Abstract
Vascular endothelia are covered with a dense glycocalix that is heavily sialylated. Sialylation of vascular glycoconjugates is involved in the regulation of cell–cell interactions, be it among endothelial cells at cell junctions or between endothelial and blood-borne cells. It also plays important roles in modulating the binding of soluble ligands and the signaling by vascular receptors. Here, we provide an overview over the sialylation-function relationships of glycoproteins expressed in the blood and lymphatic vasculature. We first describe cellular interactions in which sialic acid contributes in a stereospecific manner to glycan epitopes recognized by glycan-binding proteins. Our major focus is however on the rarely discussed examples of vascular glycoproteins whose biological functions are modulated by sialylation through other mechanisms.
Keywords: biophysical properties, endothelial cells, glycan-binding proteins, sialic acid, vascular system
Introduction
Cell membranes are studded with glycoconjugates that provide a dense layer of glycans, the glycocalyx. This glycan coat comprises the glycans of glycolipids, proteoglycans and glycoproteins. Terminal glycan positions of mammalian glycoproteins and glycolipids are typically occupied by sialic acid (Schauer 2009). The term sialic acid refers to a family of related, acidic monosaccharides. Their negative charge and exposed position predispose sialic acids for a major role in molecular and cellular interactions as they occur in physiological and pathological processes.
In the vascular system, sialic acid plays key roles in cell–cell and glycoprotein–protein interactions. In some of these processes, sialic acids are directly recognized and bound by specific glycan-binding proteins (GBP). In other instances, sialylation plays more indirect, modulatory roles, altering the molecular properties and interactions of the underlying protein.
In the present review, we first provide an overview over the functionally relevant properties of sialic acids and what is known about the regulation of sialylation. Many of the known functions of sialylation are related to the vascular system and its interactions with immune cells. We will thus mostly focus on the role of sialylation in biological processes related to vascular endothelia. For those that involve the direct recognition of sialic acids by GBP, a short overview is provided. Here, we give more room to the rarely discussed binding and signaling functions of vascular glycoproteins that are modulated by sialylation through other mechanisms.
Functionally relevant properties of sialic acids
Sialic acids are a family of acidic monosaccharides characterized by a nine-carbon basic structure with a negatively charged carboxylate (C1) and a three-carbon exocyclic side chain (C7–C9). The sialic acids found in mammalian organisms vary in their substituent at C5, which in N-acetyl neuraminic acid (Neu5Ac) is an acetylated amino group, in N-glycolylneuraminic acid (Neu5Gc) a glycolylated amino group and in 2-keto-3-deoxy-nonulosonic acid (Kdn) a hydroxyl group.
The glycosidic linkage between the C2 of sialic acid and the underlying monosaccharide may be α2,3- or α2,6- to galactose (Gal) or N-acetylgalactosamine (GalNAc), or α2,8- to another sialic acid. The different linkages of sialic acid determine its steric configuration and the ability of the linkage to bend, rotate and adopt certain topologies upon binding (Xu et al. 2009).
The type of sialic acid, further chemical modifications, as well as the configuration of the glycosidic linkage constitute stereospecific biophysical information that can be recognized by specific GBPs. The structure of the underlying glycan and the spatial organization of the sialylated glycans at the cell surface are additional factors that play important roles for the binding by GBPs and by GBP-expressing cells.
In mammals, sialic acids are very abundant and a single cell displays millions of sialic acid molecules (Varki and Gagneux 2012). Sialic acids contribute to the negative charge repulsion between cells and the regulation of cellular interactions (Born and Palinski 1985; Varki and Gagneux 2012). As an example, polysialic acid (a linear homopolymer of α2,8-linked sialic acids) on neural cell adhesion molecule (NCAM) increases the repulsion between cell membranes of adjacent cells and thus hinders NCAM- and cadherin-mediated intercellular adhesion (Johnson et al. 2005). Repulsion between sialylated glycans can also regulate the association and clustering of glycoproteins within the cell membrane and thus the avidity with which they bind their ligands (as described in the section ``lymphatic endothelial hyaluronan receptor-1''). It can furthermore affect the binding affinity of sialylated glycoprotein ligands for their receptor. The presence of highly sialylated glycans on human erythropoietin (providing 5–10 sialic acids per molecule) reduces its binding velocity and affinity for the erythropoietin receptor. Electrostatic repulsion occurs between the sialic acids of erythropoietin and its negatively charged binding sites on the erythropoietin receptor (Darling et al. 2002). Such repulsion involves long-range electrostatic forces and is due to the global electrostatic surface potential of sialylated erythropoietin. On the other hand, electrostatic interactions between sialic acids and positively charged amino acids of certain protein ligands may reinforce binding (described for the binding of vascular endothelial growth factor-A (VEGF-A) in the section ``Vascular endothelial growth factor receptor-2''). The presence of sialic acids can also affect the intensity and duration of receptor-mediated signaling (described for the release of macrophage inflammatory protein-2 (MIP-2 or CXCL2) induced by soluble intercellular adhesion molecule-1 (sICAM-1) in the section ``Intercellular adhesion molecule-1'') or the serum half-life of a given sialo-glycoprotein. It has been shown that isoforms of erythropoietin carrying more heavily sialylated glycans have an increased serum half-life and are more active in stimulating the maturation of red blood cells in vivo (Egrie and Browne 2001).
Sialylation and its regulation
Considering the multiple roles sialic acid may have in biological recognition and modulation of glycoprotein properties, it is of little surprise that sialylation is dynamically regulated in both developing and adult organisms (Cerná et al. 2002; Reiding et al. 2014; Torii et al. 2014). The rate-limiting step in sialylation is the production of the activated sialic acid donor CMP-Sia. The sialic acid precursor Neu5Ac is formed in the cytosol by condensation of phosphoenolpyruvate with N-acetyl-mannosamine-6-phosphate (ManNAc-6-P). The latter is produced by the bifunctional glucosamine (uridine diphosphate (UDP)-N-acetyl)-2-epimerase/N-acetylmannosamine kinase, encoded by the gene GNE (Hinderlich et al. 1997; Stäsche et al. 1997). CMP-Sia is then formed in the nucleus by CMP-Sia synthetase (CMAS) that transfers CMP to free sialic acid (Sellmeier et al. 2013). Rising cytosolic concentrations of CMP-Sia lead to an inhibition of the epimerization step catalyzed by GNE, which converts UDP-GlcNAc into free ManNAc (Kornfeld et al. 1964; Sommar and Ellis 1972). This is the main mechanism that regulates the amount of sialic acid that is produced in a cell (Weiss et al. 1989).
