Abstract
A common challenge in fear conditioning studies is that a relatively large proportion of individuals fail to acquire a differential conditioned skin conductance response (SCR). Researchers have identified demographic factors associated with poorer fear learning and explored the use of different fear conditioning paradigms across various populations. However, few studies have strategically aimed to enhance acquisition by manipulating the unconditioned stimulus (UCS). In the current manuscript, we examined whether demographic factors predicted failure to condition (n = 274) and explored whether modifications to the UCS enhanced fear learning (n = 143). Results indicated that race, but not age, education, or gender, predicted failure to condition. Stepwise logistic regression demonstrated that race was the most influential of these predictors; African Americans were less likely to acquire a conditioned SCR, compared to non-African Americans. Also, use of a compound UCS (i.e., electric shock combined with a scream noise) led to nearly double the rate of acquisition of a conditioned SCR. Hence, use of a compound UCS may provide a way to reduce the number of excluded individuals in studies of fear-conditioned SCR and thereby improve the representativeness of research samples.
Keywords: fear conditioning, skin conductance, acquisition, unconditioned stimulus, fear learning, race
Introduction
De novo fear conditioning is a commonly used procedure for investigating the etiology, maintenance, and treatment of anxiety disorders (Milad, Rosenbaum, & Simon, 2014). In this procedure, a neutral stimulus (conditioned stimulus; CS+) is repeatedly paired with an aversive stimulus, such as a mild, electric shock (unconditioned stimulus; UCS), during acquisition. Evidence of successful conditioning is demonstrated when presentation of the CS+ produces a conditioned response, such as an increase in skin conductance (SC), when the UCS is no longer presented. This is often evaluated by comparing the magnitude of SC responses (SCRs) to the CS+ with SCRs to a stimulus (CS−) never paired with the UCS.
The fact that a substantial percentage of individuals fail to acquire a conditioned SCR is a challenging issue for fear conditioning researchers. At times, the rate of failure can approach 60% or more of the sample (e.g., Asthana et al., 2016: 19%; Fricchione et al., 2016: 53%; Guastella, Lovibond, Dadds, Mitchell, & Richardson, 2007: 55%; Johnson & Casey, 2015: 37%; Otto et al., 2014: 43%; Schiller, Kanen, LeDoux, Monfils, & Phelps, 2013: 60%; Steinfurth et al., 2014: 50%; for review, Lonsdorf et al., 2017). This makes subject recruitment more difficult and limits representativeness of samples. Acquisition of conditioned fear responses can vary as a function of participant characteristics, with evidence for significantly decreased conditioned differential SCR among those with less education (Rosenbaum et al., 2015). Race is an additional factor, as lower rates of conditioned SCRs have been observed for African American, compared to non-African American, participants (Kredlow et al., 2017).
Meta-analyses suggest that the type of aversive stimulus (e.g., loud noise, aversive odor, painful pressure to the finger) used as the UCS does not significantly affect acquisition of a conditioned fear response (Lissek et al., 2005). However, comparisons between studies may mask specific interactive effects between populations and the aversive stimulus used as the UCS. For example, Lissek and colleagues (2008) found that using critical facial and verbal feedback as the UCS resulted in conditioned fear in social phobic, but not healthy individuals. This finding presumably reflects greater emotional sensitivity to the UCS in the socially anxious sample. Studies with children have used loud sounds, unpleasant photographs, and air puffs as UCSs, as ethical concerns preclude the use of more aversive stimuli (e.g., shock; Lau et al., 2008). Such alternative UCSs may tend to provoke minimal fear and thereby make conditioned responses more challenging to achieve. For this reason, Lau and colleagues (2008) developed and tested a protocol that involved the pairing of a facial photograph with a shrieking scream noise as the UCS, positing that this compound stimulus would be more effective and ecologically valid. The compound (face-scream) UCS produced better differential fear conditioning than other single UCSs (e.g., air puff, loud sounds, aversive pictures alone) in healthy and anxious children. In addition, Lau and colleagues (2008) found that the face-scream UCS was well tolerated as assessed by dropout rates.
Thus, the use of a compound, compared to a single, UCS may improve conditioning without increasing dropout rates. This strategy could be applied to adults by adding an additional UCS to that typically used (i.e., electric shock) in order to enhance conditioning. Another UCS variation that could enhance conditioning, but has not been explored, is the manipulation of shock duration. Although shock is the most common UCS used in fear conditioning studies, there is no standard guidance on shock duration (Lonsdorf et al., 2017) and studies vary considerably in durations used (e.g., Kredlow, Unger, & Otto, 2016). To our knowledge, no studies have examined whether variations in shock duration impact conditioning.
In the current study, we examine whether previously identified demographic factors (Rosenbaum et al., 2015; Kredlow et al., 2017) would predict failure to acquire a differential conditioned SCR and whether enhancing the UCS by combining it with a secondary UCS or increasing its duration would influence acquisition of a differential conditioned SCR. We hypothesized that using a compound UCS and lengthening the UCS duration would both increase the acquisition of differential conditioned SCRs.
Methods
Participants
Participants consisted of healthy adults recruited from the Boston University undergraduate population (n = 112) and the community (n = 42), and anxious adults recruited from the community and a treatment clinic (n = 137). Individuals were excluded if they were taking medications that potentially could influence SC or conditioned fear. Specifically, individuals taking anticholinergic medications, clonidine, or benzodiazepines; and individuals not on a stable dose, or on an as-needed dose, of other psychotropic medications were excluded. Individuals with medical conditions that contraindicated fear conditioning procedures (e.g., severe heart disease, pregnancy) were also excluded. In addition, anxious participants were required to have greater than mild-to-moderate anxiety symptom severity as assessed by a Beck Anxiety Inventory score (Beck & Steer, 1990) above 15, or score on the Fear Questionnaire above 37 (Marks & Mathews, 1979). Anxious participants were also excluded if they: 1) met DSM-5 criteria for past or present bipolar or psychotic disorder, or substance-related disorder in the last three months (other than caffeine or nicotine use disorder); 2) endorsed current suicidality, homicidality, or self-destructive acts or urges; or 3) engaged in exposure therapy the week prior to, or during, study procedures. Participants were asked to refrain from caffeine and nicotine use for two hours prior to their study visits.
