs and nsSNPs in ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8 (13).
| ABC transporter |
Number of SNPs |
Number of nsSNPs |
|---|---|---|
| ABCB1 | 1630 | 56 |
| ABCC1 | 2352 | 23 |
| ABCG2 | 854 | 17 |
| ABCG5 | 671 | 16 |
| ABCG8 | 27 |
Source: NCBI, dbSNP.
An example of such confounding results about ABCB1 variations was discussed by Haerian et al. in their meta-analysis of ABCB1 gene polymorphisms and their associated response to anti-epileptic drugs (AEDs) (16). It was initially hypothesized that there is a correlation between ABCB1 and AED response, as the P-gp gene product is highly expressed in the blood-brain barrier thereby protecting the brain from xenobiotics. Furthermore, P-gp is found to be overexpressed in epileptogenic foci, and the wide substrate specificity of P-gp includes hydrophobic compounds, such as AEDs, which are thought to be susceptible to the efflux pump (17–19). It is believed that as a consequence, limited brain uptake in spite of high plasma concentration would lead to resistance to these dmgs. Patients with drug-resistant epilepsy have demonstrated resistance to a broad range of AEDs with varied mode of action, but while some commonly prescribed AEDs have been suggested to be substrates of P-gp, the evidence for the two more common drugs, carbamazepine and valproate, is ambiguous (18, 19). Therefore, when considering specific AEDs it is difficult to ascertain whether P-gp influences the action of, or individual response to, these drugs.
There is even further ambiguity upon consideration of the implications of specific genotype variations induced by ABCB1 polymorphisms. While some studies determined that the CC genotype of C3435T is more likely to induce pharmacoresistance, others show that resistance is actually associated with the TT genotype instead, and yet others show that there is no association at all (18). We think that it is plausible to conclude that the CC genotype leads to drug resistance as it has been demonstrated that it yields increased P-gp gene product; this would presumably lead to lower intracellular concentrations and reduced anti-seizure efficacy of AEDs (18). Conversely, the TT genotype has been linked to lower P-gp expression, and thus is expected to be associated with better seizure control (13, 18, 20). However, there may be other factors influencing function of the gene product that play a role in the phenotypic characteristics and poor pharmacologic response brought about by the TT genotype in some populations.
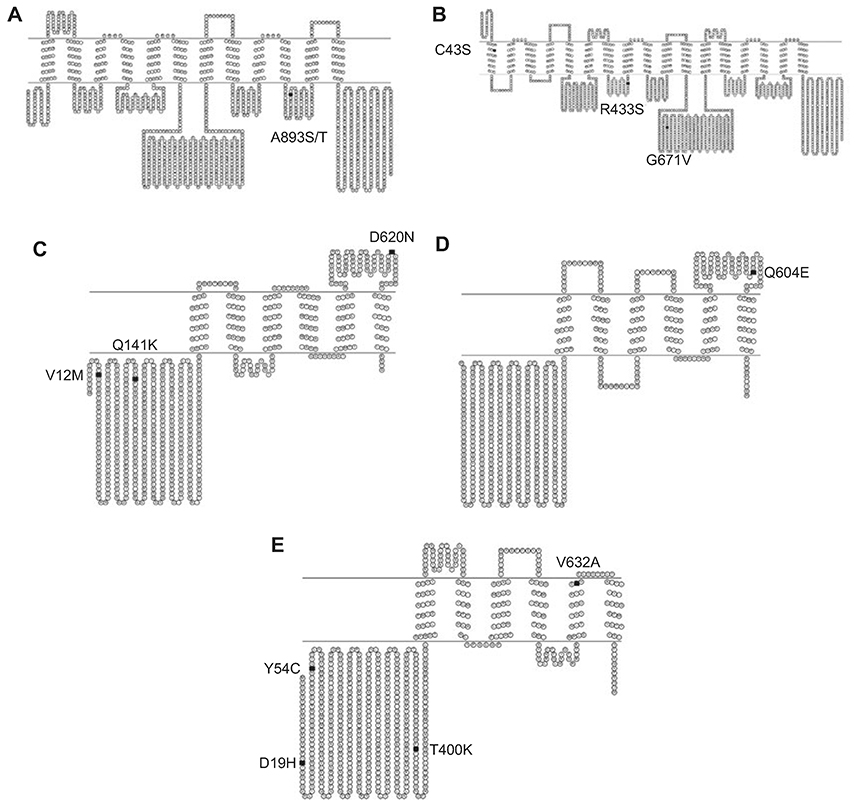
Confounding results may be due to inherent differences between the cohorts used to investigate ABCB1 polymorphisms, including age, gender, epilepsy syndrome and medication type (18). It may also be that investigators varied in their definition of treatment outcomes in terms of drug resistance and drag responsiveness (16, 21). It is also important to note that the frequency of the 3435 polymorphism varies according to ethnicity. For example, Africans have significantly higher wild-type (C) allele; the frequency distribution forms a pattern which corroborates the notion that the MDR1 genotype gradually changed in the course of human migration, which originated from Africa (1). Frequency of ABCB1 polymorphisms, which varies with race/ethnicity, may influence P-gp activity and/or AED responsiveness. These variations may be subtle or completely inverse as was found by Seo et al. when comparing their results of Japanese epileptic patients to previously determined results in European patients (19). Furthermore, some investigators contend that variable results among studies may have arisen due to specific combinations of polymorphisms that are commonly inherited together (MDR1 haplotypes), rather than by a single allele polymorphism (21). In fact, linkage analysis confirms that the C3435T SNP is associated with several other SNPs in ABCB1 including the non synonymous G2677T/A (A893S or A893T, Figure 2) in exon 21 and the synonymous C1236T in exon 12 which encodes glycine (1, 16). For these and other SNPs, patterns of linkage disequilibrium vary with race. As a consequence their haplotypes vary among different races; the CGC haplotype is predominant in people of African origin, whereas the TTT haplotype is the most predominant genotype among Asian and Indian populations (1). These differing haplotypes and allelic distributions could potentially yield variations in drug distribution and response among different races.