Sialylation of glycoconjugates occurs in the Golgi and is catalyzed by a family of 20 (human) sialyltransferases (STs) that can be subdivided into four families based on their glycan substrates and the sialic acid-linkage they form (Harduin-Lepers et al. 2001). A first family comprises the six ST3Gal (ST3Gal1–ST3Gal6) that link sialic acid in α2,3-linkage to an underlying Gal residue. The ST6Gal family includes two members that form α2,6-linkages of sialic acid to an underlying Gal residue in N-glycans as well as extended O-glycans (ST6Gal1, ST6Gal2). Third, six members of the ST6GalNAc family catalyze addition of sialic acid in α2,6-linkage to an underlying GalNAc residue. The fourth family contains six members of ST8Sia, which attach sialic acids in α2,8-linkage to a terminal sialic acid (Crespo et al. 2013). The repertoire of STs expressed in a given cell defines in which linkage and to which glycoconjugates sialic acid is added. The expression level of STs (ST3Gal5, ST6Gal1 and ST6GalNAc4) was shown to inversely correlate with the availability of CMP-Sia (Bork et al. 2017).
Another means to regulate the sialylation of glycoconjugates is the enzymatic removal of sialic acid by sialidases (formerly called neuraminidases). The mammalian genome encodes four sialidases that have similar tertiary conformations in spite of low amino acid sequence homology (NEU1–4; for recent review, see Pearce and Läubli 2015). The NEU differ in substrate specificity and cellular localization. NEU1 is most abundantly expressed in human tissues and has the lowest degree of homology to the other family members (Hata et al. 2008). NEU1 is found in the lysosome and, after transport in exovesicles, also at the cell surface, where it exclusively acts on glycoproteins (Bonten et al. 1996; Sumida et al. 2015; Machado et al. 2015). It hydrolyses α2,3-linkages of sialic acid more quickly than α2,6- or α2,8-linkages (Miyagi and Tsuiki 1984).
Sialylation-function relationships of vascular glycoproteins that involve stereospecific recognition of sialylated glycan epitopes by glycan-binding proteins
Direct binding of carbohydrates by GBPs is important in various molecular interactions between vascular endothelial cells and leukocytes (Figure 1). Such interactions are key to the dynamic regulation of the permeability of the vascular endothelium for blood-borne immune cells, thus allowing for immune responses to invading pathogens and injury. They are also crucial for adhesion of B- and T-lymphocytes to the high endothelial venules (HEVs) of peripheral lymph nodes and their homing to secondary lymphoid organs.
Fig. 1.
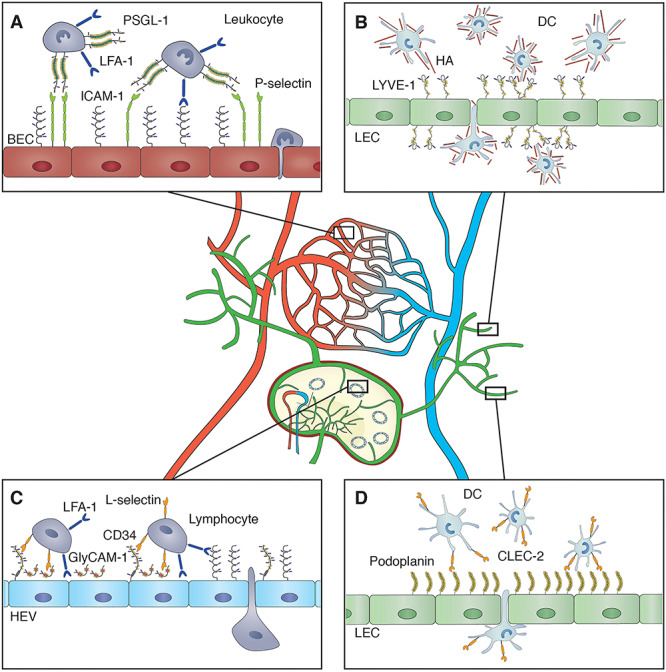
Cell–cell interactions between immune cells and vascular endothelia that depend on sialylation are shown in their microanatomical context. Lymphatic vessels entering, within and exiting a peripheral lymph node as well as small arteries (left) and veins (right) joined by a capillary network are depicted. Immune cells interacting with different subtypes of endothelial cells in various anatomical locations as well as the glycoproteins involved in such interactions are shown in the enlarged image sections. (A) Molecular interactions that are involved in leukocyte extravasation from a peripheral blood capillary into inflamed or injured tissue are shown. In the initial tethering of a leukocyte to activated BECs, binding of a sialylated glycan epitope on PSGL-1 by endothelial selectins (such as P-selectin) plays a crucial role. The subsequent firm adhesion mediated by binding of the integrin LFA-1 to ICAM-1 does however not appear to depend on the glycosylation of ICAM-1. (B) Adhesive interactions of DC with LEC are involved in their transmigration from the tissue into lymphatic capillaries. Binding of HA-coated DC by LYVE-1 that is abundantly expressed on lymphatic capillaries may be involved in this process. HA binding only occurs when LYVE-1 is present in clusters and clustering appears to be regulated by the sialylation of O-glycans present in the stalk region of LYVE-1. (C) Molecular interactions that are involved in the homing of lymphocytes into the lymph node through the postcapillary HEVs. L-selectin present on the lymphocytes binds to sialylated glycan epitopes displayed on various glycoproteins such as GlyCAM-1 and CD34. Firm adhesion occurs by binding of LFA-1 expressed by lymphocytes to endothelial ICAM-1. (D) CLEC-2 expressed on DC binds to podoplanin, which is abundantly expressed by LEC. Such binding depends on the sialylation of O-glycans on podoplanin. This figure is available in black and white in print and in color at Glycobiology online.
Endothelial cells line the lumens of blood and lymphatic vessels. Formation of cell–cell junctions between endothelial cells typically involves platelet endothelial cell adhesion molecule-1 (PECAM-1), which is used as a marker protein for endothelia. Blood vascular and lymphatic vascular endothelial cells (BECs and LECs) can be distinguished based on the expression of prospero-related homeobox-1 transcription factor (PROX-1) (Wigle and Oliver 1999), podoplanin (Breiteneder-Geleff et al. 1999) and LYVE-1 (Banerji et al. 1999) by LECs. LECs and BECs are derived from the same embryonic precursor cells, the so-called angioblasts (Adams and Alitalo 2007). Differentiation of LECs from BECs in mice is hallmarked by the expression of PROX-1 on embryonic day 9.5 (Tammela and Alitalo 2010). PROX-1 represses the expression of several blood vasculature-specific genes, but stimulates expression of genes that are associated with the lymphatic phenotype, such as LYVE-1 and podoplanin (Hong et al. 2002; Petrova et al. 2002). LYVE-1 is an N- and O-glycosylated transmembrane glycoprotein and a receptor for hyaluronan (HA) helping to anchor LECs in the ECM. Podoplanin is a heavily O-glycosylated mucin-type glycoprotein. Its binding by C-type lectin-like receptor-2 (CLEC-2) on platelets leads to platelet activation and aggregation (Suzuki-Inoue et al. 2007). Podoplanin-mediated platelet aggregation is also a crucial step during separation of the lymphatic from the blood vasculature during embryonic development (Uhrin et al. 2010).
