Abstract
Background
Primary central nervous system lymphoma (PCNSL) is a rare malignancy with few treatment options. One regimen used for induction is rituximab, high-dose methotrexate (HD-MTX), procarbazine, and vincristine (R-MPV). A common institutional practice is removing vincristine (VCR) from this regimen due to its poor CNS penetration and associated toxicities. The aim of this study was to evaluate how the omission of VCR from HD-MTX-based induction impacted clinical outcomes.
Methods
In a retrospective review, patients with PCNSL who received HD-MTX-based induction therapy between January 1, 2010 and May 31, 2018 were evaluated. Patients were stratified according to treatment into 2 groups, VCR-containing therapy versus no VCR. The primary endpoint was complete response (CR) rate following the completion of induction chemotherapy. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and adverse event rate.
Results
Twenty-nine patients were included: 16 patients in the VCR group and 13 in the non-VCR group. A CR was achieved in 7 (44%) and 5 (38%) (odds ratio [OR] = 1.24; 95% confidence interval [CI]: 0.28–5.53) patients, respectively. Median OS was 85.3 (95% CI: 20.2–85.3) versus 67.1 months (95% CI: 10.5–NR) and median PFS was 60.7 (95% CI: 9.4–NR) versus 23.7 months (95% CI: 4.7–NR) in the VCR group versus non-VCR group, respectively. The incidence of any grade peripheral neuropathy was higher in the VCR group.
Conclusions
CR rate, OS, and PFS were similar between groups regardless of VCR inclusion. Adverse events were higher in the VCR group. Larger studies are required to further evaluate the efficacy of VCR in PCNSL induction regimens.
Keywords: CNS lymphoma, complete response, induction, procarbazine
Key Points.
Vincristine removal from HD-MTX-based induction did not affect outcomes in PCNSL.
Vincristine addition to HD-MTX-based induction was associated with greater toxicity.
Importance of the Study.
The rarity of PCNSL prompts a closer look into clinical outcomes of current therapy. R-MPV is a commonly used regimen for induction, but not all patients are candidates for this full regimen. Removal of VCR may be considered in practice, but this has not been thoroughly evaluated in the literature. The findings of this study may inspire prospective research to further evaluate the utility of VCR in induction regimens for PCNSL.
Primary central nervous system lymphoma (PCNSL) is a rare malignancy which carries a poor prognosis if left untreated. Systemic high-dose methotrexate (HD-MTX) therapies have historically served as the backbone of PCNSL treatment, but the optimal regimen has yet to be clearly elucidated.1,2
One commonly used induction regimen for PCNSL is rituximab, HD-MTX, procarbazine, and vincristine (R-MPV). Current literature and evidence-based guidelines support this regimen in combination with appropriate consolidation therapy.3 In the study of Morris et al.,4 of 43 evaluable patients with PCNSL who received 5–7 cycles of R-MPV, 34 patients (79%) achieved a complete response (CR). The median overall survival (OS) was 6.6 years and the median progression-free survival (PFS) was 3.3 years. R-MPV for induction was further evaluated in a separate study of 32 patients with PCNSL by Omuro et al.5 The objective response rate was 97% with a CR rate of 66% after 5 or 7 cycles of R-MPV. The median PFS and OS were not reached in this study. The findings of these studies largely contribute to the foundation supporting the use of R-MPV in this population.