Figure 2. Selected non-synonymous SNPs in ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8.
(A) The SNP A893S/T (black square) depicted in a topological diagram of ABCB1. (B) SNPs C43S (TM1), R433S and G671V (NBD1) (black squares) located in ABCC1. (C) SNP's V12M, Q141K (NBD) and D620N (extracellular loop) (black squares) in ABCG2. (D) Topological diagram of ABCG5 depicting the SNP Q604E (black square) implicated in sitosterolemia. (E) The SNPs D19H, Y54C, T400K and V632A (black squares) in a topological diagram of ABCG8.
Equivocal results about ABCB1 polymorphisms, particularly C3435T, are not unique to epilepsy and AED response. There has been contradictory data for the role of ABCB1 variants and their implications in diseases, such as inflammatory bowel disease, Alzheimer's disease, HIV and schizophrenia (13, 22, 23). Despite confounding data about C3435T, however, it is important to note that the association of ABCB1 with pharmacoresistance in disease seems to be highly probable even when we keep in mind the effects of other polymorphisms and genetic factors in the complex interplay of disease and environment. Besides the handful of candidate genes that have been investigated as affecters of disease and/or drug response, there are many more genes that could theoretically contribute to the ailments or to the pharmacoresistance. These include the other ABC transporters that are expressed in the tissue or organs under investigation; they may contribute to reduced penetration of drugs in patients, and thus increased drug resistance and reduced drug response (18).
ABCC1
ABCC1, also known as multidrug resistance protein 1 (MRP1), is an ABC transporter which has been widely studied in the context of pharmacogenetics and cancer (24). The ABCC1 gene maps to 16p13.1, and is expressed on the basolateral membrane of epithelial cells in most tissues (13). It is expressed in most tissues in the human body with relatively higher levels in the lung, testis, kidney, muscle and peripheral mononuclear cells (25).ABCC1 has a wide range of substrates including a diverse range of organic anion conjugates, such as glutathione conjugates, glucuronides, glutathione disulfide, unconjugated anionic drugs and dyes (13).
There are 23 nsSNPs in ABCC1 (Table 1), many of which have been screened in human populations or used for expression-related efflux studies in HEK293T cells (8, 13, 26). These studies have revealed a naturally occurring mutation in exon 10, G1299T, which results in Arg433Ser (Figure 2), as a cause for altered phenotype with respect to transport of endogenous substrates and cellular resistance to doxorubicin (26). Specifically it has been determined that the G1299T polymorphism causes increased resistance to doxorubicin, and decreased transport of leukotriene C4 (LTC4), which is a signaling compound for the migration of dendritic cells (13). Another ABCC1 polymorphism of interest is G128C, which results in the Cys43Ser (Figure 2) mutation located in exon 2 (TM1). This mutation has been associated with disrupted trafficking of MRP1 to the plasma membrane of cells, and reduced resistance to vincristine and arsenite as compared to the wild type (8, 13). The remaining nsSNPs of MRP1 have not yet been definitively proven as deleterious. For example, a mutation in exon 16, G2012T (Table 2), which causes a substitution of a highly conserved glycine residue to valine at position 671 (Figure 2), has been shown not to affect transport activity of MRP1, although predictions by both SIFT and polyphen indicated the Gly671Val polymorphism to be damaging (13, 26). Though more research is warranted to absolutely determine the implications of G2012T, there are initial findings that indicate this polymorphism can alter mRNA stability of ABCC1. The variant T allele correlates to less stable transcript, which may contribute to reduced efflux, and therefore an improved chemotherapeutic outcome. Pajic et al. have demonstrated that the presence of the variant allele has been associated with an improved outcome, whereas high ABCC1 expression correlates to a poor clinical outcome for patients with neuroblastoma (26). The authors highlight that MRP1 seems to have a particular relevance in MDR for this disease. This is quite plausible as MRP1, though ubiquitous, is particularly prominent in the brain and is a causative factor for resistance to anti-cancer drugs including anthracyclines, epipodophyllotoxins, vinca alkaloids and camptothecin (13). MRP1 does not confer resistance to alkylating agents, such as cisplatin and cyclophosphamide, which are commonly used to treat neuroblastoma. However, the conjugates of these drugs can be transported by MRP1 (26).
Table 2.
