Abstract
Response assessment in light chain (AL) amyloidosis is challenging given the low level of circulating free light chains usually seen. Multi-parametric flow cytometry (MFC) from a marrow aspirate was demonstrated to retain a prognostic significance in several recent studies. In this work, 82 AL patients who had MFC study at end of therapy were analysed based on whether clonal plasma cells were detected or not. Among patients who achieved deep response (i.e. very good partial response or complete response) to first-line therapy, lack of clonal marrow plasma cells as measured by MFC was associated with improved progression-free survival (PFS) compared to patients with residual clonal plasma cells (3-year PFS 88% vs. 46%, p = .003), particularly among patients who achieved a complete response (3-year PFS 100% vs. 33%, p = .001). Absence of clonal plasma cells by MFC compared with patients with detectable clonal plasma cells among deep responders was associated with lower level of involved light chain (involved free light chain (iFLC), median 1.1 vs. 1.7 mg/dL; p = .02) and higher frequency of renal response (100% vs. 68%; p = .005). Further studies are needed to determine if MFC should be incorporated into response criteria in AL amyloidosis.
Keywords: Flow cytometry, response, survival, minimal residual disease, light chain
Introduction
Light chain amyloidosis (AL) is a systemic disease caused by deposition of abnormal immunoglobulin light chains which bear amyloidogenic properties. Clonal bone marrow plasma cells are the source of these amyloidogenic light chains [1]. Often, the clonal plasma cell disorder is of low burden, with modest bone marrow involvement (≤10% of marrow cellularity) and low circulating levels of free light chains [2]. The introduction of the serum free light chain assay more than a decade ago has improved disease recognition and facilitated diagnosis. This assay is also the key component in the haematological response assessment [3], differing from the one available for multiple myeloma [4].
Multi-parametric flow cytometry (MFC) has been used for both diagnosis and response assessment purposes in various haematological disorders [5]. It is emerging as an important tool for minimal residual disease (MRD) assessment in various haematological cancers [6], among them multiple myeloma [7]. MRD in AL amyloidosis has been explored for its prognostic value. We have previously published a comprehensive study on 7-color MFC at diagnosis and at the end of treatment (EOT) [8]. Eighty-two patients (n = 82) were assessed in the EOT cohort. We have demonstrated that minimal residual monotypic plasma cells (PCs) by MFC at EOT is associated with an improved response rate, superior progression-free survival (PFS) and overall survival (OS) compared to patients with a higher value of residual clonal PCs at EOT. Two recent studies from our group have demonstrated that among patients who achieved hematologic complete response, the absence of clonal plasma cells by flow cytometry was associated with improved PFS but not OS [9,10]. Nonetheless, these two studies used MFC at different sensitivity levels, limiting the value of the measurement of MRD.
Methods
We sought to update the results from our previous EOT cohort of 82 newly diagnosed AL patients with an extended follow-up. Our goal was to establish whether clearance of clonal plasma cells at EOT using sensitive and uniform MFC (sensitivity 1 × 10−4 to 2 × 10−5, depending on the number of analysed events, phenotype and DNA index) is associated with improved OS. The MFC method used in this analysis was previously described [8]. A total of 500,000 live cellular events were set as a target per exam (median gated events achieved 489,922, 25–75% IQR 469,765–493,662).
Haematological response was assessed using consensus criteria [3]. Organ response was assessed for heart, kidney and liver based on consensus criteria [11] and the refined response criteria for heart (a reduction of N-terminal pro b natriuretic peptide >30% of baseline level [3]) and kidney (a reduction of >30% from the baseline 24 h proteinuria in the absence of a rise in creatinine >25% [12]). PFS was defined as time from EOT until haematological progression per consensus criteria and/or institution of second-line therapy when haematological progression criteria were not met or until death from any cause. OS was defined as time from EOT until death from any cause. Survival analysis was performed using the Kaplan–Meier method, and the log-rank test to compare groups. For survival analysis, day 0 was set as the day of the MFC exam.
Results and discussion
The baseline characteristics of the EOT cohort are listed in Table 1. All patients were treated from February 2012 to November 2015. Briefly, the median age was 61 years, cardiac involvement was seen in 51% of patients. Mayo 2012 stages III/IV was seen in 25% of patients. Autologous stem cell transplantation (ASCT) was the primary therapy in 84% of patients. Twenty-four patients (n = 24, 29% of the study cohort) had no detected monotypic PCs at EOT, while 58 patients (71%) had detectable monotypic PCs by EOT. The median follow-up of the surviving patients (n = 68, 83% of the study population) from EOT was 4.6 years.
Table 1.
