Abstract
Through multiple mechanisms, regulatory B cells (Breg) have been shown to play an important role in the development of allograft tolerance. However, a careful understanding of the role of antigen-specificity in Breg-mediated allograft tolerance has remained elusive. In experimental models of islet and cardiac transplantation, it has been established that Bregs can be induced in vivo by anti-CD45RB ± anti-TIM1antibody treatment, resulting in prolonged, Breg-dependent allograft tolerance. The importance of Breg antigen recognition has been suggested but not confirmed through adoptive transfer experiments, using tolerant WT C57BL/6 animals challenged with either BALB/c or C3H grafts. However, the importance of receptor-specificity has not been formally tested. Here, we utilize the novel ovalbumin-specific B cell receptor transnuclear (OBI) mice in multiple primary tolerance and adoptive transfer experiments to establish that Breg-dependent allograft tolerance relies on antigen recognition by B cells. Additionally, we identify that this Breg-dependent tolerance relies on the function of transforming growth factor-β. Together, these experiments mark important progress toward understanding how best to improve Breg-mediated allograft tolerance.
Keywords: B cell biology, basic (laboratory) research/science, cytokines/cytokine receptors, immunobiology, tissue (nonvascularized) transplantation, tolerance, tolerance: mechanisms
1 |. INTRODUCTION
1.1 |. Regulatory B cell–mediated allograft tolerance
Classically, B cells are thought to have 2 primary functions: antigen presentation and antibody production. In the transplant setting, these features primarily contribute to allograft rejection.1 However, recent work has identified a subset of B cells that have significant suppressive functions, promoting allograft tolerance.2 Similar to experiments in autoimmune models, these regulatory B cells (Bregs) promote allograft tolerance through multiple mediators, including the upregulation of IL-10, transforming growth factor-β (TGF-β) expression, and the induction of regulatory T cells (Tregs).3–5 Interestingly, in a variety of murine models, the mechanism of the Breg-dependent tolerance appears to be graft dependent. For example, IL-10 is required for development of Breg-mediated islet tolerance after anti-CD45RB and anti-TIM1 treatment.6 In contrast, cardiac allograft tolerance is actually improved in the absence of IL-10.3,7,8 Several other factors have also been shown to play an integral role in Breg-mediated allograft tolerance, including CD45RB, major histocompatibility complex (MHC) Class II, CD40, TAP, intracellular adhesion molecule, lymphocyte function-associated antigen, TGF-β, B7.1, and B7.2.5,9
In murine models of Breg-dependent tolerance, B cells have been shown to be required during the induction of the tolerogenic phenotype. These cells do remain present in the tolerant animal for an extended period of time, as suppressive B cells have been isolated >100 days after tolerance induced by anti-CD45RB and anti-TIM1.6 Interestingly, while Bregs have been shown to be long-lived in the primary induction setting, adoptive transfer experiments have failed to show that the Bregs persist for an extended period of time after transfer, which may indicate that the Bregs are most important for the induction of tolerance and not its maintenance. Antibody-mediated depletion experiments using anti-CD25 have shown that, in Breg-dependent allograft tolerance models, depletion of Tregs will also lead to graft loss.10 These results suggest that Bregs function in a complex tolerogenic environment that includes other regulatory components, including Tregs. How the Breg population interacts with the allograft and other immunomodulatory cell populations remains unknown. Central to this question is whether recognition of the graft through the B cell receptor (BCR) and antigen-specificity are required for optimal Breg-mediated tolerance.
1.2 |. Role of antigen-specificity and BCR signaling in Breg function
Breg have been most extensively studied in models of autoimmunity. While B cells and BCR signaling have been demonstrated to be integral to the pathogenesis in some forms of autoimmunity, such as systemic sclerosis, Bregs and efficient BCR signaling have also been shown to be protective.11,12 In the EAE model, CD19-deficient mice develop a severe, nonremitting form of autoimmunity, suggesting that fully functional BCR signaling is required for adequate B cell–mediated control of autoimmunity.13,14 Furthermore, CD22−/− mice lack the CD22-mediated counter-regulation of the BCR, and these animals show an increased number of immature B cells, which may have regulatory functions.11,15 These studies suggest that BCR signaling increases the number of Bregs and the strength of their suppressive action. Some models of allergic airway disease indicate that adoptive transfer of B cells from animals exposed to a specific allergenic antigen produce allergen-specific protection.16
1.3 |. OBI mice express an OVA-specific transnuclear B cell receptor
In order to fully establish the role of BCR recognition in the development of allograft tolerance, we must demonstrate (1) that animals unable to recognize graft antigens because of a restricted BCR repertoire fail to develop graft tolerance under appropriate conditions, and (2) these same mice are capable of developing tolerance to a graft that expresses the cognate antigen. Ploegh et al have developed a novel C57BL/6-background mouse with ovalbumin (OVA)-specific BCR, termed “OBI” mice. These mice have been reported to have ≈99% full allelic exclusion with only a single BCR, specific for OVA.17,18 The OBI mice offer a unique opportunity in transplant tolerance studies given the availability of OVA-transgenic mice, which ubiquitously express OVA in all tissues, as a source of OVA+ organs/tissues for transplant and the availability of OTI and OTII T cell receptor transgenic mice specific for OVA peptides. Using animals with known BCR-specificity and tissues that express the cognate antigen, we have developed a robust system to carefully probe the importance of the BCR for successful induction of Breg-dependent tolerance.
2 |. MATERIALS AND METHODS
2.1 |. Mice
Wild-type (WT) C57BL/6 (B6, H-2b), B cell deficient C57BL/6 (μMT−/−B6, H-2b), hen egg lysozyme (HEL)–specific BCR transgenic C57BL/6 (MD4, H-2b), OVA-transgenic C57BL/6 mice (OVA+, H-2b), MHC class II variant C57BL/6 (BM12, H-2b), OTII (H-2b) and BALB/c (H-2d) mice were purchased from Jackson Laboratory (Bar Harbor, ME). OBI mice (C57BL/6 background) were generously provided by Dr Hidde Ploegh, Whitehead Institute for Biomedical Research.17 All mice were housed under specific pathogen-free conditions in the animal facility of Massachusetts General Hospital. All protocols were approved by the Institutional Animal Care and Use Committee.
2.2 |. Transplantation: skin graft and islet
Skin grafts were transplanted to mice based on the technique of Billingham and Medawar as previously described.19 Once the donor animal is euthanized and hair clipped, the full-thickness skin graft is harvested and subcutaneous fat dissected off of the deep surface. The skin is cut into 1-cm squares using a scalpel and then rinsed and kept cold in phosphate-buffered saline (PBS). The recipient is anesthetized, shaved, and a 1-cm square of skin is removed, leaving the underlying vascular bed intact. The donor skin is attached with collodion. A dressing is applied and secured with skin staples. The dressing is removed on postprocedure day 7. The skin grafts are monitored every day with blinded assessment of graft viability by an uninvolved researcher. Graft is designated as lost if >90% of the graft is nonviable or if the skin contraction has shrunk the graft to <10% of original size.