Study Design
All procedures were approved by the Boston University Institutional Review Board. Participants completed a brief screening interview; eligible individuals then provided informed consent. Healthy participants were randomized to one of four fear-conditioning procedures as represented by a 2X2 factorial combination of shock duration and presence of a compound UCS: 1) a 500-msec shock (n = 39); 2) a 1000-msec shock (n = 38); 3) a 500-msec shock and concurrent scream noise (n = 38); or 4) a 1000-msec shock and concurrent scream noise (n = 39). All anxious participants received fear-conditioning with a 500-msec shock and concurrent scream noise as the UCS. Hence, only the healthy participants who were randomized to one of the four conditions contributed to the analyses of UCS characteristics. All participants contributed to analyses of demographic predictors of conditioning; anxiety status was used as a covariate. All other aspects of the assessment procedure were the same across participant groups as described below.
Fear Conditioning Procedures
Conditioned stimuli.
Colored shapes (CS+ yellow circle, CS− white square for all participants) were used as the conditioned stimuli. The shapes were displayed on a computer monitor positioned 4 feet in front of the participant.
Unconditioned stimuli.
The electric shock was set to an intensity level that the participant deemed to be “highly annoying but not painful” (0.2 – 4.0 mA) using the duration (500-msec or 1000-msec) that corresponded with the participant’s randomization. Consistent with prior studies (e.g., Orr et al., 2000; Otto et al., 2007), the technician gave the following instructions: “For this experiment, you will set your own level of electric stimulation. You should choose a level that is highly annoying but not painful. I will start the electric stimulation at a very low level and gradually increase the level until you say “stop.” The level that you set for the electric stimulation will then be used throughout the remainder of the experiment.” The shock was generated by a Coulbourn Transcutaneous Aversive Finger Stimulator (Coulbourn Instruments, 2016) and delivered through electrodes attached to the second and third fingers of the participant’s dominant hand. The scream noise was obtained from a lab which conducted fear conditioning studies in children with anxiety (Lau et al., 2008). The scream was 95 dB, 1 s in duration, and delivered by the experimenter manually through headphones at approximately the same time as the shock during acquisition. The scream noise was not used during the shock intensity selection procedures.
Conditioning context.
All procedures took place in a sound-attenuated and electronically-shielded room. Participants were read a standard set of instructions that indicated that they “may or may not” see colored shapes and “may or may not” receive electric stimulation and/or hear an uncomfortable noise. Participants were attached to the shock electrodes and wore headphones throughout the experiment regardless of their randomization group. Participants were instructed to pay attention to the computer screen and try to figure out the relationship between the visually presented stimuli and the electric stimulation/noise.
Conditioning procedure.
Participants completed a 5-min baseline recording period followed by habituation, which consisted of 5 CS+ and 5 CS− unreinforced presentations. Following habituation, the acquisition procedure consisted of 10 CS+ and 10 CS− presentations. Stimulus presentations were pseudorandom with no more than two consecutive presentations of the same stimulus type. The CS duration was 8 s and the inter-trial interval was 11 +/− 1 s. Acquisition followed a 60% partial-reinforcement schedule, i.e., 6 of the 10 CS+ presentations were followed by the shock. For Groups 3 and 4, the concurrent scream noise was played on 5 of the 6 reinforced trials. The scream stimulus was presented on only 5, rather than all 6, of the reinforced trials in order to add an element of unpredictability, which has also been shown to enhance conditioning (Vansteenwegen, Iberico, Vervliet, Marescau, & Hermans, 2008).
Assessments
Psychophysiological assessment.
A Coulbourn Lablinc V, Human Measurement Modular Instrument System (Coulbourn Instruments, 2016) was used to measure SC level (SCL). Two 8-mm electrodes were filled with isotonic paste and attached to the hypothenar surface of the non-dominant hand per published guidelines (Fowles et al., 1981). Before beginning each study session, SCLs were checked to ensure that they were within the appropriate range and responsive to a challenge test (e.g., serial 7s). If this was not the case, electrodes were replaced and levels and responsivity were re-evaluated.
Self-report questionnaires.
Information about age, gender, educational attainment, ethnicity, and race was obtained from participants.
Outcomes
Primary outcome.
To assess the impact of demographic factors and UCSs on conditioned SCRs, our primary analyses targeted the dichotomous outcome of acquiring, or not acquiring, a differential SCR. A SCR for each CS presentation was calculated by subtracting the mean SCL during the 2-s interval immediately preceding CS onset from the peak SCL during the CS interval. Evidence of a conditioned SCR was defined as a differential SCR (CS+ minus CS−, untransformed) greater than 0.1 μS during acquisition (averaged across all acquisition trials). Although there is no standard for defining that a conditioned SCR is evident (Lonsdorf et al., 2017), our response threshold of 0.1 μS is consistent with previous work conducted across various laboratories (e.g., Fricchione et al., 2016; Otto et al., 2014; Schiller et al., 2010, 2013; Spring et al., 2015; Steinfurth et al., 2014) and the inclusion of all acquisition data for calculating the conditioned SCR is consistent with our prior work (e.g., Fricchione et al., 2016; Kredlow et al., 2017; Otto et al., 2014; Spring et al., 2015). Although this criterion may be more conservative than that used by some other researchers (e.g., Johnson & Casey, 2015; Liu et al., 2014), it is less susceptible to the occasional spurious response or the possible decline in differential SCR towards the end of acquisition (due to contingency learning) if only later trials were used to calculate the presence of a conditioned SCR.1
Secondary outcomes.