The sialylated glycan epitopes bound by selectins
The most thoroughly investigated protein–carbohydrate recognition within the vasculature involves the selectins and their sialylated binding partners. Their roles in extravasation of innate immune cells into inflamed tissues and in homing of lymphocytes to lymph nodes are not only a cornerstone of glycobiology, but also a central concept of immunology. For detailed descriptions of the selectins, their ligands and functions, we refer the reader to excellent reviews by others (Lowe 2002; Sperandio et al. 2009; Schnaar 2015). Here, we provide a brief overview and primarily focus on the role the sialylation of selectin ligands plays for selectin binding.
In the first step of leukocyte extravasation, the leukocytes are loosely tethered to the activated endothelial surface by the endothelial selectins and roll along the inner vessel wall (Figure 1A) (Girard et al. 2012). The major ligand of P-selectin on leukocytes is P-selectin glycoprotein ligand-1 (PSGL-1), a dimeric mucin-type transmembrane glycoprotein expressed by leukocytes (Table I). The P-selectin binding site is located at the N-terminus of PSGL-1 and includes a core-2-based O-glycan capped with sialyl Lewis x (sLex; Siaα2,3-Galβ1,4-(Fucα1,3)-GlcNAc). In PSGL-1-deficient mice, leukocyte rolling and recruitment mediated by P- but not E-selectin is dramatically reduced (Yang et al. 1999). Thus, E-selectin appears to also bind other sLex-carrying glycoconjugates. When it comes to binding of PSGL-1, E- and P-selectin behave differently. P-selectin displays much higher binding affinity than E-selectin and this is due to the additional contacts of basic amino acids with tyrosine sulfate residues of PSGL-1 (Wilkins et al. 1995; Leppänen et al. 1999). But when the binding affinities for sLex alone are compared, E-selectin displays 10-fold higher affinity (Poppe et al. 1997). Based on the crystal structure, this was explained by a more extensive set of hydrogen bonds formed between the carbohydrate recognition domain (CRD) of E-selectin and sLex (Somers et al. 2000). It thus appears that E- and P-selectin have differing, specialized roles in leukocyte extravasation.
Table I.
The molecular characteristics of vascular glycoproteins whose sialylated glycan epitopes are recognized by GBP
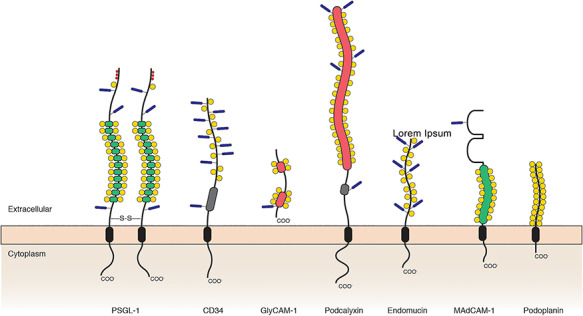
| |||||||
|---|---|---|---|---|---|---|---|
| PSGL-1 | CD34 | GlyCAM-1 | Podocalyxin | Endomucin | MAdCAM-1 | Podoplanin | |
| Protein family | Mucin | Mucin (PNAd) | Mucin (PNAd) | Mucin (PNAd) | Mucin (PNAd) CD34-like | Mucin (PNAd) Ig-like domain superfamily | – |
| Names | P-selectin glycoprotein ligand 1; Selectin P ligand | Hematopoietic progenitor cell antigen | Glycosylation-dependent cell adhesion molecule 1; Endothelial ligand for L-selectin; MC26; sulfated 50 kDa glycoprotein (SGP50) | Podocalyxin-like protein 1 (PC or PCLP-1); GCTM-2 antigen; Gp200; | Endomucin-2; Gastric cancer antigen; Ga34; Mucin-14 (MUC-14) | Mucosal addressin; cell adhesion molecule 1 | Aggrus; Glycoprotein 36 (Gp36); PA2.26 antigen; T1-alpha (T1A) |
| CD antigen | CD162 | CD34 | – | – | – | – | – |
| Gene name | SELPG | CD34 | GLYCAM1 | PODXL | EMCN | MADCAM1 | PDPN |
| Size | h: 371 aa | h: 354 aa m: 348 aa |
m: 132 aa | h: 536 aa m: 482 aa |
h: 243 aa m: 241 aa |
h: 364 aa m: 384 aa |
h: 152 aa m: 150 aa |
| N-glycans | h: 3 | h: 9 m: 7 |
m: 1 | h: 5 m: 8 |
h: 6 m: 5 |
h: 1 m: 3 |
– m: 1 |
| O-glycosylation | Heavily O-glycosylated; core-2 O-glycan with sLex at Thr 57 | Extensively O-glycosylated | Extensively O-glycosylated | Highly O-glycosylated Thr-rich domain | Highly O-glycosylated, Thr-rich domain | Ser/Thr-rich mucin-like domain O-glycosylated | h: 24 O-linked glycans m: 18 O-linked glycans |
| Ligands | P-, E- and L-selectin; Homodimer (disulfide-linked) | L-selectin | L-selectin | L-selectin CCR6 Galectin-3 Galectin-8 Galectin-9 Ficolin |
L-selectin | Homodimer; L-selectin | Homodimer; CLEC-2; CD9 (Tetraspanin) Galectin 8; HSPA9; CCL21; CD44 Moesin and Ezrin (via cytoplasmatic domain) |
| Cellular expression | Leukocytes | Endothelial cells, hematopoietic progenitor cells | Endothelial cells (HEVs) | Endothelial cells, Glomerular podocytes, glandular cells | Endothelial cells | HEVs and endothelial cells in spleen and gastro-intestinal tract | Lymphatic endothelial cells, alveolar type-1 epithelial cells, T cells, macrophages, podocytes, fibroblastic reticular cells |
The human glycoproteins are depicted with the exception of GlyCAM-1, which is not found in human tissues. The information was gathered from the human protein atlas (https://www.proteinatlas.org/) and from uniprot (https://www.uniprot.org/).
An early observation was that sialidase treatment of neutrophils led to a dramatic reduction of P-selectin-dependent rolling in vivo (Ley et al. 1995). More recently, it was shown that the sialyltransferases ST3Gal4 and ST3Gal6 are responsible for E- and P-selectin ligand formation (Ellies et al. 2002; Yang et al. 2012). The neutrophils from mice deficient in both sialyltransferases displayed significantly reduced binding to E- and P-selectin.
