Abstract
Desmoplasia plays a pivotal role in promoting pancreatic cancer progression and is associated with poor clinical outcome. Targeting the desmoplastic tumor microenvironment in combination with chemotherapy is therefore a promising strategy for pancreatic cancer therapy. Here we report a novel biodegradable copolymer to co-deliver LY2109761 (a TGF-β receptor I/II inhibitor) and CPI-613 (a novel chemotherapy agent) to desmoplastic stroma and tumor cells, respectively, in the tumor microenvironment. Hydrophobic CPI-163 is conjugated to the hydrophilic copolymer via a newly designed MMP-2-responsive linker to form a trigger-responsive nanopolyplex. LY2109761 is hydrophobic and encapsulated into the hydrophobic core of the nanopolyplex. The resulting nanopolyplex is modified with a plectin-1-targeting peptide to enhance the accumulation of the nanopolyplex in pancreatic tumors. The nanopolyplex aims to normalize the stroma by blocking the interaction between tumor cells and pancreatic stellate cells to inhibit the activation of pancreatic stellate cells and reduce the dense extracellular matrix. Normalized stroma also increases the penetration of the nanopolyplex into the tumor. The nanopolyplex shows enhanced accumulation in xenograft pancreatic tumors in a biodistribution study. Moreover, the targeted nanopolyplex markedly inhibits tumor growth in an orthotopic pancreatic cancer mouse model by dual targeting tumor cells and stroma. Overall, the multifunctional nanopolyplex is a promising platform for pancreatic cancer therapy.
Keywords: Copolymer, tumor-responsive, nanopolyplex, combinational therapy, pancreatic cancer, desmoplasia
Graphical Abstract
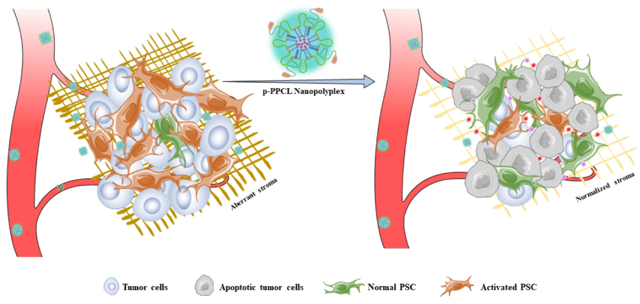
INTRODUCTION
Pancreatic ductal adenocarcinomas (PDAC) is the fourth leading cause of cancer-related death in the United States and is one of the five most lethal malignancies worldwide. In the past 30 years, the survival of patients with PDAC has not changed significantly and remains very low, approximately 6%. The median survival duration of PDAC patients is approximately 6 months after diagnosis. In 2019, 56,770 Americans will be diagnosed with pancreatic cancer, and 45,750 Americans are estimated to die from pancreatic cancer.1–2 Despite advances in conventional therapies, most patients diagnosed with early-stage PDAC ultimately experience recurrence and metastasis.2–4 Therefore, it is highly important to develop novel treatments for PDAC.
Desmoplasia, the growth of fibrotic stroma, is a fundamental characteristic of PDAC and plays a crucial role in pancreatic cancer progression. However, it is usually overlooked in pancreatic cancer therapy. Based on accumulating preclinical and clinical evidence obtained in recent years, the stromal component is not just a bystander in pancreatic cancer, it may comprise up to 80% of tumor mass.2, 5 The major cellular components of PDAC desmoplasia are activated pancreatic stellate cells (PSCs), inflammatory cells, macrophages, endothelial cells, infiltrating immune cells, and extracellular matrix (ECM), which is comprised of collagen, hyaluronan, glycoproteins and other growth factors.6–7 Desmoplasia plays important roles in tumorigenesis and aggressiveness of the tumor by promoting proliferation and metastasis of tumor cells, enhancing angiogenesis, and contributing to immune evasion.8–10 Therefore, targeting the desmoplastic tumor microenvironment along with chemotherapy is a promising strategy for pancreatic cancer therapy.
The TGF-β/SMAD pathway is one of the major signaling pathways that regulate the interaction between PSCs and pancreatic tumor cells. Particularly, TGF-β is overexpressed in pancreatic cancer, and upregulated expression of TGF-β is correlated with poor clinical outcomes.11–12 In general, pancreatic cancer cells release mitogenic and fibrogenic stimulants to activate surrounding PSCs, which are mainly responsible for the production of excess ECM. Activated PSCs in turn secret various growth factors and cytokines to promote tumor growth, metastasis, and chemoresistance.5, 13 Moreover, TGF-β also contributes to the immune evasion and angiogenesis in the tumor microenvironment.13–15 Therefore, targeting the TGF-β signaling pathway is considered a microenvironment-targeted therapy in treating PDAC. LY2109761 is a novel TGF-β receptor type I (TGF-β RI) kinase inhibitor and exhibits antitumor activity in various cancers.16 By interrupting the cross-talk between cancer cells and cancer-associated fibroblasts, LY2109761 inhibits tumor growth and metastasis. Moreover, combination of LY2109761 with chemotherapy or radiotherapy dramatically suppressed tumor progression and metastasis.17–19
Compared to normal cells, cancer cells shift energy production from oxidative phosphorylation to glycolysis during malignant progression.20 This reprogramming in cancer cells enhances the production of energy and substrates during rapid proliferation.20–21 Metabolic reprogramming is caused by mutations in the genes encoding several metabolic enzymes in the mitochondria of cancer cells.22 Therefore, researchers have developed anticancer agents to target cancer-specific metabolic changes. CPI-613 is an antimitochondrial metabolism agent, which disrupts mitochondrial metabolism in several types of cancer cells, such as pancreatic cancer and small lung cancer cells. CPI-613 inhibits two essential metabolic enzymes, which are pyruvate dehydrogenase (PDH) and alpha-ketoglutarate dehydrogenase (KGDH).23–24 Due to the specificity and selectivity for tumor cells, CPI-613 exhibited excellent antitumor activity with low side effects.22–23, 25–26 The U.S. Food and Drug Administration (FDA) recently approved CPI-613 as an orphan drug for the treatment of pancreatic cancer, acute myeloid leukemia (AML), peripheral T-cell lymphoma as well as other diseases.
Combination therapy has become a standard strategy in treating cancer in the clinic because of the heterogeneity and complexity of cancer.27 In general, combination therapy targets multiple targets or pathways simultaneously, leading to maximal antitumor efficacy with minimum side effects.28 However, simply combining two individual therapeutics together will not achieve the desired synergistic effect because the two drugs may have different pharmacokinetic properties and therefore cannot reach the tumor cells in a synergistic ratio. Therefore, combination therapy vehicles, such as polymer drug conjugate, have been developed to simultaneously deliver synergistic ratios of drugs to target tumor cells.29–30
In the present study, we develop a lysine- and poly(ethylene glycol) (PEG)-based alternating copolymer to co-deliver LY2109761 and CPI-613 to desmoplastic stroma and tumor cells, respectively, in PDAC. The hydrophilic copolymer is biodegradable and contains two pendant amine groups in each repeating unit to conjugate hydrophobic CPI-613 via a newly designed matrix metalloproteinases-2 (MMP-2)-responsive linker (LAGLVG) to form a trigger-responsive nanopolyplex (Figure 1). LY2109761 is hydrophobic and encapsulated into the hydrophobic core of the nanopolyplex. The resulting nanopolyplex is modified with a plectin-1-targeting peptide to enhance the accumulation of the nanopolyplex in pancreatic cancer cells, which overexpress plectin-1 on the cell surface.31–34 The MMP-2-responsive linker is cleaved in the tumor microenvironment to release CPI-613 from the polymer, leading to dissociation of the nanopolyplex and subsequent release of LY2109761. LY2109761, a TGF-β RI kinase inhibitor, targets the tumor stroma and inhibits the interaction between PSCs and tumor cells, while CPI-613 induces the apoptosis of tumor cells. This study presents a promising polymer-drug nanopolyplex for pancreatic cancer therapy by dual targeting tumor stroma and tumor cells.
Figure 1. Schematic illustration of the p-PPCL nanopolyplex for pancreatic cancer therapy.
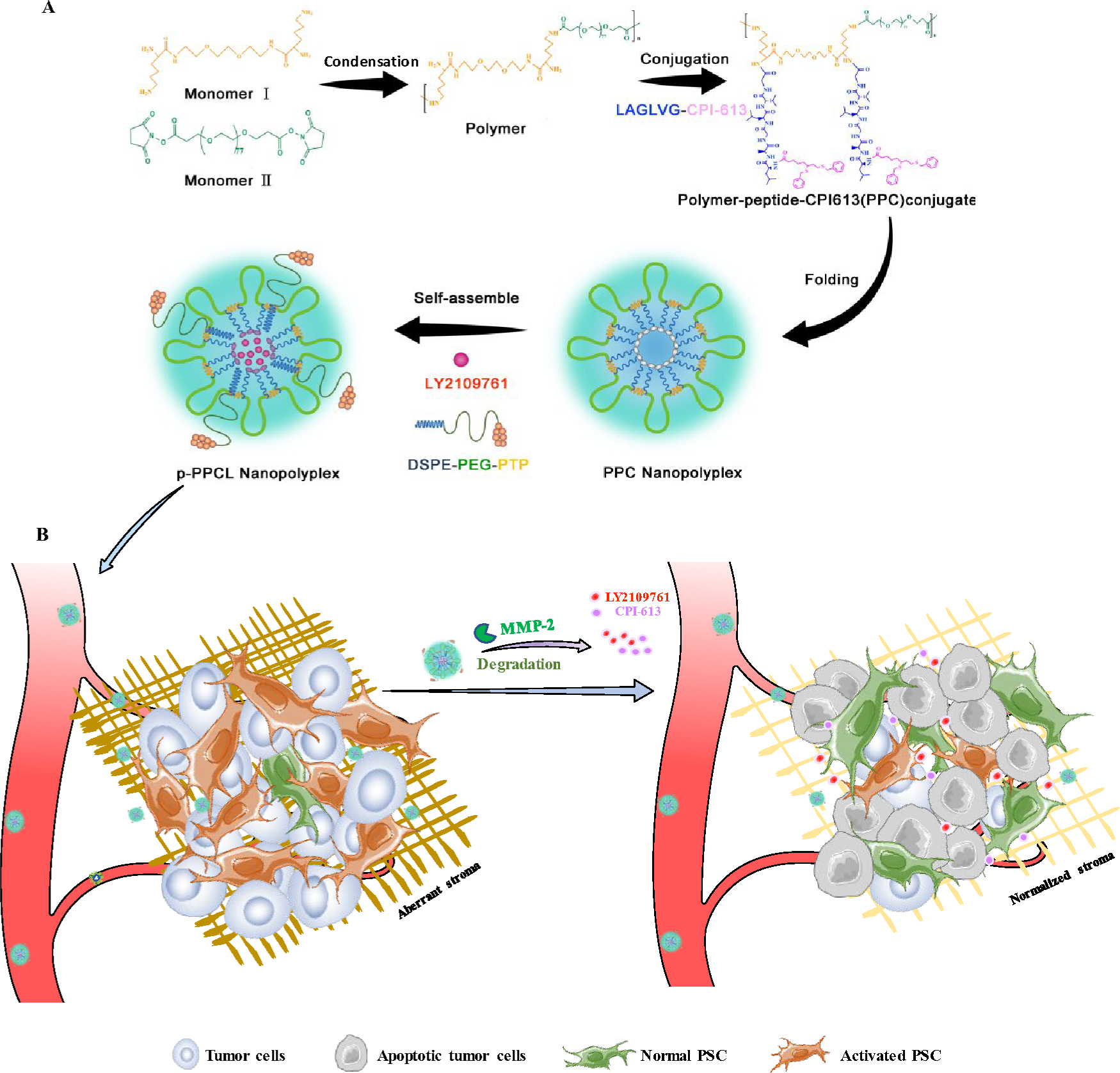
(A) The alternating copolymer is synthesized by condensation. CPI-613 is linked to the copolymer chains via MMP-2-responsive peptides (LAGLVG) to obtain the copolymer-peptide-CPI-613 (PPC) conjugate, which spontaneously folds into nanopolyplex in water. Hydrophobic LY2109761 is encapsulated into the hydrophobic core of the PPC nanopolyplex, and the amphiphilic DSPE-PEG-Plectin-1 peptide is incorporated into the PPC nanopolyplex to form the multifunctional p-PPCL nanopolyplex. (B) After systemic administration, p-PPCL nanopolyplexes accumulate in the tumor microenvironment by passive targeting and active targeting effects. MMP-2 in the tumor microenvironment triggers the release of CPI-613, leading to dissociation of the nanopolyplex and subsequent release of LY210976. CPI-613 inhibits the proliferation and migration of tumor cells by disrupting the mitochondrial metabolism. Meanwhile, LY2109761 blocks the activation of pancreatic stellate cells and normalizes the stroma to inhibit its effect on the proliferation and migration of tumor cells. Normalization of the stroma also enhances the penetration of nanopolyplexes.