One agent worth evaluating within R-MPV is vincristine (VCR) due to its dose-limiting neuropathies, which often require dose reductions or discontinuation of the induction regimen.6–8 Additionally, several studies have led to questions about whether VCR should be routinely included in chemotherapy regimens for CNS tumors. A study in pediatric patients failed to find measurable concentrations of VCR in cerebrospinal fluid after intravenous bolus dosing.9 Additionally, studies in rodent models have suggested that VCR has poor penetration into the normal brain or brain tumors, such as the investigation by Boyle et al.,10 where intracarotid administration was unable to produce adequate drug delivery of VCR to implanted gliomas in rats. Although tumor-induced disruption of the blood–brain barrier has been thought to enhance VCR penetration, the presence of drug efflux pumps such as Pgp and MRP1 may counteract this effect. The influence of blood–brain barrier integrity on VCR distribution and efflux mechanisms are intertwined and likely produce conflicting results in drug levels attained in the rodent brain.11,12
Therefore, a common practice at our institution for the treatment of newly diagnosed PCNSL is removing VCR from the R-MPV regimen. No studies to date have investigated the outcomes of removing VCR from the R-MPV regimen. The PRIMAIN study investigated rituximab, high-dose methotrexate, procarbazine (R-MP) plus lomustine (R-MPL) in 107 older patients newly diagnosed with PCNSL. Infectious complications occurred with the addition of lomustine and consequently, about half of the patients enrolled in this study received R-MP. R-MPL and R-MP were associated with CR rates of 37.7% and 31.6%, respectively, after 3 cycles. Grade 3 and 4 toxicities were shown to be lower in the R-MP group.13 It is suspected that the removal of VCR from R-MPV should yield similar efficacy results, but this has yet to be studied.
The decision to omit VCR is not a universal practice which prompts the need to evaluate if the removal of this agent impacts treatment response in PCNSL. The primary objective of this study was to evaluate clinical outcomes in patients with PCNSL who received HD-MTX-based regimens with VCR compared to those without VCR for induction therapy. We hypothesized that the clinical outcomes would not be affected regardless of VCR administration.
Materials and Methods
Study Design and Inclusion/Exclusion Criteria
This was a single-center retrospective cohort study of patients with PCNSL treated with HD-MTX-based induction therapy between January 1, 2010 and May 31, 2018 at The Ohio State University Comprehensive Cancer Center. Patients were identified by an electronic order for HD-MTX and a PCNSL diagnosis code. Patients who received less than 3 cycles or more than 7 cycles of induction chemotherapy were excluded. Based on institutional practice, patients were less likely to have completed definitive imaging to assess response prior to the completion of 3 cycles and very rarely did treatment extend beyond 7 cycles. Therefore, the typical duration of treatment of 3–7 cycles was chosen as the most appropriate assessment for efficacy and safety. Patients with T-cell CNS lymphomas or other active malignancies, prior receipt of systemic chemotherapy, evidence of systemic lymphoma, HIV-positive status, or who were incarcerated were also excluded. Data were collected from medical records and included patient demographics, induction treatment regimen, drug-related toxicities, and survival outcomes. This study was approved by the Clinical Scientific Review Committee and Institutional Review Board at The Ohio State University.
Primary and Secondary Outcomes
The primary endpoint was the CR rate defined as the absence of enhancing disease on magnetic resonance imaging following a maximum of 7 cycles of induction chemotherapy. CR was independently confirmed by the principal investigator through the interpretation of radiographic imaging for each patient. Secondary endpoints included PFS defined as the time between the start of induction treatment and disease progression, death, or censoring; OS defined as the time between the start of induction treatment and death or censoring; and the frequency and severity of adverse events. Patients were censored if they had not experienced death or progression by the cutoff date when data collection began. Patients who had notes in their electronic medical records after the cutoff date were assumed alive and progression-free at the cutoff date; otherwise, the date of the last note was used for the censoring date. The presence of neuropathy was collected based on a search for the following keywords documented in the medical chart including “numbness,” “tingling,” and/or “burning” or by Common Terminology Criteria for Adverse Events (CTCAE, version 5.0), if available. All other adverse events were graded according to the CTCAE, version 5.0.
Statistical Analysis
Descriptive statistics were compared between patients treated with VCR and those treated without VCR using frequency (%) or median (interquartile range [IQR]), as appropriate. An unadjusted odds ratio (OR) and 95% confidence interval (CI) were calculated for the primary endpoint of CR rate. Kaplan–Meier methods were used to assess PFS and OS. Outcomes were also compared in the subset of patients who did not receive a bone marrow transplant (BMT). No formal hypothesis tests were performed because the study hypothesis was of noninferiority of removal of VCR from the treatment regimen. The limited sample size lacked power for formal tests of noninferiority. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc.).