Disease associations, single nucleotide polymorphisms and corresponding amino acid changes for the ABC transporters: ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8.
| ABC transporter |
Associated disease |
Single nucleotide polymorphism (SNP) |
Amino acid position and change |
|---|---|---|---|
| ABCB1 | Infl ammatory bowel disease | C3435T | – |
| G2677T/A | A893S or A893T | ||
| C1236T | – | ||
| ABCC1 | Chronic obstructive pulmonary disease; cystic fibrosis | G1299T | R433S |
| G128C | C43S | ||
| G2012T | G671V | ||
| rs35621 [C/T] | – | ||
| rs212903 [C/T] | – | ||
| rs4148382 [A/G] | – | ||
| ABCG2 | Gout | G238A | V12M |
| C625A | Q141K | ||
| G2062A | D620N | ||
| ABCG5 | Sitosterolemia | C1950G | Q604E |
| ABCG8 | Sitosterolemia; gallstone disease | G145C | D19H |
| A251G | Y54C | ||
| C1289A | T400K | ||
| T1985C | V632A |
When considering evolutionary variation, it is also important to note that human MRP1 displays properties which vary significantly from some of its orthologs and closest homologs with respect to physiological function, substrate selectivity, tissue distribution and membrane localization (8). In humans, ABCC1 plays an important role in the efflux of xenobiotics from the lungs. In the lungs, ABCC1 is expressed in the alveolar macrophages, bronchial epithelium and hyperplastic reactive type II pneumocytes (25). Thus, it is not surprising that polymorphisms in this efflux pump have been cited to affect lung function. The frequencies of these polymorphisms show marked differences between ethnicities, and further research is warranted to understand what implications they may have among varying races (27).
In Dutch populations, Siedlinski et al. have determined that there is a significant relationship between ABCC1 SNPs and lung function. Using two independent cohorts, the authors found putative candidates for studies aiming to prevent chronic obstructive pulmonary disease (COPD), and thus highlighted targets for pharmacogenetic therapies. The authors previously demonstrated that ABCCl is expressed at a lower level in the bronchial epithelium of COPD patients than in healthy controls, with even further reduction in more COPD stages. Subsequently, Siedlinski et al. identified the TT genotype for the rs35621 SNP (intron 14) as a risk factor for excessive decline of the forced expiratory volume measured in 1 second (FEV1). The minor allele (GG genotype) for rs212093, located in the 3'untranslated region of ABCC1, was also associated with a decline in FEV1. Interestingly, Siedlinski et al. pointed out that the minor allele (AA genotype) of rs4148382 (3' untranslated region) seems to be protective, in that this SNP is significantly associated with a higher FEV1. FEV1 is an important lung function which is particularly hindered in COPD, a disease characterized by slowly progressive airflow limitation, primarily caused by inhalation of airway irritants, such as cigarette smoke. COPD is highly prevalent and is a leading cause of smokingrelated mortality worldwide, thus it is of utmost importance to understand the etiology and possible treatments of this disease (24).
Polymorphisms of ABCC1 are also believed to be associated with the severity of cystic fibrosis (CF). ABCC2 and ABCC7, the CF transnrembrane conductance regulator (CFTR) gene, share the highest homology among the ABC transporter superfamily, and MRP-CFTR regulatory interaction has been reported. Therefore, ABCCl has been suggested as a modifier gene for this disease, which is the most common fatal inherited disorder in the Caucasian population. CF is characterized by bronchopulmonary disease, pancreatic insufficiency and male infertility. Flowever, its severity can vary widely and patients with identical CF genotypes can display markedly different phenotypic expression (28). It is thus necessary to understand the complex relationship between subtle variations in genotype and consequential phenotype.
ABCG2
ABCG2, also known as breast cancer resistance protein (BCRP), is a multidrag resistance protein that is a member of the ABC family of drug transporters. It can render tumor cells resistant to the anti-cancer drugs topotecan, mitoxantrone, doxorubicin, and daunorubicin. ABCG2 maps to chromosome 4q22 and encodes a half transporter with a nucleotide-binding fold-transmembrane domain orientation (Figure 2) (29, 30). There are 17 nsSNPs in the ABCG2 transporter (Table 1) (13). Of these polymorphisms, SNPs at nucleotide G238A (V12M; exon 2), nucleotide C625A (Q141K; exon 5) and nucleotide G2062A (D620N; exon 16) have been well characterized (Figure 2). Using an unlinked sample set of 90 ethnically diverse DNA samples from the DNA Polymorphism Discovery Resource, Honjo et al. reported that the most frequently occurring polymorphisms were observed at amino acid 12 and amino acid 141. These exhibited frequencies of 22% heterozygous and 1.1% homozygous, and 10% heterozygous and 1.1% homozygous, respectively. The SNP at amino acid 620 exhibited no significant heterozygosity, with a frequency of 1.1% for the minor homozygous allele (31). Because these samples were unlinked, exact genetic origins of the samples were not made. Thus, it should be noted that there are significant differences in the frequency distribution of the enumerated ABCG2 SNPs between populations (32).