For islet transplantation: diabetes in C57BL/6 WT or MD4 BCR transgenic mice was induced by a single intraperitoneal injection of 200 mg/kg streptozocin (Sigma-Aldrich, St. Louis, MO), 4 days prior to islet transplant. Diabetes was defined as blood glucose levels >300 mg/dL for 3 consecutive days. Islets from BALB/c donors were isolated by the standard technique of collagenase digestion and Ficoll density gradient purification. Approximately 500 fresh islets were transplanted under the kidney capsule of diabetic recipient mice. Euglycemia was defined as a nonfasting blood glucose level <200 mg/dL. Rejection was diagnosed when animals became hyperglycemic posttransplant, with blood glucose >200 mg/dL for at least 2 consecutive readings. Allograft function was confirmed by nephrectomy of the kidney containing the transplanted islets on day 100.
2.3 |. Immunotherapy and adoptive transfer
Recipient mice received 100 μg anti-mouse CD45RB (clone: MB23G2, BioXCell, West Lebanon, NH) ip on days 0, 1, 3, 5, and 7 following transplantation. Islet recipient mice also received 500 μg anti-mouse TIM1 (clone RMT1–10, BioXCell, West Lebanon, NH) ip on day 0, and 300 μg on days 1 and 5 following transplantation. For the anti-TGF-β-treatment experiment, recipient mice were injected ip with 200 μg anti-TGF-β or isotype control (1D11.16.8 and MOPC-21, respectively, BioXCell, West Lebanon, NH) on days 0, 2, 4, 6, and 8 posttransplant.
For adoptive transfer experiments: after 21 days of skin graft survival, the tolerant animal is sacrificed, and spleen is isolated in sterile fashion. Spleens are disaggregated using a 70-μm nylon mesh and washed with sterile PBS. B cells are enriched by anti-CD19 magnetic bead isolation, following manufacturer protocol (Miltenyi, Auburn, CA). Purity of B cells is routinely >95%. Yield from an individual animal is typically 15–25 × 106 B cells. If a greater number of cells is needed, B cells isolated from multiple tolerant mice are pooled. 5 × 106 B cells from tolerant WT C57BL/6 or OBI mice are injected into B6.129S2-Ighmtm1Cgn/J (μMT) mice via tail or penile vein.
2.4 |. Cell preparation for in vitro studies
Lymphocytes were prepared from spleens, lymph nodes, and the peritoneal cavity of naïve and tolerant C57BL/6 or OBI mice. For cells isolated from spleens and lymph nodes, disaggregated cells were passed through a 70-μm nylon mesh. For spleens, erythrocytes were lysed with ammonium chloride buffer. For peritoneal cells, the peritoneal space was infused with 5 mL of cold PBS + 3% fetal calf serum, 3 times, and cells were pooled together from each infusion. All collected cells were washed with cold PBS and manually counted using a standard hemocytometer.
2.5 |. Cell staining, phenotyping, and flow cytometry
One million cells were suspended in PBS containing 0.1% sodium azide and 2% FBS in 96-well plates. All samples were treated with 1 μL of Fc-block (CD16/CD32 Monoclonal Antibody 93; Thermo Fisher [eBioscience], Waltham, MA). Samples were stained with the following fluorochrome-tagged antibodies: CD3-Pacific Blue (145–2C11; Biolegend), CD4-PECy7 (GK1.5; Biolegend), CD8-PE (52–6.7; Biolegend), FoxP3-Alex488 (MF-14; Biolegend), CD19-Pacific Blue (6D5; Biolegend), CD19-APC (6D5; Biolegend), CD21-fluorescein isothiocyanate (FITC) (eBio4E3; eBioscience), CD23-PECy7 (B3B4; Biolegend), IgM-PE (R6–60.2; BD Biosciences), IgG-FITC (11-4011-85; eBioscience), CD40-PE (MR1; Biolegend), CD5-PECy7 (53–7.3; Biolegend), TIM1-PE (RMT1–4; Biolegend), IL-10-APC (145–2C11; Biolegend), and/or mLAP-Brilliant Violet 421 (TW7–16B4; Biolegend). Staining for OVA-specific BCR with OVA-Alex488 (Invitrogen). Samples were run under fully stained and fluorescence minus one conditions. For B cell phenotyping, single cell suspensions of spleen, lymph node, or peritoneal cavity samples were gated on a single cell/lymphocyte gate and CD19+ gate, and then analyzed for expression of phenotypic markers. All samples were run on a FACSVerse cytometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed using FlowJo analysis software (Becton Dickinson).
For evaluation of cytokine production, single cell suspensions of purified B cells were stimulated in RPMI-based growth media with phorbol myristate acetate (PMA) (50 ng/mL, Sigma), ionomycin (1 g/mL, Sigma), lipopolysaccharide (LPS) (10 g/mL, Escherichia coli sero-type 0111:B4, Sigma), and monensin (4 g/mL, Becton Dickinson) in 6-well plates for 5 hours in a 37°C/5% CO2 incubator.5
2.6 |. In vitro suppression assay
Responder CD4+ T cells were purified from the spleen of OTII mice and labeled with Cell Trace Violet (CTV, Thermo Fisher). CTV-labeled T cell responders (150k) were cultured alone or stimulated with 150k purified, irradiated OVA splenocytes. Stimulated, CTV-labeled T cell responders were co-cultured with 300k purified splenic B cells from either naïve OBI or long-term graft survivor OBI mice. On day 4, cells were analyzed by flow cytometry for proliferation.
2.7 |. Statistical analysis
For survival studies, data were analyzed using GraphPad Prism (version 8, GraphPad Software, San Diego, CA). Graft survival between experimental groups was compared using Kaplan-Meier survival curves and significance was assessed by log-rank test. Other differences between experimental groups were analyzed using the Student t test. P values of <.05 are considered statistically significant.
3 |. RESULTS
3.1 |. A limited BCR repertoire impedes the ability of mice to develop long-standing B cell–dependent islet tolerance
If B cell recognition of a transplanted allograft is required for the optimal development of B cell–dependent tolerance, mice with a limited BCR repertoire should fail to develop graft survival when challenged with an allograft under otherwise tolerogenic conditions. MD4 mice contain a transgenic BCR on 95% of B cells, which is specific for hen egg lysozyme (HEL).20 MD4 and WT C57BL/6 mice were rendered diabetic, treated with the Breg-generating regimen of anti-CD45RB and anti-TIM1, and transplanted with BALB/c islets (Figure S1). The MD4 mice failed to manifest any long-term graft survival, with all animals rejecting by day 35. In contrast, the same tolerogenic treatment in WT mice results in 83% of transplanted islet grafts surviving to at least 100 days (Figure 1). These results suggest that lacking B cells capable of recognizing an allograft prevents the proper development of B cell–mediated tolerance.
FIGURE 1.