Results
A total of 59 patients were identified for inclusion during the study timeframe, and 29 patients met eligibility criteria. The median age was 64 (IQR: 55–69) years, 19 (65.5%) patients were female, 26 (89.7%) patients were Caucasian, and 15 (51.7%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Three patients with an ECOG performance status of 3 were included, and all were in the VCR group. There were 16 patients in the VCR group and 13 patients in the non-VCR group. In the VCR group, 14 patients received R-MPV and 2 patients received R-MV. In the non-VCR group, 4 patients received R-MP and 9 patients received R-M. Procarbazine was included in the treatment regimen of 14 (87.5%) patients in the VCR group and 4 (30.8%) patients in the non-VCR group. Cumulative methotrexate doses were similar between groups (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | Vincristine (n = 16) | No vincristine (n = 13) |
|---|---|---|
| Age at treatment (years), median (IQR) | 63 (51–69) | 64 (57–67) |
| Female, n (%) | 13 (81.3%) | 6 (46.2%) |
| Caucasian, n (%) | 14 (87.5%) | 12 (92.3%) |
| ECOG, n (%) | ||
| Grade 0 | 3 (18.8%) | 2 (15.4%) |
| Grade 1 | 6 (37.5%) | 4 (30.8%) |
| Grade 2 | 4 (25.0%) | 5 (38.5%) |
| Grade 3 | 3 (18.8%) | 0 (0.0%) |
| Days from biopsy to treatment start, median (IQR) | 10 (6–17.5) | 18 (13–22) |
| Number of treatment cycles, median (IQR) | 5 (5–5.5) | 6 (5–6) |
| Number of vincristine cycles, median (IQR) | 4.5 (2.5–5.5) | — |
| Cumulative MTX dose (mg/m2), median (IQR) | 17 500 (15 000–19 250) | 17 500 (17 500–21 000) |
| Receipt of bone marrow transplant, n (%) | 5 (31.3%) | 1 (7.7%) |
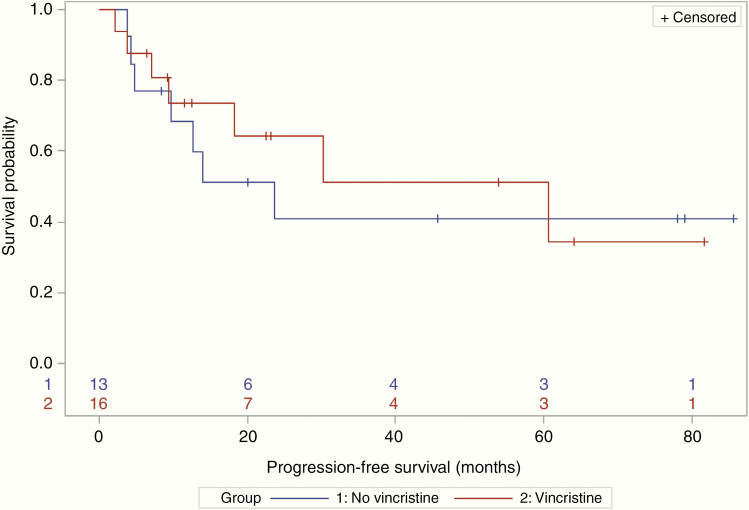
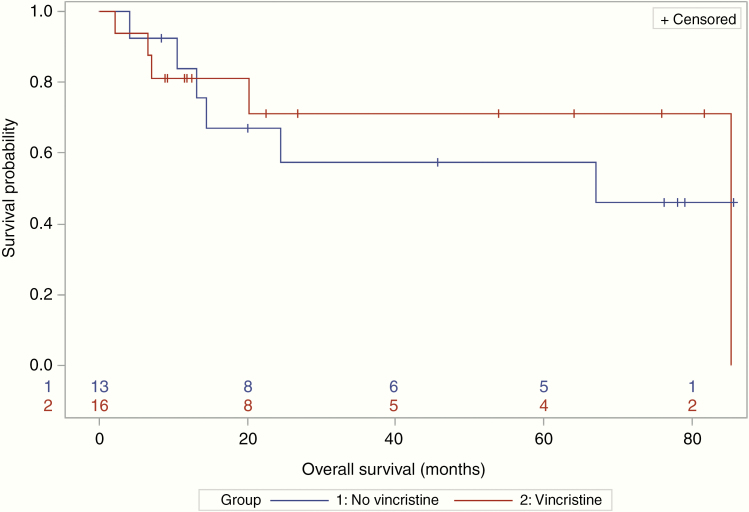
Seven (44%) patients in the VCR group and 5 (38%) in the non-VCR group achieved a CR (OR = 1.24; 95% CI: 0.28–5.53). Median PFS was 60.7 (95% CI: 9.4–NR) versus 23.7 months (95% CI: 4.7–NR) in the VCR versus non-VCR groups, respectively (Figure 1). Median OS was 85.3 (95% CI: 20.2–85.3) versus 67.1 months (95% CI: 10.5–NR) (Figure 2). One patient died with unknown progression status prior to their death. The median length of follow-up was 16.4 months for patients treated with VCR, compared to 24.5 months for patients treated without VCR.