In lymphocyte homing through the HEVs of lymph nodes, L-selectin expressed on the lymphocyte binds to its ligands on the endothelial cells (Figure 1C). The ligands of L-selectin are collectively called peripheral node addressins (PNAd) and include glycans present on the vascular sialomucin CD34, the murine glycosylated cell adhesion molecule-1 (GlyCAM-1), podocalyxin, endomucin and MAdCAM (Table I) (McEver 2002; Rosen 2004). The members of the PNAd complex are functionally redundant and thus appear to mostly function as protein scaffolds for the presentation of glycan epitopes comprising sLex and sulfated versions thereof (Mitoma et al. 2007). The 6-sulfo-sialyl Lewis x (6-sulfo-sLex) is an L-selectin ligand with particularly high affinity. The HEVs of peripheral lymph nodes display higher expression levels of genes encoding sialyl-, fucosyl- and sulfotransferases (ST3GAL4, FUT7, Chst2 and most prominently Chst4) compared to the HEVs of Peyer’s patches (Lee et al. 2014). The lower expression of these genes in Peyer’s patches is in line with earlier reports on low levels of L-selectin ligands that only allow for loose L-selectin-mediated rolling in Peyer’s patches (Butcher and Picker 1996).
The carbohydrate ligands of E- and P-selectin involved in tethering and rolling of leukocytes on activated BECs are expressed on the leukocytes, whereas the carbohydrate ligands of L-selectin involved in homing of lymphocytes to secondary lymphoid organs are presented on the HEVs (Sperandio et al. 2009). The differing cellular distribution of selectin ligands involved in extravasation depending on the location and the processes they govern within the vasculature may be a means to increase their local and functional specificity. The expression of selectin ligands on different cell types may also explain why they occur on different membrane proteins and comprise differing glycan epitopes. In homing of B- and T-lymphocytes, L-selectin binds best to ligands that include not only sLex, but also glycans containing 6-O-sulfated GlcNAc such as 6-sulfo sLex (Hernandez Mir et al. 2009). For leukocyte rolling under inflammatory conditions however, endothelial P- and E-selectin bind to sLex on the core-2-O-glycan at Thr57 of PSGL-1 (Leppänen et al. 2000).
Podoplanin
Another sialic acid-dependent interaction between blood cells and vascular endothelial cells is mediated by CLEC-2. CLEC-2 is expressed by platelets and myeloid cells (e.g. dendritic cells – DC) and appears to have an exclusive binding specificity for its one known ligand, podoplanin (Table I). Within the vasculature, podoplanin is expressed exclusively on LECs. Its binding by platelet CLEC-2 provokes platelet activation and aggregation (Suzuki-Inoue et al. 2007; Kato et al. 2008), whereas interaction with CLEC-2 on DC is important in DC migration to lymph nodes (Acton et al. 2012).
Initial studies on the role of glycosylation for the binding and activation of CLEC-2 were based on podoplanin expressed on different glycosylation mutants of CHO cells. Both human and mouse podoplanin induced aggregation of mouse platelets only when carrying sialylated O-glycans (Kaneko et al. 2004). The glycans of human podoplanin expressed by CHO cells or the glioblastoma cell line LN319 were later shown to be di- or mono-sialylated core-1 O-glycans (Kaneko et al. 2007). The CLEC-2 binding sites on human and mouse podoplanin were reported to be 47-EDDVVTPG-54 with a disialylated core-1 O-glycan at T52 and 29-EDDIVTPG-36 with a disialylated core-1 O-glycan at T34, respectively (Kaneko et al. 2006). However, later studies based on site-directed mutagenesis and peptide deletions suggested that there may be an additional CLEC-2 binding site on podoplanin (81-EDLPTSE-87 of human podoplanin) that includes negatively charged amino acids and O-glycans (Bianchi et al. 2014; Sekiguchi et al. 2015).
Podoplanin was also found to interact with galectin-8 in a glycosylation-dependent manner. Galectin-8 is a tandem-repeat galectin with an N-terminal CRD-binding α2,3-sialylated glycans with high affinity and a C-terminal CRD that does not bind sialylated glycans (Ideo et al. 2011). Galectin-8 is expressed by LECs. It was suggested that the interaction of galectin-8 with podoplanin may contribute to the anchoring of the lymphatic endothelium to the surrounding extracellular matrix, most likely together with other surface glycoproteins of LECs (Cueni and Detmar 2009). Furthermore, galectin-8 was reported to mediate crosstalk among podoplanin, vascular endothelial growth factor-C (VEGF-C) and integrin-mediated pathways in pathological lymphangiogenesis in the eye (Chen et al. 2016).
Vascular glycoproteins whose biological functions are modulated by sialylation
Besides the famous examples of sialylated glycans that are specifically bound by GBPs, there are various reports on functional roles of sialylation within the vasculature that may rely on other mechanisms.
Intercellular adhesion molecule-1
One of the most widely studied vascular adhesion molecules is ICAM-1 (Table II). Its expression on both LECs and BECs is normally low but strongly induced upon inflammatory activation (Pober et al. 1986; Johnson et al. 2006). In parallel, a soluble form of ICAM-1 is released (Rothlein et al. 1991; Budnik et al. 1996; Otto et al. 2000; Giorelli et al. 2002). The major role of membrane-bound ICAM-1 is to mediate the second step in the extravasation of immune cells into inflamed tissue occurring after the tethering and rolling mediated by P- and E-selectin, namely the firm adhesion and subsequent transmigration of leukocytes (Girard et al. 2012; Vestweber 2015). The ICAM-1 binding partners in this process are the integrins leukocyte function-associated antigen-1 (LFA-1; CD11a/CD18; Figure 1A) present on all leukocytes and macrophage antigen-1 (Mac-1; CD11b/CD18) on myeloid cells (Marlin and Springer 1987; Diamond et al. 1990). The functional roles of soluble ICAM-1 (sICAM-1) are less clear. It was proposed on the one hand that it could influence the adhesive interactions between leukocytes and endothelial cells acting as a competitive inhibitor (Rieckmann et al. 1995; Meyer et al. 1995). On the other hand, sICAM-1 was found to act as a signaling molecule inducing secretion of the CXC chemokine MIP-2 (CXCL2) by both brain-derived mouse microvascular endothelial cells and astrocytes (Otto et al. 2000).
Table II.