METHODS
Materials
CPI-613 and LY2109761 were purchased from MedKoo Biosciences (Morrisville, NC). Bis-succinimidyl polyethylene glycol ester (NHS-PEG3400-PEG) was purchased from NANOCS Inc. (New York, NY). 1,8-diamino-3,6-dioxaoctane, N,N-Dimethylformamide (DMF), Dichloromethane (DCM), N,N-Diisopropylethylamine (DIPEA) and 4-Dimethylaminopyridine (DMAP) were ordered from Acros Organics (Morris Plains, NJ). Piperazine, polymer-bound was ordered from Sigma-Aldrich (St. Louis, MO). O-(7-Aza-1H-benzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphat (HATU) and 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) were obtained from Alfa Aesar (Haverhill, MA). Fmoc-Lys(Boc)-OH was purchased from Chem-Impex International, Inc. (Wood Dale, IL). KTLLPTPC-PEG2k-DSPE was ordered from LifeTein LLC (Somerset, NJ). Custom peptides were ordered from United BioSystems Inc. (Herndon, VA).
Cell Culture
PANC-1 cell line was purchased from American Type Culture Collection (Manassas, VA) and cultured in DMEM with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin. Human pancreatic stellate cells were purchased from iXCells Biotechnologies (San Diego, CA) and maintained in stellate cell growth medium from iXCells Biotechnologies (San Diego, CA). The cells were incubated in a humidified atmosphere at 37°C with 5% CO2.
Synthesis of the Polymer
Synthesis of the polymer was started from Fmoc-Lys(Boc)-OH and 1,8-diamino-3,6-dioxaoctane. First, seven hundred milligrams of Fmoc-Lys(Boc)-OH (1.49 mmol) was dissolved in anhydrous DCM, followed by adding 431 mg of EDCI (2.25 mmol), 70 μL of 1,8-diamino-3,6-dioxaoctane (0.49 mmol), and DMAP (0.049 mmol) into the solution. After stirring overnight at room temperature, the solution was concentrated and purified by silica gel chromatography with ethyl acetate/methanol (100/1 – 25/1, v/v). The purified compound was dissolved in TFA/DCM (1:1, v/v) and then stirred at room temperature for 30 min to remove the Boc group. The solvent was dried under nitrogen, and ice-cold diethyl ether was added to precipitate the product. The white precipitate was collected by filtration and dried under vacuum to get the monomer.
Thirty milligrams of the monomers were dissolved in 200 μL anhydrous DMF and transferred to a dry 5-mL flask, followed by adding DIPEA and stirring for 10 min under nitrogen atmosphere. Ninety-eight milligrams of NHS-PEG3400-NHS (98 mg) was also dissolved in 200 μL anhydrous DMF and then added into the monomer solution drop by drop under nitrogen. After stirring at 50°C for 2 h, the solution was stirred at room temperature for 24–72 h. The solution was transferred to ice-cold diethyl ether to precipitate the polymer product. The white precipitate was collected by filtration, diluted with distilled water, and dialyzed against distilled water in a dialysis tube (MWCO 14,000 Da). The polymer solution was lyophilized and pure polymer was collected. Molecular weights (Mw and Mn) of the polymer were determined by gel permeation chromatography (GPC).
Synthesis of the Polymer-Peptide-CPI-613 (PPC) Conjugate
The MMP-2 sensitive peptide (LAGLVG) was conjugated to CPI-613 using HATU/DIPEA. Briefly, CPI-613 and the peptide (2:1 molar ratio) were dissolved in dry DMF, followed by the addition of HATU and DIPEA. After stirring at room temperature for 24 h, the solution was concentrated, and the compound was purified using a CombiFlash Rf system (Teledyne Isco Inc., NE) with a C18 column. Acetonitrile/methanol (9:1, v/v) was used as the eluting solvent, and the flow rate was set at 2 mL/min. The pure product was collected and lyophilized for next reaction.
After deprotection of the Fmoc groups using polymer-bound piperazine, the polymer was mixed with the MMP-2 sensitive peptide CPI-613 conjugate (LAGLVG-CPI-613) in 1:4 molar ratio, followed by the addition of HATU and DIPEA. The resulting mixture was stirred at room temperature for 48 h, followed by concentration and precipitation in cold diethyl ether. The precipitate was dissolved in distilled water, and insoluble solids were removed by filtration through a 0.45 μm micro filter. The solution was dialyzed against distilled water (MWCO 14,000 Da) for 48 h. The final product PPC was freeze dried to provide the product as a white solid.
Characteristics of PPC, PPCL and p-PPCL Nanopolyplexes
Chemical structure of the PPC conjugate was determined by 1H-NMR. The percentage of CPI-613 conjugated to the polymer was calculated by ultraviolet-visible (UV) spectrophotometry (λmax=250 nm). The critical micelle concentration (CMC) of the PPC conjugate was determined as we described before35–36. Briefly, iodine solution was prepared by dissolving 0.05 g of iodine and 0.1 g of potassium iodine in 5 mL of distilled water. A series of PPC solutions with different concentrations ranging from 0.025 ng/mL to 0.5 mg/mL were prepared. Ten microliters of iodine solution was added to each PPC solution and incubated in the dark at room temperature for 15 h. The UV absorbance of each sample at 460 nm was measured and plotted against the logarithm of the sample concentrations. The CMC value was estimated at the cross point when extrapolating the absorbance at low and high concentration regions.
The PPCL nanopolyplex was prepared using the solvent evaporation method. Briefly, 10 mg PPC conjugate and 4 mg LY2109761 were dissolved in 1 mL DCM, evaporated under vacuum, reconstituted in 1 mL water, and then sonicated on ice for 10 min to form the LY2109761-loaded PPCL nanopolyplexes. Unloaded LY2109761 was removed by centrifugation and filtered by a 0.45 μm membrane. Drug loading (wt %) of LY2109761 was determined by HPLC.
Plectin-1 targeting peptide (KTLLPTPC)-PEG-DSPE and freeze-dried PPCL (1:10 weight ratio) were mixed, dissolved in water, and sonicated in a bath sonicator for 30 min to form the plectin-1 targeting peptide-modified PPCL nanopolyplexes (p-PPCL).
The particle size and zeta potential of the nanopolyplexes were determined using dynamic light scattering (DLS). The morphology of the nanopolyplexes was evaluated by transmission electron microscopy (TEM).
Cleavage Study of the New-Designed MMP-2-responsive Peptide
The peptide ESLAGLVGES, which contains the new-designed MMP-2 sensitive peptide sequence LAGLVG, was dissolved in pH 7.5 Tris buffer at 0.5 mg/mL and incubated with recombinant human MMP-2 protein (25 nM) at 37°C for 1h.37 Cleavage of the peptide was then analyzed by LC-MS.
In Vitro Cell Viability Assay
PANC-1 cells were seeded in a black 96-well plate at a density of 5000 cells/well. After 12 h, the cells were incubated with CPI-613, CPI-613 plus LY2109761, and nanopolyplexes containing CPI-613 and LY2109761 at different concentrations of CPI-613 (1, 10 and 50 μM) at 37 °C for 72 h. The molar ratio of CPI-613:LY2109761 is 1:1.16. Cell viability was detected using the CellTiter-Glo® kit (Promega, WI) as per manufacturer’s instructions.
Two-Chamber Migration Assay
PSCs (50,000 cells/well) were seeded into a 24-well plate in DMEM with 10% FBS, and PANC-1 cells (20,000 cells/well) were seeded into transwell inserts with an 8.0 μm pore size in DMEM with 0.5% FBS. PANC-1 cells alone were used as the control. Both chambers were filled with culture medium containing formulations. After incubation for 24 h with LY2109761, LY2109761 plus CPI-613, PPCL, or PPCL plus MMP-2 (5 ng/μL) at 5 μM LY2109761 and 4.3 μM CPI-613, the cells that did not migrate through the membrane were carefully cleaned using a cotton swab. The migrated cells were fixed in 4% paraformaldehyde and stained with 0.05% crystal violet for 5 min. The stained cells were then counted under an optical microscopy (Leica DMI3000B, Germany).
Immunofluorescence Staining
PSCs were cultured in a 96-well black plate at a density of 5,000 cell/well for 12 h. After treatment with LY2109761, LY2109761 plus CPI-613, PPCL, and PPCL plus MMP-2 (5 ng/μL) for 48 h at 5 μM LY2109761 and 4.3 μM CPI-613, the cells were fixed with 4% paraformaldehyde for 20 min and blocked with 3% BSA for 2 h. A rabbit polyclonal antihuman collagen I antibody (Abcam, MA) diluted in 3% BSA solution was added and incubated with the cells overnight at 4 °C. After washing, a goat anti-rabbit secondary antibody conjugated with Alexa 488 (Invitrogen, CA) was incubated with the PSCs for 1 h. The cells were evaluated under a fluorescence microscope (Leica DMI3000B, Germany).
3D Tumor Spheroid Penetration Study
3D spheroids of PANC-1 cells were prepared using Spheroids Formation ECM (Amsbio, Cambridge, MA) as we previously described.30, 38 Briefly, 3000 PANC-1 cells were suspended in 50 μL Spheroids Formation ECM and added into the Corning™ 96-well Ultra-low attachment microplates (Pittsburgh, PA). After centrifugation at 200 g for 3 min at 4 °C, the cells were incubated at 37 °C for 7 days to form spheroids. The PANC-1 spheroids were incubated with coumarin 6 (C6)-loaded PPC and p-PPC nanopolyplexes for 2 h, followed by washing with PBS and fixing with 10% paraformaldehyde. The spheroids were subjected to confocal microscopy (Leica TCS SP5, Germany) to evaluate the penetration of the nanopolyplexes.
Biodistribution of DiR-loaded PPC and p-PPC Nanopolyplexes
The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Missouri-Kansas City. Approximately 2×106 PANC-1 cells (200 μL) were subcutaneously implanted to the lower back of male nude mice. When the tumor size reached approximately 200 mm3, 100 μL of DiR-loaded PPC or p-PPC nanopolyplexes were injected via the tail vein at a dose of 1.6 mg nanopolyplex/kg. At 1, 2, 4, 6, and 24 h after the injection, the mice were anesthetized for fluorescence imaging with a Bruker MS FX PRO imaging system (Billerica, CA). After the last in vivo imaging at 24 h post-injection, the mice were euthanized, and major organs, including the liver, spleen, lungs, kidneys, heart, muscle, and tumor were harvested for ex vivo fluorescence images.
In Vivo Anti-Tumor Activity
To establish a desmoplastic pancreatic cancer mouse model, 8×105 PANC-1/Luc tumor cells were orthotopically injected into the tail of the pancreas. Eight days after the inoculation, tumor-bearing mice were randomly divided into 5 groups and intravenously injected saline, CPI-613, CPI-613 plus LY2109761, PPCL, and p-PPCL (CPI-613 10 mg/kg, LY2109761 13.2 mg/kg) every 5 days for a total of 6 injections. Tumor bioluminescence was monitored using the Bruker MS FX PRO imaging system. Sixty days after the tumor inoculation, the mice were euthanized to harvested the tumors for following studies.