It has been found that the VI2M and Q141K SNPs (Figure 2 and Table 2) were the most frequent polymorphisms in various ethnic and racial groups, including Caucasian, Asian and Swedish populations. The V12M SNP was found from as high as 100% in the Mexican-Indian population to as low as 4.7% in the Caucasian population. The Q141K SNP was the most prevalent allele in both the Japanese and Chinese populations with an allelic frequency of 35% (33). Because of their considerable frequency, investigation of the impact of these SNPs on ABCG2 expression and behavior is of importance (34). Of the three nsSNPs, V12M and Q141K are located in the region of the ATP binding domain (Figure 2); the polymorphism at amino acid 12 is located near the ATG start site, while amino acid 141 lies between the Walker A motif and the C signature region. D620N is located between the last two transmembrane segments, and may be extracellular (Figure 2). At these sites, it is believed that there is little impact on substrate binding. Therefore, although it was found that ABCG2 is well conserved, it was concluded by Honjo et al. that the described amino acid polymorphisms were unlikely to alter transporter stability or function (31).
Using predictive studies it seemed unlikely that any of the nsSNPs would affect ABCG2 expression and function. However, further investigation suggests that the Q141K SNP affects transport efficiency of ABCG2 and may result in altered pharmacokinetics or drug resistance profiles in clinical oncology (34). Using transfected embryonic kidney cells (HEK-293), Morisaki et al. compared behavior of ABCG2 variants to the wild type. Through four-day cytotoxicity assays, they demonstrated that cells expressing Q141K ABCG2 had IC50 values for mitoxantrone, topotecan, SN-38 and diflomotecan that were as much as 5-fold lower than those expressing comparable levels of wild-type or V12M ABCG2. By examining drug accumulation and ATPase activity, this group further elucidated that the Q141K polymorphism impaired die activity of the ABCG2 protein (34). These results were corroborated by Mizuarai et al., who also concluded that Q141K exhibited reduced drug resistance activity and increased drug accumulation in polarized LLC-PK1 cells (35). Using this cell line, the Mizuarai group also found that V12M transfected cells exhibited increased sensitivity to ABCG2 substrate drugs, which deviates from the findings of Morisaki et al., who demonstrated that Q141K was the only polymorphism that affected pharmacogenetics of ABCG2. The porcine kidney LLC-PK1 cells were thought to be an appropriate system to study the possible pharmacological implications of ABCG2 SNPs. Since ABCG2 protein localizes to the apical side of cells in several normal tissues, it was believed that the localization of ABCG2 in LLC-PK1 may have mimicked the physiological state of the transporter accurately. However, the different findings for the V12M polymorphism observed between the HEK293 and LLC-PK1 systems point to differences in the experimental systems as regulation of protein expression differs among organisms, tissues and cell lines (35). Thus, these differences may be inherently due to variations in the cellular context, selective pressure and/or evolution of the species from which the cell lines are derived.
Further clinical studies have implicated Q141K in affecting drug metabolism. The polymorphism, which is caused by the C625A [also referred to as 421C>A by Sparreboom et al. (30)] SNP, has been identified by Sparreboom et al. to significantly affect the pharmacokinetics of diflomotecan. Using a cohort of 22 adult white patients with cancer, Sparreboom et al. (30) demonstrated that patients carrying a defective ABCG2 C625A allele have elevated plasma concentrations of diflomotecan as compared with those patients with two wild-type alleles. These results are consistent with in vitro work, which suggested that carriers of the ABCG2 C625A allele may have decreased clearance and/or increased oral bioavailability of ABCG2 substrate drugs. It should be noted that these results were specific to ABCG2 genotype, as diflomotecan levels were not significantly influenced by variants in ABCB1, ABCC2, cytochrome P450 (CYP) 3A4, and CYP3A5 genes. Due to the small size and ethnic uniformity of this cohort, further investigation into the effects of ABCG2 variants on drug disposition in other populations is warranted. However, these preliminary findings corroborate the notion that a high-frequency A allele in certain ethnic groups may have therapeutic and prognostic implications for individuals treated with ABCG2 substrate drugs (30).
The Q141K polymorphism in ABCG2 is not only thought to affect inter-individual variability for drug metabolism. Using a genonre-wide association study, Dehghan et al. have identified the Q141K polymorphism as a risk factor for gout (36). This genetic variation in ABCG2 encodes a uric acid transporter, and its association to gout was subsequently evidenced in diverse populations. ABCG2 is a urate efflux transporter, and Q141K is a loss-of-function mutation which has been associated with hyperuricemia and gout in Japanese, Pacific Islanders, Han Chinese, Caucasian and AfricanAmerican populations (36–40). The lysine allele of Q141K encodes a transporter with approx. 50% reduced activity. This leads to elevated uric acid levels and prevalent gout (37, 38). Hyperuricemia is not only a key risk factor for gout, but it has also been associated with cardiovascular conditions and diseases, such as hypertension, diabetes, metabolic syndrome, stroke and coronary heart disease (37). Understanding the genetic association of ABCG2 polymorphisms and hyperuricemia may be a key to mitigating the negative consequences of this physiological condition.