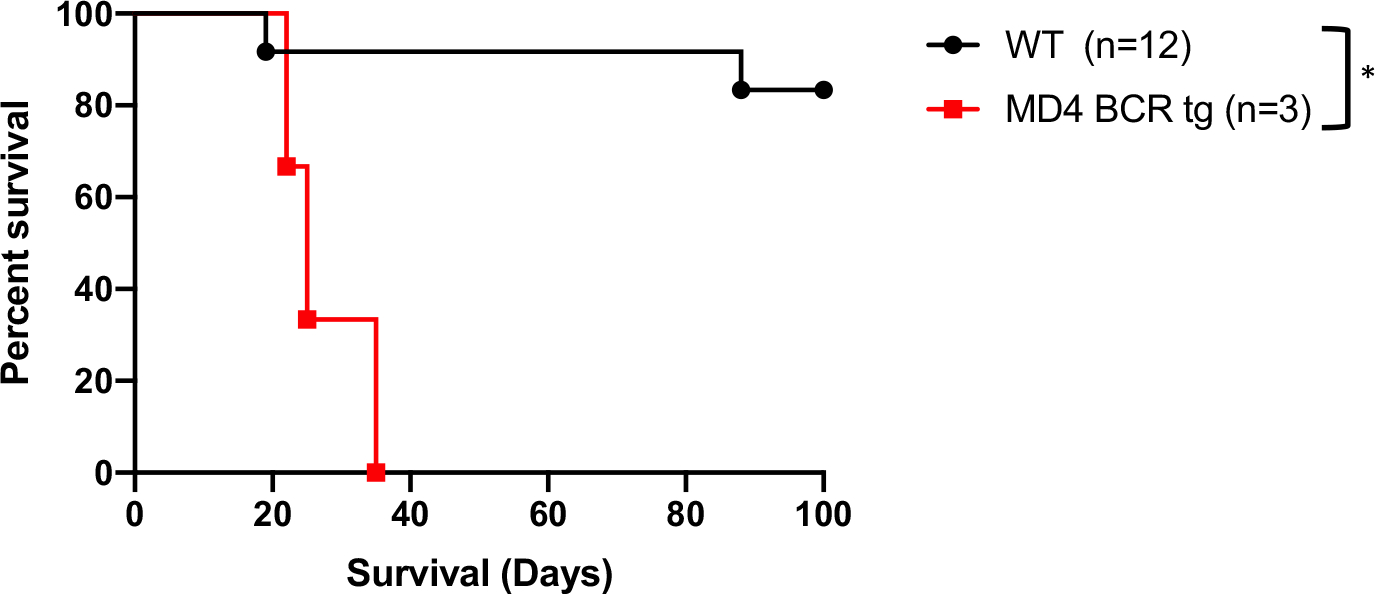
C57BL/6 transgenic mice expressing the hen egg lysozyme–specific, MD4 BCR fail to develop tolerance to BALB/c islets when treated with anti-CD45RB and anti-TIM1, compared to WT C57BL/6, which reliably develop tolerance post antibody treatment. *P < .05. BCR, B cell receptor; WT, wild type
3.2 |. OBI naïve mouse B cell and T cell development is similar to WT C57BL/6
Prior to using the OBI mice to study B cell–dependent tolerance models, we confirmed the presence of the primary lymphocyte components in these animals. First, we compared both B and T cell lineages. Splenocytes isolated from OBI mice had a slightly higher number of CD3+ T cells (32% vs 37%) and slightly lower number of CD19+ B cells (58% vs 53%) compared to WT splenocytes without statistical significance (Figure 2A–C). Among CD3+ T cells, there were no statistically significant differences in the CD4/CD8 ratio nor the proportion of FoxP3+ cells between these 2 groups (Figure 2C). These results indicate that both primary T cell and B cell development of OBI mice are comparable to that of WT C57BL/6 mice.
FIGURE 2.
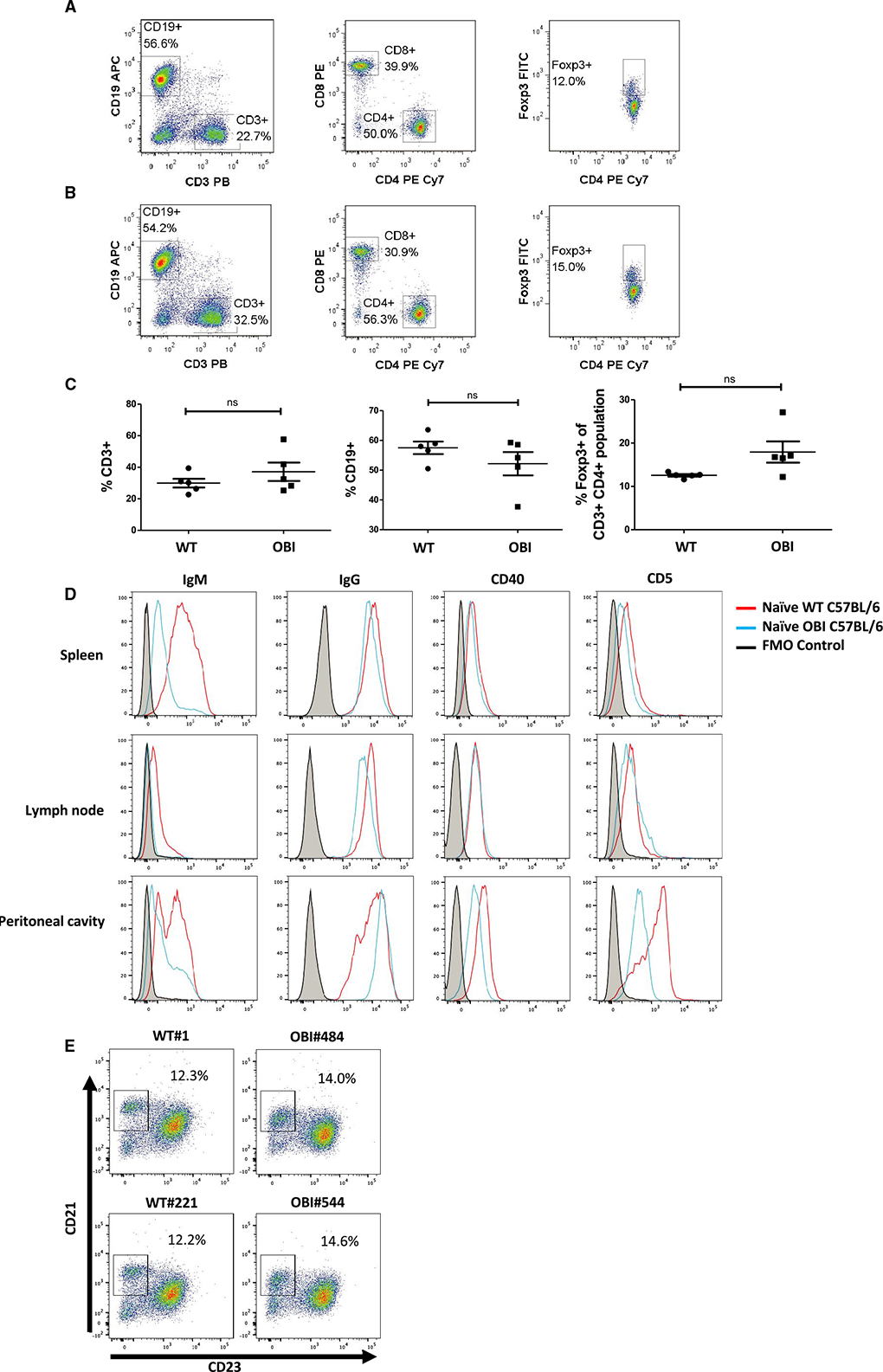
B cell, CD4+ T cell, CD8+ T cell, and Treg compartments show similar proportions in (A) naïve WT C57BL/6 and (B) naïve OBI splenocytes. C, Analysis of spleen samples from multiple OBI and WT C57BL/6 mice. D, Naïve WT and OBI B cells express similar levels of IgG, CD40, and CD5 in spleen, LN, and PC. OBI B cells express a lower level of IgM compared to WT B cells in all 3 compartments. Spleen data represent 5 individual experiments. LN and PC data represent 3 individual experiments. E, CD19+ B cells isolated from the spleen from OBI and WT mice show present populations of marginal zone and follicular B cells. Data from 2 independent experiments are shown (left column: WT, right column: OBI). FITC, fluorescein isothiocyanate; LN, lymph node; OBI, ovalbumin (OVA)-specific BCR mice; PC, peritoneal cavity; Treg, regulatory T cell; WT, wild type
For CD19+ B cells, samples were collected from spleens, lymph nodes (LN), and peritoneal cavity washings in naïve WT and OBI mice. OBI B cells from all 3 compartments show a high degree of binding to OVA peptide, whereas WT B cells do not (Figure S2). OBI mice manifest significantly lower levels of IgM expression, which is expected given these mice have enforced expression of an already class-switched BCR (Figure 2D). Peritoneal B cells from OBI mice do show a higher level of IgM expression compared to spleen or LN, which is consistent with previous characterization of OBI mice.17 Levels of IgG and CD40 are similar among the B cells from naïve WT and OBI mice in all compartments (Figure 2D). CD5 expression is slightly lower in OBI splenic B cells and significantly lower in peritoneal B cells compared to the WT mice, which is again consistent with their initial characterization.17 CD21 and CD23 staining show similar levels of splenic marginal zone B cells (Figure 2E).