Cell apoptosis was performed using a TUNEL assay kit (Invitrogen, CA) according to the manufacturer’s instructions. Nine representative images from each treatment group were evaluated under an inverted fluorescence microscope (Leica DMI3000B, Germany) and quantified using Image J. Collagen expression and activation of PSCs were studied using Picro-Sirius Red staining and immunohistochemical staining (IHC) of α-SMA, respectively. Nine representative images from each treatment group were evaluated under an optical microscope (Olympus, Japan) and quantified using Image J.
Statistical Analysis
Data were presented as the mean ± standard deviation (SD). Two-tailed t-test and oneway ANOVA with Tukey’s post hoc test were used to compare the difference between two groups and multiple groups, respectively. P<0.05 was considered statically significant.
RESULTS AND DISCUSSION
Desmoplasia is the most significant characteristic of PDAC and plays critical roles in tumorigenesis, metastasis, and drug resistance. The crosstalk between pancreatic cancer cells and stroma cells has therefore been extensively investigated. In the largest study so far, the expression of α-SMA, a marker of PSC activity, was evaluated in the tumor specimen from 233 PDAC patients. The results showed a high association between PSC activity and clinical outcomes, suggesting the importance of stroma in pancreatic cancer therapy. Patients with the highest PSC activity had the worst survival rate.39 Recently, the prognostic role of stroma in PDAC was studied 145 patients treated with surgery followed by chemotherapy. A moderate-to-strong α-SMA expression was associated with longer survival in early-stage (T1-T2) but not later-stage (T3-T4) tumors, suggesting the complex roles of stroma in PDAC.40 While it was initially believed that depleting stroma may enhance drug delivery to pancreatic cancer cells and subsequently enhance the therapeutic index, stroma-depleting clinical trials have failed.41–42 It is now believed that stroma acts not only as a barrier for therapy but also as a defense against metastasis.2 Instead of completely depleting stroma, it could be a more efficient therapy to modulate the stroma or regulate the crosstalk between cancer cells and the stroma. Our strategy is to normalize the stroma by blocking the TGF-β pathway and keep PSCs in a quiescent state, thus reducing the dense product of ECM. As a result, we aim to co-deliver CPI-613 and LY2109761 to target pancreatic cancer cells and desmoplastic stroma, respectively. CPI-613 is a tumor-specific anti-mitochondrial agent, which only down-regulates the gene expression of tumor cells, but not non-transformed NIH 3T3 fibroblast cells.25 Thus, our strategy maintains the integrity of the tumor microenvironment to lock the tumor cells at “nest,” leading to less metastasis.
The major challenge of combination therapy is how to simultaneously deliver two different drugs to the same site to achieve the highest synergistic effect. One solution is to encapsulate two drugs in a same drug carrier. Polymer-drug conjugate has become an attractive strategy in combination therapy because of its ability to control drug-loading, deliver multiple drugs simultaneously, prolong blood circulation time, and improve the accumulation of drugs in tumor site via the enhanced penetration and retention effect.29, 43–44
In this study, we designed a biodegradable and high drug-loading (26 wt%) alternating copolymer to deliver CPI-613 and LY2109761 for the treatment of PDAC. The lysine-based comonomer serves as the backbone for drug conjugation, while the PEG3400 comonomer facilitates the nanopolyplex formation of the copolymer. To increase the drug loading of the copolymer, we developed a hybrid drug loading strategy by conjugating CPI-613 to the polymer and then encapsulating LY2109761 into the nanopolyplex. In addition, the strategy can precisely control the ratio of chemically conjugated drug and the physically encapsulated drug.
Synthesis and characterization of the nanopolyplex
We hypothesize that a polymer-drug conjugate of the novel anti-cancer specific metabolic agent CPI-613 can be used to improve its solubility and biodistribution profile and prolong its duration of action. A lysine- and PEG-based alternating copolymer was synthesized by alternating NHS-PEG3400-NHS and bis(Oct-Lys-NHFmoc)1,8-diamino-3,6-dioxazocctane. The mass spectra of the intermediate compounds were illustrated in Figure S1–3. As shown in Figure 1A and Figure 2A, in each repeating unit, bis(Oct-Lys-NHFmoc)1,8-diamino-3,6-dioxazocctane contains two functional groups for grafting CPI-613 molecules that were separated from the next bis(Oct-Lys-NHFmoc)1,8-diamino-3,6-dioxazocctane unit by PEG3400. This copolymer architecture not only enhances drug loading but also evenly distributes the drug along the polymer chain, minimizing heterogeneity and aggregation.
Figure 2. Cleavage assay of the MMP-2-responsive peptide.
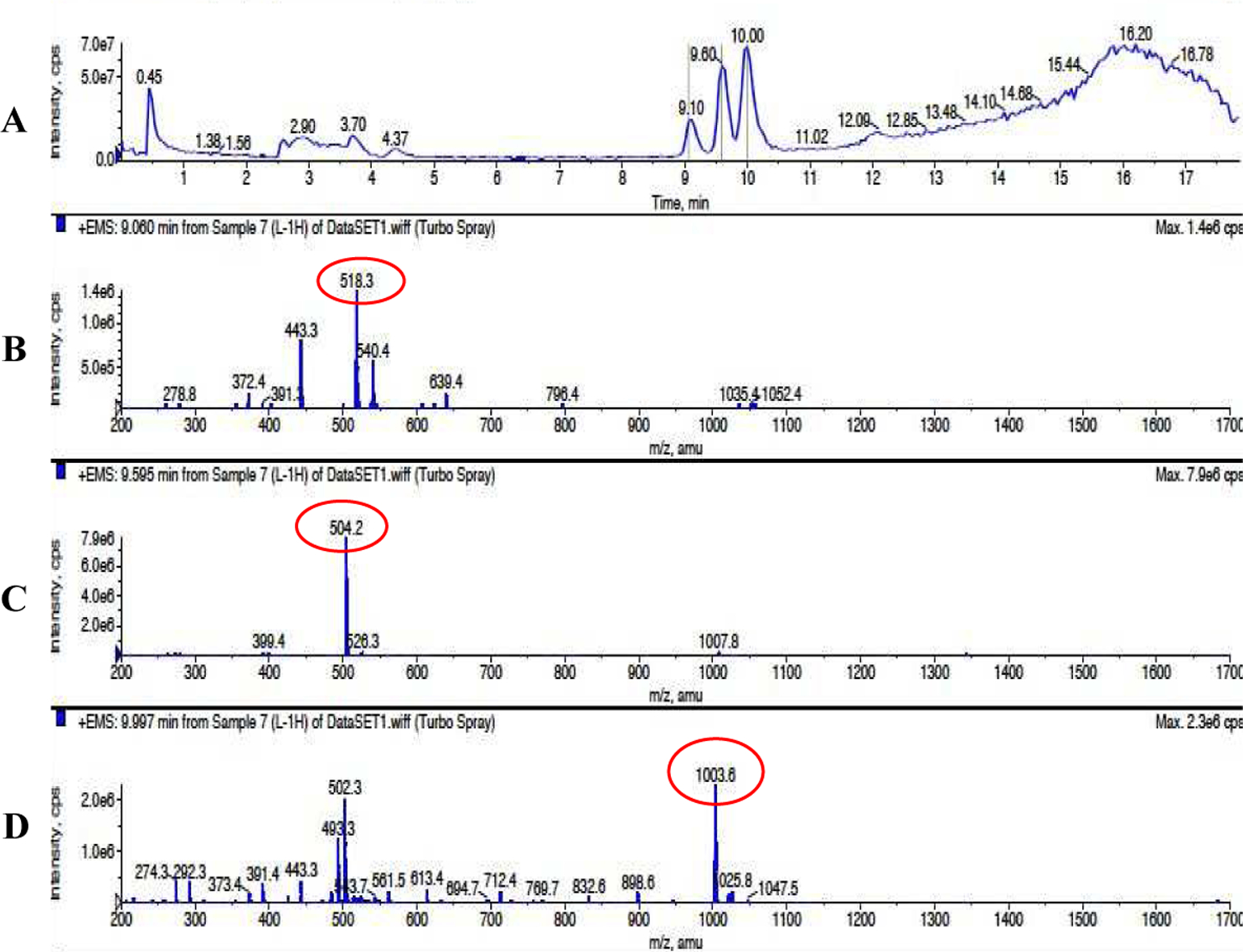
The peptide (ESLAGLVGES) was incubated with MMP-2 (25 nM) at 37 °C for 1h. (A) There are three peaks on the LC/MS chromatogram. (B) represents the degraded fragment ESLAG ([M1+H]+ 518.3). (C) represents the degraded fragment LVGES ([M1+H]+ 504.2). (D) represents the intact peptide ESLAGLVGES ([M1+H]+ 1003.8).
The multiblock copolymer poly[bis(Oct-Lys)1,8-diamino-3,6-dioxazocctane] was synthesized by condensation of the comonomer bis(Oct-Lys-NHFmoc)1,8-diamino-3,6-dioxazocctane and the comonomer NHS-PEG3400-NHS in the presence of an organic base. The comonomer bis(Oct-Lys-NHFmoc)1,8-diamino-3,6-dioxazocctane was synthesized according to the scheme in Figure 2A. The viscosity of the solution increased with the reaction time, indicating elongation of the polymer chain. At the beginning of the reaction, a higher temperature, 50°C, was used to reduce the viscosity of the reaction to increase the condensation efficiency and avoid gelation.44 As shown in Table 1, the molecular weight of the polymer increased with reaction time, while the polydispersity index (PDI) decreased with reaction time.
Table 1.
Molecular weight and polydispersity index of polymer
| Reaction time | Mw (kDa) | Mn (kDa) | PDI (Mw/Mn) |
|---|---|---|---|
| 24 h | 21.7 | 16.5 | 1.31 |
| 48 h | 24.6 | 18.9 | 1.30 |
| 72 h | 35.1 | 27.5 | 1.28 |
In pancreatic cancer, MMP-2 is highly expressed by stromal cells in the tumor microenvironment. The expression and activation of MMP-2 in pancreatic carcinoma tissue was significantly higher than that in chronic pancreatitis and normal pancreatic tissue. Moreover, MMP-2 plays an essential role in invasion and metastasis of pancreatic tumor cells.45–46 We, therefore, designed a novel MMP-2-responsive peptide (LAGLVG) to link CPI-613 to the polymer in order to specifically release the drug and dissociate the nanopolyplex in the tumor microenvironment. Previous studies indicated that the critical residues at the cleavage site of MMP-2-responsive peptides are hydrophobic Glycine (G) and Leucine (L). The other residues of the peptide LAGLVG were designed based on the frequencies of appearance of amino acids at various positions of MMP-2 substrate.47 The sequence also meets the criteria in another study, in which the substrates containing L/IXX↓Xhydrophobic consensus sequence exhibit higher selectivity for MMP-2 over MMP-9.37 In addition, hydrophobicity of the peptide also enhances the hydrophobic interaction of the core of the nanopolyplex, leading to lower CMC. The responsiveness of the peptide ESLAGLVGES to recombinant human MMP-2 was confirmed by LC-MS. After incubation with MMP-2 (25 nM) at 37°C for 1 h, there were two degradation fragments ESLAG ([M1+H]+ 518.3) and LVGES ([M2+H]+ 504.2) in the sample. This result confirmed that the newly designed peptide was sensitive to the MMP-2 enzyme and specifically cleaved between G and L as expected (Figure 2).