Yamagishi et al. have demonstrated the importance of this research in the Japanese population, in which they have shown the frequency of the risk allele to be as high as 31%. Their studies have further shown that carriers of the risk allele in the Japanese population have statistically significant higher levels of uric acid than major allele homozygotes. The association of the causal ABCG2 variant C625A (leading to Q141K) and gout was thus confirmed in the sample of Japanese ancestry. Interestingly, Yamagishi et al. also pointed out that traditionally gout was rarely observed among Japanese people, but presumably due to rapid changes in lifestyle after World War II, as more Japanese adopted Western lifestyles, there was a concomitant increase in the prevalence of gout. The authors believe their findings are indicative of potential gene-environment interaction in relation to gout/uric acid resulting in an increased incidence of gout (37).
Phipps-Green et al. have demonstrated similar effects of the selective pressure of the environment on genetic variation and susceptibility to disease. By studying the effect of Polynesian migrations on the prevalence of gout, this group concluded that the ABCG2 gout risk allele (C625A) has a strong effect only in people of Western Polynesian ancestry (Tonga, Samoa, Niue, Tokelau) as opposed to Eastern Polynesians (Maori and Cook Islands). These results indicate that there are specific sub-population differences, which ought to be accounted for when undertaking biomedical genetic research. Although a group may be defined by geographical region and shared ancestry, migratory events create bottlenecks and alter genetic structure in the founder populations. The authors point out that the differences observed between Eastern and Western Polynesia at ABCG2 are more likely due to neutral processes, such as migration and genetic drift than to adaptation (38). Thus, the conclusions of the Yamigishi and Phipps-Green groups have highlighted the importance of environmental pressures on the evolution, penetrance and behavior of SNPs in genes for various populations.
ABCG5 and ABCG8
ABCG5 and ABCG8 genes encode for proteins of 651 and 673 amino acids, respectively, which share 28% identity. These two members of the ABC transporter family are half transporters that each contain an N-terminal ATP-binding motif (Walker A and Walker B motifs), and six transmembrane segments in the terminus (Figure 2). ABCG5 and ABCG8 are located in close proximity, in a head-to-head configuration on chromosome 2p21. The close proximity and opposite orientation of these two genes suggest that they have a bidirectional promoter and share common regulatory elements (41). Both transporters are expressed mainly in the liver and intestine, and may form hetrodimers; they may cooperate to regulate intestinal absorption and biliary excretion of sterols (5, 42).
There are 16 nsSNPs in ABCG5 and 27 nsSNPs in ABCG8 (13) (Table 1). Individuals who carry mutations in ABCG5 and ABCG8 are predisposed to sterol accumulation, atherosclerosis, coronary artery disease and gallstone disease (41, 43, 44). Several of these mutations have been found in patients with sitosterolemia (5). Sitosterolemia is a rare inherited disease which is characterized by elevated levels of plant or fish sterols in plasma, most importantly sitosterol (24-ethyl cholesterol) (13). Although mutations that cause sitosterolemia are extremely rare, more common sequence variants in these genes may have more subtle effects on sterol metabolism, and may contribute to inter-individual variation in the plasma concentrations of plant sterols (45). However, unlike patients with other forms of hyperlipidemia, Berge et al. report that sitosterolemic subjects respond to restriction in dietary cholesterol and to bile acid resin treatment with considerable reductions in plasma cholesterol levels (41). This result strongly suggests that the disease is correlated to an altered, diminished or loss of function of the transporters, as opposed to other exogenous factors to which the condition could be attributed. Furthermore, it should be noted that hyperabsorption and inefficient secretion are not limited to plant sterols. Sitosterolemic subjects also absorb a higher fraction of dietary cholesterol and secrete less cholesterol into bile than normal subjects. It was thus hypothesized by Berge et al. that there is a lack of gene product in patients with sitosterolemia that normally limits the absorption and accelerates the biliary excretion of sterols (41).
Researchers have sought after the cause of the genetic mishap which causes sitosterolemia and its associated effects. Weggemans et al. have investigated one such cand;idate in a polymorphism in ABCG5. They studied the association between the ABCG5 SN;P, C1950G, which encodes for Gln604Glu, and blood cholesterol concentrations in a clinical cohort of 486 subjects. Their findings indicated that the response of serum total cholesterol to dietary cholesterol tended to be larger in subjects with the homozygous G genotype as compared to carriers of the homozygous major C allele. The authors demonstrated that subjects with the G/G genotype had higher baseline cholesterol than carriers of the C allele, and that the response of total serum cholesterol to dietary cholesterol tended to increase with the number of G alleles. Though the trend was not statistically significant, it increased corroboration for the argument that differences in responsiveness to dietary cholesterol are caused by differences in absorption efficiency. It is thus predicted that the G allele of the polymorphism is associated with higher cholesterol absorption than the C allele (42).