3.3 |. Antigen specificity in B cell–dependent tolerance induced by anti-CD45RB treatment
Our previous work has demonstrated that treatment with anti-CD45RB at the time of cardiac allograft transplant results in long-term B cell–dependent graft survival in ≈75% of animals receiving full MHC mismatch grafts (C3H into C57BL/6).6
For the purposes of understanding the importance of BCR recognition of the allograft tissue, we utilized a similar tolerance induction regimen by treating OBI and WT C57BL/6 mice with anti-CD45RB at the time of skin graft placement from donor OVA+ mice that ubiquitously express OVA in all tissues (Figure 3A). OVA+ skin grafts were transplanted to WT or OBI recipients with or without anti-CD45RB treatment. OVA+ skin grafts all rejected by untreated WT and OBI recipients in 19.6 ± 1.5 and 24.4 ± 2.3 days. When OVA+ skin grafts were transplanted to untreated μMT recipients, which lack B cells, grafts were rejected in an accelerated manner compared to WT or OBI recipients (16.3 ± 1.3 days; P < .05; Figure 3C). Short-term anti-CD45RB antibody treatment induced tolerance to OVA+ skin grafts in both WT and OBI recipients, with only 1 graft in the OBI cohort not surviving to at least 100 days and no graft loss in the WT cohort. However, this same treatment failed to produce long-term graft tolerance in μMT recipients (Figure 3C). These results indicate that anti-CD45RB antibody-induced skin graft tolerance is B cell dependent, similar to the previous work in islet and cardiac allograft tolerance. Furthermore, OBI mice are capable of developing graft tolerance through this protocol.
FIGURE 3.
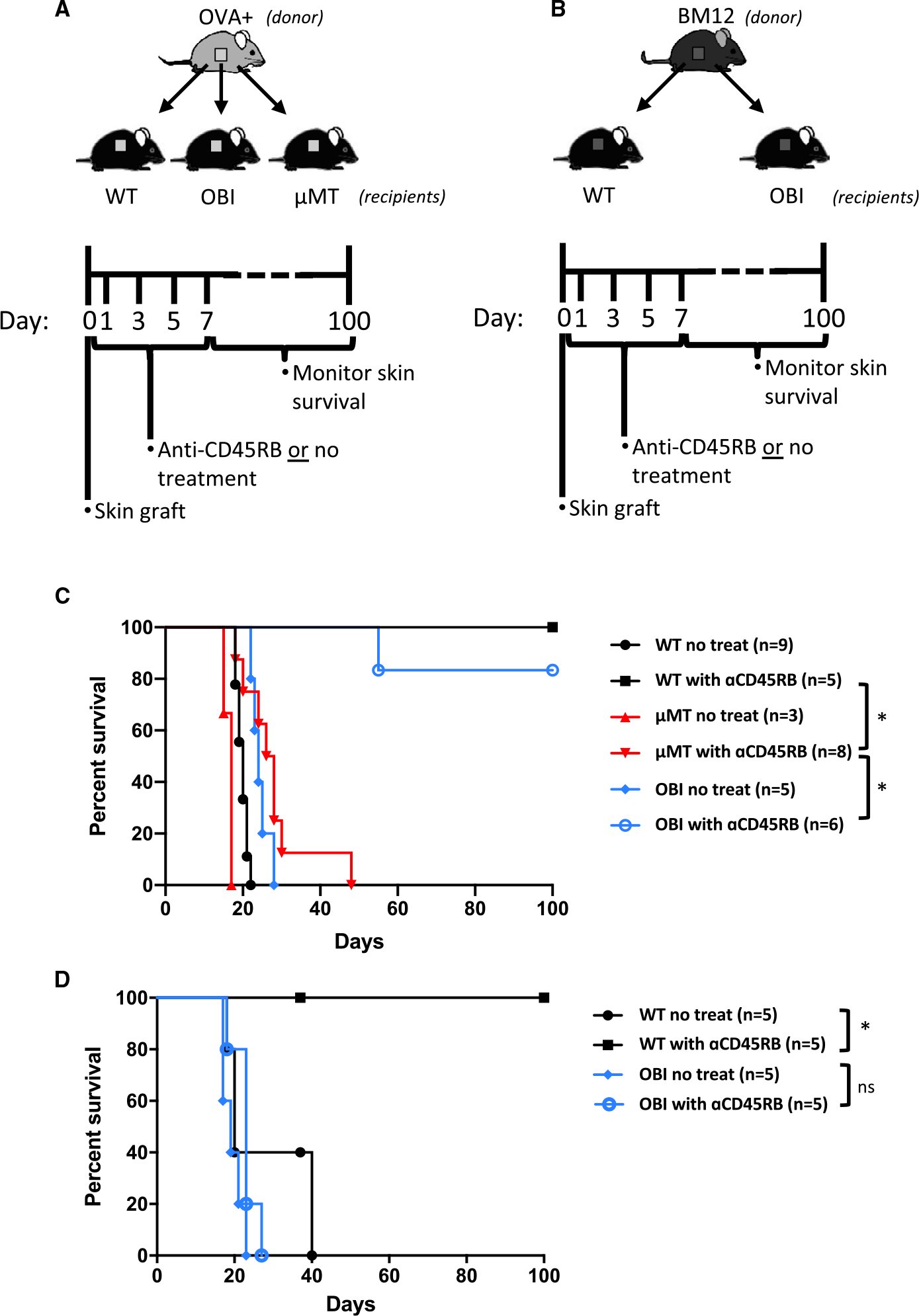
Tolerance induction to OVA+ and third-party (BM12) skin grafts. Diagrams and timelines for in vivo (A) OVA+ and (B) BM12 graft experiments. C, WT C57BL/6 and OBI mice treated with anti-CD45RB, on days 0, 1, 3, 5, and 7, develop long-standing tolerance to OVA+ skin grafts. μMT mice, which lack B cells, fail to develop tolerance to the skin grafts. D, WT C57BL/6 mice develop tolerance to BM12 grafts after anti-CD45RB treatment, while OBI mice fail under the same conditions. WT C57BL/6, but not OBI mice, develop tolerance to third-party BM12 skin grafts following anti-CD45RB treatment. ns, nonsignificant. *P < .05. OBI, ovalbumin (OVA)-specific BCR mice; OVA, ovalbumin; WT, wild type; μMT, B6.129S2-Ighmtm1Cgn/J