The molecular characteristics of the vascular glycoproteins whose biological functions are modulated by sialylation
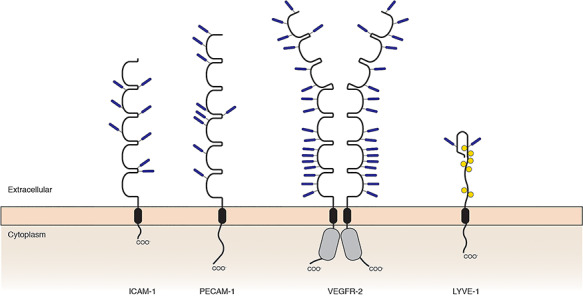
| ||||
|---|---|---|---|---|
| ICAM-I | PECAM-1 | VEGFR-2 | LYVE-1 | |
| Protein family | Ig-like domain superfamily | Ig-like domain superfamily | Ig-like domain superfamily; tyrosine kinase receptor | C-type lectin-like/link domain superfamily |
| Names | Intercellular adhesion molecule-1; Major group rhinovirus receptor | Platelet endothelial cell adhesion molecule; EndoCAM; GPIIA’; PECA1 | Vascular endothelial growth factor receptor 2; Fetal liver kinase (FLK-1); Kinase insert domain receptor (KDR); Protein-tyrosine kinase receptor flk-1 | Lymphatic vessel endothelial hyaluronic acid receptor 1; Cell surface retention sequence-binding protein 1 (CRSBP-1); Extracellular link domain-containing protein 1; Hyaluronic acid receptor |
| CD antigen | CD54 | CD31 | CD309 | – |
| Gene name | ICAM1 | PECAM1 | KDR | LYVE1 |
| Size |
h: 505 aa
m: 510 aa |
h: 711 aa
m: 710 aa |
h: 1337 aa
m: 1348 aa |
h: 303 aa
m: 295 aa |
| N-glycans |
h: 8
m: 9 |
h: 7
m: 9 |
h: 18
m: 17 |
h: 2
m: 2 |
| O-glycosylation | ND | ND | ND | Sialylated O-linked glycans in the Ser/Thr-rich stalk region |
| Ligands | LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18) MUC1 CD81, CD247, CD9 | PECAM-1 (trans-homodimer) | VEGF-A, VEGF-C and VEGF-D Forms homodimer or heterodimers with VEGFR-1 and VEGFR3 | LYVE-1 (disulfide-linked homodimer); hyaluronan |
| Cell expression | Endothelial cells, pneumocytes and subsets of lymphoid cells. | Endothelial cells and smooth muscle cells. | Endothelial cells | Lymphatic endothelial cells and macrophage subsets |
The human glycoproteins are depicted. The information was gathered from the human protein atlas (https://www.proteinatlas.org/) and from uniprot (https://www.uniprot.org/). ND, not determined.
Glycosylation affects the different functions of ICAM-1 differentially. Binding to LFA-1 is not influenced by glycosylation of both, human and mouse ICAM-1 (Diamond et al. 1991; Otto et al. 2004). Binding to Mac-1 is however favored if ICAM-1 carries oligomannose N-glycans rather than complex-type N-glycans (Diamond et al. 1991). Neuraminidase treatment of transfected L-cells expressing human ICAM-1 does not change their interaction with Mac-1 suggesting that it is not the sialylation of complex N-glycans that hinders Mac-1 binding. The MIP-2-inducing capacity of murine ICAM-1 in mouse astrocytes (not expressing LFA-1 or Mac-1) strongly depends on its sialylation. Whereas sICAM-1 expressed in CHO cells had potent signaling activity, nonsialylated sICAM-1 expressed in the Lec-2 mutant of CHO cells was much less active (Otto et al. 2004). MIP-2 induction was further reduced if sICAM-1 lacked both sialic acid and galactose, or if it contained only high-mannose-type N-glycans. All these glycoforms of mouse sICAM-1 retained a normal ability to bind LFA-1 on lymphocytes. The ligand of sICAM-1 on mouse astrocytes has not been identified. However, the affinity of sICAM-1 containing either sialylated complex-type or oligomannose N-glycans for the astrocyte surface did not differ (Ki of roughly 300 nM as measured using a competitive radioligand binding assay), suggesting that there was no direct involvement of the sialylated sICAM-1 glycans in binding. The kinetics of the MIP-2 induction were clearly different with fully sialylated sICAM-1 inducing a more rapid, more pronounced and more persistent MIP-2 response than ICAM-1 containing oligomannose N-glycans (Schürpf et al. 2008). Thus, the intensity and kinetics of signaling induced by sICAM-1 in astrocytes was regulated by the completeness of N-glycosylation and in particular of sialylation.
Platelet endothelial cell adhesion molecule-1
PECAM-1 (Table II) is concentrated at the cell–cell junctions of endothelia. Homophilic interactions between PECAM-1 on neighboring endothelial cells play important roles in the formation and regulation of endothelial cell junctions (Chistiakov et al. 2016). PECAM-1 is also involved in the transmigration of leukocytes across endothelial junctions (Muller 2014; Vestweber 2015). The major binding sites in homophilic PECAM-1 interactions lie in the first N-terminal Ig-like domain and involve five charged amino acids (Asp11, Asp33, Lys50, Asp51 and Lys89), but the second Ig-like domain is also required for binding (Paddock et al. 2016). Homophilic interactions occur between PECAM-1 molecules present on the same cell (clustering) as well as on opposing cells. In spite of 79% homology at the amino acid level (Xie and Muller 1993), mouse and human PECAM-1 do not bind to each other (Sun, DeLisser, et al. 1996; Sun, Williams, et al. 1996). Indeed, 18 amino acids that differ between human and mouse PECAM-1 are located within the IgD1-homophilic binding interface and include N25, which carries an N-glycan in human PECAM-1 (Paddock et al. 2016).
PECAM-1 from mouse lung homogenates contains α2,6-sialylated glycans, which are required for its homophilic binding and binding is inhibited by linear, sialylated oligosaccharides containing Neu5Gc or Neu5Ac in α2,6-linkage (Kitazume et al. 2014).
PECAM-1 on human pulmonary microvascular endothelial cells (HPMECs) carries α2,6-sialylated glycans (Lee, Liu et al. 2014). Sialylation was found to be important for the ability of HPMECs to form capillary-like tubes in matrigel, an in vitro surrogate for one of the key processes in angiogenesis. EC tube formation was inhibited upon NEU1 expression and restored upon ST6GAL1 overexpression.
Recently, the role of sialylation of human PECAM-1 for its homophilic binding was investigated more closely. Presence of α2,6-linked sialic acid on PECAM-1-Fc chimera appeared to inhibit binding to PECAM-1-expressing REN cells (Lertkiatmongkol et al. 2016). Based on in-silico docking studies of α2,6- and α2,3-sialylated lactosamine to the crystal structure of the first two Ig domains of human PECAM-1, it was proposed that sialic acid in α2,6-linkage to the N57-glycan could provoke an “autoinhibition” of the PECAM-1 molecule by an intradomain electrostatic interaction with Lys89. In contrast, an α2,3-linked sialic acid on the N25-glycan could bind to the Lys89 of the opposing PECAM-1 thus reinforcing homophilic binding. In such a model, the binding sites of the glycans containing sialic acid in either α2,3- or α2,6- linkage differ, even though they both comprise the Lys89 that engages into electrostatic interactions with sialic acid, either on the same or an opposing PECAM-1 molecule. Deletion of the N25-glycan on PECAM-1 of REN cells did not affect homophilic binding or endothelial barrier function under steady state conditions; it however slowed down the recovery of the endothelial barrier after thrombin treatment.