Based on various studies, there are currently contrasting opinions on the extent of clinical implications of polymorphisms in ABCG5 and ABCG8. After conducting a meta-analysis of these studies, and investigating five common ABCG5/G8 polymorphisms (Q604E, D19H, Y54C, T400K, and A632V) Jakulj et al. dismissed current findings as unsubstantial (46). It is also debated whether the polymorphisms influence disease directly or if they act in conjunction with other genes, or perhaps through linkage disequilibrium with other polymorphisms (44, 45). However, in spite of these concerns and critiques, it is commonly accepted that ABCG5 and ABCG8 play a major role in proper lipid metabolism, and therefore could be implicated in diseases which lack that function. For example, various studies have shown that the SNPs D19H and T400K are associated with cholesterol gallstones in patients (44,45). These results were corroborated for D19H in ABCG8 using a clinical study of gallstone disease (GSD) in an Indian population. Gallstones are mainly cholesterol monohydrate crystals, and consequently precipitate if the amounts of cholesterol or bilirubin in the gallbladder exceed solubility (44). Siddapurum et al. point out that there are significant differences in GSD prevalence in various populations, which cannot be completely explained by environmental factors. In fact the authors hypothesize that populations sharing the same environment with high differences in prevalence of the disease can only be explained by genetic predisposition. Their study demonstrated that the heterozygous variant allele of SNP D19H was significantly higher in the GSD patients as compared to individuals who were disease free. Further, the mutant allelic (heterozygous+mutant homozygous) distribution was also found to be more statistically significant in GSD patients than in controls. It is thus hypothesized that the histidine allele of the polymorphism leads to increased ABCG8 transporter activity and may be associated with more efficient transport of cholesterol into the bile (44, 45). Such supersaturation of bile with cholesterol represents a common defect in patients with cholesterol gallstones (44).
In considering the genetics and evolution of ABCG5 and ABCG8, it is interesting to note that there are heritable inter-individual variations in the plasma concentrations of sterols, which these genes metabolize. These results were determined by Berge et al. who demonstrated that their results are strongly influenced by genetic factors, which were not confounded by shared environmental factors (45). Although immediate environmental factors are unlikely to influence individual response to lipid metabolism, it is important to consider how individual environment, in the form of available diet, has impacted the evolution of the ABCG5 and ABCG8 transporters which act in food digestion. Populations evolving in different environments, thus consuming different diets (perhaps varying types or levels of sterols), may consequently develop varying polymorphisms to aid in the processing of their foods. This hypothesis may be corroborated by the theory that genetic variation in selected SNPs, haplotypes and copy number variants can have a dramatic effect on the response to dietary components, food preferences and their optimal utilization (47). It is also believed that micronutrients and botanicals can interact with the genome, modify gene expression, alter protein and metabolite composition within cells, and even participate in the DNA repair and replication process. These concepts subtend the studies of nutrigenomics and nutrigenetics, which have been reviewed in great detail by Subbiah (3, 47). Properly understanding these fields may lead to better mechanisms and/or ethnopharmacologic approaches to mediate the effects of polymorphisms in ABCG5 and ABCG8.
Conclusions
In studying the evolution of ABC transporters and the implications of their polymorphisms, it is clear that there are complex gene-environment factors which affect;disease and drug metabolism in genetically susceptible individuals (37). Pharmacogenetic approaches could be the source of detangling the labyrinth of human genotype and human health; optimizing treatments according to genotype and phenotype would increase the efficiency of treatment, reduce morbidity and mortality, and reduce health costs overall (48). Understanding the genotype of ABC transporter polymorphisms may even provide nutraceutical avenues for the control of disease and maintenance of health.
As has been demonstrated through our analysis of ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8, there are a plethora of consequences for changes in genotype. Depending on the identity and location of the SNP, the mechanism of their genetic influence would differ, ranging from gene expression, stability, structure, resulting amino acid, or function of the protein. Phenotypically this could manifest in variations in individual side effects to drugs, altered drug efficacy, multidrug resistance profile or the emergence of disease (49). Diversity in haplotype and LD patterns may cause further changes and add to the complex interactions of genes, the environment and the individual response (16).
In this short review we have evaluated a limited number of the vast possibilities of consequences for polymorphisms in ABC transporters. Though some of the results are not definitive, it is indisputable that the genes have evolved, and continue to evolve, bringing about critical influences on human health and treatment. From our review it is evident that partial isolation of human populations, migration, diet, and other environmental and cultural pressures have allowed genotypes to evolve uniquely; an allele may become more frequent in one population, but not in another (16). As these genes evolve, and globalization yields additional migration and racial admixtures, further investigations are warranted to completely explore and comprehend the complex implications ABC transporters have for pharmacogenetics and disease in humans.