To investigate further the antigen specificity of B cell–dependent tolerance in this model, third-party (BM12) skin grafts were transplanted to WT C57BL/6 or OBI recipients with or without anti-CD45RB treatment (Figure 3B). Without treatment, all BM12 skin grafts were rejected by WT mice with slightly longer and more variable survival length (27 ± 10.5 days) than the OVA+ skin grafts (19.6 ± 1.5 days). Treatment of WT mice with anti-CD45RB resulted in all BM12 skin grafts surviving long-term (Figure 3D). In contrast, BM12 skin grafts transplanted to treated and untreated OBI mice exhibited similar lengths of survival (19.4 ± 2.6 days; 22.8 ± 3.2 days, P = .07), and none of the OBI mice achieved long-term BM12 graft tolerance (Figure 3B). Paralleling the results with HEL recipients above, these data indicate that lack of a BCR capable of recognizing the BM12 skin grafts impedes the ability of these animals to develop B cell–mediated tolerance (Figure 1).
3.4 |. Adoptive transfer of tolerant B cells into B cell KO mice induced tolerance
We hypothesized that if donor antigen recognition was requisite for Breg function, a population with a greater frequency of donor reactive cells would be more potent at inducing tolerance. To test this thesis, naïve μMT mice were adoptively transferred with B cells isolated from tolerant WT or OBI mice previously rendered tolerant to OVA+ skin grafts through anti-CD45RB treatment (Figure 4A). This adoptive transfer of either tolerant WT or OBI B cells confers tolerance to μMT recipients of OVA+ skin grafts (Figure 4B). All 5 recipients adoptively transferred OB1 B cells demonstrated long-term graft survival, whereas only 4 of 6 treated with tolerant WT B cells did so. These same B cells from mice tolerant to OVA+ grafts fail to provide tolerance to BM12 skin grafts (Figure 4B). Additionally, the ability of B cells from tolerant mice to suppress an immune response is demonstrated by an in vitro mixed lymphocyte reaction showing that B cells isolated from tolerant OBI mice are capable of partially inhibiting growth of OTII (OVA-specific) T cells when stimulated with OVA+ splenocytes in vitro, while B cells from naïve OBI mice failed to do so (Figure 4C,D).
FIGURE 4.
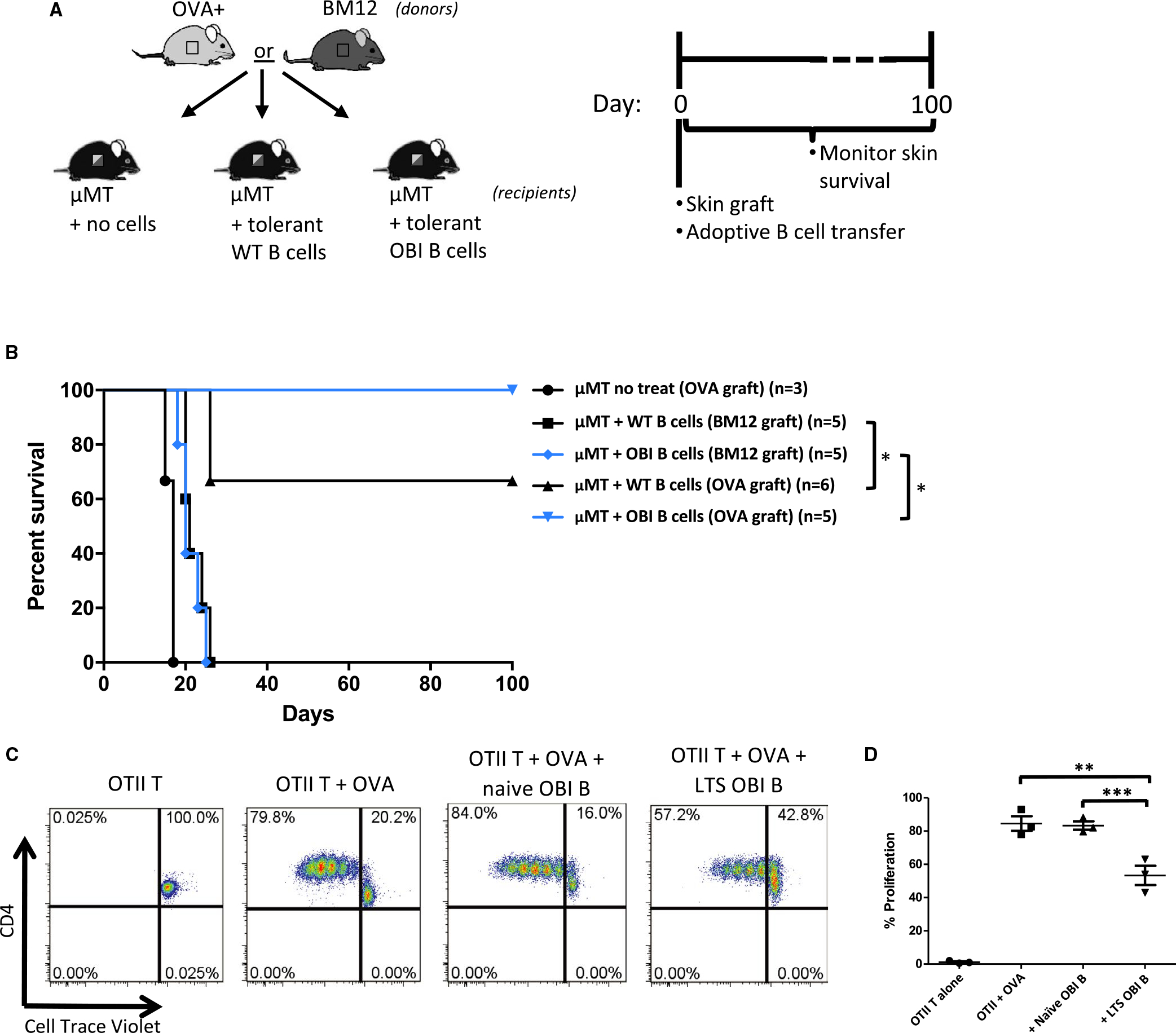
A, Diagram and timeline for B cell adoptive transfer experiments. Skin from OVA+ or BM12 mice is grafted at the same time as 5 × 106 B cells from tolerant mice (mice previously made tolerant to OVA+ skin grafts). Monitored for 100 d. B, OVA+ or BM12 skin graft survival after adoptive transfer of tolerant B cells. B cells isolated from WT C57BL/6 and OBI mice previously grafted with OVA+ skin, confer tolerance to OVA+ skin grafts when adoptively transferred into μMT mice (denoted with “OVA graft”). μMT mice that receive skin grafts from BM12 donors (denoted with “BM12 graft”) fail to develop tolerance after adoptive transfer of tolerant WT or OBI B cells. OVA+ skin