Baseline patients’ characteristics (n = 82).
| Age in years, median (range) | 61 (43–77) |
| Male sex, n (%) | 56 (68%) |
| No. of involved organs | |
| Median (range) | 2 (1–4) |
| Cardiac involvement | 42 (51%) |
| Renal involvement | 51 (62%) |
| Peripheral/autonomic neuropathy | 17 (26%) |
| Liver involvement | 10 (12%) |
| GI involvement | 6 (9%) |
| Lambda restriction, n (%) | 55 (67%) |
| dFLC, mg/dL, median (range) | 21 (0.7–1498) |
| Monotypic PCs percentage, median (range) | |
| By MFC | 2 (0–41.8) |
| By aspiration/biopsy | 10 (2–80) |
| 2004 Mayo stage, n (%) | |
| I | 37 (45%) |
| II | 32 (39%) |
| III | 13 (16%) |
| 2012 Mayo stage, n (%) | |
| I | 34 (42%) |
| II | 27 (33%) |
| III | 6 (7%) |
| IV | 15 (18%) |
| FISH abnormalities, n (%) | n = 70 |
| t (11;14) | 35 (50%) |
| del (13q) | 28 (40%) |
| Any trisomy(ies) | 17 (24%) |
| First-line treatment | |
| ASCT | 69 (84%) |
| Bortezomib-based | 10 (12%) |
| Melphalan-based | 3 (4%) |
Patients with no residual monotypic PCs by MFC at EOT exhibited longer PFS compared to patients with any level of detected clonal PCs (3-year PFS 88% vs. 28%, P < .001; Supplementary Figure 1a). However, OS difference was not seen between groups (3-year OS 96% vs. 84%, P = .17; Supplementary Figure 1b). We then restricted analysis to the subgroup of patients who achieved VGPR/CR at the time MFC was performed (n = 54; VGPR 38 patients, CR 16 patients). In these patients, MFC would have a greater value in response assessment than in patients who had less than a VGPR and would be likely to have substantial numbers of residual clonal plasma cells. In this subgroup of patients, 24 patients (44%) had no residual monotypic PCs, while 30 patients (56%) had detectable clonal PCs at EOT. Among patients who achieved VGPR/CR, PFS was superior in those with no residual clonal plasma cells by MFC (MRD−) compared to those with any level of residual clonal plasma cells (MRD+)(3-year PFS 88% vs. 46%, P = .003; Figure 1(A)). This PFS advantage was striking among those who achieved haematologic CR and were MRD− (n = 8), in whom PFS was 100% at the 3-year mark compared to 33% in those who achieve CR but had residual clonal plasma cells (Figure 1(B)). In contrast, while there was some evidence for PFS advantage among those who achieved VGPR and were MRD− compared to those who achieved VGPR but were MRD+ by EOT, this difference did not reach statistical significance (P = .14; Figure 1(C)).
Figure 1.
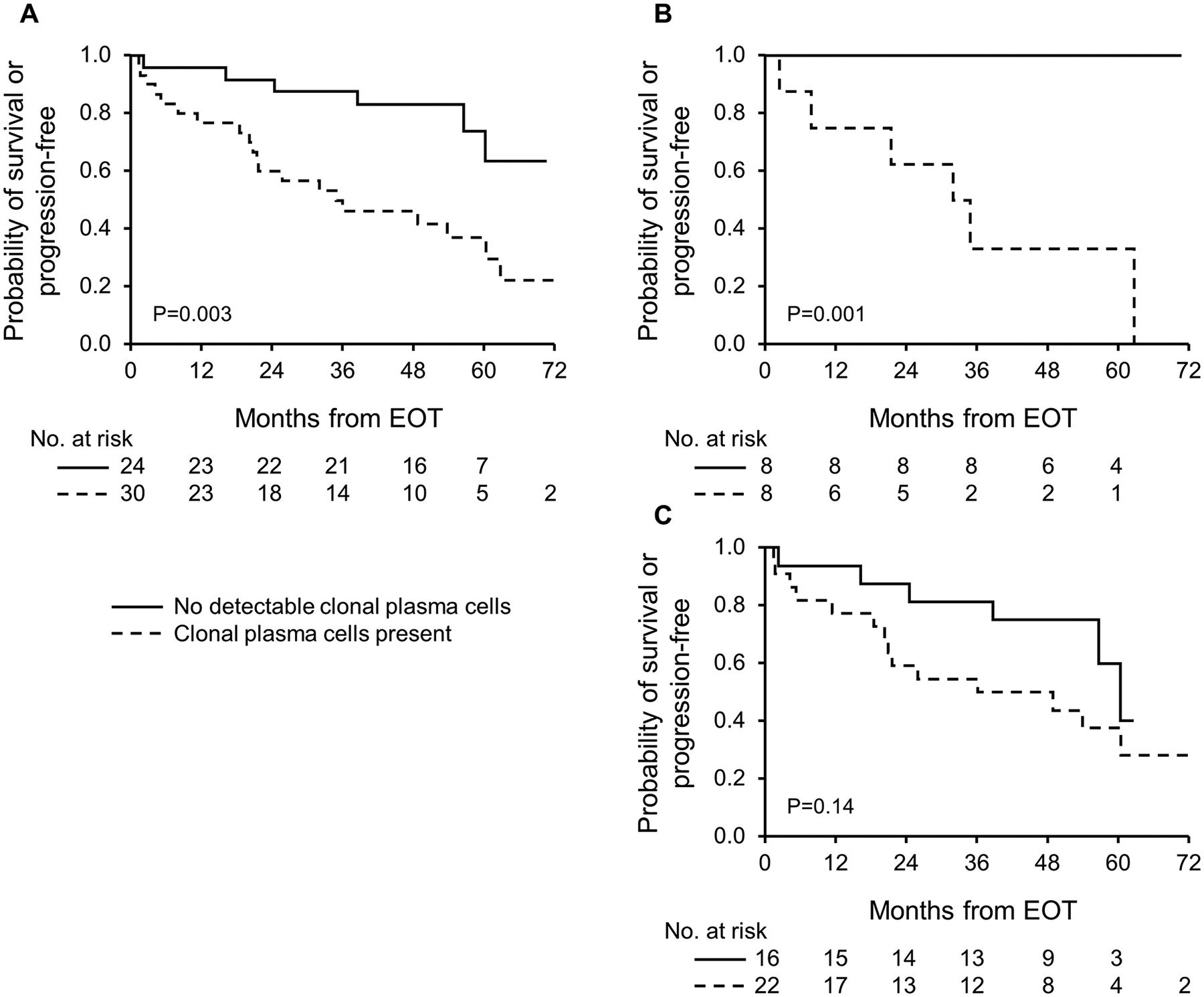
Progression-free survival from end of treatment (EOT) among subgroup of patients who achieved very good partial response (VGPR) or better following first line treatment: (A) whole subgroup; (B) patients who attained a complete response; (C) patients who attained a VGPR.