As shown in Figure 3, CPI-613 was conjugated to the polymer chains via the following steps: (i) conjugation of CPI-613 with the MMP-2-responsive peptide LAGLVG, (ii) deprotection of Fmoc from polymer chains to liberate the amine groups, and (iii) conjugation of the CPI-613-peptide to the polymer chains to obtain the final polymer-peptide-CPI-613 (PPC) conjugate. Proton NMR (1H-NMR) demonstrated the presence of CPI-613 in the PPC conjugate. As shown in Figure 3C, the peaks (a) between 7.0 and 7.5 ppm correspond to the protons of benzene in CPI-613. Similarly, the peak between 3.0 and 3.5 ppm (b’) corresponds to the protons of methylene in the polymer (Figure 3C). The 1H-NMR of PPC showed characteristic peaks representing both CPI-613 and the polymer (Figure 3C), indicating the presence of CPI-613 in the PPC conjugate. Moreover, the multiplet at 1.0–1.5 ppm (e) in the spectrum of CPI-613 is shifted upfield to ~0.9 ppm in the spectrum of the polymer-peptide-CPI-613 conjugate. Similar shifts were also found in peaks (d). This could be due to the fact that upon conjugation, the strongly deshielded carboxylic acid group which is in close proximity to the methylene groups in question is converted to a less deshielded CONH2 (amide). The drug loading of CPI-613 in the polymer is approximately 15.13% (wt %). To study the formation and characteristics of the self-assembled PPC nanopolyplex, the particle size, zeta potential, critical micelle concentration (CMC), and morphology were studied (Figure 4). The z-average particle size of the PPC nanopolyplex was 96.74 nm with a PDI of 0.159. The CMC value determined by UV spectrophotometry was 25.7 μg/mL (Figure 4D), suggesting that the PPC nanopolyplex as a drug carrier can maintain good stability under diluted conditions in the blood circulation.48–49 The spherical morphology and uniform size distribution of the PPC nanopolyplex were confirmed by TEM (Figure 4A). The particle size detected by TEM was similar as that detected by DLS.
Figure 3. Synthetic scheme.
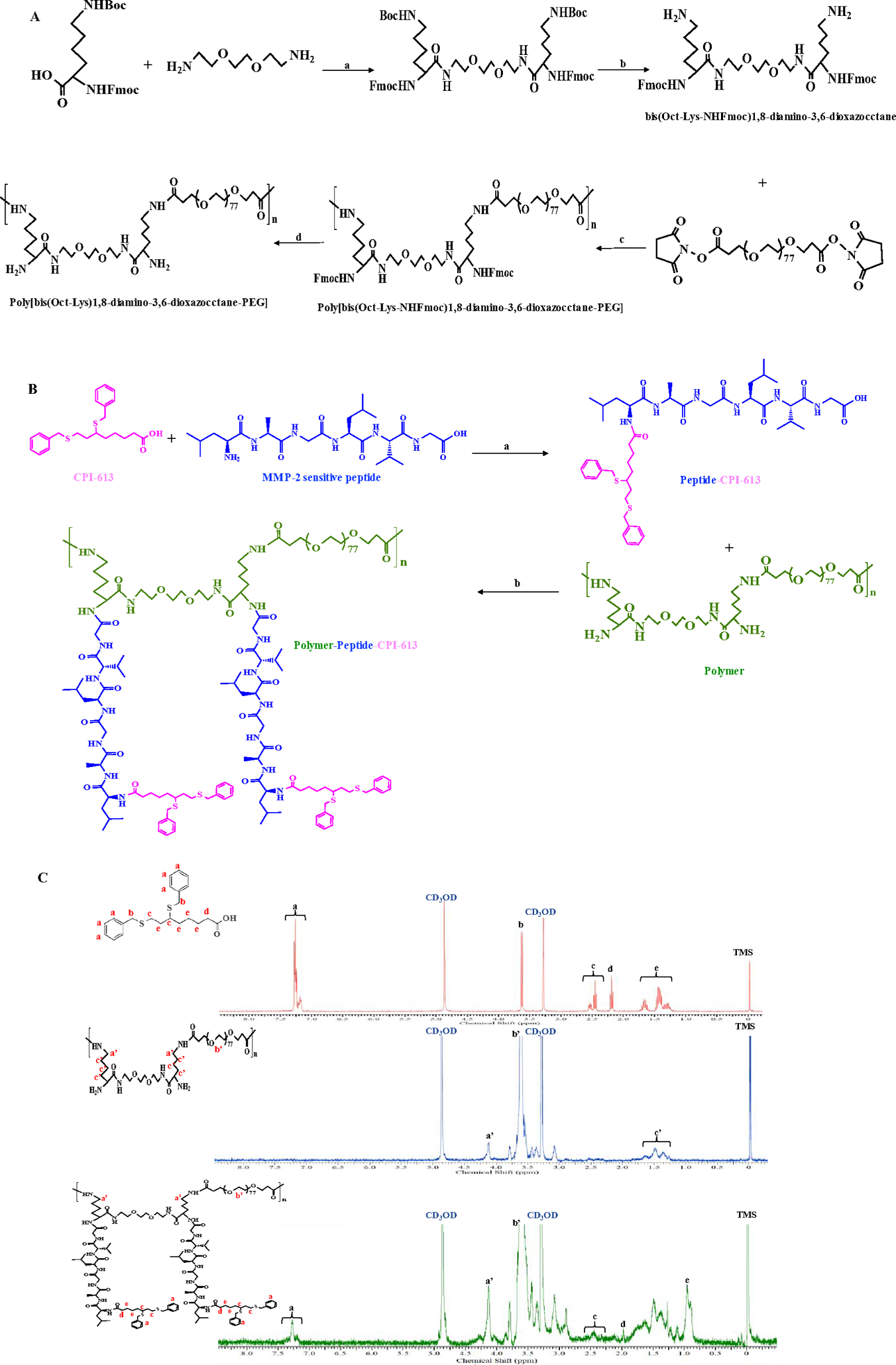
(A) Synthetic scheme of poly[bis(Oct-Lys-NHFmoc)1,8-diamino-3,6-dioxazocctane-PEG]. Reagents and conditions: (a) EDCI, DMAP; (b) TFA/DCM; (c) DIPEA; (d) polymer-bound piperazine. (B) Synthetic scheme of the polymer-peptide-CPI-613 conjugate. Reagents and conditions: (a) HATU, DIPEA; (b) HATU, DIPEA. (C) 1H-NMR spectra of CPI-613, polymer, and polymer-peptide-CPI-613 conjugate.
Figure 4. Characterization of the PPC, PPCL, and p-PPCL nanopolyplexes.
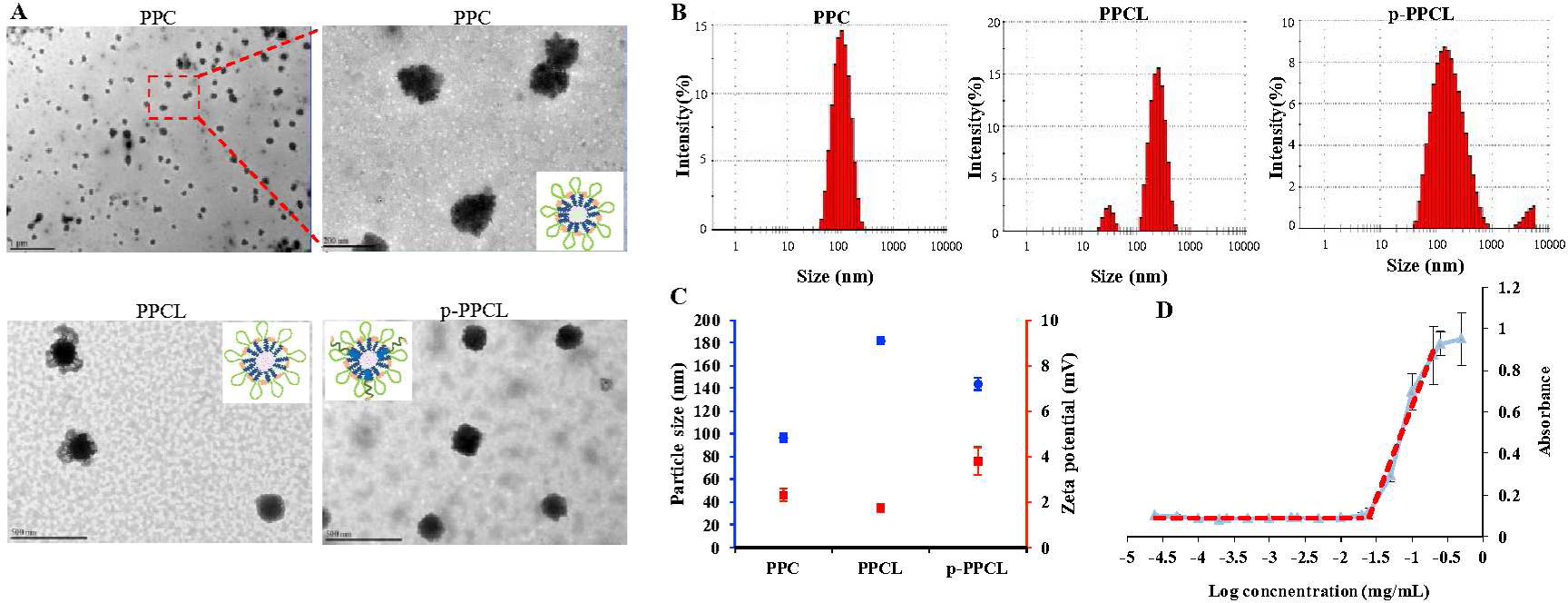
(A) TEM images. The scale bar in PPC images represents 1 μm (left) and 200 nm (right), respectively. The scale bars in both PPCL and p-PPCL images represent 500 nm. (B) Particle size and zeta potential. (C) DLS results. (D) CMC value of the PPC nanopolyplex. The results are presented as the mean ± SD. (n=3 independent samples)
As shown in Figure 4A, TEM images of the LY2109761-loaded PPCL nanopolyplexes showed a “flower-like” structure with high electron intensity in the core but low electron intensity in surrounding shell. This is consistent with the hypothetical structure in Figure 1 A. Similar “flower-like” structure was also observed in micelles formed by PCL-PEG-PCL and PLA-PEG-polyHis.50–51 The z- average particle size of the PPCL nanopolyplex was 181.63 nm and PDI was 0.360, which was larger than that of PPC nanopolyplex. The zeta potential of the PPCL nanopolyplex was 1.74 mV, which was similar to that of the PPC nanopolyplex (2.33 mY) (Figure 4B and 4C). The drug loading (wt %) of LY2109761 in the PPCL nanopolyplex was 15.01 %. The z-average particle size of the p-PPCL nanopolyplex, which includes the DSPE-PEG-plectin-1 targeting peptide, was 144.13 nm with PDI value of 0.357, and its zeta potential was 3.79 mV (Figure 4B and 4C).
Anti-tumor efficacy in vitro
The in vitro anti-tumor effects of CPI-613 and nanopolyplexes were tested on PANC-1 cells. As shown in Figure 5A, CPI-613 alone and CPI-613 plus LY2109761 exhibited similar cytotoxicity at different concentrations. The cytotoxicity of LY2109761 on PANC-1 cells was performed (Figure S4). LY2109761 did not show significant cytotoxicity on PANC-1 cells at low concentrations. Only a minor toxicity was observed at 50 μM as compared to untreated group (P<0.05). Considering the fact that the concentration of LY2109761 in in vitro studies is 5 μM, the cytotoxicity observed in Figure 5 is induced by CPI-613. To further evaluate the cytotoxicity of CPI-613-MMP-2 sensitive peptide, we also compared viability of the cells treated with CPI-613 and CPI-613/MMP-2 sensitive peptide plus MMP-2 (Figure S5). In the presence of MMP-2, the CPI-613-MMP-2 sensitive peptide conjugate showed similar anti-tumor activity as CPI-613. The cytotoxicity of PPCL and p-PPCL nanopolyplexes was lower than that of free drugs because the encapsulated drugs have to be released to exert their activity.52 By contrast, in the presence of MMP-2, the PPCL nanopolyplex showed comparable cytotoxicity as free drugs, indicating MMP-2-responsive drug release is essential for the nanopolyplex’s antitumor activity. Moreover, the PPCL and p-PPCL nanopolyplexes showed concentration-dependent cytotoxicity in the presence of MMP-2. To further demonstrate the MMP-2-responsive activity of the PPCL nanopolyplex, we incubated the nanopolyplex with ARP-100, an MMP-2 inhibitor, and observed diminished cytotoxicity of the nanopolyplex. This result further confirmed that the cleavage of the MMP-2 sensitive linker is essential to release drugs to exhibit their antitumor activity.