grafts on untreated μMT mice fail to survive beyond 20 days. C, B cells from OBI mice with long-term surviving grafts (LTS) are capable of suppressing proliferation of CD4+ OTII T cells when stimulated by OVA-expressing splenocytes in vitro. OTII T cells are labeled with Cell Trace Violet and co-cultured with irradiated OVA+ splenocytes with or without an equal number of B cells from either naive OBI mice or from LTS OBI mice. D, Summary of 3 experiments. *P < .05; **P = .0128, ***P = .009. OBI, ovalbumin (OVA)-specific BCR mice; OVA, ovalbumin; WT, wild type; μMT, B6.129S2-Ighmtm1Cgn/J
3.5 |. Tolerant OBI mice show a greater proportion of B cells expressing TGF-β than naïve OBI mice
Flow cytometry–based phenotyping of naïve and tolerant OBI B cells demonstrates similar levels of known pro-tolerogenic surface factors, TIM1 and CD5. CD40, which has also been associated with suppressive B cell functioning,21 is modestly increased (Figure 5A). The 2 cytokines most closely linked to B cell–mediated allograft protection are IL-10 and TGF-β.3,5,10 In this model of graft tolerance, there is an increase in the proportion of B cells that express LAP (a marker of TGF-β production) upon stimulation (Figure 5B). In contrast, no difference is seen in the level of IL-10 between tolerant and naïve OBI B cells (Figure 5C).
FIGURE 5.
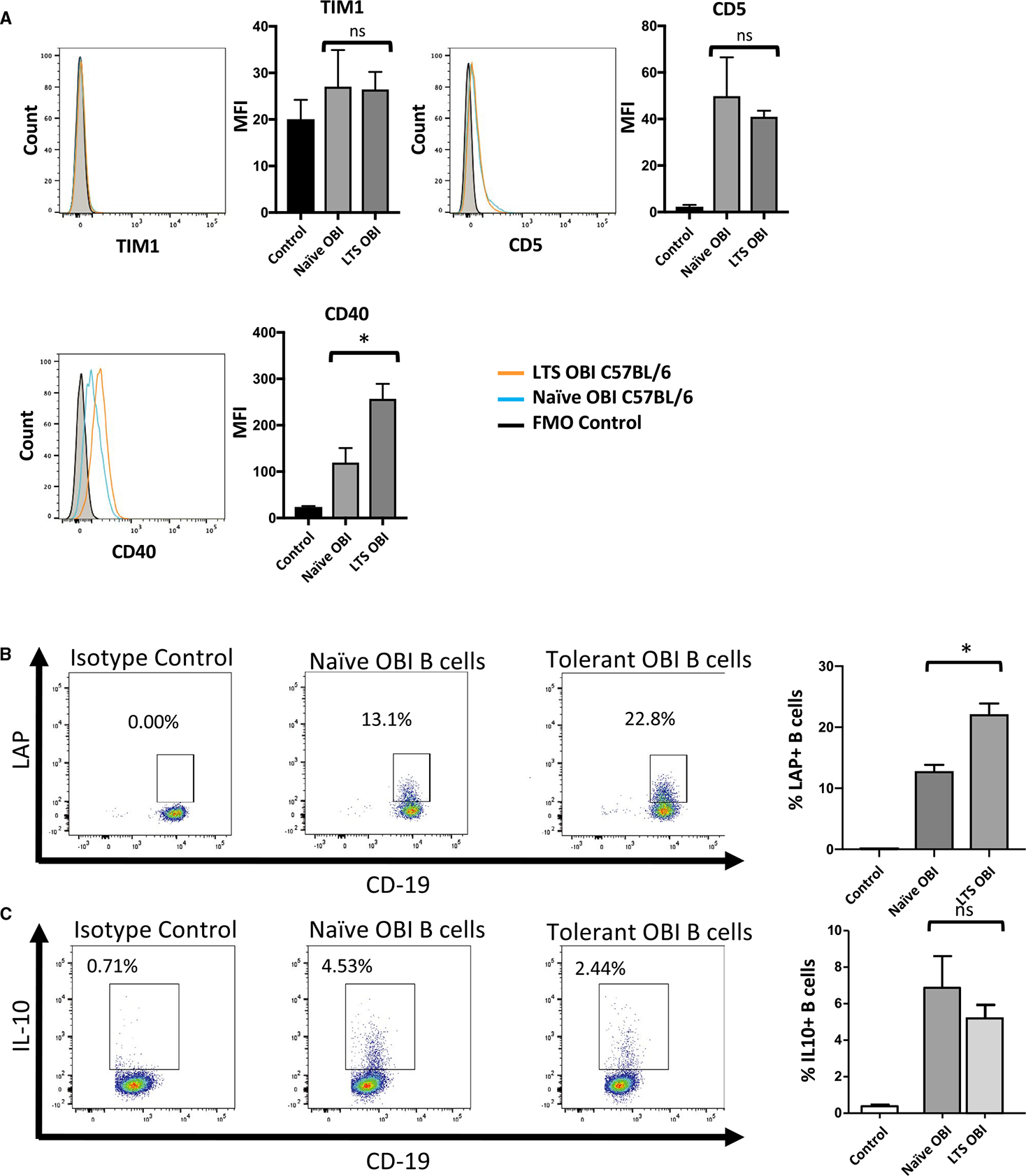
Phenotype of B cells from naive and long-term graft survivor (LTS) OBI mice. A, Surface staining of B cells from naive and LTS OBI mice show similar levels of TIM1 and CD5 and higher levels of CD40. B, Tolerant OBI B cells show an increased proportion of LAP+ (a marker for TGF-β production) B cells upon stimulation with LPS, PMA, and ionomycin. C, Tolerant OBI B cells, however, do not show upregulation of IL-10 under the same stimulation protocol. Data are representative of 3 experiments. ns, nonsignificant. *P < .05. LPS, lipopolysaccharide; MFI, median flourescence intensity; OBI, ovalbumin (OVA)-specific BCR mice; PMA, phorbol myristate acetate; TGF, transforming growth factor
3.6 |. Pharmacological blockade of TGF-β prevents tolerance induction of OVA skin grafts in OBI mice
To test whether TGF-β is required for B cell–dependent skin graft tolerance after treatment with anti-CD45RB, OBI mice grafted with OVA+ skin grafts were treated with anti-CD45RB to induce tolerance and concurrently administered either a blocking anti-TGF-β or control antibody, using a regimen previously found to abrogate Breg-dependent tolerance (Figure 6A).3 Mice receiving the anti-TGF-β antibody universally failed to develop long-term graft survival, with all graft loss by 30 days, while mice receiving anti-CD45RB alone or with the control antibody successfully accepted the OVA+ skin graft (Figure 6B,C).