Figure 1.
Of the 14 patients who died during the follow-up period, 13 had disease progression prior to their death. For patients who received vincristine, median progression-free survival was 60.7 months (95% CI: 9.4–NR), whereas median progression-free survival was 23.7 months (95% CI: 4.7–NR) for patients who did not receive vincristine.
Figure 2.
For patients who received vincristine, median survival was 85.3 months (95% CI: 20.2–85.3). For patients who did not receive vincristine, median survival was 67.1 months (95% CI: 10.5–NR, upper bound not reached).
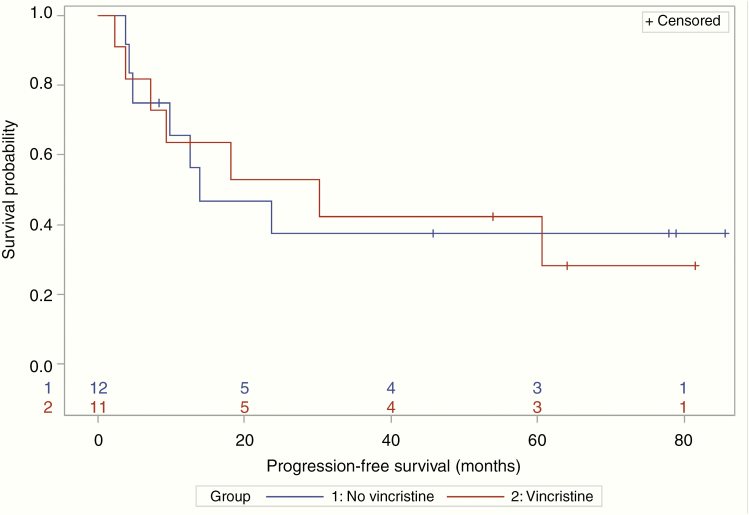
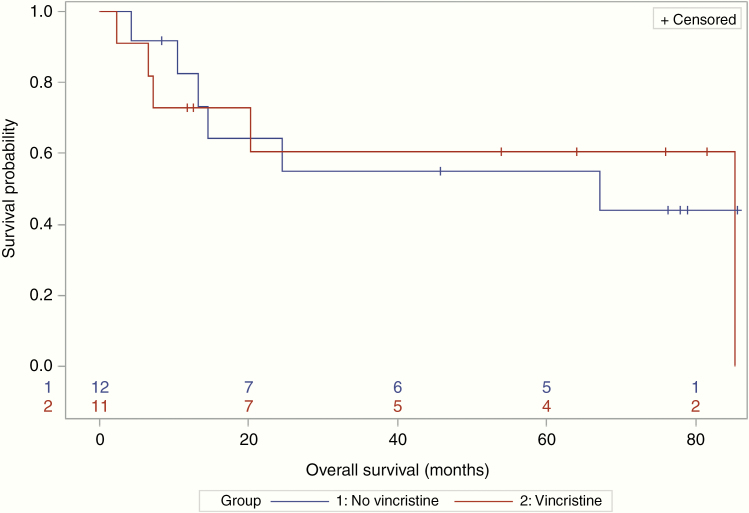
A total of 17 patients received consolidation therapy (11 patients in the VCR group and 6 patients in the non-VCR group). Three patients had missing data due to lost to follow-up or insufficient documentation. The majority of patients were consolidated with high-dose cytarabine-based therapy (82% in the VCR group and 50% in the non-VCR group). Of these 17 patients, 6 patients proceeded to BMT. Five (83%) patients who underwent a BMT received VCR with their induction regimen. In patients without a BMT, 8 of 23 (35%) achieved a CR. This subgroup had a median PFS of 18.2 months (95% CI: 9.4–NR) and a median OS of 85.3 months (95% CI: 13.2–NR). Median PFS was 30.2 (95% CI: 3.8–NR) versus 13.9 months (95% CI: 4.3–NR) and median OS was 85.3 (95% CI: 6.6–85.3) versus 67.1 months (95% CI 10.5–NR), in the VCR versus non-VCR groups, respectively (Figures 3 and 4).