Figure 5. In vitro anti-tumor activity and tumor penetration of the nanopolyplexes.
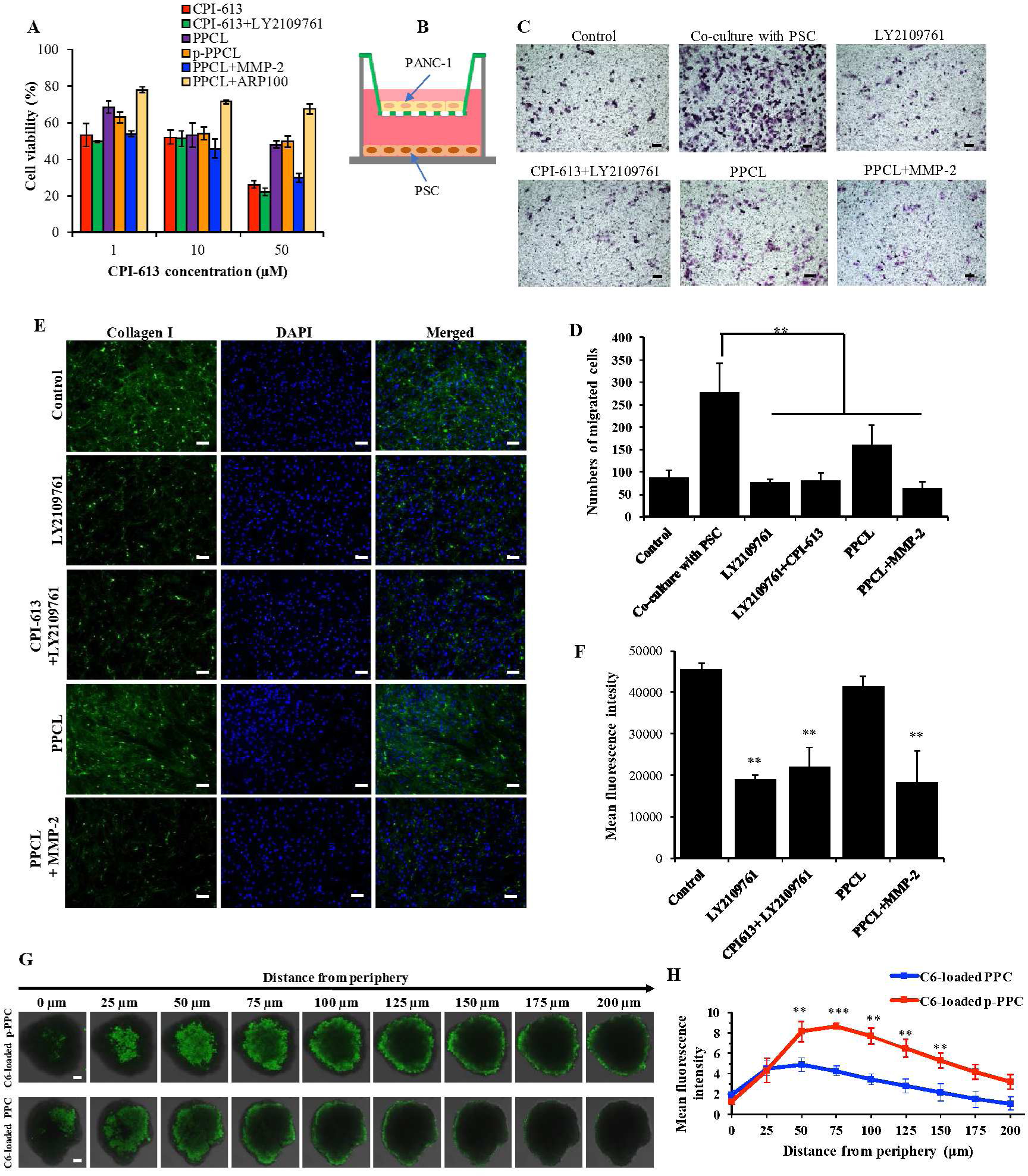
Cytotoxicity and migration of PANC-1 cells and inhibition of Collagen I expression of PSCs and penetration of nanopolyplexes through the PANC-1 multicellular spheroids (MCSs). (A) Cell viability of PANC-1 cells incubated with the nanopolyplexes for 72 h (n=5). (B) Co-culture of PANC-1 cells with PSCs in a transwell double chamber system. PANC-1 cells were seeded in the upper chamber of a transwell insert with 8 pm pore size membrane. PSCs were seeded in the lower chamber of a 24-well plate. (C) Migration of the PANC-1 cells co-cultured with PSCs for 24 h. PANC-1 alone was used as a control. The nanopolyplexes were added into the upper chamber. The scale bar represents 200 μm. (D) Quantitative analysis of migrated PANC-1 cells. (n=5, ** p<0.01) (E) Immunostaining of type I collagen in PSCs after 48 h treatment with the free drug and nanopolyplexes. The scale bar represents 100 μm. (F) Quantitative expression of type I collagen in PSCs (n=5, **P<0.01). (G) Representative z-stack confocal images of the PANC-1 spheroids after 2 h incubation with coumarin 6-loaded nanopolyplexes. The scale bar is 50 μm. (H) Mean fluorescence intensity of the z-stacked confocal images vs. the distance from the periphery of the spheroids (n=3, **P<0.01).
Migration of neighboring tumor cells induced by co-cultured PSCs
The active bidirectional interaction between pancreatic tumor cells and PSCs is receiving increasing attention in pancreatic cancer therapy.8, 53 Emerging evidence suggests that PSCs not only inhibit the apoptosis of tumor cells, but also promote the cancer stem cell-like phenotypes that play an essential role in chemoresistance and disease recurrence.53 Moreover, PSCs are able to travel from the primary tumor site to remote metastatic sites and assist the growth of metastatic cancer cells.54 As a TGF-beta receptor inhibitor, LY2109761 was found to inhibit the invasion of PDAC cells when the cells were co-cultured with fibroblast cells.19
The migration of pancreatic cancer cells PANC-1 was determined in a two-chamber co-culture system (Figure 5B) to investigate the interaction between pancreatic cancer cells and PSCs. As shown in Figure 5C, the presence of PSCs led to approximately 3-fold greater numbers of PANC-1 cells migrating to the lower chamber. The number of migrated cells was significantly reduced in the presence of LY2109761, CPI-613 plus LY2109761, PPCL nanopolyplex, and PPCL nanopolyplex plus recombinant MMP-2. Similar cell motility was observed both in the LY2109761 and CPI-613 plus LY2109761 groups relative to the control without PSC co-culturing. Although the PPCL nanopolyplex alone exhibited effective reduction in migration, the presence of MMP-2 in PPCL nanopolyplex resulted in much lower cell migration relative to PPCL nanopolyplex alone, suggesting that MMP-2 was essential for the drug release to inhibit cancer cell migration. These results indicated that PSCs induce the migration of pancreatic cancer cells, and the PPCL nanopolyplex (with or without MMP-2) can inhibit this neighboring effect.
Downregulation of collagen expression in PSCs
PSCs are the major cellular components in the PDAC tumor microenvironment, occupying over 80% of tumor mass. PSCs contribute to tumorigenesis and metastasis by overexpressing numerous growth factors, proteases and cytokines.3, 5, 55 Among them, overexpressed type I collagen plays an important role in preventing drug penetration as well as promoting tumor proliferation and metastasis9. Here, we investigated the regulatory effects of the nanopolyplex on the expression of collagen in PSCs with immunostaining (Figure 5E and 5F). The expression of type I collagen from PSCs treated with LY2109761 was reduced by 58.2% relative to the control group. As a TGF-β receptor inhibitor, LY2109761 inhibits the phosphorylation of Smad2 and Smad3 and subsequently downregulates the expression of type I collagen. Treatment of PSCs with LY2109761 plus CPI-613 and PPCL nanopolyplex plus MMP-2 also inhibited the expression of type I collagen by 51.8% and 59.9%, respectively. Collagen expression in PSCs is promoted by the phosphorylation of Smad2 and Smad3, which are the downstream proteins in the TGF-β pathway.56 The PPCL nanopolyplex alone negligibly downregulated the expression of type I collagen. In the absence of MMP-2, the amount of LY2109761 released from the PPCL nanopolyplex was too low to inhibit the TGF-β pathway to reduce the expression of type I collagen.
Three-dimensional (3D) tumor spheroid penetration study
The dense ECM in PDAC is the major barrier that limits the penetration of anti-cancer therapeutics into the tumor microenvironment. Multicellular spheroid (MCS) provides a unique structure to simulate the physiological characteristics of solid tumors, and they have been widely as an in vitro model to evaluate the tumor penetration efficacy of various therapeutics. A 3D tumor spheroid model of PANC-1 cells was developed to study tumor penetration of the coumarin-6-loaded nanopolyplexes. After 2 h incubation, coumarin-6-loaded PPC nanopolyplex was mostly located in the periphery areas of the spheroids, while coumarin-6-loaded p-PPC nanopolyplex penetrated deeper into the spheroids (Figure 5G and 5H). These results suggested that the penetration capacity of the p-PPC nanopolyplex was improved because of the plectin-1 peptide, which binds to plectin-1 on pancreatic cancer cells.
Biodistribution of the nanopolyplexes in a subcutaneous xenograft pancreatic cancer mouse model
A recent report revealed that the expression of plectin-1 is positive in all PDACs but negative in benign tissues.31 Moreover, plectin-1 is exclusively located in the cytoplasm in normal physiology, but it is expressed on the surface of pancreatic cancer cells.34 We, therefore, modify the nanopolyplex with a plecin-1 targeting peptide to enhance its tumor accumulation.
To visualize the biodistribution of the PPCL and p-PPCL nanopolyplexes in vivo, the hydrophobic fluorescence dye DiR, rather than LY2109761, was loaded into the nanopolyplexes using the solvent evaporation method. In contrast to DiR-loaded PPC nanopolyplex, DiR-loaded p-PPC nanopolyplex exhibited increased tumor accumulation over the time from 1 h to 24 h post-injection. The p-PPC nanopolyplex quickly accumulated at the tumor site within 1 h and retained in the tumor for up to 24 h (Figure 6A). Moreover, the fluorescence signals of harvested tumors from DiR-loaded p-PPC nanopolyplex group obtained at 24 h post-injection were 2.7-fold stronger than those from the DiR-loaded PPC nanopolyplex group (Figure 6B and 6C). Both DiR-loaded PPC and DiR-loaded p-PPC nanopolyplexes showed a high distribution in the liver and spleen. Livers collected from the p-PPC nanopolyplex group showed similar fluorescence signal strength relative to the PPC nanopolyplex group. However, DiR-loaded p-PPC nanopolyplex exhibited lower accumulation in the spleen than DiR-loaded PPC nanopolyplex (Figure 6B and 6C). No significant fluorescence was observed in the lung, kidney, heart and muscle, suggesting negligible accumulation of nanopolyplexes in those organs. The results demonstrated that modification of the PPC nanopolyplexes with the plectin-1 targeting peptide improved accumulation in pancreatic cancer cells.
Figure 6. Biodistribution of the DiR-loaded nanopolyplexes in a subcutaneous xenograft pancreatic cancer mouse model.