Acknowledgments
We would like to thank Alexander Borsa (National Cancer Institute at Frederick, MD) for assistance with the figures. This research was supported (in part) by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest statement
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article. Research support played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
References
- 1.Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta 2009;1794:860–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr 2001;33:453–8. [DOI] [PubMed] [Google Scholar]
- 3.Subbiah MT. Personalizing our diet to improve our health: the potential impact of nutrigenomics. Pers Med 2007;4:233–6. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Chong SS, Lee CG. Characterization of single nucleotide polymorphisms in 13 members of the ABC drug transporter genes in three different populations. Open Pharmacol J 2007;1:1–12. [Google Scholar]
- 5.Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, et al. Catalog of 605 single nucleotide polymorphisms (SNPs) among 13 genes encoding human ATP-binding cassette transporters: ABCA4, ABCA7, ABCA8, ABCD1, ABCD3, ABCD4, ABCE1, ABCF1, ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8. J Hum Genet 2002;47:285–310. [DOI] [PubMed] [Google Scholar]
- 6.Yee SW, Chen L, Giacomini KM. Pharmacogenomics of membrane transporters: past, present and future. Pharmacogenomics 2010;11:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moitra K, Dean M. Evolution of ABC transporters by gene duplication and their role in human disease, Biol Chem 2011:392: 29–37. [DOI] [PubMed] [Google Scholar]
- 8.Letoumeau IJ, Deeley RG, Cole SP. Functional characterization of non-synonymous single nucleotide polymorphisms in the gene encoding human multidrug resistance protein 1 (MRP1/ABCC1). Pharmacogenet Genom 2005;15:647–57. [DOI] [PubMed] [Google Scholar]
- 9.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, et al. Acquired mutations in the MXR/BCRP/ ABCP gene alter substrate specificity in MXR/BCRP/ABCPoverexpressing cells. Cancer Res 2001;61:6635–9. [PubMed] [Google Scholar]
- 10.Kroetz DL, Yee SW, Giacomini KM. The pharmacogenomics of membrane transporters project: research at the interface of genomics and transporter pharmacology. Clin Pharmacol Ther 2010;87:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annilo T, Chen ZQ, Shulenin S, Costantino J, Thomas L, Lou H, et al. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics 2006;88:1–11. [DOI] [PubMed] [Google Scholar]
- 12.Ansermot N, Rebsamen M. Chabert J, Fathi M, Gex-Fabry M, Daali Y, et at Influence of ABCB1 gene polymorphisms and P-glycoprotein activity on cyclosporine pharmacokinetics in peripheral blood mononuclear cells in healthy volunteers. Drug Metab Lett [Controlled Clinical Trial Research Support, Non-U.S. Govť] 2008;2:76–82. [DOI] [PubMed] [Google Scholar]
- 13.Wang LL, Liu YH, Meng LL, Li CG, Zhou SF. Phenotype prediction of non-synonymous single-nucleotide polymorphisms in human ATP-binding cassette transporter genes. Basic Clin Pharmacol Toxicol 2011:108:94–114. [DOI] [PubMed] [Google Scholar]
- 14.Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. Silent polymorphisms speak: how they affect pharmacogenomics and the treatment of cancer. Cancer Res 2007;67:9609–12. [DOI] [PubMed] [Google Scholar]
- 15.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science 2007;315:525–8. [DOI] [PubMed] [Google Scholar]
- 16.Haerian BS, Lim KS, Tan CT, Raymond AA, Mohamed Z. Association of ABCB1 gene polymorphisms and their haplotypes with response to antiepileptic drugs: a systematic review and meta-analysis. Pharmacogenomics 2011;12:713–25. [DOI] [PubMed] [Google Scholar]
- 17.Meng H, Guo G, Ren J, Zhou H, Ge Y, Guo Y. Effects of ABCB1 polymorphisms on plasma carbamazepine concentrations and pharmacoresistance in Chinese patients with epilepsy. Epilepsy Behav 2011;21:27–30. [DOI] [PubMed] [Google Scholar]
- 18.Szoeke C, Sills GJ, Kwan P, Petrovski S, Newton M, Hitiris N, et al. Multidrug-resistant genotype (ABCBl) and seizure recurrence in newly treated epilepsy: data from international pharmacogenetic cohorts. Epilepsia 2009;50:1689–96. [DOI] [PubMed] [Google Scholar]
- 19.Seo T, Ishitsu T, Ueda N, Nakada N, Yurube K, Ueda K, et al. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics 2006;7:551–61. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000;97:3473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bournissen FG, Moretti ME, Juurlink DN, Koren G, Walker M, Finkelstein Y. Polymorphism of the MDR1/ABCB1 C3435T drug-transporter and resistance to anticonvulsant drugs: a meta-analysis. Epilepsia 2009;50:898–903. [DOI] [PubMed] [Google Scholar]
- 22.Onnie CM, Fisher SA, Pattni R, Sanderson J, Forbes A, Lewis CM, et al. Associations of allelic variants of the multidrug resistance gene (ABCBl or MDR1) and inflammatory bowel disease and their effects on disease behavior: a case-control and meta-analysis study. Inflamm Bowel Dis 2006;12:263–71. [DOI] [PubMed] [Google Scholar]