Figure 3.
No patients who had a bone marrow transplant progressed or died during the follow-up period (progression-free survival [PFS] censoring times for the 6 transplant patients ranged from 6.5 to 23.1 months). In 23 nontransplant patients, median PFS was 13.9 months (95% CI: 4.3–NR) without vincristine and 30.2 months (95% CI: 3.8–NR) with vincristine.
Figure 4.
No patients who had a bone marrow transplant died during the follow-up period (overall survival [OS] censoring times for the 6 transplant patients ranged from 8.8 to 26.7 months). In 23 nontransplant patients, the median OS was 67.1 months (95% CI: 10.5–NR) without vincristine and was 85.3 months (95% CI: 6.6–85.3) with vincristine.
More patients in the VCR group experienced grade 3 or 4 neutropenia compared to those who did not receive VCR (5 patients vs 1 patient, respectively). Peripheral neuropathy was reported in 12 (75%) patients in the VCR group compared to 1 (7.7%) patient in the non-VCR group (Table 2). The median number of VCR-containing cycles was 4.5 cycles in the VCR group. More patients in the VCR group experienced a treatment delay due to toxicity than in the non-VCR group (8 vs 3 patients, respectively).
Table 2.
Patient Adverse Events
| Variable | Vincristine (n = 16) | No vincristine (n = 13) |
|---|---|---|
| Peripheral neuropathy, any grade, n (%) | 12 (75.0) | 1 (7.7) |
| Neutropenia, grade 3/4, n (%) | 5 (31.3) | 1 (7.7) |
| Elevated ALT or AST, grade 3/4, n (%) | 4 (25.0) | 1 (7.7) |
| Increased serum creatinine, grade 3/4, n (%) | 2 (12.5) | 0 (0.0) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
R-MPV is a frequently used induction regimen for PCNSL. Unfortunately, VCR has toxicities that often result in its omission from the regimen in patients with lower performance status or baseline neuropathy.
The CR rate was similar between both groups. Trends in PFS and OS probability over the early portion of the Kaplan–Meier curves were relatively similar regardless of whether or not patients received VCR. There was some separation around 20 months in both curves. Although, trends become less informative as the number of patients remaining in each group declined over time. However, this cohort was not large enough to test noninferiority of VCR inclusion with adequate statistical power. The results could have also been influenced by a variety of factors. The difference in follow-up between the VCR and non-VCR groups was likely caused by more frequent use of VCR in recent years. Most of the patients who received a BMT also had VCR in their induction regimen. There were also more patients in the VCR group with an ECOG of 0 or 1 than the non-VCR group, 9 versus 6 patients (56.3% vs 46.2%, respectively). When patients who received a BMT were removed from the PFS and OS analyses, PFS in both groups decreased but OS did not change.
The majority of patients who received VCR also received procarbazine (n = 14, 87.5%), but only 4 patients (30.8%) received procarbazine in the non-VCR group; therefore, procarbazine was a confounding factor. Many patients who did not receive VCR also did not receive procarbazine. A definitive explanation cannot be given to the role of procarbazine in this study, but it appears that its addition could have benefited the VCR group as well with a higher number of patients receiving procarbazine compared to the non-VCR group (88% vs 31%). The omission of procarbazine may have been multifactorial with reasons including tolerability, performance status, and cost.