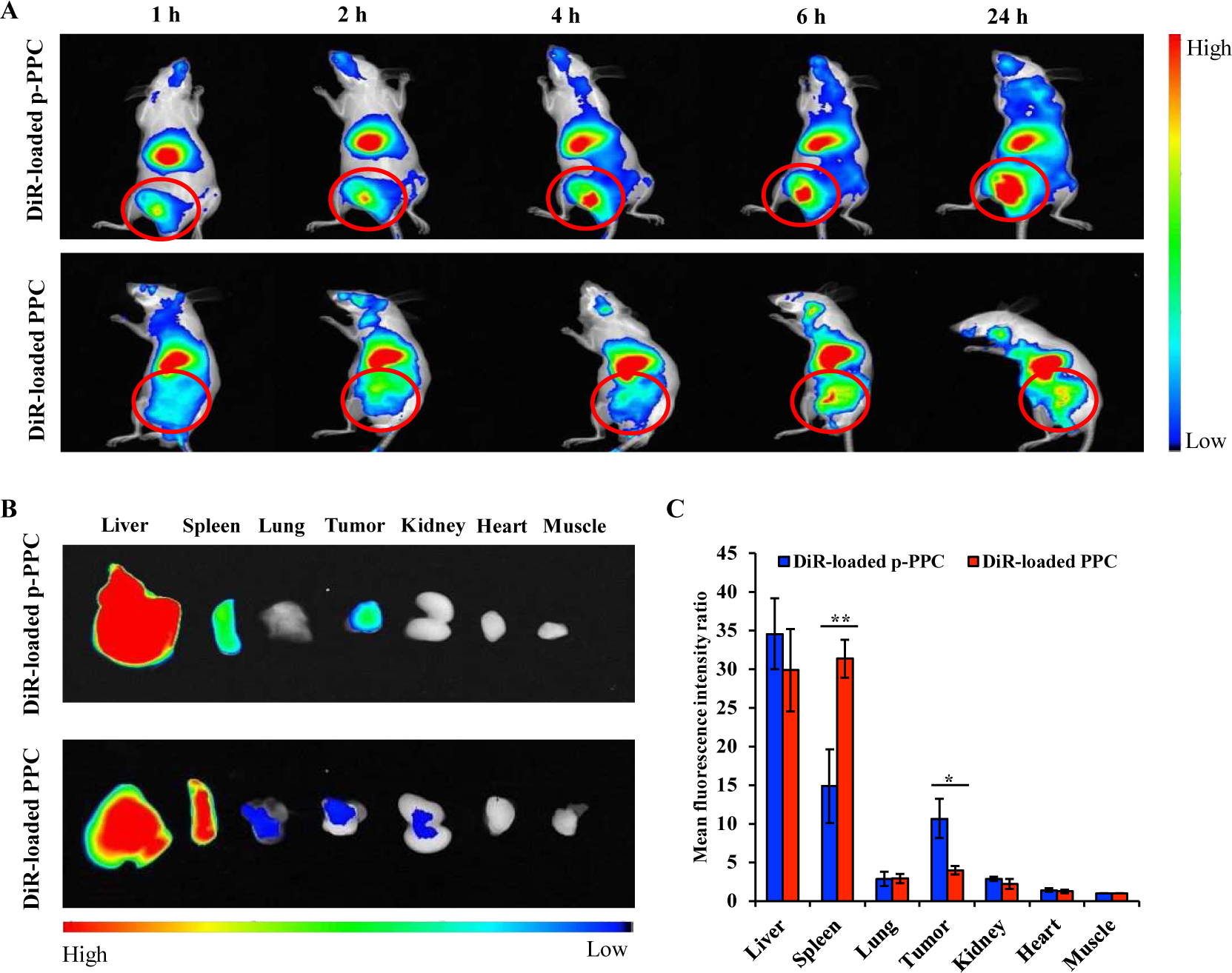
The mice bearing PANC-1 xenograft tumors were administrated DiR-loaded PPC and p-PPC nanopolyplexes by the tail vein. (A) Representative fluorescence imaging of the mice at 1, 2, 4, 6, and 24 h post-administration. Red circles indicate the implanted tumors. (B) Representative fluorescence images of the liver, spleen, lung, tumor, kidney, heart, and muscle from the mice 24 h post-administration. (C) Mean fluorescence intensity of each organ was normalized to that of the muscle. The results are presented as the mean ± SD. (n=3 mice per group, *P<0.05, **P<0.01).
In vivo antitumor efficacy
The antitumor efficacy of the nanopolyplexes was evaluated in mice with orthotopic implantation of PANC-1/Luc cells (Figure 7A). As compared to commonly used subcutaneous xenograft mouse models of PDAC, orthotopic models mimic the stromal-rich desmoplastic tumor microenvironment in human patients. Tumor growth was monitored by quantifying the luminescence intensity of the tumors using a small animal imaging system. While free drugs, such as CPI-163 or CPI-613 plus LY2109761, inhibited tumor growth, the p-PPCL nanopolyplex exhibited the most significant tumor growth inhibition. The luminescence intensity of the mice treated with saline is 11-fold higher than that treated with the p-PPCL nanopolyplex. The PPCL nanopolyplex also exhibited superior antitumor efficacy than free drugs and saline. In addition, tumor bioluminescence in the mice treated with CPI-613 plus LY2109761 was decreased by 34.41% compared to that in the mice treated with CPI-613 alone. The weights (Figure 7D and 7E) of harvested tumor tissues are consistent with the above luminescence imaging results.
Figure 7. Antitumor activity of the nanopolyplexes in an orthotopic pancreatic cancer mouse model.
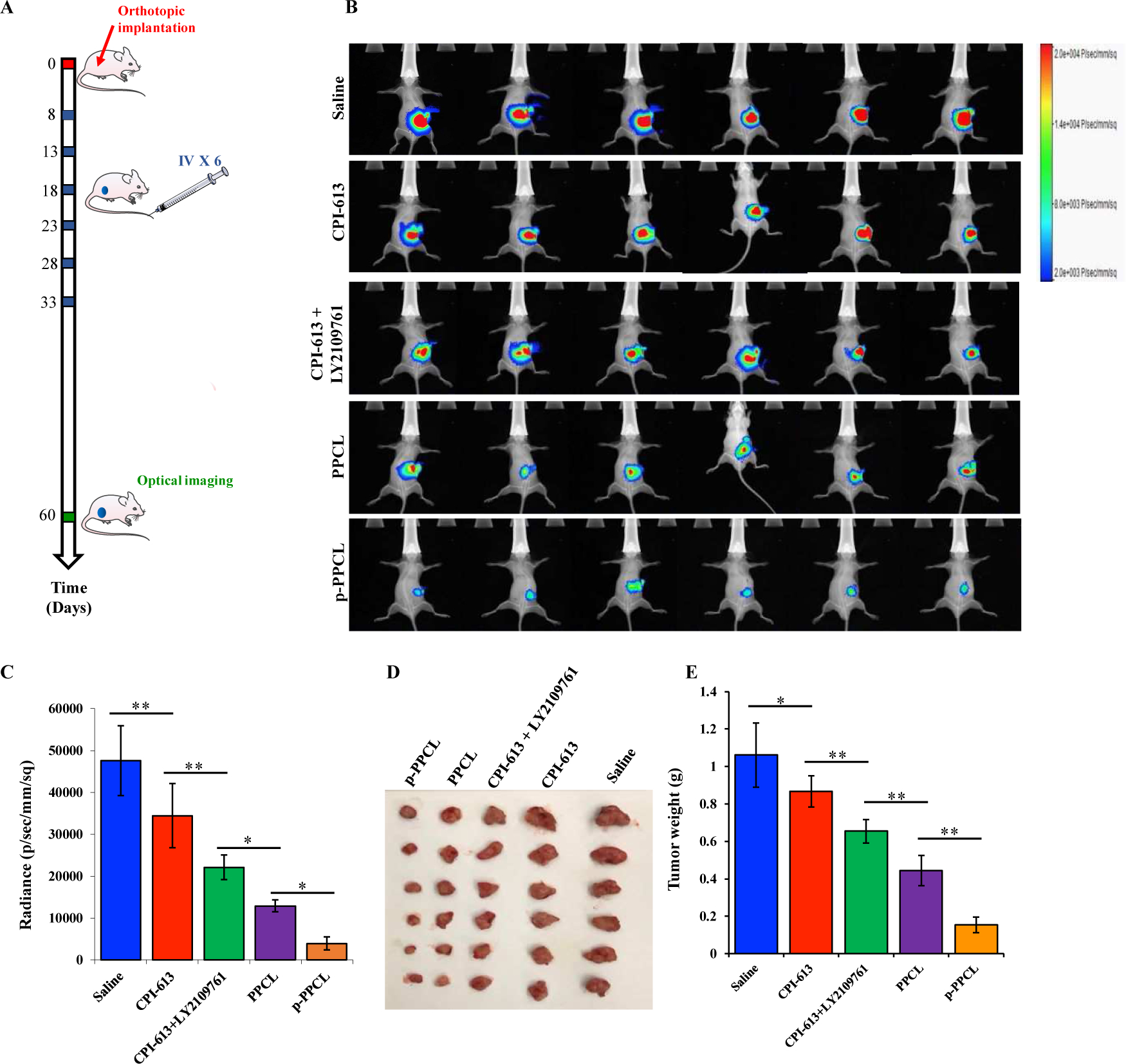
(A) Eight days after orthotopic implantation of PANC-1/Luc cells, the mice were administrated saline, CPI-613, CPI-613 plus LY2109761, PPCL nanopolyplex, and p-PPCL nanopolyplex by the tail vein every 5 days for a total of 6 doses. The mice were euthanized at day 60 post-implantation. (B) In vivo bioluminescence imaging of PANC-1/Luc cells. (C) Quantification of bioluminescence from the orthotopic tumors at Day 60 post-implantation. (D) Images of harvested tumors. (E) Tumor weight. The results are presented as mean ± SD (n=6 mice per group, *P<0.05, **P<0.01).
A TUNEL assay was performed to detect cell apoptosis in tumor tissues (Figure 8A and 8B). The combination of CPI-613 and LY2109761 effectively enhanced the apoptosis of tumor cells than CPI-613 alone. Encapsulation of the drugs in the PPCL nanopolyplex exhibited higher apoptosis than free drugs. Most importantly, the percentage of apoptotic cells in the p-PPCL nanopolyplex group was 19.35%, which is the highest among all the groups.
Figure 8. Histological examination of tumor tissues.