Among patients who achieved haematological VGPR or better, cardiac, renal and hepatic responses were available for evaluation in 20, 33 and 4 patients, respectively. Of the 20 patients available for cardiac response, all patients with MRD− disease (n = 8) achieved a cardiac response, compared to 10/12 (83%) with residual clonal plasma cells at EOT (P = .13). The rate of renal response was higher among patients with MRD− compared to those with MRD+ [100% (14/14) vs. 68% (13/19); P = .005]. Of the 4 patients available for hepatic response, the one patient with MRD− achieved hepatic response compared to 2 out of 3 patients with residual clonal plasma cells at EOT who achieved hepatic response.
We compared serum free light chain assay values at the time MFC was performed among the VGPR/CR patients, stratified by MRD status. Patients with MRD− had lower involved free light chain (iFLC, median 1.1 vs. 1.7 mg/dL; P = .02) and lower difference between involved to uninvolved light chains (dFLC, median 0.1 vs. 0.7 mg/dL; P = .005) compared to patients with MRD+. Although these differences were statistically significant, they were small and within the normal range of the assay. This highlights the fact that at a deep response level, one cannot ascertain based on the serum free light chain assay alone the presence of clonal marrow residual disease. Moreover, we recently demonstrated in a large cohort of patients that a lower iFLC at EOT translates into improved PFS and OS in patients achieving a deep response to therapy [13]. Achieving MRD− at EOT predicts lower iFLC, and a response and survival advantage has been demonstrated in a larger patient population.
We believe that this study as well as prior work raises the need to perform a bone marrow aspiration and biopsy at end of therapy in AL amyloidosis patients who achieved a deep hematologic response to treatment, i.e. VGPR or better. Currently, this is not routinely recommended as part of consensus criteria for response assessment [3]. However, at the time consensus haematological response criteria were formulated, the role of MFC was not clear. Nonetheless, the authors in the consensus response criteria paper commented that ‘the utility of refined bone marrow studies (e.g. multi-parameter flow cytometry, molecular studies) to assess risk of relapse remains to be established’. We believe this new technology developed after the response criteria were published should be assessed by other groups. If other studies will confirm these results it will appropriate to incorporated MFC into revised response criteria for amyloidosis.
The above findings are observational. We are also limited by selection bias and retrospective chart review. It should be noted that 84% of the patients underwent ASCT, a selected population with better baseline organ function and better prognosis. Therefore, the impact on PFS and OS based on MRD status may be different than patients not eligible for ASCT, who are more likely to succumb to their disease even in the face of deep haematological response due to advanced organ involvement. Moreover, fit patients are more likely to face a disease relapse and resume therapy than those who are less fit. It should also be acknowledged that whether interventions to further reduce residual PCs in those who already achieved a deep response to therapy would be beneficial remains to be assessed in prospective clinical trials.