FIGURE 6.
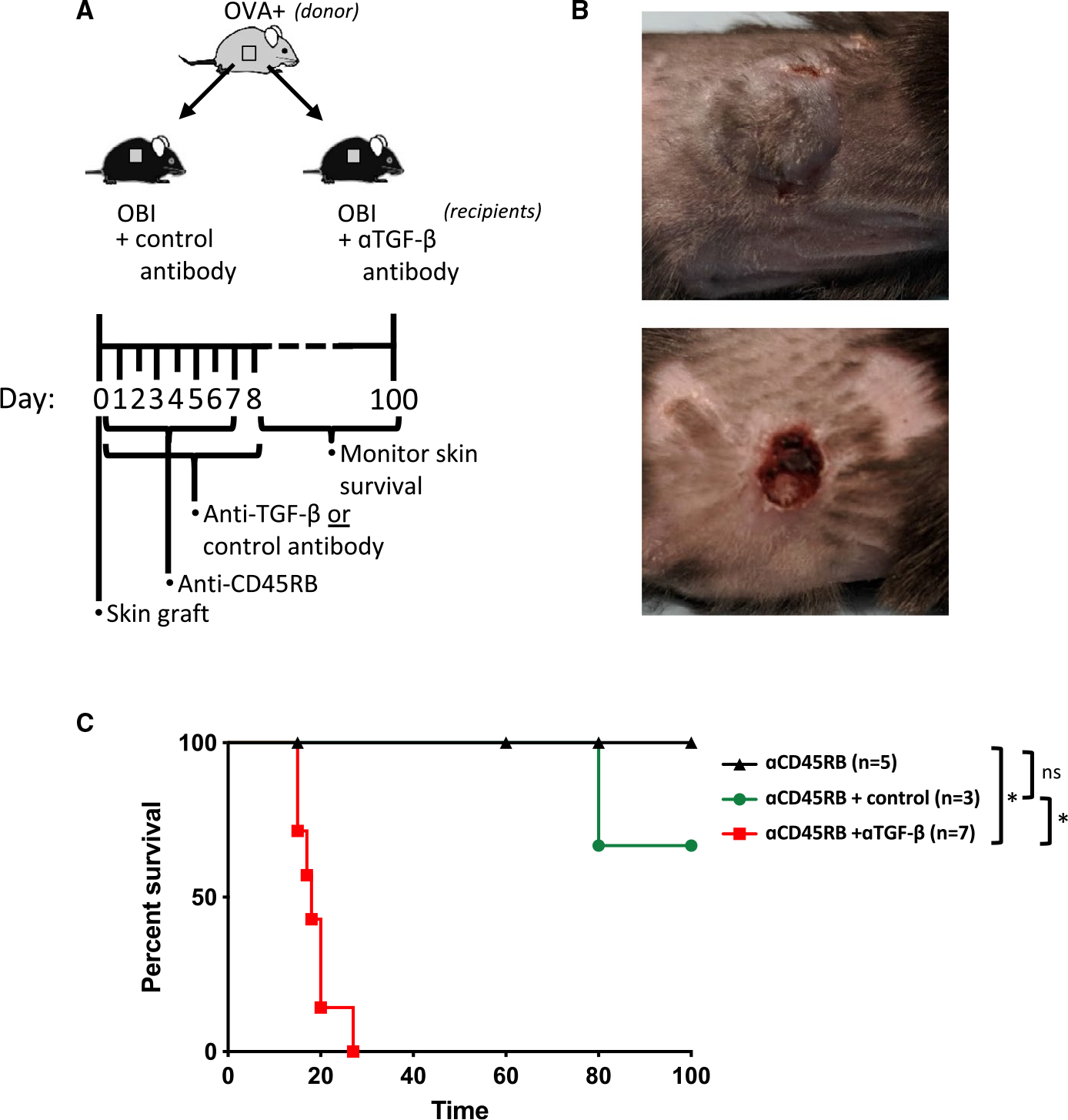
A, Diagram for in vivo skin graft experiments using blocking anti-TGF-β antibody. B, Representative skin graft sites at day 20 for anti-CD45RB treatment alone (upper panel) and anti-CD45RB+ anti-TGF-β (bottom panel). C, Control antibody (mIgG1, clone MOPC-21)–treated mice demonstrate long-term graft survival. Concurrent administration of neutralizing anti-TGFβ antibody with anti-CD45RB prevents the development of tolerance to OVA+ skin grafts in OBI mice. ns, nonsignificant. *P < .05. OBI, ovalbumin (OVA)-specific BCR mice; OVA, ovalbumin; TGF, transforming growth factor
4 |. DISCUSSION
Since Wolf et al first discovered an immune-suppressive effect of B cells in 1996 by the observation that B cell–deficient mice were unable to recover from experimental autoimmune encephalitis,22 multiple demonstrations of suppressive B cells have been reported, with various phenotypes and suppressive mechanisms implicated in their action.23 Furthermore, Breg-mediated tolerance can require the function of other cell populations, such as regulatory T cells, as demonstrated by previous work showing that anti-CD25 antibody treatment can mitigate Breg-mediated islet allograft survival.10 Thus, Bregs can play an important role in the development of a tolerogenic environment, requiring the interaction of several immune cell types and interactions with the graft, itself.
Central to understanding this tolerogenic state is the question of whether Bregs require, or at least benefit from, antigen recognition. While antigen-recognition has been established in regulatory T cells, the literature is more limited on the topic of Breg specificity. Several studies have identified specific subpopulations of B cells as immunoregulators in contact hypersensitivity or autoimmunity models, which may require appropriate antigen recognition.9,13,24–26 The work reported herein, however, provides the most complete demonstration of recognition being required for allograft tolerance. Because of the marked clonality of the BCR in MD4 and OBI mice,17,18 we are able to definitively show that a severely limited BCR repertoire is capable of producing tolerance when the appropriate antigen is present (OVA+ skin grafts) and that allograft tolerance is not achieved when the allograft lacks a recognizable antigen (BM12 skin grafts). Collectively, these findings show that the BCR is vital for proper Breg functioning in transplantation models.