Vascular endothelial growth factor receptor-2
VEGFR2 is primarily expressed on endothelial and hematopoietic cells. It is a tyrosine kinase receptor of VEGF-A and importantly involved in the regulation of angiogenesis, vascular development and vascular permeability (Shalaby et al. 1995; Gille et al. 2001; Koch and Claesson-Welsh 2012) (Table II). Its seven Ig-like domains are heavily N-glycosylated (Chandler et al. 2017). The VEGFR2 of HUVEC cells was shown to predominantly carry α2,6-sialylated glycans. Such sialylation was important in supporting binding of the VEGF-A165 isoform, but not of the VEGF121 isoform that lacks the cationic heparin-binding domain (Chiodelli et al. 2017). Pretreatment with Sambucus nigra agglutinin or neuraminidase reduced VEGF-A165 binding and VEGFR2 autophosphorylation. Interestingly, silencing of the ST6Gal1 gene was accompanied by a compensatory upregulation of ST3Gal1 expression and increased α2,3-sialylation. VEGFR2 carrying α2,3-sialylated glycans was well able to bind VEGF-A165. Only concomitant silencing of ST6Gal1 and ST3Gal1 abolished VEGF-A165-induced autophosphorylation of VEFR2. It thus seems that only the negatively charged sialic acid, but not its stereochemical presentation, was important to support VEGFR2 activation by VEGF-A165.
Lymphatic vessel endothelial hyaluronan receptor-1
LYVE-1 is primarily expressed by lymphatic endothelial cells (LEC; Table II). It has long been known as a receptor for HA and is closely related to the HA receptor CD44 of leukocytes (Banerji et al. 1999). More recent studies have revealed that it preferentially binds large, polyvalent HA-protein complexes (Jackson 2014; Lawrance et al. 2016). Thus, LYVE-1 may primarily function as a lymphatic trafficking receptor that mediates docking of HA-coated leukocytes to lymphatic vessels (Figure 1B) (Lawrance et al. 2016; Johnson et al. 2017).
The ability of LYVE-1 to bind HA is regulated by the degree of its sialylation (Nightingale et al. 2009). When expressed on glycosylation mutants of CHO cells, those lacking sialic acid on both N- and O-glycans (Lec-2 cells) showed strongly increased binding to HA, whereas the cells expressing glycoproteins that carry normal O-glycans but only nonsialylated oligomannose N-glycans (as expressed by Lec-1 cells) showed reduced binding. It thus appears that the two complex-type N-glycans—including their sialylation—on the HA-binding domain are rather supportive of HA binding, whereas the sialylated O-glycans present in the membrane-proximal domain hinder HA binding. For HA binding to occur, LYVE-1 needs to be present in clusters (Lawrance et al. 2016). It was thus proposed that sialylation of the membrane-proximal O-glycans hinders LYVE-1 self-association and clustering and thus also HA binding (Nightingale et al. 2009; Jackson 2019). Sialylation of the LYVE-1 stalk region may therefore allow for the regulation of the avidity of LYVE-1 for its ligand HA.
Taken together
Many of the cellular and molecular interactions in which sialylation plays a regulatory role occur in the vascular system. These are on the one hand cell–cell contacts in which very specific glycan- or glycopeptide structures are directly bound by GBPs. In these bindings, molecular properties such as sialic acid substituents and the linkage to the underlying glycan, the identity of the neighboring monosaccharides as well as side chains of the underlying peptide are of key importance. The most famous and best investigated examples are the glycan epitopes related to sLex (that may also include nearby amino acid side chains) present on leukocytes that are recognized by endothelial P- and E-selectin. Also, the interaction of CLEC-2 expressed on platelets and DC with podoplanin on lymphatic endothelia most likely depends on direct binding of sialylated core-1 O-glycans. On the other hand, there are various examples in which sialylation plays more modulatory roles and in which the more general, biophysical properties of sialic acid appear to be important. The MIP-2 production induced by soluble ICAM-1 in mouse astrocytes is most intense and prolonged if sICAM-1 is sialylated. Its affinity for the unknown receptor on astrocytes is however not higher than the affinity of nonsialylated sICAM-1 with poor MIP-2 inducing capacity. Thus, the dynamics and intensity of the signaling elicited appears to be altered if sICAM-1 is sialylated. Binding of VEGF-A165 by the VEGFR2 is enhanced if the receptor is sialylated. It does however not matter whether sialic acid is α2,6- or α2,3-linked. This suggests that it is not sophisticated stereo-electronic features, but the global negative charge of the sialoglycan that is important to support binding to the positively charged heparin-binding domain of VEGF-A165. The negative charges of sialylated mucin-type O-glycans in the stalk region of LYVE-1 were proposed to hinder clustering of LYVE-1-molecules at the cell surface through electrostatic repulsion. As a consequence, HA binding is hindered due to a loss in avidity. With the current analytical methods, it is hardly possible to characterize the biophysical impact of sialylation on the overall biological properties of a glycoconjugate in mechanistic detail. Therefore, such roles of sialylation remain less well defined than those that involve direct binding. As a consequence, they are only rarely described in review articles. The present text was aimed at paying its tribute to these “orphan functions” of sialylation thus adding an additional facet to the abundant review literature on sialic acid.
Acknowledgements
This work was supported by OPO foundation, Zurich, Switzerland.
Conflict of interest statement
None declared.