Next-generation MRD assessment in plasma cell disorders is being performed in AL amyloidosis in the clinical practice [14]. This is expected to further improve our ability to detect residual clonal PCs following therapy. By this, identifying patients with MRD-negative disease using more sensitive techniques should further increase our ability to predict haematological long-term disease control.
Supplementary Material
Disclosure statement
Eli Muchtar reports no disclosure. Angela Dispenzierireceived research funding from Celgene, Millennium, Pfizer, and Janssen and travel grant from Pfizer. Dragan Jevremovic reportsno disclosure. David Dingli received research funding from Karyopharm Therapeutics, Amgen, and Millenium Pharmaceuticals. Francis Buadi reports no disclosure. Martha Q. Lacy received research funding from Celgene. Wilson Gonsalves, Rahma Warsame, Taxiarchis Kourelis and Suzanne R. Hayman report no disclosure. Prashant Kapoor received research funding from Takeda, Celgene, and Amgen. Nelson Leung, Stephen Russel, John A. Lust, Yi Lin, Ronald S. Go, Steven Zeldenrust, Robert A. Kyle and Vincent Rajkumar report no disclosure. Shaji Kumar received consultancy from Celgene, Millennium, Onyx, Janssen, and BMS and research funding from Celgene, Millennium, Novartis, Onyx AbbVie, Janssen, and BMS. Morie A. Gertz received consultancy from Milleniu and honoraria from Celgene, Millenium, Onyx, Novartis, Smith Kline, Prothena, Ionis.
Abbreviations:
- AL
light chain amyloid protein
- ASCT
autologous stem cell transplantation
- CR
complete response
- dFLC
difference between involved to uninvolved free light chain
- EOT
end of treatment
- iFLC
involved free light chain
- IQR
interquartile range
- MFC
multi-parametric flow cytometry
- MRD
minimal residual disease
- OS
overall survival
- PCs
plasma cells
- PFS
progression-free survival
- VGPR
very good partial response
Footnotes
Supplemental data for this article can be accessed here.
References
- [1].Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. [DOI] [PubMed] [Google Scholar]
- [2].Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15): 2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac bio-markers: impact on survival outcomes. J Clin Oncol. 2012; 30(36):4541–4549. [DOI] [PubMed] [Google Scholar]
- [4].Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- [5].Braylan RC. Impact of flow cytometry on the diagnosis and characterization of lymphomas, chronic lymphoproliferative disorders and plasma cell neoplasias. Cytometry A. 2004;58: 57–61. [DOI] [PubMed] [Google Scholar]
- [6].Ben Lassoued A, Nivaggioni V, Gabert J. Minimal residual disease testing in hematologic malignancies and solid cancer. Expert Rev Mol Diagn. 2014;14(6):699–712. [DOI] [PubMed] [Google Scholar]
- [7].Paiva B, Garcia-Sanz R, San Miguel JF. Multiple myeloma minimal residual disease. Cancer Treat Res. 2016;169:103–122. [DOI] [PubMed] [Google Scholar]
- [8].Muchtar E, Jevremovic D, Dispenzieri A, et al. The prognostic value of multiparametric flow cytometry in AL amyloidosis at diagnosis and at the end of first-line treatment. Blood. 2017; 129(1):82–87. [DOI] [PubMed] [Google Scholar]
- [9].Sidana S, Tandon N, Dispenzieri A, et al. The importance of bone marrow examination in patients with light chain amyloidosis achieving a complete response. Leukemia. 2018;32(5): 1243–1246. [DOI] [PubMed] [Google Scholar]
- [10].Sidiqi MH, Aljama MA, Jevremovic D, et al. Prognostic significance of stringent complete response after stem cell transplantation in immunoglobulin light chain amyloidosis. Bone Marrow Transplant. 2019;54(3):442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79(4):319–328. [DOI] [PubMed] [Google Scholar]
- [12].Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124(15):2325–2332. [DOI] [PubMed] [Google Scholar]
- [13].Muchtar E, Dispenzieri A, Leung N, et al. Optimizing deep response assessment for AL amyloidosis using involved free light chain level at end of therapy: failure of the serum free light chain ratio. Leukemia. 2019;33(2):527–531. [DOI] [PubMed] [Google Scholar]
- [14].Kastritis E, Kostopoulos IV, Terpos E, et al. Evaluation of minimal residual disease using next-generation flow cytometry in patients with AL amyloidosis. Blood Cancer J. 2018;8(5):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