The most common adverse event in the VCR group was peripheral neuropathy. However, it did not lead to discontinuation of therapy in the majority of cases, as the median number of cycles received in the VCR group was 4.5 cycles. Grade 3 and 4 neutropenia were higher in the VCR group which was likely due to increased procarbazine use.
For newly diagnosed patients with PCNSL who are or are not candidates for BMT, induction chemotherapy containing VCR may not provide additional benefit, although this study was not powered to detect a statistical difference. While VCR may not offer a survival advantage, this assessment is confounded by the omission of procarbazine. Additionally, VCR-containing therapy was associated with greater toxicity.
There were several limitations identified with this study. This was a retrospective study with a small sample size at a single institution and lacked adequate power to test noninferiority. There was an inherent selection bias that may have influenced treatment outcomes as those who consolidated with a BMT tended to receive VCR-containing therapy. Procarbazine use was a confounding factor given the disparity in its use between the VCR and non-VCR treatment groups.
Conclusions
In summary, the CR rate and survival outcomes were similar between groups. However, this study was insufficiently powered to test noninferiority. For patients with newly diagnosed PCNSL, VCR-containing induction chemotherapy was associated with increased toxicity. Even though it appears that VCR-containing therapy may offer increased toxicity and little additional benefit, providers should carefully consider the available evidence and evaluate patients prior to omitting VCR from induction therapy. Larger studies are required to further evaluate the efficacy of VCR in PCNSL induction regimens.
Acknowledgments
None declared.
Funding
None.
Conflict of interest statement. P.G. received research funding from BioMimetix. V.P. received research funding from AbbVie, Bexion, Beigene, Celldex, DNAtrix, and Novartis. V.P. has stock in Gilead and Amarin.
Authorship statement. Gathered primary data: T.F., C.S.L., and M.A.S.; performed primary statistical analysis of the data: E.M.M.; and analyzed data and contributed to the writing of the manuscript: T.F., C.S.L., M.A.S., E.M.M., V.P., P.G., and J.G.
References
- 1. Phillips EH, Fox CP, Cwynarski K. Primary CNS lymphoma. Curr Hematol Malig Rep. 2014;9(3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prodduturi P, Bierman PJ. Current and emerging pharmacotherapies for primary CNS lymphoma. Clin Med Insights Oncol. 2012;6:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Primary Central Nervous System Lymphoma. Clinical practice guidelines in oncology: primary central nervous system lymphoma. National Comprehensive Cancer Network. Version 2. 2020. [Google Scholar]
- 4. Morris PG, Correa DD, Yahalom J, et al. . Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omuro A, Correa DD, DeAngelis LM, et al. . R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9): 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Said R, Tsimberidou AM. Pharmacokinetic evaluation of vincristine for the treatment of lymphoid malignancies. Exp Opin Drug Metab Toxicol. 2014;10(3):483–494. [DOI] [PubMed] [Google Scholar]
- 7. Gogia A, Das CK, Kumar L, et al. . Diffuse large B-cell lymphoma: an institutional analysis. South Asian J Cancer. 2018;7(3):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kellie SJ, Barbaric D, Koopmans P, Earl J, Carr DJ, de Graaf SS. Cerebrospinal fluid concentrations of vincristine after bolus intravenous dosing: a surrogate marker of brain penetration. Cancer. 2002;94(6):1815–1820. [DOI] [PubMed] [Google Scholar]
- 10. Boyle FM, Eller SL, Grossman SA. Penetration of intra-arterially administered vincristine in experimental brain tumor. Neuro Oncol. 2004;6(4):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang F, Zhou F, Kruh GD, Gallo JM. Influence of blood-brain barrier efflux pumps on the distribution of vincristine in brain and brain tumors. Neuro Oncol. 2010;12(10):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burkhart CA, Watt F, Murray J, et al. . Small-molecule multidrug resistance-associated protein 1 inhibitor reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma. Cancer Res. 2009;69(16):6573–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fritsch K, Kasenda B, Schorb E, et al. . High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia. 2017;31(4):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]