In order to better understand the impact of the different UCSs on fear conditioning, we examined two continuous variables as secondary outcomes: 1) the average differential SCR, calculated by subtracting the average SCR (square root transformed) across all CS− acquisition trials from the average SCR (square root transformed) across all CS+ trials, with follow up tests to examine each component (i.e., SCR to CS+ and SCR to CS−), and 2) the average magnitude of SCRs to the UCSs, calculated by subtracting the mean SCL during the last 2 s of the CS+ interval from the peak SCL during the 6-s interval following the UCS offset (square root transformed) (e.g., Orr et al., 2000).
Statistical Analyses
Demographic predictors of SCR conditioning.
We conducted a series of logistic regression analyses to examine the significance of demographic predictors identified in previous studies (Rosenbaum et al., 2015; Kredlow et al., 2017). The dependent variable was the dichotomous outcome of acquiring or not acquiring a differential SCR during acquisition. The predictors examined were age (younger vs. older group), gender (male vs. female), education (completed vs. did not complete college), and race (African American vs. not-African American). Because the distribution was not normal, age was dichotomized to create a younger group (≤ 20 years old) and older group (> 20 years old), using a median split. Individuals who identified as half African American and half another race (n = 2) were coded as African American. Anxiety status (healthy vs. anxious) was included as a covariate.
Effect of UCS on SCR conditioning.
Data from the healthy participants (n = 154) were used to examine whether the compound UCS and/or shock duration influenced SCR conditioning. The respective UCS groups were compared on demographic variables and shock intensity selection. Logistic regression (for primary outcomes) and ANOVA (for secondary outcomes) were used to compare the main and interaction effects of a compound vs single UCS and 500-msec vs 1000-msec shock duration on the primary and secondary outcomes. Significant predictors of conditioning from the demographic analyses were used as covariates in these analyses. In situations of non-normal distributions where extreme outliers (i.e., values greater than three times the interquartile range) were present, analyses were repeated examining ordinal data using a Mann-Whitney U test.
Results
Demographic Predictors of SCR Conditioning
Preliminary analyses and exclusions.
Participants’ data were excluded from analyses if they displayed unmeasurable SCLs (n = 2) or were not responsive to the UCS (n = 4) two known factors that preclude the use of SC data (for definitions see Kredlow et al., 2017). Seven participants were also excluded from analyses because they withdrew from the study midway through the habituation or acquisition phase; four participants’ data were unusable due to equipment failure. In total, 274 participants’ data were included in the analyses of demographic predictors of conditioning.
Participant characteristics.
The mean age of participants was 24 years (SD = 9.7, range 18–64), with 143 (52%) participants in the younger group and 131 (48%) participants in the older group. Most participants were female (65%, n = 177) and non-Hispanic (90%, n = 246); a minority of participants had completed college (28%, n = 77). Forty-three percent of participants identified as White (n = 118), 39% identified as Asian (n = 108), 6% identified as Black or African American (n = 16), 5% identified as more than one race (n = 13), and 7% identified as other (n = 19).
Primary outcome.
We first conducted logistic regression analyses with anxiety status as a covariate and each demographic predictor considered individually. Race significantly predicted failure to acquire a differential SCR (p < .05), whereas age (p = .86), education (p = .31) and gender (p = .25) did not. Anxiety status alone was also not predictive of failure to acquire a differential SCR (p = .26). The direction of these effects suggested poorer conditioning for participants who identified as African American. A forward stepwise logistic regression analysis that included anxiety status and all demographic predictors was significant (Model χ2(1) = 4.80, p < .05) and correctly classified 55.3% of participants. Race was the only significant non-redundant predictor of failure to acquire a differential conditioned SCR (Wald = 4.29, p < .05). The odds of failing to acquire a differential conditioned response was 3.07 greater in participants who identified as African American, compared to participants who identified as non-African American. Seventy-two percent (13/18) of African American participants failed to evidence a differential conditioned SCR, compared to 46% (118/256) of non-African American participants.2,3
Effect of UCS on SCR Conditioning
Preliminary analyses and exclusions.
In total, 143 of the 154 healthy participants who were part of the study that involved randomization to one of the four UCS conditions were included in the following analyses. Participants’ data were excluded from analyses if they displayed unmeasurable SCLs (n = 1) or were non-responsive to the UCS (n = 3) as described above. Five participants were also excluded from analyses because they withdrew from the study midway through the habituation or acquisition phase; two participants’ data were unusable due to equipment failure.
Preliminary analyses.
Preliminary analyses evaluated two potential confounds, the shock level selected by participants and differential SCR during habituation. Factorial ANOVA indicated that participants who were later randomized to receive the compound UCS had selected a higher shock intensity level (M = 1.92, SD = 0.83), compared to participants who were randomized to the single UCS (M = 1.65, SD = 0.53; F(1, 138) = 5.14, p < .05). Because shock intensity might influence the acquisition of conditioned fear (Rescorla & Wagner, 1972), all participants in the compound UCS group who had selected the highest shock intensity (4.0 mA, n = 8) were removed from analysis to equate the study groups4. This led to almost identical mean shock intensities for the resulting compound (n = 65) and single (n = 70) UCS groups (M = 1.66 and 1.65 mA, respectively), with no significant differences remaining in the factorial ANOVA for shock intensities. Preliminary analyses indicated there were no main or interactive effects for the UCS conditions (single vs compound UCS or 500 vs 1000-msec shock) on differential SCR during the habituation phase (ps > .74); hence, there were no pre-existing differences in SCR to confound the evaluation of acquisition SCRs.