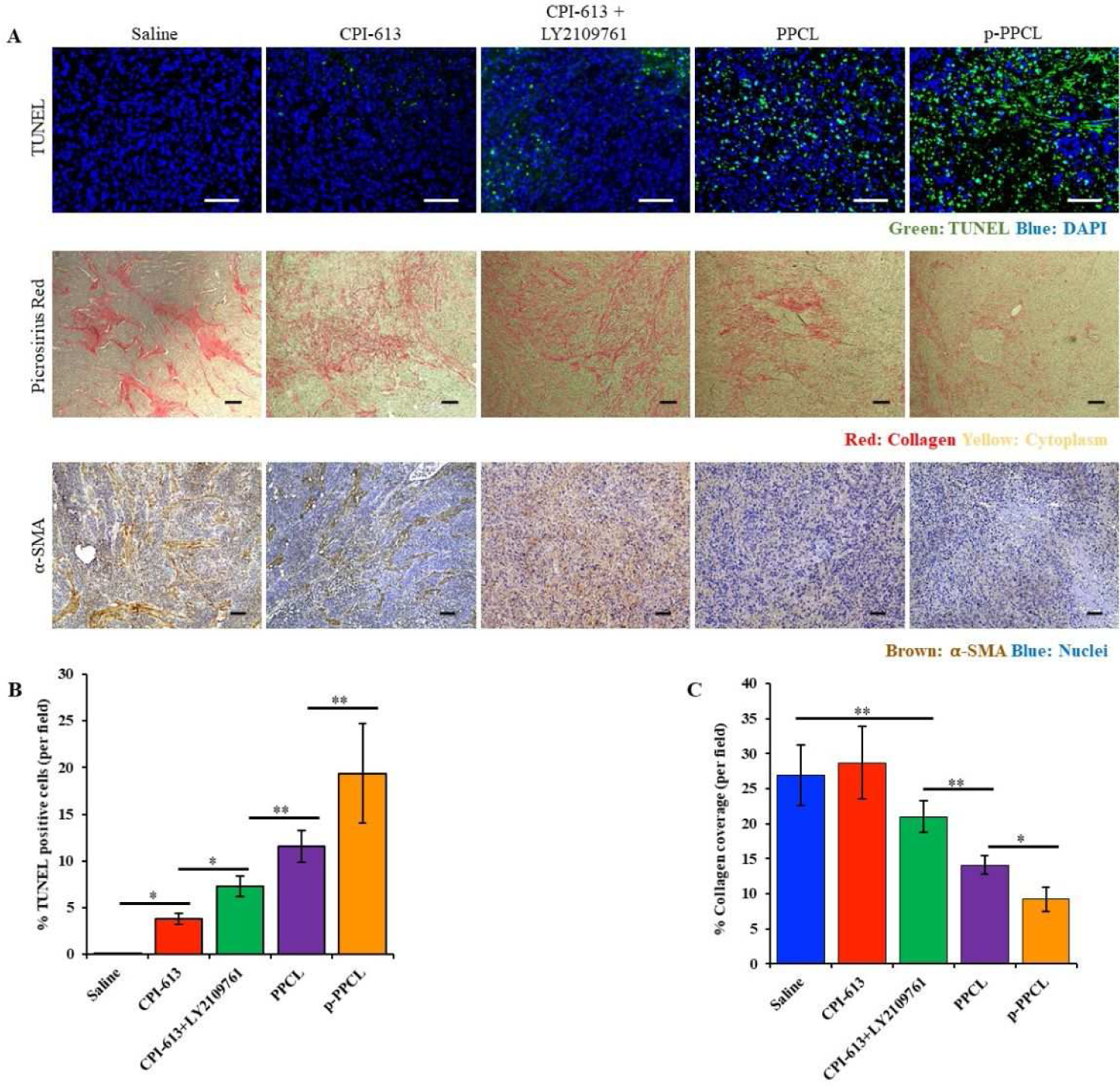
(A) TUNEL assay, Picro-Sirius Red, and α-SMA immunohistochemistry staining of tumor specimen. The scale bar represents 100 μm. (B) Quantitative analysis of cell apoptosis in TUNEL assay. The scale bar represents 100 μm. (C) Quantitative analysis of Picro-Sirius Red stained areas using ImageJ. We randomly selected three tumor slides from each group and took three pictures of each slide from randomly selected areas (mean ± SD, n=9, *P<0.05, **P<0.01). The scale bar represents 100 μm.
Expression of collagen and activation of PSCs in tumors
Collagen is the major component of ECM in PDAC. Overexpression of collagen enhances proliferation and metastasis of PDAC cells as well as reduces the penetration of drugs. Picro-Sirius Red staining was, therefore, used to evaluate the expression of collagen in the tumor tissues (Figure 8A and 8C). The expression of collagen in the tumors treated with CPI-613 alone showed no significant difference as compared to the saline group. By contrast, the combination of LY2109761 and CPI-613 greatly suppressed the expression of collagen in tumors, suggesting that LY2109761 is responsible for the reduction of collagen in the tumor. Encapsulation of LY2109761 and CPI-613 in the PPCL nanopolyplex improved their activity and inhibited the expression of collagen by 47.6 %. Moreover, treatment with the p-PPCL nanopolyplex exhibited the highest inhibitory effect on the expression of collagen.
To further confirm the inhibitory effect of the nanopolyplexes on tumor stroma, the expression of α-SMA, the bio-marker of activated PSCs, in tumors was examined by immunohistochemistry staining (Figure 8A). The dense and strong expression of α-SMA was observed in both control and CPI-613-treated groups. However, after treatment with LY210961 plus CPI-613, the level of α-SMA was significantly reduced, which is consistent with the results of collagen expression. The treatment with p-PPCL showed the most significant down-regulation of α-SMA in tumors.
CONCLUSIONS
In the present study, we developed a lysine- and PEG-based alternating copolymer to co-deliver LY2109761, a TGF-β RI kinase inhibitor, and CPI-613, a novel chemotherapy drug, to desmoplastic stroma and tumor cells, respectively. Hydrophobic CPI-613 is conjugated to the hydrophilic copolymer via a newly designed MMP-2-responsive linker to form a trigger-responsive nanopolyplex. LY2109761 is hydrophobic and encapsulated into the hydrophobic core of the nanopolyplex. The resulting nanopolyplex is modified with a plectin-1-targeting peptide to enhance the accumulation of the nanopolyplex in pancreatic cancer cells. The MMP-2-responsive linker is cleaved in the tumor microenvironment to release CPI-613 from the polymer, leading to dissociation of the nanopolyplex and subsequent release of LY2109761.
By interfering with the interaction between the tumor cells and stroma, the nanopolyplex blocks the activation of PSCs, and normalizes the stroma to inhibit the proliferation and migration of tumor cells. Normalization of the stroma also increases the penetration of the nanopolyplex into the tumor. The nanopolyplex shows enhanced accumulation in xenograft pancreatic tumors in a biodistribution study. Moreover, the targeted nanopolyplex markedly inhibits tumor growth in an orthotopic pancreatic cancer mouse model by dual targeting tumor cells and stroma. This study presents a promising polymer-drug nanopolyplex for pancreatic cancer therapy by dual targeting tumor cells and stroma.
Supplementary Material
Acknowledgments
Yuanke Li is supported by the Chinese Scholarship Council (CSC). This work was supported in part by the National Institutes of Health (R01CA23109901, R01GM121798).
Footnotes
Supporting Information
Mass spectrum of intermediate compounds; Cell viability of PANC-1 cells incubated with LY2109761, CPI-613 or CPI-613-peptide plus MMP-2.
Reference
- (1).Siegel RL; Miller KD; Jemal A Cancer Statistics, 2019. CA Cancer J Clin 2019, 69 (1), 7–34, DOI: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- (2).Erkan M; Hausmann S; Michalski CW; Fingerle AA; Dobritz M; Kleeff J; Friess H The Role of Stroma in Pancreatic Cancer: Diagnostic and Therapeutic Implications. Nat Rev Gastroenterol Hepatol 2012, 9 (8), 454–467, DOI: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- (3).Kota J; Hancock J; Kwon J; Korc M Pancreatic Cancer: Stroma and Its Current and Emerging Targeted Therapies. Cancer Lett 2017, 391, 38–49, DOI: 10.1016/j.canlet.2016.12.035. [DOI] [PubMed] [Google Scholar]
- (4).Winter JM; Brennan MF; Tang LH; D’Angelica MI; Dematteo RP; Fong Y; Klimstra DS; Jarnagin WR; Allen PJ Survival after Resection of Pancreatic Adenocarcinoma: Results from a Single Institution over Three Decades. Ann Surg Oncol 2012, 19 (1), 169–175, DOI: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- (5).Apte MV; Wilson JS; Lugea A; Pandol SJ A Starring Role for Stellate Cells in the Pancreatic Cancer Microenvironment. Gastroenterology 2013, 144 (6), 1210–1219, DOI: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).von Ahrens D; Bhagat TD; Nagrath D; Maitra A; Verma A The Role of Stromal Cancer-Associated Fibroblasts in Pancreatic Cancer. J Hematol Oncol 2017, 10 (1), 76, DOI: 10.1186/s13045-017-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Xu Z; Pothula SP; Wilson JS; Apte MV Pancreatic Cancer and Its Stroma: A Conspiracy Theory. World J Gastroenterol 2014, 20 (32), 11216–11229, DOI: 10.3748/wjg.v20.i32.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kleeff J; Korc M; Apte M; La Vecchia C; Johnson CD; Biankin AV; Neale RE; Tempero M; Tuveson DA; Hruban RH; Neoptolemos JP Pancreatic Cancer. Nat Rev Dis Primers 2016, 2, 16022, DOI: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- (9).Whatcott CJ; Posner RG; Von Hoff DD; Han H Desmoplasia and Chemoresistance in Pancreatic Cancer In Pancreatic Cancer and Tumor Microenvironment; Grippo PJ; Munshi HG, Eds.; Trivandrum (India), 2012. [PubMed] [Google Scholar]
- (10).Neesse A; Michl P; Frese KK; Feig C; Cook N; Jacobetz MA; Lolkema MP; Buchholz M; Olive KP; Gress TM; Tuveson DA Stromal Biology and Therapy in Pancreatic Cancer. Gut 2011, 60 (6), 861–868, DOI: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- (11).Friess H; Yamanaka Y; Buchler M; Ebert M; Beger HG; Gold LI; Korc M Enhanced Expression of Transforming Growth Factor Beta Isoforms in Pancreatic Cancer Correlates with Decreased Survival. Gastroenterology 1993, 105 (6), 1846–1856. [DOI] [PubMed] [Google Scholar]
- (12).Rucki AA; Zheng L Pancreatic Cancer Stroma: Understanding Biology Leads to New Therapeutic Strategies. World J Gastroenterol 2014, 20 (9), 2237–2246, DOI: 10.3748/wjg.v20.i9.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Neuzillet C; Tijeras-Raballand A; Cohen R; Cros J; Faivre S; Raymond E; de Gramont A Targeting the Tgfbeta Pathway for Cancer Therapy. Pharmacol Ther 2015, 147, 22–31, DOI: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- (14).Yang L; Pang Y; Moses HL Tgf-Beta and Immune Cells: An Important Regulatory Axis in the Tumor Microenvironment and Progression. Trends Immunol 2010, 31 (6), 220–227, DOI: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Li MO; Wan YY; Sanjabi S; Robertson AK; Flavell RA Transforming Growth Factor-Beta Regulation of Immune Responses. Annu Rev Immunol 2006, 24, 99–146, DOI: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- (16).Herbertz S; Sawyer JS; Stauber AJ; Gueorguieva I; Driscoll KE; Estrem ST; Cleverly AL; Desaiah D; Guba SC; Benhadji KA; Slapak CA; Lahn MM Clinical Development of Galunisertib (Ly2157299 Monohydrate), a Small Molecule Inhibitor of Transforming Growth Factor-Beta Signaling Pathway. Drug Des Devel Ther 2015, 9, 4479–4499, DOI: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Flechsig P; Dadrich M; Bickelhaupt S; Jenne J; Hauser K; Timke C; Peschke P; Hahn EW; Grone HJ; Yingling J; Lahn M; Wirkner U; Huber PE Ly2109761 Attenuates Radiation-Induced Pulmonary Murine Fibrosis Via Reversal of Tgf-Beta and Bmp-Associated Proinflammatory and Proangiogenic Signals. Clin Cancer Res 2012, 18 (13), 3616–3627, DOI: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- (18).Mazzocca A; Fransvea E; Dituri F; Lupo L; Antonaci S; Giannelli G Down-Regulation of Connective Tissue Growth Factor by Inhibition of Transforming Growth Factor Beta Blocks the Tumor-Stroma Cross-Talk and Tumor Progression in Hepatocellular Carcinoma. Hepatology 2010, 51 (2), 523–534, DOI: 10.1002/hep.23285. [DOI] [PubMed] [Google Scholar]
- (19).Melisi D; Ishiyama S; Sclabas GM; Fleming JB; Xia Q; Tortora G; Abbruzzese JL; Chiao PJ Ly2109761, a Novel Transforming Growth Factor Beta Receptor Type I and Type Ii Dual Inhibitor, as a Therapeutic Approach to Suppressing Pancreatic Cancer Metastasis. Mol Cancer Ther 2008, 7 (4), 829–840, DOI: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Vander Heiden MG; Cantley LC; Thompson CB Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324 (5930), 1029–1033, DOI: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dorsam B; Fahrer J The Disulfide Compound Alpha-Lipoic Acid and Its Derivatives: A Novel Class of Anticancer Agents Targeting Mitochondria. Cancer Lett 2016, 371 (1), 12–19, DOI: 10.1016/j.canlet.2015.11.019. [DOI] [PubMed] [Google Scholar]