Based on this insight that antigen recognition is essential, one can speculate that Bregs may function through initial recognition of the allograft and local production of tolerogenic cytokines, through specific antibody production, or through presentation of allograft-specific antigens that have been internalized through cognate BCR-mediated capture. All of these are exciting avenues for future research. Work using B cells that lack costimulatory molecules (such as CD80/86) or MHC-II will help elucidate whether antigen presentation is involved and knowing specific target antigens may be beneficial in tracking Breg localization. Insight into the role of Breg antigen specificity also has the potential to guide therapy development as newer technologies such as Chimeric Antigen Receptor (CAR)–based lymphocyte development may be able to produce cellular therapies based on targeted Bregs, similar to ongoing work with CAR-Tregs.27
Additionally, these results establish the OBI mouse as a valuable tool for studying the functions of Bregs in allograft tolerance. There are some slight differences between WT and OBI B cell compartments, notably fewer IgM+ B cells, lower CD5, and small differences in marginal zone (CD21+, CD23−low; Figure 2E). Importantly, OBI mice behave similarly to WT mice in the tolerance of OVA+ skin grafts. Furthermore, the adoptive transfer experiments, which use only splenic B cells, eliminate concern over differences in other B cell compartments such as the peritoneal cavity. With the availability of OVA-expressing mice, as well as mice expressing OVA-specific CD8+ or CD4+ T cells (OTI and OTII mice, respectively), there is an entire framework for future experiments using this OVA-antigen-based system to uncover antigen-specific interactions between B and T cells in the development of tolerance. Through these anticipated studies, we may learn how better to combine tolerogenic strategies to develop more robust and safer means of clinically applicable tolerance.
This model of Breg-dependent skin graft tolerance also provides another demonstration of TGF-β-dependent allograft survival, which is similar to the findings from other groups working with models of autoimmunity.28–31 TGF-β is known to be vital for Treg induction and function, and we have previously shown that TGF-β-producing B cells can be important for Treg induction in islet graft tolerance.5 The current experiments do not definitively show that the anti-TGF-β-blocking antibody is impacting graft survival through inhibition of B cell–produced TGF-β per se. Future work will require OBI mice crossed with mouse strains deficient in specific cytokine or cell surface mediators to provide significant insight into the underlying mechanism of antigen-specific tolerance. The in vitro suppression assay shows that the OBI Bregs are capable of direct T cell suppression, but the in vivo significance of this finding remains to be fully explored. Interestingly, CD40 is reproducibly higher in tolerant OBI mice, suggesting that CD40-high B cells are preferentially selected in the setting of tolerogenesis or that CD40 is upregulated. As work in autoimmune disease has demonstrated the function of CD40 in suppression,9 future work will explore the importance of this costimulatory molecule in allograft tolerance by crossing OBI with CD40−/− mice. We do not see some of the classic markers of suppressive B cells, such as TIM1, CD5, and IL-10 expression in this model, highlighting the varied Breg mechanisms of immune suppression.3,10 This raises the prospect that multiple Breg subtypes could be produced to synergistically provide allograft protection.
Above all else, this first clear demonstration of antigen specificity in the development of Breg-mediated tolerance will help steer future work that will provide a better understanding of the various immunomodulatory cells, how they interact, and how we can best harness their function to produce transplant-preserving and lifesaving tolerance.
Supplementary Material
Funding information
National Institute of Allergy and Infectious Diseases, Grant/Award Number: 5R01AI057851-13
Abbreviations:
- μMT
B6.129S2-Ighmtm1Cgn/
- B6
C57BL/6
- BCR
B cell receptor
- Breg
regulatory B cell
- HEL
hen egg lysozyme
- LN
lymph node
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- OVA
ovalbumin
- Treg
regulatory T cell
- WT
wild type
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Chong AS, Rothstein DM, Safa K, Riella LV. Outstanding questions in transplantation: B cells, alloantibodies, and humoral rejection. Am J Transplant. 2019;19(8):2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng S, Moore DJ, Huang X, et al. Antibody-induced transplantation tolerance that is dependent on thymus-derived regulatory T cells. J Immunol. 2006;176:2799–2807. [DOI] [PubMed] [Google Scholar]
- 3.Zhao G, Moore DJ, Lee KM, et al. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am J Transplant. 2010;10:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JI, OʼConnor MR, Duff PE, et al. Generation of adaptive regulatory T cells by alloantigen is required for some but not all transplant tolerance protocols. Transplantation. 2011;91:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KM, Stott RT, Zhao G, et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol. 2014;44:1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng S, Moore DJ, Huang X, et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178:6028–6032. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Moore DJ, Mohiuddin M, et al. Inhibition of ICAM-1/LFA-1 interactions prevents B-cell-dependent anti-CD45RB-induced transplantation tolerance. Transplantation. 2008;85:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichardt P, Dornbach B, Rong S, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. [DOI] [PubMed] [Google Scholar]
- 9.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. [DOI] [PubMed] [Google Scholar]
- 10.Lee KM, Kim JI, Stott R, et al. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12:2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedder TF, Poe JC, Haas KM. CD22: a multifunctional receptor that regulates B lymphocyte survival and signal transduction. Adv Immunol. 2005;88:1–50. [DOI] [PubMed] [Google Scholar]
- 12.Saito E, Fujimoto M, Hasegawa M, et al. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J Clin Invest. 2002;109:1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanaba K, Bouaziz J-D, Matsushita T, Magro CM, St.Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita T, Fujimoto M, Hasegawa M, et al. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168: 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng B, Ming Y, Yang C. Regulatory B cells: the cutting edge of immune tolerance in kidney transplantation. Cell Death Dis. 2018;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh A, Carson WF, Secor ER, et al. Regulatory role of B cells in a murine model of allergic airway disease. J Immunol. 2008;180:7318–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougan SK, Ogata S, Hu C-CA, et al. IgG1+ ovalbumin-specific B-cell transnuclear mice show class switch recombination in rare allelically included B cells. Proc Natl Acad Sci USA. 2012;109:13739–13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avalos AM, Bilate AM, Witte MD, et al. Monovalent engagement of the BCR activates ovalbumin-specific transnuclear B cells. J Exp Med. 2014;211:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. [DOI] [PubMed] [Google Scholar]
- 20.Mason DY, Jones M, Goodnow CC. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Int Immunol. 1992;4:163–175. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol. 2000;12:597–605. [DOI] [PubMed] [Google Scholar]
- 22.Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. [DOI] [PubMed] [Google Scholar]
- 24.Aviszus K, MacLeod MKL, Kirchenbaum GA, et al. Antigen-specific suppression of humoral immunity by anergic Ars/A1 B cells. J Immunol. 2012;189:4275–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald KG, Hoeppli RE, Huang Q, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Yu H-J, Wang N, et al. Clara cell 10-kDa protein inhibits TH17 responses through modulating dendritic cells in the setting of allergic rhinitis. J Allergy Clin Immunol. 2013;131(2):387–394.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan P, Singh A, McNamara JT, et al. Regulatory B cells from hilar lymph nodes of tolerant mice in a murine model of allergic airway disease are CD5+, express TGF-beta, and co-localize with CD4+Foxp3+ T cells. Mucosal Immunol. 2012;5:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