- 23.Kohen R, Shofer JB, Korvatska O, Petrie EC, Wang LY, Schellenberg GD.et al. ABCBl genotype and CSF {betaj-amyloid in alzheimer disease, J Geriatr Psychiatry Neurol 2011;24:63–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siedlinski M, Boezen HM, Boer JM, Smit HA, Postma DS. ABCC1 polymorphisms contribute to level and decline of lung function in two population-based cohorts. Pharmacogenet Genom 2009;19:675–84. [DOI] [PubMed] [Google Scholar]
- 25.Conseil G, Deeley RG, Cole SP. Polymorphisms of MRP1 (ABCC1) and related ATP-dependent drug transporters. Pharmacogenet Genom 2005;15:523–33. [DOI] [PubMed] [Google Scholar]
- 26.Pajic M, Murray J, Marshall GM. Cole SP, Norris MD, Haber M. ABCC1 G2012T single nucleotide polymorphism is associated with patient outcome in primary neuroblastoma and altered stability of the ABCC1 gene transcript. Pharmacogenet Genom 2011;21:270–9. [DOI] [PubMed] [Google Scholar]
- 27.Oselin K, Mrozikiewicz PM, Gaikovitch E, Pahkla R, Roots I. Frequency of MRP1 genetic polymorphisms and their functional significance in Caucasians: detection of a novel mutation G816A in the human MRP1 gene. Eur J Clin Pharmacol 2003:59:347–50. [DOI] [PubMed] [Google Scholar]
- 28.Mafficini A, Ortombina M, Sermet-Gaudelius I, Lebecque P, Leal T, Iansa P, et al. Impact of polymorphism of multidrug resistance-associated protein 1 (ABCC1) gene on the severity of cystic fibrosis. J Cyst Fibros 2011;10:228–33. [DOI] [PubMed] [Google Scholar]
- 29.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst 2000;92:1651–6. [DOI] [PubMed] [Google Scholar]
- 30.Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P, et al. Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther 2004;76:38–44. [DOI] [PubMed] [Google Scholar]
- 31.Honjo Y, Morisaki K, Huff LM, Robey RW, Hung J, Dean M, et al. Single-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1). Cancer Biol Ther 2002;1:696–702. [DOI] [PubMed] [Google Scholar]
- 32.Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol 2006;25:231–59. [DOI] [PubMed] [Google Scholar]
- 33.Zamber CP, Lamba JK, Yasuda K, Famum J, Thummel K, Schuetz JD, et al. Natural allelic variants of breast cancer resist tance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics 2003;13:19–28. [DOI] [PubMed] [Google Scholar]
- 34.Morisaki K, Robey RW, Ozvegy-Laczka C, Honjo Y, Polgar O, Steadman K, et al. Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol 2005:56:161–72. [DOI] [PubMed] [Google Scholar]
- 35.Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int J Cancer 2004:109:238–16. [DOI] [PubMed] [Google Scholar]
- 36.Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 2008;372:1953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamagishi K, Tanigawa T. Kitamura A, Kottgen A, Folsom AR, Iso H. The rs2231142 variant of the ABCG2 gene is associated with uric acid levels and gout among Japanese people. Rheumatology (Oxford) 2010;49:1461–5. [DOI] [PubMed] [Google Scholar]
- 38.Phipps-Green AJ, Hollis-Moffatt JE, Dalbeth N, Merriman ME, Topless R, Gow PJ, et al. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, Case and control sample sets. Hum Mol Genet 2010;19:4813–9. [DOI] [PubMed] [Google Scholar]
- 39.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA 2009:106:10338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B, Miao Z, Liu S, Wang J, Zhou S, Han L, et al. Genetic analysis of ABCG2 gene C421A polymorphism with gout disease in Chinese Han male population. Hum Genet [Research Support, Non-U.S. Govť] 2010;127:245–6. [DOI] [PubMed] [Google Scholar]
- 41.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 2000;290:1771–5. [DOI] [PubMed] [Google Scholar]
- 42.Weggemans RM, Zock PL, Tai ES, Qrdovas JM, Molhuizen HO, Katan MB. ATP binding cassette G5 C1950G polymorphism may affect blood cholesterol concentrations in humans. Clin Genet 2002;62:226–9. [DOI] [PubMed] [Google Scholar]
- 43.Hubacek JA, Berge KE, Stefkova J, Pitha J, Skodova Z, Lanska V, et al. Polymorphisms in ABCG5 andABCG8 transporters and plasma cholesterol levels. Physiol Res 2004;53:395–401. [PubMed] [Google Scholar]
- 44.Siddapuram SP, Mahurkar S, Duvvuru NR, Mitnala S, Guduru VR, Rebala P, et al. Hepatic cholesterol transporter ABCG8 polymorphisms in gallstone disease in an Indian population. J Gastroenterol Hepatol 2010;25:1093–8. [DOI] [PubMed] [Google Scholar]
- 45.Berge KE, von Bergmann K, Lutjohann D, Guerra R, Grundy SM, Hobbs HH, et al. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res 2002;43:486–94. [PubMed] [Google Scholar]
- 46.Jakulj L, Vissers MN, Tanck MW, Hutten BA, Stellaard F, Kastelein JJ, et al. ABCG5/G8 polymorphisms and markers of cholesterol metabolism: systematic review and meta-analysis. J Lipid Res 2010;51:3016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subbiah MT. Understanding the nutrigenomic definitions and concepts at the food-genome junction. Omics 2008;12: 229–35. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson LR, Shelling AN, Browning BL, Fluebner C, Petennann I. Genes, diet and inflammatory bowel disease. Mutat Res 2007;622:70–83. [DOI] [PubMed] [Google Scholar]
- 49.Wolf SJ, Bachtiar M, Wang J, Sim TS, Chong SS, Lee CG. An update on ABCB1 pharmacogenetics: insights from a 3D model into the location and evolutionary conservation of residues corresponding to SNPs associated with drug pharmacokinetics. Pharmacogenomics J 2011;11:315–25. [DOI] [PubMed] [Google Scholar]