- (22).Pardee TS; Lee K; Luddy J; Maturo C; Rodriguez R; Isom S; Miller LD; Stadelman KM; Levitan D; Hurd D; Ellis LR; Harrelson R; Manuel M; Dralle S; Lyerly S; Powell BL A Phase I Study of the First-in-Class Antimitochondrial Metabolism Agent, Cpi-613, in Patients with Advanced Hematologic Malignancies. Clin Cancer Res 2014, 20 (20), 5255–5264, DOI: 10.1158/1078-0432.CCR-14-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Zachar Z; Marecek J; Maturo C; Gupta S; Stuart SD; Howell K; Schauble A; Lem J; Piramzadian A; Karnik S; Lee K; Rodriguez R; Shorr R; Bingham PM Non-Redox-Active Lipoate Derivates Disrupt Cancer Cell Mitochondrial Metabolism and Are Potent Anticancer Agents in Vivo. J Mol Med (Berl) 2011, 89 (11), 1137–1148, DOI: 10.1007/s00109-011-0785-8. [DOI] [PubMed] [Google Scholar]
- (24).Stuart SD; Schauble A; Gupta S; Kennedy AD; Keppler BR; Bingham PM; Zachar Z A Strategically Designed Small Molecule Attacks Alpha-Ketoglutarate Dehydrogenase in Tumor Cells through a Redox Process. Cancer Metab 2014, 2 (1), 4, DOI: 10.1186/2049-3002-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lee KC; Maturo C; Perera CN; Luddy J; Rodriguez R; Shorr R Translational Assessment of Mitochondrial Dysfunction of Pancreatic Cancer from in Vitro Gene Microarray and Animal Efficacy Studies, to Early Clinical Studies, Via the Novel Tumor-Specific Anti-Mitochondrial Agent, Cpi-613. Ann Transl Med 2014, 2 (9), 91, DOI: 10.3978/j.issn.2305-5839.2014.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lycan TW; Pardee TS; Petty WJ; Bonomi M; Alistar A; Lamar ZS; Isom S; Chan MD; Miller AA; Ruiz J A Phase Ii Clinical Trial of Cpi-613 in Patients with Relapsed or Refractory Small Cell Lung Carcinoma. PLoS One 2016, 11 (10), e0164244, DOI: 10.1371/journal.pone.0164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Mayer LD; Janoff AS Optimizing Combination Chemotherapy by Controlling Drug Ratios. Mol Interv 2007, 7 (4), 216–223, DOI: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- (28).Xiao B; Ma L; Merlin D Nanoparticle-Mediated Co-Delivery of Chemotherapeutic Agent and Sirna for Combination Cancer Therapy. Expert Opin Drug Deliv 2017, 14 (1), 65–73, DOI: 10.1080/17425247.2016.1205583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Vogus DR; Evans MA; Pusuluri A; Barajas A; Zhang M; Krishnan V; Nowak M; Menegatti S; Helgeson ME; Squires TM; Mitragotri S A Hyaluronic Acid Conjugate Engineered to Synergistically and Sequentially Deliver Gemcitabine and Doxorubicin to Treat Triple Negative Breast Cancer. J Control Release 2017, 267, 191–202, DOI: 10.1016/j.jconrel.2017.08.016. [DOI] [PubMed] [Google Scholar]
- (30).Zhao Z; Li Y; Shukla R; Liu H; Jain A; Barve A; Cheng K Development of a Biocompatible Copolymer Nanocomplex to Deliver Vegf Sirna for Triple Negative Breast Cancer. Theranostics 2019, 9 (15), 4508–4524, DOI: 10.7150/thno.34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bausch D; Thomas S; Mino-Kenudson M; Fernandez-del CC; Bauer TW; Williams M; Warshaw AL; Thayer SP; Kelly KA Plectin-1 as a Novel Biomarker for Pancreatic Cancer. Clin Cancer Res 2011, 17 (2), 302–309, DOI: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kelly KA; Bardeesy N; Anbazhagan R; Gurumurthy S; Berger J; Alencar H; Depinho RA; Mahmood U; Weissleder R Targeted Nanoparticles for Imaging Incipient Pancreatic Ductal Adenocarcinoma. PLoS Med 2008, 5 (4), e85, DOI: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Konkalmatt PR; Deng D; Thomas S; Wu MT; Logsdon CD; French BA; Kelly KA Plectin-1 Targeted Aav Vector for the Molecular Imaging of Pancreatic Cancer. Front Oncol 2013, 3, 84, DOI: 10.3389/fonc.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Shin SJ; Smith JA; Rezniczek GA; Pan S; Chen R; Brentnall TA; Wiche G; Kelly KA Unexpected Gain of Function for the Scaffolding Protein Plectin Due to Mislocalization in Pancreatic Cancer. Proc Natl Acad Sci U S A 2013, 110 (48), 19414–19419, DOI: 10.1073/pnas.1309720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Saxena V; Hussain MD Poloxamer 407/Tpgs Mixed Micelles for Delivery of Gambogic Acid to Breast and Multidrug-Resistant Cancer. Int J Nanomedicine 2012, 7, 713–721, DOI: 10.2147/IJN.S28745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wei Z; Hao J; Yuan S; Li Y; Juan W; Sha X; Fang X Paclitaxel-Loaded Pluronic P123/F127 Mixed Polymeric Micelles: Formulation, Optimization and in Vitro Characterization. Int J Pharm 2009, 376 (1–2), 176–185, DOI: 10.1016/j.ijpharm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- (37).Chen EI; Kridel SJ; Howard EW; Li W; Godzik A; Smith JW A Unique Substrate Recognition Profile for Matrix Metalloproteinase-2. J Biol Chem 2002, 277 (6), 4485–4491, DOI: 10.1074/jbc.M109469200. [DOI] [PubMed] [Google Scholar]
- (38).Liu H; Zhao Z; Zhang L; Li Y; Jain A; Barve A; Jin W; Liu Y; Fetse J; Cheng K Discovery of Low-Molecular Weight Anti-Pd-L1 Peptides for Cancer Immunotherapy. J Immunother Cancer 2019, 7 (1), 270, DOI: 10.1186/s40425-019-0705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Erkan M; Michalski CW; Rieder S; Reiser-Erkan C; Abiatari I; Kolb A; Giese NA; Esposito I; Friess H; Kleeff J The Activated Stroma Index Is a Novel and Independent Prognostic Marker in Pancreatic Ductal Adenocarcinoma. Clin Gastroenterol Hepatol 2008, 6 (10), 1155–1161, DOI: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- (40).Wang LM; Silva MA; D’Costa Z; Bockelmann R; Soonawalla Z; Liu S; O’Neill E; Mukherjee S; McKenna WG; Muschel R; Fokas E The Prognostic Role of Desmoplastic Stroma in Pancreatic Ductal Adenocarcinoma. Oncotarget 2016, 7 (4), 4183–4194, DOI: 10.18632/oncotarget.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Gore J; Korc M Pancreatic Cancer Stroma: Friend or Foe? Cancer Cell 2014, 25 (6), 711–712, DOI: 10.1016/j.ccr.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhan HX; Zhou B; Cheng YG; Xu JW; Wang L; Zhang GY; Hu SY Crosstalk between Stromal Cells and Cancer Cells in Pancreatic Cancer: New Insights into Stromal Biology. Cancer Lett 2017, 392, 83–93, DOI: 10.1016/j.canlet.2017.01.041. [DOI] [PubMed] [Google Scholar]
- (43).Camacho KM; Menegatti S; Mitragotri S Low-Molecular-Weight Polymer-Drug Conjugates for Synergistic Anticancer Activity of Camptothecin and Doxorubicin Combinations. Nanomedicine (Lond) 2016, 11 (9), 1139–1151, DOI: 10.2217/nnm.16.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Tai W; Chen Z; Barve A; Peng Z; Cheng K A Novel Rapamycin-Polymer Conjugate Based on a New Poly(Ethylene Glycol) Multiblock Copolymer. Pharm Res 2014, 31 (3), 706–719, DOI: 10.1007/s11095-013-1192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Koshiba T; Hosotani R; Wada M; Miyamoto Y; Fujimoto K; Lee JU; Doi R; Arii S; Imamura M Involvement of Matrix Metalloproteinase-2 Activity in Invasion and Metastasis of Pancreatic Carcinoma. Cancer 1998, 82 (4), 642–650. [DOI] [PubMed] [Google Scholar]
- (46).Bramhall SR; Neoptolemos JP; Stamp GW; Lemoine NR Imbalance of Expression of Matrix Metalloproteinases (Mmps) and Tissue Inhibitors of the Matrix Metalloproteinases (Timps) in Human Pancreatic Carcinoma. J Pathol 1997, 182 (3), 347–355, DOI: . [DOI] [PubMed] [Google Scholar]
- (47).Turk BE; Huang LL; Piro ET; Cantley LC Determination of Protease Cleavage Site Motifs Using Mixture-Based Oriented Peptide Libraries. Nat Biotechnol 2001, 19 (7), 661–667, DOI: 10.1038/90273. [DOI] [PubMed] [Google Scholar]
- (48).Yao J; Zhang L; Zhou J; Liu H; Zhang Q Efficient Simultaneous Tumor Targeting Delivery of All-Trans Retinoid Acid and Paclitaxel Based on Hyaluronic Acid-Based Multifunctional Nanocarrier. Mol Pharm 2013, 10 (3), 1080–1091, DOI: 10.1021/mp3005808. [DOI] [PubMed] [Google Scholar]
- (49).Li Y; Wu Y; Huang L; Miao L; Zhou J; Satterlee AB; Yao J Sigma Receptor-Mediated Targeted Delivery of Anti-Angiogenic Multifunctional Nanodrugs for Combination Tumor Therapy. J Control Release 2016, 228, 107–119, DOI: 10.1016/j.jconrel.2016.02.044. [DOI] [PubMed] [Google Scholar]
- (50).Zhang L; Chen Z; Wang H; Wu S; Zhao K; Sun H; Kong D; Wang C; Leng X; Zhu D Preparation and Evaluation of Pcl-Peg-Pcl Polymeric Nanoparticles for Doxorubicin Delivery against Breast Cancer. RSC Advances 2016, 6 (60), 54727–54737, DOI: 10.1039/C6RA04687H. [DOI] [Google Scholar]
- (51).Lee ES; Oh KT; Kim D; Youn YS; Bae YH Tumor Ph-Responsive Flower-Like Micelles of Poly(L-Lactic Acid)-B-Poly(Ethylene Glycol)-B-Poly(L-Histidine). J Control Release 2007, 123 (1), 19–26, DOI: 10.1016/j.jconrel.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zhu L; Wang T; Perche F; Taigind A; Torchilin VP Enhanced Anticancer Activity of Nanopreparation Containing an Mmp2-Sensitive Peg-Drug Conjugate and Cell-Penetrating Moiety. Proc Natl Acad Sci U S A 2013, 110 (42), 17047–17052, DOI: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Hamada S; Masamune A; Takikawa T; Suzuki N; Kikuta K; Hirota M; Hamada H; Kobune M; Satoh K; Shimosegawa T Pancreatic Stellate Cells Enhance Stem Cell-Like Phenotypes in Pancreatic Cancer Cells. Biochem Biophys Res Commun 2012, 421 (2), 349–354, DOI: 10.1016/j.bbrc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- (54).Xu Z; Vonlaufen A; Phillips PA; Fiala-Beer E; Zhang X; Yang L; Biankin AV; Goldstein D; Pirola RC; Wilson JS; Apte MV Role of Pancreatic Stellate Cells in Pancreatic Cancer Metastasis. Am J Pathol 2010, 177 (5), 2585–2596, DOI: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Heinemann V; Reni M; Ychou M; Richel DJ; Macarulla T; Ducreux M Tumour-Stroma Interactions in Pancreatic Ductal Adenocarcinoma: Rationale and Current Evidence for New Therapeutic Strategies. Cancer Treat Rev 2014, 40 (1), 118–128, DOI: 10.1016/j.ctrv.2013.04.004. [DOI] [PubMed] [Google Scholar]
- (56).Neuzillet C; de Gramont A; Tijeras-Raballand A; de Mestier L; Cros J; Faivre S; Raymond E Perspectives of Tgf-Beta Inhibition in Pancreatic and Hepatocellular Carcinomas. Oncotarget 2014, 5 (1), 78–94, DOI: 10.18632/oncotarget.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