Participant characteristics.
Table 1 presents demographic characteristics for the four groups, with no significant differences evident for any of the demographic factors examined. In brief, the mean age of participants was 23 years (SD = 9.8, range 18–64). The majority of participants were female (59%, n = 80), had completed some college (70%, n = 95), identified as non-Hispanic (93%, n = 125), and either Asian (44%, n = 60) or White (43%, n = 58).
Table 1.
Participant characteristics for sample used to examine effect of UCS variations on SCR conditioning (n = 135)
| Participant Characteristics | Factor 1: Single UCS vs. Compound UCS | Factor 2:500-msec Shock vs 1000-msec Shock | ||||
|---|---|---|---|---|---|---|
| Single UCS (n = 70) | Compound UCS (n = 65) | Comparison of two groups | 500-msec Shock (n = 69) | 1000-msec Shock (n = 66) | Comparison of two groups | |
| Age {Mean (SD), years} | 22.9 (10.0) | 23.5 (10.0) | t(133) = −0.39, p = .70 | 23.7 (10.4) | 22.7 (9.1) | t(133) = 0.58, p = .56 |
| Gender (% female, n) | 54%, n = 38 | 65%, n = 42 | χ2(1, 135) = 1.49, p = .22 | 61%, n = 42 | 58%, n = 38 | χ2(1, 135) = 0.15, p = .70 |
| Education (% completed college, n) | 14%, n = 10 | 20%, n = 13 | χ2(1, 135) = 0.78, p = .38 | 16%, n = 11 | 18%, n = 12 | χ2(1, 135) = 0.12, p = .73 |
| Ethnicity (% Hispanic, n) | 4%, n = 3 | 11%, n = 7 | Fischer’s exact p = .20 | 9%, n = 6 | 6%, n = 4 | Fischer’s exact p = .74 |
| Race (% African American, n) | 6%, n = 4 | 3%, n = 2 | Fischer’s exact p = .68 | 4%, n = 3 | 5%, n = 3 | Fischer’s exact p = 1.00 |
| Unconditioned Stimulus Intensity {Mean(SD) mA} | 1.6 (0.5) | 1.7 (0.4) | F(1, 130) < 1, p = ns | 1.6 (0.4) | 1.7 (0.5) | F(1, 130) < 1, p = ns |
Primary outcome.
Results of logistic regression showed that participants who received the compound UCS were significantly more likely to acquire a differential SCR during the acquisition phase (62%, 40/65), compared to participants who received the single UCS (34%, 24/70; Wald = 9.64, p < .01). The odds of acquiring a differential SCR was 3.11 greater for participants in the compound UCS group. Participants who received the 1000-msec shock (44%, 29/66) were not more likely to acquire a differential SCR, compared to participants who received the 500-msec shock (51%, 35/69; Wald = 0.69, p = .41). The interaction effect between the two factors (single vs compound UCS, 500 vs 1000-msec shock) also was not significant (Wald = 1.12, p = .29).5
Secondary outcomes.
Differential SCR.
Participants who received the compound UCS did not display a larger mean differential SCR, compared to participants who received the single UCS (F(1,130) = 2.26, p = .14). Although not reaching significance, the small effect size (d = .26) did favor the compound UCS group. Because the data were not normally distributed and extreme outliers were present for the outcome of mean differential SCR, a Mann-Whitney U test was conducted, thereby minimizing the effect of the outliers. Participants who received the compound UCS displayed significantly larger mean rank differential SCR, compared to participants who received the single UCS (U = 1740, p < .05). Follow-up tests to separately examine SCRs to CS+ and SCRs to CS− revealed that the compound UCS resulted in significantly larger SCRs to both the CS+ (F(1, 130) = 11.64, p < .001, d = .60) and the CS− (F(1,130) = 6.22, p < .05; d = .43), compared to the single UCS. Because the data for SCR to the CS− were not normally distributed and extreme outliers were also present, analyses were repeated with a Mann-Whitney U test. The effect of the compound UCS on SCR to the CS− remained significant (U = 1770, p < .05). Participants who received the 500-msec shock did not display a larger mean differential SCR compared to participants who received the 1000-msec shock (F(1,130) < 1, p = ns). The effect size was small (d = 0.12) and in favor of the 500-msec shock group; it remained non-significant when examining ordinal data (U = 1996, p = .22). Follow up tests revealed that the 500-msec shock resulted in a significantly larger SCR to both the CS+ (F(1, 130) = 7.13, p < .01, d = .47) and CS− (F(1,130) = 6.53, p < .05; d = .44), compared to the 1000-msec shock. SCR to the CS− remained significant when examining ordinal data (U = 1759, p < .05). There was no interaction between the two factors (single vs compound UCS, 500 vs 1000-msec shock) in predicting differential SCR (F(1,130) < 1, p = ns). See Figures 1–4 for trial by trial data.
Figure 1.

Acquisition SCRs for single UCS group. This figure presents CS+ (solid line) and CS− (dotted line) trial by trial SCR data during acquisition for the group of participants who received the single UCS (n = 70).
Figure 4.