References
- Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG et al. 2012. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 37:276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 8:464–478. [DOI] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX et al. 1999. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 144:789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Fischer E, Yuen D et al. 2014. Mutation of threonine 34 in mouse podoplanin-fc reduces CLEC-2 binding and toxicity in vivo while retaining antilymphangiogenic activity. J Biol Chem. 289:21016–21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonten E, Van Der Spoel A, Fornerod M, Grosveld G, d'Azzo A. 1996. Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev. 10:3156–3169. [DOI] [PubMed] [Google Scholar]
- Bork K, Weidemann W, Berneck B et al. 2017. The expression of sialyltransferases is regulated by the bioavailability and biosynthesis of sialic acids. Gene Expr Patterns. 23–24:52–58. [DOI] [PubMed] [Google Scholar]
- Born GVR, Palinski W. 1985. Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br J Exp Pathol. 66:543. [PMC free article] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Kowalski H et al. 1999. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. AJPA. 154:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik A, Grewe M, Gyufko K, Krutmann J. 1996. Analysis of the production of soluble ICAM-1 molecules by human cells. Exp Hematol. 24:352–359. [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. 1996. Lymphocyte homing and homeostasis. Science. 272:60–66. [DOI] [PubMed] [Google Scholar]
- Cerná A, Janega P, Martanovic P, Lisý M, Babál P. 2002. Changes in sialic acid expression in the lung during intrauterine development of the human fetus. Acta Histochem. 104:339–342. [DOI] [PubMed] [Google Scholar]
- Chandler KB, Leon DR, Meyer RD, Rahimi N, Costello CE. 2017. Site-specific N-glycosylation of endothelial cell receptor tyrosine kinase VEGFR-2. J Proteome Res. 16:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-S, Cao Z, Sugaya S et al. 2016. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat Commun. 7:11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodelli P, Rezzola S, Urbinati C et al. 2017. Contribution of vascular endothelial growth factor receptor-2 sialylation to the process of angiogenesis. Oncogene. 36:6531–6541. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Orekhov AN, Bobryshev YV. 2016. Endothelial PECAM-1 and its function in vascular physiology and atherogenic pathology. Exp Mol Pathol. 100:409–415. [DOI] [PubMed] [Google Scholar]
- Crespo HJ, Lau JTYP, Videira PAP. 2013. Dendritic cells: a spot on sialic acid. Front Immunol. 4, article 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni LN, Detmar M. 2009. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp Cell Res. 315:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling RJ, Kuchibholta U, Glaesner W, Micanovic R, Witcher DR, Beals JM. 2002. Glycosylation of erythropoietin affects receptor binding kinetics: role of electrostatic interactions. Am Chem Soc. 41:14524–14531. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Staunton DE, Fougerolles AR et al. 1990. ICAM-1 (CD54): a counter-receptor for mac-1 (CD11b/CD18). J Cell Biol. 111:3129–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Staunton DE, Marlin SD, Springer TA. 1991. Binding of the integrin mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 65:961–971. [DOI] [PubMed] [Google Scholar]
- Egrie JC, Browne JK. 2001. Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer. 84:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellies LG, Sperandio M, Underhill GH et al. 2002. Sialyltransferase specificity in selectin ligand formation. Blood. 100:3618–3625. [DOI] [PubMed] [Google Scholar]
- Gille H, Kowalski J, Li B et al. 2001. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). J Biol Chem. 276:3222–3230. [DOI] [PubMed] [Google Scholar]
- Giorelli M, De Blasi A, Defazio G et al. 2002. Differential regulation of membrane bound and soluble ICAM 1 in human endothelium and blood mononuclear cells: effects of interferon beta-1a. Cell Commun Adhes. 9:259–272. [DOI] [PubMed] [Google Scholar]
- Girard J-P, Moussion C, Förster R. 2012. HEVs, Lymphatics and Homeostatic Immune Cell Trafficking in Lymph Nodes. Nat Rev Immunol 12: 762–773. [DOI] [PubMed] [Google Scholar]
- Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi M-A, Samyn-Petit B, Julien S, Delanoy P. 2001. The human sialyltransferase family. Biochimie. 83:727–737. [DOI] [PubMed] [Google Scholar]
- Hata K, Koseki K, Yamaguchi K et al. 2008. Limited inhibitory effects of oseltamivir and zanamivir on human sialidases. Antimicrob Agents Chemother. 52:3484–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Mir G, Helin J, Skarp K-P et al. 2009. Glycoforms of human endothelial CD34 that bind L-selectin carry sulfated sialyl Lewis x capped O- and N-glycans. Blood. 114:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderlich S, Stäsche R, Zeitler R, Reutter W. 1997. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. J Biol Chem. 272:24313–24318. [DOI] [PubMed] [Google Scholar]
- Hong Y-K, Harvey N, Noh Y-H et al. 2002. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 225:351–357. [DOI] [PubMed] [Google Scholar]
- Ideo H, Matsuzaka T, Nonaka T, Seko A, Yamashita K. 2011. Galectin-8-N-domain recognition mechanism for sialylated and sulfated glycans. J Biol Chem. 286:11346–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG. 2014. Lymphatic regulation of cellular trafficking. J Clini Cell Immunol. 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG. 2019. Hyaluronan in the lymphatics: the key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol. 78–79:219–235. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Fujimoto I, Rutishauser U, Leckband DE. 2005. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J Biol Chem. 280:137–145. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Banerji S, Lawrance W et al. 2017. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat Immunol. 18:762–770. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. 2006. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 203:2763–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko MK, Kato Y, Kameyama A et al. 2007. Functional glycosylation of human podoplanin: glycan structure of platelet aggregation-inducing factor. FEBS Lett. 581:331–336. [DOI] [PubMed] [Google Scholar]
- Kaneko MK, Kato Y, Kitano T, Osawa M. 2006. Conservation of a platelet activating domain of Aggrus/podoplanin as a platelet aggregation-inducing factor. Gene. 378:52–57. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kato Y, Kunita A, Fujita N, Tsuruo T, Osawa M. 2004. Functional sialylated O-glycan to platelet aggregation on Aggrus (T1α/Podoplanin) molecules expressed in chinese hamster ovary cells. J Biol Chem. 279:38838–38843. [DOI] [PubMed] [Google Scholar]
- Kato Y, Kaneko MK, Kunita A et al. 2008. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 99:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazume S, Imamaki R, Kurimoto A et al. 2014. Interaction of platelet endothelial cell adhesion molecule (PECAM) with α2,6-sialylated glycan regulates its cell surface residency and anti-apoptotic role. J Biol Chem. 289:27604–27613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Claesson-Welsh L. 2012. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2:a006502–a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Kornfeld R, Neufeld EF, O'Brien PJ. 1964. The feedback control of sugar nucleotide biosynthesis in the liver. Proc Natl Acad Sci USA. 52:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrance W, Banerji S, Day AJ, Bhattacharjee S, Jackson DG. 2016. Binding of hyaluronan to the native lymphatic vessel endothelial receptor LYVE-1 is critically dependent on receptor clustering and hyaluronan organization. J Biol Chem. 291:8014–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Liu A, Miranda-Ribera A et al. 2014. NEU1 sialidase regulates the sialylation state of CD31 and disrupts CD31-driven capillary-like tube formation in human lung microvascular endothelia. J Biol Chem. 289:9121–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kiefel H, LaJevic MD et al. 2014. Transcriptional programs of lymphoid tissue capillary and high endothelium reveal control mechanisms for lymphocyte homing. Nat Immunol. 15:982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen A, Mehta P, Ouyang YB et al. 1999. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J Biol Chem. 274:24838–24848. [DOI] [PubMed] [Google Scholar]