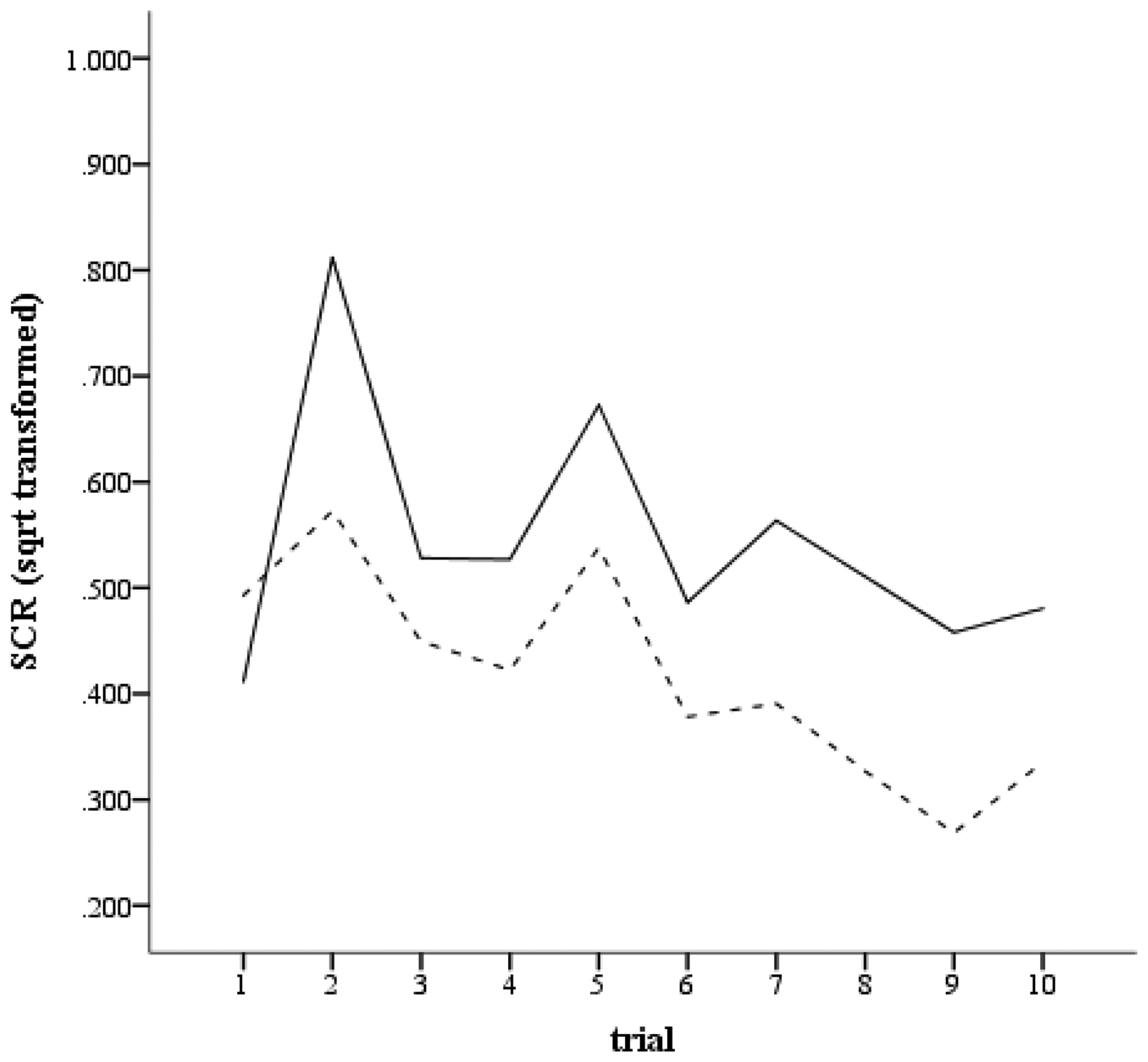
Acquisition SCRs for 1000-msec UCS group. This figure presents CS+ (solid line) and CS− (dotted line) trial by trial SCR data during acquisition for the group of participants who received the 1000-msec UCS (n = 66).
Magnitude of unconditioned SCR.
Magnitude of the unconditioned SCR did not significantly differ but was larger at a trend-level for participants who received the compound versus single UCS (F(1,130) = 3.62, p = .06). The effect size was small (d = 0.33) and favored larger responses to the compound UCS. The magnitude of unconditioned SCR also did not differ for participants who received the 500 vs 1000-msec shock (F(1,130) = 0.87, p = .35). The effect size was small (d = .16) and favored larger responses in the 1000-msec shock group. There was no interaction between the two factors (single vs compound UCS, 500 vs 1000-msec shock) in predicting the unconditioned SCR (F(1,130) < 1, p = ns).
A summary of results for UCS effects on SCR conditioning can be found in Table 2.
Table 2.
Outcomes for effect of UCS variations on SCR conditioning (n = 135)
| Outcomes | Factor 1: Single UCS vs. Compound UCSa | Factor 2: Short Shock vs Long Shocka | ||
|---|---|---|---|---|
| Single UCS (n = 70) | Compound UCS (n = 65) | Short Shock (n = 69) | Long Shock (n = 66) | |
| Primary Outcome | ||||
| Acquired Differential SCR (%, n) | 34%, n = 24 | 62%, n = 40** | 51%, n = 35 | 44%, n = 29 |
| Secondary Outcomes | ||||
| Differential SCR {Mean(SE) μS}b | 0.11(0.03) | 0.18(0.03)*c | 0.16(0.03) | 0.13(0.03)c |
| • SCR to CS+ {Mean(SE) μS}b | 0.53(0.04) | 0.70(0.04)*** | 0.68(0.04) | 0.55(0.04)** |
| • SCR to CS− {Mean(SE) μS}b | 0.42(0.03) | 0.52(0.03)*c | 0.52(0.03) | 0.42(0.03)*c |
| Unconditioned SCR {Mean(SE) μS}b | 1.42(0.07) | 1.62(0.08)t | 1.47(0.07) | 1.57(0.08) |
Note.
There were no significant interactions between Factor 1 and Factor 2;
Means and standard errors are based on factorial ANOVA estimated marginal means covarying for race;
Significance is based on Mann-Whitney U test.
p = .06
p < .05
p < .01
p < .001
Tolerability.
Of the five participants who withdrew midway through the habituation or acquisition phase, two indicated that it was because they could not tolerate hearing the scream noise, one was uncomfortable not knowing what the noise would be, and two withdrew for reasons unrelated to the UCS. After completing the acquisition phase, two participants reported being unable to tolerate the scream noise and withdrew from the study and one participant in the compound UCS condition withdrew because the procedures were reported to be stressful, in general. Thus, 4 of 77 participants who heard the scream noise specifically reported withdrawing from procedures because of it, as compared to an estimated 18 additional participants (based on 34% conditioning rate applied to the compound UCS sample size of 65) that met conditioning threshold because of the scream stimulus.
Discussion
The current study provides additional evidence for the influence of demographic variables on the likelihood that participants will acquire a conditioned response in a de novo fear-conditioning paradigm. Consistent with the findings of Kredlow et al. (2017), we found that African American participants were less likely to meet the threshold criterion for acquisition of a fear-conditioned SCR, compared to non-African American participants (reflecting a medium effect size; Odds ratio = 3.07). The percentage of African American participants in our sample who did not meet the threshold criterion for acquisition of a fear-conditioned SCR (72%), is consistent with rates presented in Kredlow et al., 2017 (33.3–71.4%). Nonetheless, these results are tentative as our sample of African American participants was much smaller than our sample of non-African American participants; further research with larger samples of African American participants prospectively recruited to examine the influence of race on conditioning is needed. We did not observe a significant relationship between education and fear-conditioned SCR, unlike Rosenbaum et al. (2015). However, Rosenbaum et al. compared participants who had completed college with those having higher degrees; only a small portion (9%) of our sample had higher degrees. Consequently, it is possible that the influence of education on conditioning is specific to higher ranges of education, an association that we had limited ability to detect given the characteristics of our sample.
We found that a compound UCS, consisting of a shock and scream, improved fear conditioning as assessed by SCR. This was demonstrated by roughly twice as many individuals meeting our criterion for a fear-conditioned SCR when the compound stimulus was used. Furthermore, the compound UCS was tolerated by most participants; only 5% exposed to this stimulus withdrew from the study because of reported difficulty tolerating the scream stimulus. This would seem to be an acceptable loss given the observed 28% increase in included participants with the use of the compound UCS. As such, using the compound shock and scream UCS has the potential to substantively expand the evaluable participant sample in de novo fear conditioning studies.
Possible explanations for the improved acquisition rates for the compound UCS include enhancements in the perceived intensity, relevance, or novelty/unpredictability of the UCS. We were unable to confirm that the improved acquisition rate was due to increased UCS intensity, at least as measured by physiological reactivity, given that we did not observe a significant difference in the unconditioned SCR to the compound, compared to single, UCS, although the results were suggestive at a trend-level. Alternatively, and consistent with research showing an effect for the personal relevance of the UCS (Lissek et al., 2008), part of the effect may reflect greater fear relevance of the scream stimulus, or possibly broader relevance overall, i.e., the relevance of the shock stimulus for some participants and of the scream stimulus for others. Prior research has also shown that novelty can enhance memory (Ranganath & Gregor, 2003). In our study, participants were not told that the “uncomfortable noise” would be a scream sound, so the novelty of this stimulus may have played a role in the improved acquisition rates. Additionally, as noted above, the scream stimulus was presented on 5 of the 6 reinforced trials (as compared to 6 out of 6 for the shock stimulus), which may have enhanced the unpredictability of the UCS. Unpredictability has been shown to enhance conditioning (Vansteenwegen et al., 2008). However, given that the one reinforced trial that contained only the shock did not occur until late in acquisition (the 5th reinforced trial), it seems unlikely that unpredictability was a major factor in improving acquisition. In sum, our design does not allow us to identify which specific characteristic of the compound UCS, e.g., intensity, relevance, or novelty/unpredictability, was responsible for the significant increase in acquisition rates.
It is interesting that although the compound UCS clearly reduced the number of poorly responding participants, it did not substantially increase the mean differential SCRs for the samples. Trends indicated only a small effect size for mean differential SCRs for those who received the single versus the compound stimulus. This was in part due to outliers, but also due to enhanced SCRs to both the CS− and CS+, per the follow-up analyses. This finding is consistent with research suggesting that fear generalization is more likely to occur with stronger UCSs (Dunsmoor, Kroes, Braren, & Phelps, 2017). In some instances, generalization may interfere with differential conditioning (i.e., the participant responds nearly as strongly to the CS− as to the CS+), which diminishes the differential SCR. In the current study, although the compound UCS enhanced the SCR to the CS−, as well as the CS+, a differential SCR was still maintained. As noted, the differential SCR was sufficiently enhanced that a substantially greater proportion of participants met the differential SCR criterion for responder status.