- Leppänen A, White SP, Helin J, McEver RP, Cummings RD. 2000. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 275:39569–39578. [DOI] [PubMed] [Google Scholar]
- Lertkiatmongkol P, Paddock C, Newman DK, Zhu J, Thomas MJ, Newman PJ. 2016. The role of sialylated glycans in human platelet endothelial cell adhesion molecule 1 (PECAM-1)-mediated trans homophilic interactions and endothelial cell barrier function. J Biol Chem. 291:26216–26225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Zakrzewicz A, Hanski C, Stoolman LM, Kansas GS. 1995. Sialylated O-glycans and L-selectin sequentially mediate myeloid cell rolling in vivo. Blood. 85:3727–3735. [PubMed] [Google Scholar]
- Lowe JB. 2002. Glycosylation in the control of selectin counter-receptor structure and function. Immunol Rev. 186:19–36. [DOI] [PubMed] [Google Scholar]
- Machado E, White-Gilbertson S, Vlekkert D et al. 2015. Regulated lysosomal exocytosis mediates cancer progression. Sci Adv. 1:e1500603–e1500603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin SD, Springer TA. 1987. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 51:813–819. [DOI] [PubMed] [Google Scholar]
- McEver RP. 2002. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 14:581–586. [DOI] [PubMed] [Google Scholar]
- Meyer DM, Dustin ML, Carron CP. 1995. Characterization of intercellular adhesion molecule-1 ectodomain (sICAM-1) as an inhibitor of lymphocyte function-associated molecule-1 interaction with ICAM-1. J Immunol. 155:3578–3584. [PubMed] [Google Scholar]
- Mitoma J, Bao X, Petryanik B et al. 2007. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 8:409–418. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Tsuiki S. 1984. Rat-liver lysosomal sialidase. Eur J Biochem. 141:75–81. [DOI] [PubMed] [Google Scholar]
- Muller WA. 2014. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol. 184:886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale TD, Frayne MEF, Clasper S, Banerji S, Jackson DG. 2009. A mechanism of sialylation functionally silences the hyaluronan receptor LYVE-1 in lymphatic endothelium. J Biol Chem. 284:3935–3945. [DOI] [PubMed] [Google Scholar]
- Otto VI, Heinzel-Pleines UE, Gloor SM, Trentz O, Kossmann T, Morganti-Kossmann MC. 2000. sICAM-1 and TNF-alpha induce MIP-2 with distinct kinetics in astrocytes and brain microvascular endothelial cells. J Neurosci Res. 60:733–742. [DOI] [PubMed] [Google Scholar]
- Otto VI, Schürpf T, Folkers G, Cummings RD. 2004. Sialylated complex-type N-glycans enhance the signaling activity of soluble intercellular adhesion molecule-1 in mouse astrocytes. J Biol Chem. 279:35201–35209. [DOI] [PubMed] [Google Scholar]
- Paddock C, Zhou D, Lertkiatmongkol P, Newman PJ, Zhu J. 2016. Structural basis for PECAM-1 homophilic binding. Blood. 127:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce OMT, Läubli H. 2015. Sialic acids in cancer biology and immunity. Glycobiology. 26:111–128. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Mäkelä TP et al. 2002. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21:4593–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Gimbrone MA, Lapierre LA et al. 1986. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 137:1893–1896. [PubMed] [Google Scholar]
- Poppe L, Brown GS, Philo JS, Pandurang V, Nikrad A, Shah BH. 1997. Conformation of sLex tetrasaccharide, free in solution and bound to E-, P-, and L-selectin. J Am Chem Soc. 119:1727–1736. [Google Scholar]
- Reiding KR, Blank D, Kuijper DM, Deelder AM, Wuhrer M. 2014. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal Chem. 86:5784–5793. [DOI] [PubMed] [Google Scholar]
- Rieckmann P, Michel U, Albrecht M, Brück W, Wöckel L, Felgenhauer K. 1995. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 60:9–15. [DOI] [PubMed] [Google Scholar]
- Rosen SD. 2004. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 22:129–156. [DOI] [PubMed] [Google Scholar]
- Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. 1991. A form of circulating ICAM-1 in human serum. J Immunol. 147:3788–3793. [PubMed] [Google Scholar]
- Schauer R. 2009. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 19:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL. 2015. Glycans and glycan-binding proteins in immune regulation: a concise introduction to glycobiology for the allergist. J Allergy Clin Immunol. 135:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürpf T, Callewaert N, Meyer MJT et al. 2008. Consequences of soluble ICAM-1 N-glycan alterations on receptor binding and signaling kinetics in mouse astrocytes. Open Glycosci. 1:40–51. [Google Scholar]
- Sekiguchi T, Takemoto A, Takagi S et al. 2015. Targeting a novel domain in podoplanin for inhibiting platelet-mediated tumor metastasis. Oncotarget. 7:3934–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellmeier M, Weinhold B, Münster-Kühnel A. 2013. CMP-sialic acid synthetase: the point of constriction in the sialylation pathway In: Topics in Current Chemistry. Berlin, Heidelberg: Springer; p. 139–168. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Janet R, Yamaguchi TP et al. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 376:62–66. [DOI] [PubMed] [Google Scholar]
- Somers WS, Tang J, Shaw GD, Camphausen RT. 2000. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to sLex and PSGL-1. Cell. 103:467–479. [DOI] [PubMed] [Google Scholar]
- Sommar KM, Ellis DB. 1972. Uridine diphosphate N-acetyl-D-glucosamine 2-epimerase from rat liver. Biochim Biophys Acta Enzymol. 268:581–589. [DOI] [PubMed] [Google Scholar]
- Sperandio M, Gleissner CA, Ley K. 2009. Glycosylation in immune cell trafficking. Immunol Rev. 230:97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäsche R, Hinderlich S, Weise C et al. 1997. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. J Biol Chem. 272:24319–24324. [DOI] [PubMed] [Google Scholar]
- Sumida M, Hane M, Yabe U et al. 2015. Rapid trimming of cell surface polysialic acid (PolySia) by exovesicular Sialidase triggers release of preexisting surface neurotrophin. J Biol Chem. 290:13202–13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. 1996. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 271:18561–18570. [DOI] [PubMed] [Google Scholar]
- Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. 1996. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 271:11090–11098. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Kato Y, Inoue O et al. 2007. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 282:25993–26001. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. 2010. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 140:460–476. [DOI] [PubMed] [Google Scholar]
- Torii T, Yoshimura T, Narumi M et al. 2014. Determination of major sialylated N-glycans and identification of branched sialylated N-glycans that dynamically change their content during development in the mouse cerebral cortex. Glycoconj J. 31:671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrin P, Zaujec J, Breuss JM et al. 2010. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 115:3997–4005. [DOI] [PubMed] [Google Scholar]
- Varki A, Gagneux P. 2012. Multifarious roles of sialic acids in immunity. Ann NY Acad Sci. 1253:16–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. 2015. How leukocytes cross the vascular endothelium. Nat Publ Group. 15:692–704. [DOI] [PubMed] [Google Scholar]
- Weiss P, Tietze F, Gabl WA, Seppala R, Ashwell G. 1989. Identification of the metabolic defect in sialuria. J Biol Chem. 264:17635–17636. [PubMed] [Google Scholar]
- Wigle JT, Oliver G. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell. 98:769–778. [DOI] [PubMed] [Google Scholar]
- Wilkins PP, Moore KL, McEver RP, Cummings RD. 1995. Tyrosine sulfation of P-selectin glycoprotein ligand-1 is required for high affinity binding to P-selectin. J Biol Chem. 270:22677–22680. [DOI] [PubMed] [Google Scholar]
- Xie Y, Muller WA. 1993. Molecular cloning and adhesive properties of murine platelet/endothelial cell adhesion molecule 1. Proc Natl Acad Sci. 90:5569–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Newhouse EI, Amaro RE et al. 2009. Distinct glycan topology for avian and human sialopentasaccharide receptor analogues upon binding different hemagglutinins: a molecular dynamics perspective. J Mol Biol. 387:465–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hirata T, Croce K et al. 1999. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 190:1769–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Nussbaum C, Grewal PK, Marth JD, Sperandio M. 2012. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood. 120:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]