Regarding limitations, our study did not allow for an adequately-powered examination of whether UCS stimulus characteristics would differentially rescue the small SCRs observed in African American participants. Only nine African American participants enrolled in the healthy-subject study, leaving too few participants across groups for meaningful evaluation of interaction effects between race and UCS condition. Additionally, given our goal of replicating Kredlow et al.’s (2017) findings regarding African American race, an examination of differences in SCRs between other racial and ethnic groups was beyond the scope of this manuscript. We did explore rates of failure to acquire of a fear-conditioned SCR between Asian and White participants and found that they did not differ. Also, our study provided no encouragement that increasing shock duration has an impact on fear acquisition, although we studied only two durations of shock: 500-msec and 1000-msec. Currently, there is no standard criterion or cut-off score for defining an acquired differential SCR in the literature (Lonsdorf et al., 2017). Thus, the criterion we used for our primary outcome in the current manuscript, although consistent with some studies (Fricchione et al., 2016; Kredlow et al., 2017; Otto et al., 2014; Spring et al., 2015), differs from that used by other researchers (e.g., Asthana et al., 2016; Johnson & Casey, 2015). Given the impact of the compound UCS on the primary as well as secondary outcomes, it is unlikely that the use of different acquisition criteria would impact results. Our post-hoc analyses using a different acquisition criterion led to relatively consistent findings. Lastly, minor limitations of our conditioning procedure should be noted. First, we did not counterbalance the conditioned stimuli used during acquisition, which would have further controlled for any pre-existing SCR differences to the stimuli. Given that no SCR differences were observed during habituation, it is unlikely that this limitation impacted our findings. Second, as described above, the scream stimulus was delivered manually. Although no errors in delivery of the scream stimulus were noted by research staff, we acknowledge that manually controlled procedures pose a greater risk to reliability than automated procedures.
In conclusion, the failure to acquire a conditioned SCR during fear conditioning is a common reason for excluding participant data from studies of fear extinction. This is important because rates of acquisition influence the sample size required to obtain sufficient statistical power to examine questions related to extinction. In addition, if large portions of participants do not acquire a conditioned fear response, research questions may only be examined in exceptionally good fear learners and, thereby, have limited generalizability. Moreover, the failure to acquire a conditioned SCR has become more problematic as an increasing number of studies use partial reinforcement schedules (i.e., delivering the UCS on only a portion of the CS+ trials). The aim of using partial reinforcement is to slow the rate of extinction (Grady, Bowen, Hyde, Totsch, & Knight, 2016) to allow for better examination of extinction-related processes. A complication when using partial reinforcement schedules is that, in contrast to constant reinforcement schedules, higher numbers of individuals may fail to acquire a conditioned-fear response (Grady et al., 2016). The use of a compound UCS may help to address this limitation. In sum, the compound UCS used in the present work provides a stimulus that holds promise for improving fear-conditioning of SCR and may thereby improve the representativeness of research samples and reduce costs associated with recruitment.
Figure 2.

Acquisition SCRs for compound UCS group. This figure presents CS+ (solid line) and CS− (dotted line) trial by trial SCR data during acquisition for the group of participants who received the compound UCS (n = 65).
Figure 3.

Acquisition SCRs for 500-msec UCS group. This figure presents CS+ (solid line) and CS− (dotted line) trial by trial SCR data during acquisition for the group of participants who received the 500-msec UCS (n = 69).
Highlights.
African-American participants displayed lower rates of fear conditioning
Age, gender, and education did not influence rates of fear conditioning
Use of a compound UCS nearly doubled the rate of fear conditioning
Use of a longer shock duration did not influence the rate of fear conditioning
Acknowledgements
This work was supported by the National Institute of Mental Health [grant number F31MH103969] and a Clara Mayo Memorial Research Fellowship from the Department of Psychological and Brain Sciences, Boston University to M.A.K.. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Post-hoc analyses were conducted to examine whether the same pattern of results would be obtained when an alternative criterion for acquiring a differential SCR was used. Based on prior publications (e.g., Schiller et al., 2010; 2013; Steinfurth et al., 2014), this criterion required a differential SCR (CS+ minus CS−, untransformed) greater than 0.1 μS during acquisition when averaged across the last half of acquisition trials.
Given that the non-African American comparison group consisted largely of White (n = 118) and Asian (n = 108) participants, additional follow up analyses were conducted to explore whether rates of failure to acquire a differential conditioned SCR differed between White and Asian participants. The rate of failure to acquire a differential conditioned SCR in Asian participants (53/108) was similar to that in White participants (50/118), and Asian vs. White race did not predict failure to acquire a differential conditioned SCR (Wald = 0.75, p = .39).
Using the alternative criterion for acquiring a differential SCR, viz., a differential SCR greater than 0.1 μS during acquisition when averaged across the last half of acquisition trials, the results were not changed. Race significantly predicted failure to acquire a differential SCR (p < .05), whereas age (p = .47), education (p = .27), gender (p = .75), and anxiety status (p = .96) did not. The direction of these effects suggested poorer conditioning for participants who identified as African American. A forward stepwise logistic regression analysis that included anxiety status and all demographic predictors was significant (Model χ2(1) = 7.87, p < .01) and correctly classified 57.1% of participants. Race was the only significant non-redundant predictor of failure to acquire a differential conditioned SCR (Wald = 6.50, p < .05). The odds of failing to acquire a differential conditioned response was 4.40 greater in participants who identified as African American, compared to participants who identified as non-African American.”
Outcome analyses were later repeated with these 8 participants included and results did not change.
Using the alternative criterion for acquiring a differential SCR, viz., a differential SCR greater than 0.1 μS during acquisition when averaged across the last half of acquisition trials, results of logistic regression showed that participants who received the compound UCS were more likely to acquire a differential SCR at a trend level during the last half of the acquisition phase (60%, 39/65), compared to participants who received the single UCS (43%, 30/70; Wald = 3.49, p = .06). The odds of acquiring a differential SCR was 1.96 greater for participants in the compound UCS group. Participants who received the 1000-msec shock (48%, 32/66) were not more likely to acquire a differential SCR, compared to participants who received the 500-msec shock (54%, 37/69; Wald = 0.40, p = .53). The interaction effect between the two factors (single vs compound UCS, 500 vs 1000-msec shock) also was not significant (Wald = 0.07, p = .79).
References
- Asthana MK, Brunhuber B, Mühlberger A, Reif A, Schneider S, & Herrmann MJ (2016). Preventing the return of fear using reconsolidation update mechanisms depends on the met-allele of the brain derived neurotrophic factor Val66Met polymorphism. International Journal of Neuropsychopharmacology, 19(6). doi: 10.1093/ijnp/pyv137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1990). Manual for the Beck anxiety inventory. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Coulbourn Instruments, A Harvard Apparatus Company (2016). Available from http://www.coulbourn.com
- Dunsmoor JE, Kroes MC, Braren SH, & Phelps EA (2017). Threat intensity widens fear generalization gradients. Behavioral Neuroscience, 131(2), 168. doi: 10.1037/bne0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, & Venables PH (1981). Publication recommendations for electrodermal measurements. Psychophysiology, 18(3), 232–239. Doi: 10.1111/j.1469-8986.1981.tb03024.x [DOI] [PubMed] [Google Scholar]
- Fricchione J, Greenberg MS, Spring J, Wood N, Mueller-Pfeiffer C, Milad MR, … & Orr SP (2016). Delayed extinction fails to reduce skin conductance reactivity to fear-conditioned stimuli. Psychophysiology, 53(9), 1343–1351. doi: 10.1111/psyp.12687 [DOI] [PubMed] [Google Scholar]
- Grady AK, Bowen KH, Hyde AT, Totsch SK, & Knight DC (2016). Effect of continuous and partial reinforcement on the acquisition and extinction of human conditioned fear. Behavioral Neuroscience, 130(1), 36. doi: 10.1037/bne0000121 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, & Richardson R (2007). A randomized controlled trial of the effect of d-cycloserine on extinction and fear conditioning in humans. Behaviour Research and Therapy, 45(4), 663–672. doi: 10.1016/j.brat.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Johnson DC, & Casey BJ (2015). Extinction during memory reconsolidation blocks recovery of fear in adolescents. Scientific Reports, 5, 8863. doi: 10.1038/srep08863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredlow MA, Pineles SL, Inslicht SS, Marin MF, Milad MR, Otto MW, & Orr SP (2017). Assessment of skin conductance in African American and Non–African American participants in studies of conditioned fear. Psychophysiology, 54(11), 1741–1754. doi: 10.1111/psyp.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredlow MA, Unger LD, & Otto MW (2016). Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychological Bulletin, 142(3), 314–336. doi: 10.1037/bul0000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, … & Pine DS (2008). Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. Journal of the American Academy of Child & Adolescent Psychiatry, 47(1), 94–102. doi: 10.1097/chi.0b01e31815a5f01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, & Grillon C (2008). Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. American Journal of Psychiatry, 165(1), 124–132. doi: 10.1176/appi.ajp.2007.06091513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, & Pine DS (2005). Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy, 43(11), 1391–1424. doi: 10.1016/j.brat.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Liu J, Zhao L, Xue Y, Shi J, Suo L, Luo Y, … & Bao Y (2014). An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biological Psychiatry, 76(11), 895–901. doi: 10.1016/j.biopsych.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, … & Drexler SM (2017). Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience & Biobehavioral Reviews, 77, 247–285. doi: 10.1016/j.neubiorev.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Marks IM, & Matthews AM (1979). Fear questionnaire. Psychiatric Annals, 64, 6848. [Google Scholar]
- Milad MR, Rosenbaum BL, & Simon NM (2014). Neuroscience of fear extinction: Implications for assessment and treatment of fear-based and anxiety related disorders. Behaviour Research and Therapy, 62, 17–23. doi: 10.1016/j.brat.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, & Pitman RK (2000). De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology, 109(2), 290. doi: 10.1037//0021-843X.109.2.290 [DOI] [PubMed] [Google Scholar]
- Otto MW, Leyro TM, Christian K, Deveney CM, Reese H, Pollack MH, & Orr SP (2007). Prediction of “fear” acquisition in healthy control participants in a de novo fear-conditioning paradigm. Behavior Modification, 31(1), 32–51. doi: 10.1177/0145445506295054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Moshier SJ, Kinner DG, Simon NM, Pollack MH, & Orr SP (2014). De novo fear conditioning across diagnostic groups in the affective disorders: Evidence for learning impairments. Behavior Therapy, 45(5), 619–629. doi: 10.1016/j.beth.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, & Gregor R (2003). Neural mechanisms for detecting and remembering novel events. Nature Reviews Neuroscience, 4(3), 193. doi: 10.1038/nrn1052 [DOI] [PubMed] [Google Scholar]
- Rosenbaum BL, Bui E, Marin MF, Holt DJ, Lasko NB, Pitman RK, … Milad MR (2015). Demographic factors predict magnitude of conditioned fear. International Journal of Psychophysiology, 98(1), 59–64. doi: 10.1016/j.ijpsycho.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils MH, & Phelps EA (2013). Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proceedings of the National Academy of Sciences U S A, 110, 20040–20045. doi: 10.1073/pnas.1320322110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, & Phelps EA (2010). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature, 463, 49–53. doi: 10.1038/nature08637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfurth EC, Kanen JW, Raio CM, Clem RL, Huganir RL, & Phelps EA (2014). Young and old Pavlovian fear memories can be modified with extinction training during reconsolidation in humans. Learning & Memory, 21(7), 338–341. doi: 10.1101/lm.033589.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteenwegen D, Iberico C, Vervliet B, Marescau V, & Hermans D (2008). Contextual fear induced by unpredictability in a human fear conditioning preparation is related to the chronic expectation of a threatening US. Biological Psychology, 77(1), 39–46. doi: 10.1016/j.biopsycho.2007 [DOI] [PubMed] [Google Scholar]


